Abstract
High-altitude retinopathy (HAR) is an ocular manifestation of acute oxygen deficiency at high altitudes. Although the pathophysiology of HAR has been revealed by many studies in recent years, the molecular mechanism is not yet clear. Our study aimed to systematically identify the genes and microRNA (miRNA) and explore the potential biomarkers associated with HAR by integrated bioinformatics analysis. The mRNA and miRNA expression profiles were obtained from the Gene Expression Omnibus database. We performed Gene Ontology functional annotations and Kyoto Encyclopedia of Genes and Genomes pathway analysis. Potential target gene analysis and miRNA–mRNA network analysis were also conducted. Quantitative RT-PCR (qRT-PCR) was used to validate the results of the bioinformatics analysis. Through a series of bioinformatics analyses and experiments, we selected 16 differentially expressed miRNAs (DE-miRNAs) and 157 differentially expressed genes related to acute mountain sickness (AMS) and constructed a miRNA–mRNA network containing 240 relationship pairs. The hub genes were filtered from the protein-protein interaction network: IL7R, FOS, IL10, FCGR2A, DDX3X, CDK1, BCL11B and HNRNPH1, which were all down-regulated in the AMS group. Then, nine up-regulated DE-miRNAs and eight hub genes were verified by qRT-PCR in our hypoxia-induced HAR cell model. The expression of miR-3177-3p, miR-369-3p, miR-603, miR-495, miR-4791, miR-424-5p, FOS, IL10 and IL7R was consistent with our bioinformatics results. In conclusion, FOS, IL10, IL-7R and 7 DE-miRNAs may participate in the development of HAR. Our findings will contribute to the identification of biomarkers and promote the effective prevention and treatment of HAR in the future.
Keywords: Bioinformatics analysis, Differentially expressed genes, High-altitude retinopathy, MiRNA-mRNA network
Introduction
High-altitude retinopathy (HAR) is one clinical entity of acute high-altitude illness (AAI), which also includes acute mountain sickness (AMS), high-altitude pulmonary edema (HAPE) and high-altitude cerebral edema (HACE) [1,2]. As retinopathy is caused by the inability to adapt to acute oxygen deficiency at high altitudes, HAR mainly manifests as leakage of the peripheral retinal vessels, retinal hemorrhages, tortuous retinal vessels, optic disc edema and macular edema [3,4]. With the development of the economy and tourism in the plateau area, the incidence of HAR is up to 79% and is increasing [5]. However, the pathogenesis of HAR remains unclear, and there is a lack of effective prevention and treatment measures.
Current studies suggest that hypoxia is the main reason for the pathophysiological change in high-altitude situations [6,7]. However, under the same hypoxic conditions, some high-altitude residents will remain in good condition, while others may have AAI [8]. Several genes, including ACE, EDN1, ACYP2, RTEL1, and VEGF, are related to hypoxia [9–11]. In hypoxic environments, transcription of various genes, such as endothelial PAS domain-containing protein 1 (EPAS1) and prolyl hydroxylase domain-containing protein 2 (PHD2), is initiated by hypoxia-related pathways [12]. Also, genetic susceptibility has been reported as one of the major determinants of HAPE and AMS. The oxidative stress-related genes CYBA and GSTP1, contributing to endothelial damage under hypobaric hypoxia, are potential candidate genes for HAPE [13–17]. However, there are a few genetic studies on HAR.
MicroRNAs (miRNAs) can inhibit mRNA translation or induce degradation of target mRNAs by binding to complementary sequences in target regions [18,19]. Huang et al. revealed that saliva miR-134-3p and miR-15b-5p could be used as noninvasive biomarkers to predict AMS individuals exposed to high altitude in advance [19]. In a hypoxic atmosphere, the expression levels of miR-16, 20b, 22, 206, and 17/92 are reduced, which can inhibit ion channels, increase pulmonary artery pressure, and cause vascular dysfunction and the loss of cell integrity, promoting the occurrence of HAPE, and miR-155, 23b, 26a can inhibit TGF signals and promote pulmonary pressure, miR-210 can inhibit mitochondrial function. These may promote the occurrence of HAPE [20]. However, the differential expression of miRNAs has not been reported in HAR.
In the present study, we aimed to explore HAR-related miRNAs and mRNAs via analyzing the GSE90500 and GSE75665 datasets from the Gene Expression Omnibus (GEO). Gene Ontology (GO) functional annotations, Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway analysis, potential target gene analysis, and construction of potential miRNA–mRNA network were also conducted. Besides, the HAR cell model was established to validate the results of the bioinformatics analysis via quantitative RT-PCR (qRT-PCR). The present study may help elucidate the pathogenic mechanism and identify valuable diagnostic biomarkers for HAR.
Materials and methods
Data source
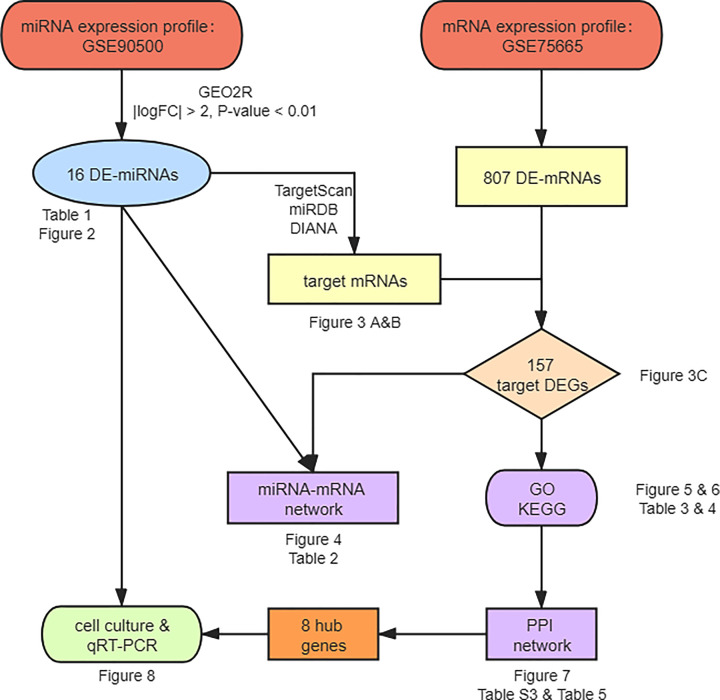
The miRNA expression profile GSE90500 and the mRNA expression profile GSE75665 were obtained from the GEO database. The RNA-seq data of GSE90500 were based on the platform of GPL18058 (Exiqon miRCURY LNA microRNA array, 7th generation) and contained 13 patients with AMS and 9 non-AMS volunteers. The microarray data of GSE75665 were based on the platform GPL11154 (Illumina HiSeq 2000) and contained 5 patients with AMS and 5 non-AMS volunteers. Figure 1 shows the workflow for the study.
Figure 1. The workflow of bioinformatics analysis.
Identification of the differentially expressed miRNA and differentially expressed mRNA
The GEO2R online tool (https://www.ncbi.nlm.nih.gov/geo/geo2r/) based on the R software ‘LIMMA’ package was used to identify differentially expressed miRNA (DE-miRNA) between AMS and non-AMS blood samples of GSE90500 with thresholds of |logFC| > 2 and P-value <0.01. A volcano plot was generated to visualize the significant miRNA expression changes using GraphPad Prism 8.0 (GraphPad, San Diego, CA, USA). For GSE75665, differentially expressed mRNAs (DE-mRNAs) related to AMS were obtained from the study of Liu et al. [21].
The prediction of target genes of DE-miRNA
We used three prediction tools to predict the target mRNAs of DE-miRNA: TargetScan (http://www.targetscan.org/), miRDB (http://mirdb.org/), and DIANA-microT (http://www.microrna.gr/microT). The genes predicted by at least two programs were chosen as the targets of DE-miRNA. We selected the overlapping genes between targets and DE-mRNAs as target differentially expressed genes (DEGs). A heatmap was performed using the R package ‘Pheatmap’. Then, using Cytoscape software [22], the miRNA–mRNA regulatory network was constructed.
Functional enrichment analysis of miRNA-target DEG
GO annotation and KEGG pathway enrichment analyses were performed by utilizing the Database for Annotation, Visualization, and Integrated Discovery (DAVID, http://david.abcc.Ncifcrf.gov/) online software [23]. P<0.05 was set as the cut-off value. The GO analysis included three categories: biological process (BP), cellular component (CC), and molecular function (MF). Heatmaps of DEGs significantly enriched KEGG pathways was performed by the R package ‘Pheatmap’.
Construction of the protein–protein interaction network
By putting the target DEGs in the Search Tool for the Retrieval of Interacting Genes (STRING, http://string-db.org/) database [24], the interaction relationships among DEGs at the protein level were screened. Then, the protein–protein interaction (PPI) networks for up- and down-regulated target DEGs were constructed under the criterion of a combined score > 0.4. Cytoscape software was used to visualize the networks. The connectivity degrees were calculated through network statistical methods.
Establishment of HAR cell model
Human retinal microvascular endothelial cells (HRMECs) were purchased from Cell Systems Corporations (catalog no. ACBRI181; Kirkland, WA, USA) [25]. The HRMECs were cultured in M199 medium supplemented with 20% fetal bovine serum, 3 ng/ml FGF-basic, 10 units/ml heparin, and 1% streptomycin/penicillin at 37°C. To establish the hypoxia model, cell culture was performed in a hypoxia chamber filled with an anaerobic gas mixture of 94% N2, 5% CO2, and 1% O2 for 12 h. Control groups were cultured in 95% air and 5% CO2.
Quantitative real-time PCR
Total RNA was extracted from HRMECs by using TRIzol reagent. RNA was reverse-transcribed into complementary DNA using PrimeScript RT Master Mix or miRNA First-Strand Synthesis kits (TaKaRa, Kumamoto, Japan), following the manufacturer’s protocols. qRT-PCR was performed by TB Green Premix ExTaq (TaKaRa, Kumamoto, Japan). The oligonucleotide primers used for PCR amplification were purchased from BioSune Biotechnology (Shanghai, China) and are listed in Supplementary Table S1. ACTB and U6 served as internal control for gene and miRNA expression in analysis, respectively. The relative expression of the DE-miRNAs and the DE-mRNAs was calculated using the 2−ΔΔCt method.
Statistical analysis
The data in the present study are presented as the mean ± SD. The results were analyzed by the two-tailed Student’s t test, and P<0.05 was considered to show a statistical difference.
Results
Identification of the DE-mRNA and the DE-miRNA
According to the analysis of Liu et al. for GSE75665 [21], a total of 807 genes were differentially expressed between the non-AMS and AMS groups, with 271 up-regulated and 535 down-regulated DE-mRNAs (Supplementary Table S2). During an analysis of the GSE90500 dataset, we obtained 16 DE-miRNAs (8 up-regulated and 8 down-regulated) by using the GEO2R online tool (P<0.01 and |logFC| > 2) (Table 1). These DE-miRNAs are indicated by a volcano plot (Figure 2). MiR-495 includes miR-495-3p and miR-495-5p, and we discussed them separately in the study, so there were 17 DE-miRNAs (9 up-regulated and 8 down-regulated).
Table 1. Up- and down-regulated DE-miRNA.
| DE-miRNA | LogFC | T | B | P-value | Regulation |
|---|---|---|---|---|---|
| hsa-miR-3177-3p | −6.20491 | −5.3524 | −3.024 | 4.01E-03 | Up |
| hsa-miR-369-3p | −5.90352 | −6.64136 | −2.0516 | 7.64E-04 | Up |
| hsa-miR-138-2-3p | −4.5548 | −4.49122 | −2.6156 | 4.98E-03 | Up |
| hsa-miR-603 | −4.25215 | −4.52661 | −2.6024 | 4.81E-03 | Up |
| hsa-miR-495 | −4.19332 | −3.61489 | −2.5279 | 7.51E-03 | Up |
| hsa-miR-4791 | −3.41691 | −4.26991 | −1.8136 | 2.33E-03 | Up |
| hsa-miR-424-5p | −3.19308 | −4.15642 | −2.4021 | 4.90E-03 | Up |
| hsa-miR-449b-3p | −2.47931 | −3.29887 | −2.5743 | 9.89E-03 | Up |
| hsa-miR-23b-5p | 3.31787 | 4.21334 | −1.7154 | 2.53E-03 | Down |
| hsa-miR-1304-3p | 3.26306 | 3.3816 | −2.3317 | 7.43E-03 | Down |
| hsa-miR-1183 | 3.14353 | 5.14318 | 0.0349 | 2.74E-04 | Down |
| hsa-miR-1255b-5p | 2.77088 | 4.48947 | −0.2807 | 5.49E-04 | Down |
| hsa-miR-15b-5p | 2.59052 | 2.88696 | −2.4875 | 8.20E-03 | Down |
| hsa-miR-1258 | 2.47358 | 3.60538 | −2.2692 | 6.18E-03 | Down |
| hsa-miR-3144-3p | 2.32697 | 3.15168 | −2.1737 | 6.34E-03 | Down |
| hsa-miR-155-5p | 2.20884 | 3.06764 | −2.1221 | 5.35E-03 | Down |
Figure 2. Volcano plot of DE-miRNAs.
Red dots indicate up-regulated miRNAs and green dots indicate down-regulated miRNAs with P<0.01 and |logFC| > 2. Black dots indicate the genes which were not differentially expressed in AMS vs. non-AMS.
MiRNA–mRNA network
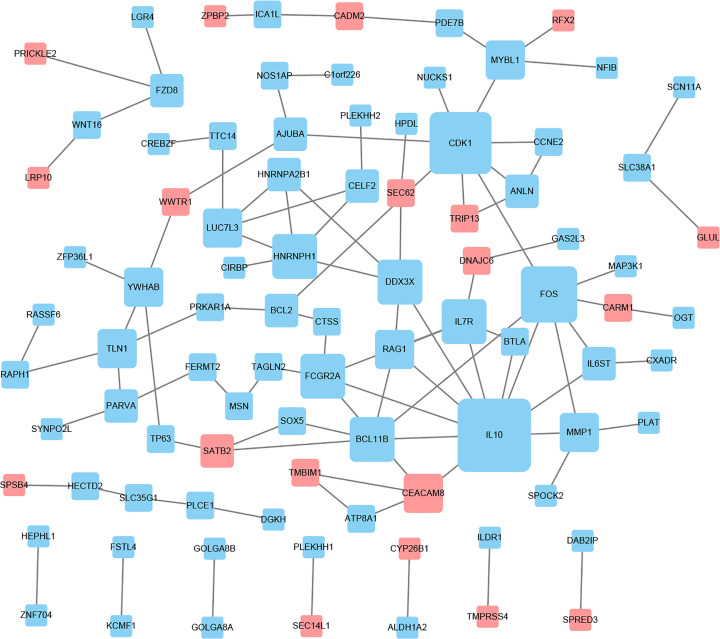
The target mRNAs of the DE-miRNAs were predicted by the three programs. Besides, by comparing the targets with DE-mRNAs, we only selected the overlapping genes as target DEGs. As shown in Figure 3A and B, 122 out of 5652 predicted targets of up-regulated DE-miRNAs overlapped down-regulated DE-mRNAs, and 37 out of 3857 predicted targets of down-regulated DE-miRNAs overlapped up-regulated DE-mRNAs. After removing 1 duplicate, we got 157 target DEGs, and the expression of these DEGs was visualized in Figure 3C. Then, we constructed the miRNA–mRNA regulatory network (Figure 4), which consisted of 240 miRNA–mRNA pairs in total. Among them, there were 196 up-regulated miRNA–mRNA pairs and 44 down-regulated miRNA–mRNA pairs. Among the target DEGs, there were 29 transcription factors and 128 non-transcription factors. Table 2 listed the target DEGs of DE-miRNAs.
Figure 3. The mRNAs related to AMS.
(A) Overlapped genes between targets of up-regulated DE-miRNAs and down-regulated DE-mRNAs. (B) Overlapped genes between targets of down-regulated DE-miRNAs and up-regulated DE-mRNAs. (C) Heatmap plot of 157 target DEGs. Green means down-regulation, while red means up-regulation.
Figure 4. The miRNA–mRNA network in AMS.
The diamonds stand for DE-miRNAs, and the ellipses stand for target DEGs. The red and blue colors represent up-regulation and down-regulation, respectively. The yellow ellipses represent transcription factors. The lines indicate the regulation relationship between DE-miRNAs and target DEGs.
Table 2. The target DEGs of DE-miRNA.
| MiRNA | Up/Down | Count | Target DEGs |
|---|---|---|---|
| hsa-miR-3177-3p | Up | 1 | HNRNPH1 |
| hsa-miR-369-3p | Up | 18 | IL10, IL7R, ANLN, CCNE2, CDH20, CELF2, CXADR, KCMF1, KL, MDFIC, MSN, NFIB, PDE7B, PTAR1, STEAP2, TLN1, TP63, WNT16 |
| hsa-miR-138-2-3p | Up | 36 | FCGR2A, DDX3X, ATAD1, ATP6AP1L, BTLA, CCDC83, CREBZF, CTSS, DGKH, FAM179B, FER, GPR173, HOXA5, HPDL, ICA1L, IL6ST, LGR4, MAP3K1, MYBL1, NFIB, NUCKS1, PLAT, PLCE1, PRKAR1A, PSIP1, PTAR1, RIMKLA, SCN11A, SLC35G1, SLC38A1, SPAG17, TC2N, TTC14, YWHAB, ZC3H12C, ZNF780B |
| hsa-miR-603 | Up | 37 | FOS, ALDH1A2, BTLA, CD164, CELF2, CHD5, DGKH, ELOVL4, ESM1, FAM110C, FER, FZD8, GNAI1, GPR173, HLF, HNRNPA2B1, IL6ST, KCMF1, KCNK10, LGR4, LUC7L3, MDFIC, NEBL, NFIB, OGT, PARVA, PKIB, PLAT, PSIP1, RAG1, RAPH1, RIMKLA, SPOCK2, SYNPO2L, TP63, ZNF226, ZNF704 |
| hsa-miR-495-3P | Up | 41 | DDX3X, HNRNPH1, CDK1, AJUBA, ANLN, ATP8A1, C21ORF62, CCNE2, CD164, CELF2, CREBZF, CTSS, DGKH, ESM1, FER, FERMT2, FZD8, GAS2L3, HECTD2, KCMF1, LUC7L3, MAP3K1, MYEF2, NEBL, NFIB, NTN4, NUCKS1, PRKAR1A, PTAR1, RAI2, RAPH1, RBMS3, RCAN3, SLC12A5, SOX5, SYT15, TNC, WWTR1, ZC3H12C, ZNF644, ZNF704 |
| hsa-miR-495-5p | Up | 10 | DDX3X, DGKH, GOLGA8A, IL6ST, KL, SOX5, TPBG, WWTR1, ZC3H12C, ZFP36L1 |
| hsa-miR-4791 | Up | 7 | FOS, CXADR, FZD8, NUCKS1, RASSF6, SLC38A1, ZNF460 |
| hsa-miR-424-5p | Up | 19 | IL7R, DDX3X, BCL11B, ANLN, BCL2, BTLA, FAM110C, FERMT2, FSTL4, HEPHL1, ILDR2, MYBL1, MYEF2, NEBL, NRN1, PLEKHH1, SLC35G1, SYDE1, ZNF704 |
| hsa-miR-449b-3p | Up | 27 | BCL2, C1ORF226, CD164, CELF2, CIRBP, DAB2IP, DGKH, EXPH5, FOXD, GOLGA8B, ICA1L, IGDCC4, ILDR1, KL, LGR4, LUC7L3, MAP3K, MMP1, NOS1AP, PLEKHH2, RPL22, SLC13A1, SLC38A1, TAGLN2, TLN1, TNC, TNFRSF19 |
| hsa-miR-23b-5p | Down | 3 | SCUBE1, SPRED3, RFX2 |
| hsa-miR-1304-3p | Down | 10 | C2ORF48, CDC42EP4, GALNT5, LRP10, NDUFV3, PRICKLE2, RNF152, SATB2, CEACAM8, SEC62 |
| hsa-miR-1183 | Down | 8 | CADM2, DNAJC6, GALNT5, GLUL, ZPBP2, CYP26B1, HDLB, SEC62 |
| hsa-miR-1255b-5p | Down | 1 | NEK11 |
| hsa-miR-15b-5p | Down | 8 | BACE2, GDPD5, SPSB4, SYT3, ZBTB44, CARM1, CYP26B1, LYNX1 |
| hsa-miR-1258 | Down | 4 | SEC14L1, TMBIM1, ZBTB44, TMPRSS4 |
| hsa-miR-3144-3p | Down | 3 | GALNT5, SATB2, FAM166B |
| hsa-miR-155-5p | Down | 7 | CDC42EP4, GLUL, MARVELD3, SATB2, ZDHHC2, MYO1D, TRIP13 |
Functional annotation analysis of the target DEGs
To further explore the biological function of the target DEGs, GO enrichment and KEGG pathway analysis were performed using DAVID. GO analysis results showed that acrosome assembly was the only significantly enriched BP for the up-regulated target DEGs. As shown in Figure 5A and Table 3, the down-regulated target DEGs were mainly enriched in the process of RNA expression, such as positive regulation of transcription from RNA polymerase II promoter, positive regulation of gene expression at BP level. On the CC level, the down-regulated DEGs were mainly enriched in the nucleus, cytoplasm, and plasma membrane. For the MF, the down-regulated DEGs were mainly enriched in sequence-specific DNA binding, transcriptional activator activity, and RNA polymerase II core promoter proximal region sequence-specific binding. In addition, KEGG pathway analyses indicated that down-regulated DEGs were significantly enriched in the Hippo signaling pathway (Figure 5B and Table 4), which is associated with the proliferation and migration of vascular endothelial cells [26,27] and can be deactivated by hypoxia [28]. In addition, as shown in Figure 6A–D, heatmaps illustrated the allocation of down-regulated DEGs significantly enriched four KEGG pathways.
Figure 5. GO enrichment and KEGG pathway analysis of the down-regulated target DEGs.
(A) Significant enriched GO terms of down-regulated target DEGs. Green bars stand for the BP, blue bars stand for the CC, and red bars stand for the MF. (B) KEGG pathway analysis of the down-regulated target DEGs.
Table 3. GO enrichment terms of down-regulated target DEGs in AMS.
| GO ID | GO Term | Category | Count | P-value | FDR |
|---|---|---|---|---|---|
| GO:0045944 | Positive regulation of transcription from RNA polymerase II promoter | BP | 15 | 5.22E-03 | 7.91E+00 |
| GO:0006366 | Transcription from RNA polymerase II promoter | BP | 9 | 2.05E-02 | 2.78E+01 |
| GO:0010628 | Positive regulation of gene expression | BP | 8 | 1.78E-03 | 2.77E+00 |
| GO:0033077 | T-cell differentiation in thymus | BP | 5 | 2.82E-05 | 4.45E-02 |
| GO:0034097 | Response to cytokine | BP | 4 | 4.91E-03 | 7.47E+00 |
| GO:0030182 | Neuron differentiation | BP | 4 | 2.51E-02 | 3.30E+01 |
| GO:0010468 | Regulation of gene expression | BP | 4 | 2.86E-02 | 3.67E+01 |
| GO:0035264 | Multicellular organism growth | BP | 4 | 1.60E-02 | 2.24E+01 |
| GO:0048041 | Focal adhesion assembly | BP | 3 | 1.09E-02 | 1.58E+01 |
| GO:0048538 | Thymus development | BP | 3 | 3.28E-02 | 4.09E+01 |
| GO:0042100 | B-cell proliferation | BP | 3 | 1.89E-02 | 2.60E+01 |
| GO:1900740 | Positive regulation of protein insertion into mitochondrial membrane involved in apoptotic signaling pathway | BP | 3 | 1.67E-02 | 2.33E+01 |
| GO:0008625 | Extrinsic apoptotic signaling pathway via death domain receptors | BP | 3 | 2.61E-02 | 3.41E+01 |
| GO:0045727 | Positive regulation of translation | BP | 3 | 4.80E-02 | 5.39E+01 |
| GO:0031532 | Actin cytoskeleton reorganization | BP | 3 | 3.86E-02 | 4.62E+01 |
| GO:0034446 | Substrate adhesion-dependent cell spreading | BP | 3 | 2.61E-02 | 3.41E+01 |
| GO:0032835 | Glomerulus development | BP | 3 | 1.51E-03 | 2.36E+00 |
| GO:0072577 | Endothelial cell apoptotic process | BP | 2 | 4.54E-02 | 5.19E+01 |
| GO:0046632 | α-β T-cell differentiation | BP | 2 | 3.90E-02 | 4.66E+01 |
| GO:0005634 | Nucleus | CC | 48 | 3.07E-03 | 3.67E+00 |
| GO:0005737 | Cytoplasm | CC | 46 | 4.66E-03 | 5.52E+00 |
| GO:0005886 | Plasma membrane | CC | 36 | 1.88E-02 | 2.07E+01 |
| GO:0005856 | Cytoskeleton | CC | 8 | 7.63E-03 | 8.90E+00 |
| GO:0005925 | Focal adhesion | CC | 8 | 1.00E-02 | 1.15E+01 |
| GO:0005913 | Cell–cell adherens junction | CC | 7 | 1.42E-02 | 1.60E+01 |
| GO:0030027 | Lamellipodium | CC | 5 | 1.66E-02 | 1.84E+01 |
| GO:0015629 | Actin cytoskeleton | CC | 5 | 4.44E-02 | 4.24E+01 |
| GO:0030018 | Z disc | CC | 4 | 3.54E-02 | 3.56E+01 |
| GO:0044300 | Cerebellar mossy fiber | CC | 2 | 3.01E-02 | 3.11E+01 |
| GO:0043565 | Sequence-specific DNA binding | MF | 9 | 1.81E-02 | 2.14E+01 |
| GO:0001077 | Transcriptional activator activity, RNA polymerase II core promoter proximal region sequence-specific binding | MF | 8 | 8.05E-04 | 1.05E+00 |
| GO:0098641 | Cadherin binding involved in cell-cell adhesion | MF | 7 | 1.08E-02 | 1.34E+01 |
| GO:0000166 | Nucleotide binding | MF | 7 | 2.44E-02 | 2.77E+01 |
| GO:0003779 | Actin binding | MF | 6 | 3.31E-02 | 3.58E+01 |
| GO:0003730 | mRNA 3′-UTR binding | MF | 4 | 3.79E-03 | 4.87E+00 |
Table 4. Enriched KEGG pathway of down-regulated target DEGs associated with AMS.
| KEGG ID | KEGG Term | Count | FDR | Genes |
|---|---|---|---|---|
| hsa04390 | Hippo signaling pathway | 6 | 2.65E+00 | AJUBA, FZD8, WNT16, RASSF6, YWHAB, WWTR1 |
| hsa05161 | Hepatitis B | 6 | 2.23E+00 | CCNE2, FOS, DDX3X, BCL2, MAP3K1, YWHAB |
| hsa05200 | Pathways in cancer | 7 | 3.27E+01 | CCNE2, FZD8, FOS, WNT16, GNAI1, BCL2, MMP1 |
| hsa05203 | Viral carcinogenesis | 5 | 3.60E+01 | CCNE2, CDK1, DDX3X, IL6ST, YWHAB |
Figure 6. Heatmaps of down-regulated DEGs significantly enriched four KEGG pathways.
Viral carcinogenesis (A), pathways in cancer (B), Hepatitis B (C), and Hippo signaling pathway (D). Green means down-regulation, while red means up-regulation.
PPI network of the target DEGs
Based on information from the STRING database, the PPI network was constructed and contained 59 nodes and 99 interactions (Figure 7). The details of the PPI network are described in Supplementary Table S3. In this network, with the criteria of filtering degree > 5, eight genes were defined as hub genes, including IL10, CDK1, FOS, HNRNPH1, IL7R, BCL11B, FCGR2A, and DDX3X (Table 5).
Figure 7. PPI network construction and module analysis.
The red nodes represent the up-regulated DEGs. The blue nodes represent the down-regulated DEGs. The size of the nodes indicates the degree of the DEGs, and the lines represent the interaction between gene-encoded proteins.
Table 5. Top eight highest degree nodes.
| Node | IL10 | CDK1 | FOS | HNRNPH1 | IL7R | BCL11B | FCGR2A | DDX3X |
|---|---|---|---|---|---|---|---|---|
| Degree | 10 | 8 | 7 | 5 | 5 | 5 | 5 | 5 |
| Type | Down | Down | Down | Down | Down | Down | Down | Down |
Validation of hub genes in HAR cell model
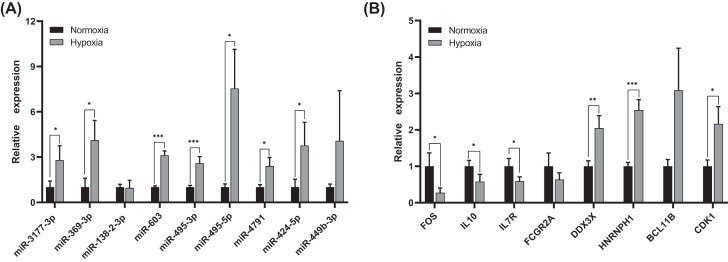
To validate the results of bioinformatics analysis, we chose the nine up-regulated DE-miRNAs and eight hub genes for qRT-PCR under normoxia and hypoxia in HRMECs. As shown in Figure 8A and B, miR-3177-3p, miR-369-3p, miR-603, miR-495-3p, miR-495-5p, miR-4791, and miR-424-5p were significantly up-regulated in hypoxia-cultured HRMECs compared with normoxia (P<0.05). FOS, IL10, and IL7R were strongly down-regulated under hypoxia conditions (P<0.05), which was also consistent with our bioinformatics results. However, the expression of DDX3X, BCL1B, HNRNPH1, and CDK1 was higher than that in normoxia-cultured HRMECs.
Figure 8. Relative expression levels of DE-miRNAs and hub genes in HRMECs under normoxia and hypoxia conditions by qRT-PCR.
(A) The expression of nine up-regulated miRNAs. (B) The expression of eight DEGs. *P<0.05; **P<0.01; ***P<0.001.
Discussion
HAR is an ocular manifestation of AAI [1], and studies have found a statistically significant correlation between HAR and HACE [3]. Some researchers have proposed that AMS is a mild form of HACE [29,30]. Since the retina and optic nerve act as the directly visible part of the brain, belonging to the central nervous system due to its embryonic origin [31,32], we obtained the DE-miRNAs and DE-mRNAs of HAR by analyzing the AMS datasets and then explored the possible potential target genes of HAR.
MiRNAs can regulate many BPs by negatively regulating the expression of target genes and are thus important targets for studying the genetic susceptibility of diseases [19,33]. Through a series of bioinformatics analyses and experiments, we selected 16 DE-miRNAs and 157 target DEGs related to AMS from GSE90500 and GSE75665 and constructed an miRNA–mRNA network containing 240 relationship pairs. The top eight hub genes—IL7R, FOS, IL10, FCGR2A, DDX3X, CDK1, BCL11B and HNRNPH1—filtered from the PPI network may serve as candidate biomarkers of HAR. The expression of miR-3177-3p, miR-369-3p, miR-603, miR-495-3p, miR-495-5p, miR-4791, miR-424-5p, FOS, IL10, and IL7R were confirmed by qRT-PCR in our HAR cell model to have the same trend as that found in our bioinformatics results. These miRNAs and mRNAs may play an important role in the development of HAR.
FOS, consisting of c-Fos, FosB, Fra1, and Fra2 [34], was significantly down-regulated in our hypoxia-cultured HRMECs. In the lung tissues of hypoxic rats, the expression of c-Fos and FosB was significantly decreased [35], which is consistent with our results. c-Fos promotes the development of inflammatory diseases such as arthritis [36], but it also inhibits inflammation in myeloid and lymphoid cell lineages [37]. In studies on retinal development, it has been found that c-Fos induces inflammatory signals in photoreceptor cells, leading to an increase in VEGF, thus promoting the formation of new blood vessels [38]. Therefore, FOS may be associated with the leakage of the peripheral retinal vessels and retinal hemorrhages in HAR through the regulation of inflammatory constituents. The bioinformatics analysis showed that miR-603 and miR-4791 may down-regulate FOS, and miR-603 can promote the growth of glioma cells via the Wnt/β-catenin pathway [39]. The Wnt pathway mediates many complex biological and pathological processes of retinopathy [40], but there are no reports about miR-603 and this pathway in the development of HAR. Further research about the role of FOS and miR-603 in HAR is needed.
Another inflammation-related gene, IL10, could reduce tissue damage and promote immune tolerance [41]. It was down-regulated in our study, and maybe serve as a biomarker in HAR. Under hypoxic conditions, the decrease in IL10 indicates hypoxia reduces the ability to inhibit immunity and inflammation [42]. However, in the study of Kang et al., mice adapted to the low oxygen environment (10% O2) had higher IL10 levels [43]. Therefore, the role of anti-inflammatory markers IL10 in HAR needs further investigation.
IL7R mediates the signaling of IL7, affecting the role of IL7 in regulating the development of T and B cells and the balance of the internal environment of T cells [44], and IL7R is involved in the pathogenesis of several autoimmune diseases, such as rheumatoid arthritis, multiple sclerosis, and systemic lupus erythematosus etc. [45] High altitude also could cause changes in different immune cells [46]. According to our bioinformatics analysis, we found that IL7R was regulated by miR-369-3p and miR-424-5p. It is noted that the induction of miR-369-3p expression inhibits the release of inflammatory cytokines [47]. Furthermore, up-regulated miR-424-5p in hypoxic vascular endothelial cells was shown to stabilize hypoxia-inducible factor-1α (HIF-1α) and fine-tune VEGF, promoting angiogenesis and preserving the integrity of the endothelial barrier [48]. Although the down-regulated IL7R and the up-regulated miR-369-3p and miR-424-5p may play an important role in the progression of HAR, the specific mechanism is not clear and remains to be clarified.
High-altitude retinal hemorrhages and vascular leakage may be caused by the retinal microcirculation disorder under hypoxia conditions at high altitude, but the specific mechanism is not clear. The expression of DDX3X was found to be correlated with overexpression of HIF-1α in breast cancer, indicating the oncogenic role of DDX3X [49]. CDK1 has been shown to promote tumor angiogenesis by stabilizing HIF-1α, and its disruption could inhibit retinal angiogenesis by inducing cell cycle arrest and apoptosis [50]. However, inhibition of miR-495 improves angiogenesis and blood flow recovery after ischemia [51]. Although the expression of DDX3X and CDK1 did not down-regulate in HRMECs as predicted, these genes had high degrees in the PPI network, indicating their potential roles in the development of HAR. It worth mentioning that very little was reported on miR-3177-3p, so the specific function of miR-3177-3p needs further research.
To our knowledge, our study is the first to explore the DE-miRNAs and DEGs of HAR and establish the miRNA–mRNA network through comprehensive bioinformatics analyses. However, there are still some limitations in the present study. First, the data of the GSE90500 and GSE75665 are collected from blood samples, which may be the reason why the expression of several hub genes in HRMECs was inconsistent with the bioinformatics analyses. Then, there are some differences between the analytical methods of GSE90500 and GSE75665, which reduces the reliability of this study to a certain extent. Third, the miRNA–mRNA relationships in HAR were based on target prediction and statistical evidence, which need experimental investigations. Thus, Further studies are required to verify the specific functions of the identified DE-miRNAs and hub genes in HAR.
In conclusion, the present study identified FOS, IL10, IL7R and seven miRNAs as candidate biomarkers of HAR, which may participate in the development of HAR through possible pathways. It has been proposed that HAR is related to individual sensitivity, promoting the effective prevention, and treatment of HAR in the future. Nevertheless, we carried out the systematic and comprehensive bioinformatics analyses to identify the new miRNA and genes of HAR and performed experimental validation in the HAR cell model, which is helpful for understanding the gene changes in HAR and provides novel candidate biomarkers for the diagnosis of HAR.
Supplementary Material
Abbreviations
- AAI
acute high-altitude illness
- AMS
acute mountain sickness
- BP
biological process
- CC
cellular component
- DAVID
Database for Annotation, Visualization, and Integrated Discovery
- DE-miRNA
differentially expressed miRNA
- DE-mRNA
differentially expressed mRNA
- DEG
differentially expressed gene
- GEO
Gene Expression Omnibus
- GO
Gene Ontology
- HACE
high-altitude cerebral edema
- HAPE
high-altitude pulmonary edema
- HAR
high-altitude retinopathy
- HIF-1α
hypoxia-inducible factor-1α
- HRMEC
human retinal microvascular endothelial cell
- KEGG
Kyoto Encyclopedia of Genes and Genomes
- MF
molecular function
- miRNA
microRNA
- qRT-PCR
uantitative RT-PCR
- STRING
Search Tool for the Retrieval of Interacting Genes
Contributor Information
Zhi Zheng, Email: zzheng88@sjtu.edu.cn.
Qinghua Qiu, Email: qinghuaqiu@163.com.
Data Availability
All data associated with the present paper are already included in the manuscript.
Competing Interests
The authors declare that there are no competing interests associated with the manuscript.
Funding
This work was supported by the National Natural Science Foundation of China [grant numbers 81970811, 81770947]; the National Science and Technology Major Project of China [grant number 2017ZX09304010]; the National Key R&D Program of China [grant numbers 2016YFC0904800, 2019YFC0840607]; and the Tibet Natural Science Foundation of China [grant numbers XZ2017ZR-ZYZ09, XZ2018ZRG-95, XZ2018ZRG-100(Z)].
Author Contribution
All authors contributed significantly to this work. Tong Su, Chufeng Cu, and Deji Draga were involved in all aspects of research, analyzed data and wrote the paper. Chuandi Zhou performed the experiments. Thashi Lhamo was involved in research design. Qinghua Qiu and Zhi Zheng contributed to research design, supervision of research, data analysis and revised the manuscript. All authors have read and approved the final manuscript.
References
- 1.Houston C.S. (1976) High altitude illness. Disease with protean manifestations. JAMA 236, 2193–2195 10.1001/jama.1976.03270200031025 [DOI] [PubMed] [Google Scholar]
- 2.Seth R.K. and Adelman R.A. (2010) High-altitude retinopathy and optical coherence tomography findings. Semin. Ophthalmol. 25, 13–15 10.3109/08820538.2010.481560 [DOI] [PubMed] [Google Scholar]
- 3.Wiedman M. and Tabin G.C. (1999) High-altitude retinopathy and altitude illness. Ophthalmology 106, 1924–1926, discussion 1927 10.1016/S0161-6420(99)90402-5 [DOI] [PubMed] [Google Scholar]
- 4.Tian X. et al. (2018) Retinal changes following rapid ascent to a high-altitude environment. Eye (Lond.) 32, 370–374 10.1038/eye.2017.195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Honigman B. et al. (2001) High altitude retinal hemorrhages in a Colorado skier. High Alt. Med. Biol. 2, 539–544 10.1089/152702901753397117 [DOI] [PubMed] [Google Scholar]
- 6.McFadden D.M. et al. (1981) High-altitude retinopathy. JAMA 245, 581–586 10.1001/jama.1981.03310310023016 [DOI] [PubMed] [Google Scholar]
- 7.Li K. et al. (2017) Transcriptome reveals the overexpression of a kallikrein gene cluster (KLK1/3/7/8/12) in the Tibetans with high altitude-associated polycythemia. Int. J. Mol. Med. 39, 287–296 10.3892/ijmm.2016.2830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.MacInnis M.J. and Koehle M.S. (2016) Evidence for and against genetic predispositions to acute and chronic altitude illnesses. High Alt. Med. Biol. 17, 281–293 10.1089/ham.2016.0024 [DOI] [PubMed] [Google Scholar]
- 9.Jin T. et al. (2019) Association between the IL1R2 rs2072472 polymorphism and high-altitude pulmonary edema risk. Mol. Genet. Genom. Med. 7, e542 10.1002/mgg3.542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hanaoka M. et al. (2009) Polymorphisms of human vascular endothelial growth factor gene in high-altitude pulmonary oedema susceptible subjects. Respirology 14, 46–52 10.1111/j.1440-1843.2008.01420.x [DOI] [PubMed] [Google Scholar]
- 11.Rong H. et al. (2017) Association between regulator of telomere elongation helicase1 (RTEL1) gene and HAPE risk: a case-control study. Medicine (Baltimore) 96, e8222 10.1097/MD.0000000000008222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pan H. et al. (2018) Mutations in EPAS1 in congenital heart disease in Tibetans. Biosci. Rep. 38, 6 10.1042/BSR20181389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.He Y. et al. (2016) Telomere length-related gene ACYP2 polymorphism is associated with the risk of HAPE in Chinese Han population. J. Gene Med. 18, 244–249 10.1002/jgm.2896 [DOI] [PubMed] [Google Scholar]
- 14.Mishra A. et al. (2012) CYBA and GSTP1 variants associate with oxidative stress under hypobaric hypoxia as observed in high-altitude pulmonary oedema. Clin. Sci. (Lond.) 122, 299–309 10.1042/CS20110205 [DOI] [PubMed] [Google Scholar]
- 15.Richalet J.P. et al. (2012) Physiological risk factors for severe high-altitude illness: a prospective cohort study. Am. J. Respir. Crit. Care Med. 185, 192–198 10.1164/rccm.201108-1396OC [DOI] [PubMed] [Google Scholar]
- 16.Schneider M. et al. (2002) Acute mountain sickness: influence of susceptibility, preexposure, and ascent rate. Med. Sci. Sports Exerc. 34, 1886–1891 10.1097/00005768-200212000-00005 [DOI] [PubMed] [Google Scholar]
- 17.Bloch K.E. et al. (2009) Effect of ascent protocol on acute mountain sickness and success at Muztagh Ata, 7546 m. High Alt. Med. Biol. 10, 25–32 10.1089/ham.2008.1043 [DOI] [PubMed] [Google Scholar]
- 18.Lou W. et al. (2018) MicroRNA regulation of liver cancer stem cells{Lou, 2018 #42}. Am J. Cancer Res. 8, 1126–1141 [PMC free article] [PubMed] [Google Scholar]
- 19.Nunez-Iglesias J. et al. (2010) Joint genome-wide profiling of miRNA and mRNA expression in Alzheimer’s disease cortex reveals altered miRNA regulation. PLoS ONE 5, e8898 10.1371/journal.pone.0008898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Alam P., Saini N. and Pasha M.A. (2015) MicroRNAs: an apparent switch for high-altitude pulmonary edema. MicroRNA 4, 158–167 10.2174/2211536604666151103121633 [DOI] [PubMed] [Google Scholar]
- 21.Liu B. et al. (2017) IL-10 dysregulation in acute mountain sickness revealed by transcriptome analysis. Front. Immunol. 8, 628 10.3389/fimmu.2017.00628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Smoot M.E. et al. (2011) Cytoscape 2.8: new features for data integration and network visualization. Bioinformatics 27, 431–432 10.1093/bioinformatics/btq675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Huang da W., Sherman B.T. and Lempicki R.A. (2009) Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat. Protoc. 4, 44–57 10.1038/nprot.2008.211 [DOI] [PubMed] [Google Scholar]
- 24.Szklarczyk D. et al. (2011) The STRING database in 2011: functional interaction networks of proteins, globally integrated and scored. Nucleic Acids Res. 39, D561–D568 10.1093/nar/gkq973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gu C. et al. (2019) miR-590-3p inhibits pyroptosis in diabetic retinopathy by targeting NLRP1 and inactivating the NOX4 signaling pathway. Invest. Ophthalmol. Vis. Sci. 60, 4215–4223 10.1167/iovs.19-27825 [DOI] [PubMed] [Google Scholar]
- 26.Moon K.H. and Kim J.W. (2018) Hippo signaling circuit and divergent tissue growth in mammalian eye. Mol. Cells 41, 257–263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kim J. et al. (2017) YAP/TAZ regulates sprouting angiogenesis and vascular barrier maturation. J. Clin. Invest. 127, 3441–3461 10.1172/JCI93825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ma B. et al. (2015) Hypoxia regulates Hippo signalling through the SIAH2 ubiquitin E3 ligase. Nat. Cell Biol. 17, 95–103 10.1038/ncb3073 [DOI] [PubMed] [Google Scholar]
- 29.Bartsch P. et al. (2004) Acute mountain sickness: controversies and advances. High Alt. Med. Biol. 5, 110–124 10.1089/1527029041352108 [DOI] [PubMed] [Google Scholar]
- 30.Hackett P.H. and Roach R.C. (2001) High-altitude illness. N. Engl. J. Med. 345, 107–114 10.1056/NEJM200107123450206 [DOI] [PubMed] [Google Scholar]
- 31.Hughes S., Yang H. and Chan-Ling T. (2000) Vascularization of the human fetal retina: roles of vasculogenesis and angiogenesis. Invest. Ophthalmol. Vis. Sci. 41, 1217–1228 [PubMed] [Google Scholar]
- 32.Patton N. et al. (2005) Retinal vascular image analysis as a potential screening tool for cerebrovascular disease: a rationale based on homology between cerebral and retinal microvasculatures. J. Anat. 206, 319–348 10.1111/j.1469-7580.2005.00395.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Beermann J. et al. (2016) Non-coding RNAs in development and disease: background, mechanisms, and therapeutic approaches. Physiol. Rev. 96, 1297–1325 10.1152/physrev.00041.2015 [DOI] [PubMed] [Google Scholar]
- 34.Tkach V. et al. (2003) Role of the Fos family members, c-Fos, Fra-1 and Fra-2, in the regulation of cell motility. Oncogene 22, 5045–5054 10.1038/sj.onc.1206570 [DOI] [PubMed] [Google Scholar]
- 35.Singh M. et al. (2018) The MAPK-activator protein-1 signaling regulates changes in lung tissue of rat exposed to hypobaric hypoxia. J. Cell. Physiol. 233, 6851–6865 10.1002/jcp.26556 [DOI] [PubMed] [Google Scholar]
- 36.Shiozawa S. and Tsumiyama K. (2009) Pathogenesis of rheumatoid arthritis and c-Fos/AP-1. Cell Cycle 8, 1539–1543 10.4161/cc.8.10.8411 [DOI] [PubMed] [Google Scholar]
- 37.Ray N. et al. (2006) c-Fos suppresses systemic inflammatory response to endotoxin. Int. Immunol. 18, 671–677 10.1093/intimm/dxl004 [DOI] [PubMed] [Google Scholar]
- 38.Sun Y. et al. (2017) Inflammatory signals from photoreceptor modulate pathological retinal angiogenesis via c-Fos. J. Exp. Med. 214, 1753–1767 10.1084/jem.20161645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Guo M. et al. (2015) miR-603 promotes glioma cell growth via Wnt/beta-catenin pathway by inhibiting WIF1 and CTNNBIP1. Cancer Lett. 360, 76–86 10.1016/j.canlet.2015.02.003 [DOI] [PubMed] [Google Scholar]
- 40.Huang H.M. et al. (2015) Activating the Wnt/beta-catenin pathway did not protect immature retina from hypoxic-ischemic injury. Invest. Ophthalmol. Vis. Sci. 56, 4300–4308 10.1167/iovs.14-16176 [DOI] [PubMed] [Google Scholar]
- 41.Hilgenberg E. et al. (2014) Interleukin-10-producing B cells and the regulation of immunity. Curr. Top. Microbiol. Immunol. 380, 69–92 10.1007/978-3-662-43492-5_4 [DOI] [PubMed] [Google Scholar]
- 42.Yuhai G.U. and Zhen Z. (2015) Significance of the changes occurring in the levels of interleukins, SOD and MDA in rat pulmonary tissue following exposure to different altitudes and exposure times. Exp. Ther. Med. 10, 915–920 10.3892/etm.2015.2604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kang J.G. et al. (2016) Low ambient oxygen prevents atherosclerosis. J. Mol. Med. (Berl.) 94, 277–286 10.1007/s00109-016-1386-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mai H.L. et al. (2014) IL-7 receptor blockade following T cell depletion promotes long-term allograft survival. J. Clin. Invest. 124, 1723–1733 10.1172/JCI66287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wang X.S. et al. (2013) Perspectives of the relationship between IL-7 and autoimmune diseases. Clin. Rheumatol. 32, 1703–1709 10.1007/s10067-013-2360-x [DOI] [PubMed] [Google Scholar]
- 46.Khanna K. et al. (2018) High-altitude-induced alterations in gut-immune axis: a review. Int. Rev. Immunol. 37, 119–126 10.1080/08830185.2017.1407763 [DOI] [PubMed] [Google Scholar]
- 47.Galleggiante V. et al. (2019) Quercetin-induced miR-369-3p suppresses chronic inflammatory response targeting C/EBP-beta. Mol. Nutr. Food Res. 63, e1801390 10.1002/mnfr.201801390 [DOI] [PubMed] [Google Scholar]
- 48.Ghosh G. et al. (2010) Hypoxia-induced microRNA-424 expression in human endothelial cells regulates HIF-alpha isoforms and promotes angiogenesis. J. Clin. Invest. 120, 4141–4154 10.1172/JCI42980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bol G.M. et al. (2013) Expression of the RNA helicase DDX3 and the hypoxia response in breast cancer. PLoS ONE 8, e63548 10.1371/journal.pone.0063548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gao X. et al. (2019) Cyclin-dependent kinase 1 disruption inhibits angiogenesis by inducing cell cycle arrest and apoptosis. Exp. Ther. Med. 18, 3062–3070 10.3892/etm.2019.7883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Welten S.M. et al. (2014) Inhibition of 14q32 microRNAs miR-329, miR-487b, miR-494, and miR-495 increases neovascularization and blood flow recovery after ischemia. Circ. Res. 115, 696–708 10.1161/CIRCRESAHA.114.304747 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data associated with the present paper are already included in the manuscript.