Key Points
Question
What are the most effective systemic treatments for metastatic castration-sensitive prostate cancer?
Findings
This network meta-analysis of 7 randomized clinical trials including 7287 patients noted that, combined with androgen-deprivation therapy, treatments associated with significantly improved overall survival included abiraterone acetate, apalutamide, and docetaxel; treatments associated with significantly improved radiographic progression-free survival included enzalutamide, abiraterone acetate, apalutamide, and docetaxel, ordered from the agent with the greatest to least effectiveness according to the results of clinical trials. Docetaxel was associated with substantially increased serious adverse events, abiraterone with slightly increased serious adverse events, and other treatments with no increase in serious adverse events.
Meaning
This network meta-analysis suggests that abiraterone acetate and apalutamide may provide the largest and most consistent overall survival benefits with relatively low serious adverse event risks among metastatic castration-sensitive prostate cancer treatments.
Abstract
Importance
Multiple systemic treatments are available for metastatic castration-sensitive prostate cancer (mCSPC), with unclear comparative effectiveness and safety and widely varied costs.
Objective
To compare the effectiveness and safety determined in randomized clinical trials of systemic treatments for mCSPC.
Data Sources
Bibliographic databases (MEDLINE, Embase, and Cochrane Central), regulatory documents (US Food and Drug Administration and European Medicines Agency), and trial registries (ClinicalTrials.gov and European Union clinical trials register) were searched from inception through November 5, 2019.
Study Selection, Data Extraction, and Synthesis
Eligible studies were randomized clinical trials evaluating the addition of docetaxel, abiraterone acetate, apalutamide, or enzalutamide to androgen-deprivation therapy (ADT) for treatment of mCSPC. Two investigators independently performed screening. Discrepancies were resolved through consensus. A Cochrane risk-of-bias tool was used to assess trial quality. Relative effects of competing treatments were assessed by bayesian network meta-analysis and survival models. The Preferred Reporting Items for Systematic Reviews and Meta-analyses guideline was used.
Main Outcomes and Measures
Overall survival, radiographic progression-free survival, and serious adverse events (SAEs).
Results
Seven trials with 7287 patients comparing 6 treatments (abiraterone acetate, apalutamide, docetaxel, enzalutamide, standard nonsteroidal antiandrogen, and placebo/no treatment) were identified. Ordered from the most to the least effective determined by results of clinical trials, treatments associated with improved overall survival when added to ADT included abiraterone acetate (hazard ratio [HR], 0.61; 95% credible interval [CI], 0.54-0.70), apalutamide (HR, 0.67; 95% CI, 0.51-0.89), and docetaxel (HR, 0.79; 95% CI, 0.71-0.89); treatments associated with improved radiographic progression-free survival when added to ADT included enzalutamide (HR, 0.39; 95% CI, 0.30-0.50), apalutamide (HR, 0.48; 95% CI, 0.39-0.60), abiraterone acetate (HR, 0.51; 95% CI, 0.45-0.58), and docetaxel (HR, 0.67; 95% CI 0.60-0.74). Docetaxel was associated with substantially increased SAEs (odds ratio, 23.72; 95% CI, 13.37-45.15), abiraterone acetate with slightly increased SAEs (odds ratio, 1.42; 95% CI, 1.10-1.83), and other treatments with no significant increase in SAEs. Risk of bias was noted for 4 trials with open-label design, 3 trials with missing data, and 2 trials with potential unprespecified analyses.
Conclusions and Relevance
In this network meta-analysis, as add-on treatments to ADT, abiraterone acetate and apalutamide may provide the largest overall survival benefits with relatively low SAE risks. Although enzalutamide may improve radiographic progression-free survival to the greatest extent, longer follow-up is needed to examine the overall survival benefits associated with enzalutamide.
This systematic review and network meta-analysis compares the results of randomized clinical trials that evaluated the use of medications used in the treatment of metastatic castration-resistant prostate cancer.
Introduction
Prostate cancer is the most common cancer and the second leading cause of cancer death among men in the US.1 Although 76% of the patients with prostate cancer were first diagnosed with localized cancer,2 approximately 30% of these patients experience disease recurrence after definitive treatments.3 Most recurrent prostate cancers initially respond to androgen-deprivation therapy (ADT) but eventually develop resistance, transforming from castration-sensitive to castration-resistant prostate cancer.4 Metastatic castration-resistant prostate cancer is associated with high mortality: a 5-year survival rate of 30%.2
Progress in research has led to several promising treatments that, when added to ADT, delay disease progression to metastatic castration-resistant prostate cancer (mCRPC). These drugs include the taxane docetaxel,5,6 androgen synthesis inhibitor abiraterone acetate,7,8,9,10 and androgen receptor inhibitors apalutamide and enzalutamide.11,12 The availability of these drugs has improved prostate cancer survival. However, owing to the lack of head-to-head trials comparing active treatments, little is known about the optimal choice weighing effectiveness and safety. As a result, clinical guideline committees hesitate to recommend one drug over others.13,14
In addition, drug costs vary widely. The range of drug acquisition costs for patients to complete all planned courses of treatment (18 weeks for docetaxel and a median treatment duration of 2 years for other drugs) is $627 for docetaxel, $62 714 for generic abiraterone acetate, $175 438 for enzalutamide (Xtandi), and $231 789 for apalutamide (Erleada).15 This study aimed to compare the effectiveness and safety of systemic treatments for mCSPC determined in randomized clinical trials (RCTs) to inform decision-making.
Methods
Eligibility Criteria
The study protocol was registered in PROSPERO (the International Prospective Register of Systematic Reviews, CRD42020160839). We included RCTs of parallel design for mCSPC and excluded cluster and dose-escalation trials. The interventions of interest were docetaxel, abiraterone acetate, apalutamide, and enzalutamide; the comparator of interest was any active drug, placebo, or no treatment—all in addition to ADT. Androgen-deprivation therapy includes orchiectomy, luteinizing hormone-releasing hormone agonists and antagonists, and estrogen. We combined different dose regimens of the same drug, combined placebo with no treatment, and required a median follow-up of at least 12 months. Trial registrations without results, published trial protocols, and abstracts were excluded. This study followed the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) reporting guideline and its extension for network meta-analysis.16,17
Data Sources and Extraction
We searched bibliographic databases (MEDLINE [PubMed interface], EMBASE [OVID interface]), the Cochrane Central Register of Controlled Trials (CENTRAL, Wiley interface), trial registries (ClinicalTrials.gov and the EU Clinical Trials Register), and regulatory documents (US Food and Drug Administration and European Medicines Agency review packets) from inception to November 5, 2019, with no language or date restrictions. Search strategies in eMethods 1 in the Supplement.
We used Sysrev for title and abstract screening and Endnote X9 (Clarivate Analytics) for full-text screening. Two investigators (L.W. and A.D.F.) independently performed the screening. One investigator (L.W.) extracted data from included trials and the second investigator (C.J.P.) checked the extracted data. Discrepancies were resolved through consensus. Data extracted included trial design, interventions, outcomes, baseline characteristics, and results (eTable 1 in the Supplement). The efficacy outcomes of interest were overall survival (OS) and radiographic progression-free survival (rPFS), defined as time from randomization to radiographic progression or death from any cause, whichever occurred first. The safety outcome of interest was any serious adverse events (SAEs).
Risk of Bias Assessment
We assessed the risk of bias of individual trials for effectiveness outcomes using the Cochrane Collaboration’s tool (version 2.0).18 The overall bias of a trial was assessed from 5 domains: randomization process, deviation from intended interventions, missing outcome data, measurement of the outcome, and selection of reported results. The overall bias was judged to be low if all domains were at low risk of bias and high if at least 1 domain was at high risk of bias or multiple domains raised concerns. Judgments were made independently by 2 investigators (L.W. and G.C.A.). Disagreements were resolved by discussion. Risk of bias assessment was incorporated into our interpretation of results.
Statistical Analysis
Bayesian network meta-analysis was performed using generalized linear models.19 We used multivariate normal distribution to account for between-arm correlations in multiarm trials.19,20 We fitted fixed- and random-effects models, with the latter accounting for between-study heterogeneity. We report results from fixed-effects models because, for treatment comparisons examined in RCTs, 4 of the 6 comparisons were examined in only 1 trial. In addition, the results from fixed- and random-effects models were consistent, with only wider 95% credible intervals (CIs) noted for the random-effects models.
As primary analysis for OS and rPFS, time-invariant hazard ratios (HRs) between treatment arms from individual trials were analyzed to estimate the overall HRs. For patient subgroups consistently examined across trials, we performed subgroup analyses to evaluate how comparative effectiveness varied by patient characteristics. For SAEs, the number of events in individual trial arms was analyzed to estimate the overall odds ratios (ORs) between treatments.
As secondary analysis for OS and rPFS, we estimated time-varying HRs by bayesian parametric survival network meta-analysis and compared expected survival curves across treatments. Specifically, published Kaplan-Meier curves were digitalized using Web PlotDigitizer, version 4.2.21 The individual level time-to-event data were reconstructed using Guyot algorithms.22 and the Stata, version (StataCorp LLC) command ipdfc.23 We fit a series of first-order fractional polynomial models with power parameters −2, −1, −0.5, 0.5, 1, 2, and 3, which include common survival distributions, such as Weibull (power parameter = 0) and Gompertz (power parameter = 1).24 The deviance information criterion was used to assess model fit.25
Bayesian models estimate treatment effects via Markov chain Monte Carlo algorithms. Noninformative priors were used to allow the observed trial data to explain effect estimates.19 For primary analysis, we used the gemtc package26 in R, version 3.6.2,27 with 4 parallel Markov chains consisting of 100 000 samples after a 5000-sample burn-in. For secondary analysis, we used WinBUGS, version 1.4.3,28 with 3 parallel Markov chains consisting of 50 000 samples after a 5000-sample burn-in. We checked the statistical consistency between direct (head-to-head RCTs) and indirect (treatments sharing common comparators) evidence by fitting node-splitting models via the R gemtc package and the z test.26,29,30 Convergence of Markov chains was checked by trace plots and Gelman-Rubin diagnostic statistics.31,32 The significance level was α = .05 for statistical tests. Statistical models and WinBUGS code are available in eMethods 2 in the Supplement).
Results
Study Selection and Network Geometry
A total of 8424 unique study records were identified, including 7582 publication citations, 800 trial registrations, and 42 trial regulatory records. Full-text screening was done for 103 publication citations and all trial registrations and trial regulatory records (eFigure 1 in the Supplement).
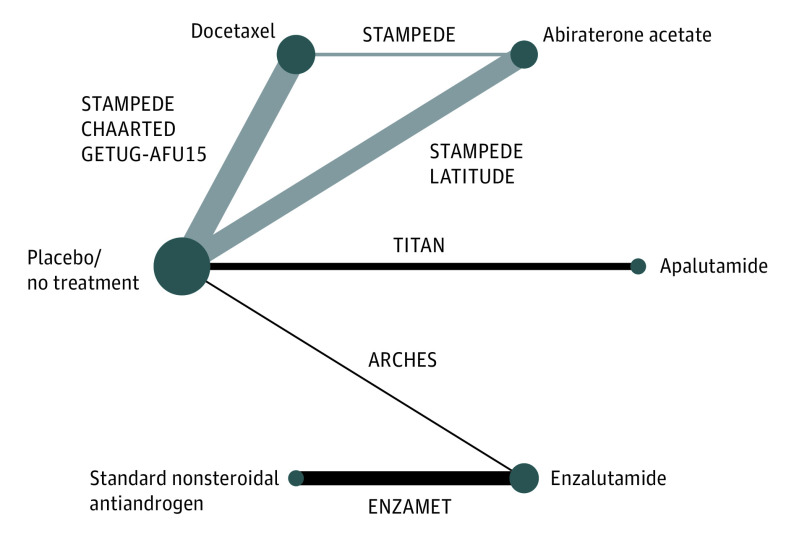
Seven trials comparing 6 treatments were analyzed (Table 1),5,6,7,8,9,10,11,12,33,34,35,36,37,38,39,40,41,42 including placebo/no treatment, a standard nonsteroidal antiandrogen (bicalutamide, nilutamide, or flutamide), docetaxel, abiraterone acetate, enzalutamide, and apalutamide. A network graph of treatment comparisons is presented in Figure 1, with nodes representing competing treatments and edges representing RCTs for pairs of treatments. The most studied treatments were docetaxel (3 trials), abiraterone acetate (2 trials), and enzalutamide (2 trials). Six RCTs5,7,11,12,33,34 used placebo/no treatment as the comparator and 1 trial35 used standard nonsteroidal antiandrogen therapy. Active treatments have not been compared in head-to-head trials except for docetaxel and abiraterone acetate, which were compared in the only multiarm RCT: the Systemic Therapy in Advancing or Metastatic Prostate Cancer: Evaluation of Drug Efficacy (STAMPEDE) trial.33
Figure 1. Network Graph of Treatment Comparison.
Graph depicts underlying evidence base of this study. Nodes (circles) represent competing treatments added to androgen-deprivation therapy and edges (lines) show which treatments have been compared. Node size proportional to the number of trials evaluating each treatment, edge thickness proportional to precision (the inverse of the variance of hazard ratios of overall survival) of each direct comparison. The labels on the edges are randomized clinical trials of pairs of treatments. Edges with gray color represent multiarm Systemic Therapy in Advancing or Metastatic Prostate Cancer: Evaluation of Drug Efficacy (STAMPEDE) trial. Study names are expanded in the footnotes to Table 1.
Characteristics of Included Trials
The 7 included trials were multicenter phase 3 RCTs published between 2013 and 2019, involving a total of 7287 patients (Table 1). The median sample size was 1125 (range, 385-1586) patients; the median duration of follow-up was 52 months (range, 14-84). The main eligibility criteria entailed newly diagnosed prostate adenocarcinoma with radiologic evidence of metastatic disease and adequate performance status. Previous chemotherapy and hormone therapy in the metastatic setting were either prohibited or restricted. The STAMPEDE trial recruited a broader patient population; we used only mCSPC data in this analysis. The LATITUDE trial required at least 2 high prognostic risk factors for eligibility7,9; we assessed the association between these factors and outcomes by subgroup analysis.
Table 1. Characteristics of Included Trials.
| Trial | Registration and documentation | No. of countries | Main eligibility criteria | Interventiona | mCSPC cases, No. | Efficacy outcomes of interestb | Median duration of follow-up, mo |
|---|---|---|---|---|---|---|---|
| GETUG-AFU1534,38 | Trial registration: NCT00104715 (no result posted); regulatory document: EMA/647024/2019 | 2 (Europe) | (1) Prostate adenocarcinoma with metastasis, (2) ADT <2 mo without disease progression, (3) ECOG 0-2, no previous chemotherapy, (4) previous hormone therapy in metastatic setting within the past 2 mo allowed | Docetaxel vs no treatment | 385 | OS, radiographic progression-free survival | 84 |
| CHAARTED5,36,37 | Trial registration: NCT00309985; regulatory document: EMA/647024/2019 | 1 (North America) | (1) Prostate cancer with metastasis, (2) ADT <4 mo without disease progression, (3) ECOG 0-2, (4) no previous chemotherapy, (4) no previous hormone therapy in metastatic setting | Docetaxel vs no treatment | 790 | OS, clinical progression-free survival | 54 |
| STAMPEDE6,8,10,33,42c | Trial registration: NCT00268476 (no result posted), 2004-000193-31; regulatory document EMA/647024/2019 | 2 (Europe) | Prostate adenocarcinoma: high-risk, newly diagnosed, nonmetastatic, node negative; newly diagnosed, metastatic or node positive; previously radically treated, now relapsing; (2) WHO performance 0-22; (3) no previous chemotherapy; (4) no previous long-term hormone therapy | Docetaxel vs no treatment | 362 vs 724 | OS, progression-free survival | 78 |
| Abiraterone vs no treatment | 500 vs 502 | 42 | |||||
| LATITUDE7,9,39,40 | Trial registration: NCT01715285; 2012-002940-26; regulatory document: FDA drug label (06/2019); EMA/816845/2017 | 34 (South Africa, Asia, Europe, North America, South America, Oceania) | (1) Newly diagnosed prostate adenocarcinoma with metastasis, (2) ADT <3 mo without disease progression, (3) number of high-risk prognostic factors ≥2,d (4) ECOG 0-2, (5) no previous chemotherapy, (6) previous hormone therapy in metastatic setting within the past 3 mo allowed | Abiraterone vs placebo | 1199 | OS, radiographic progression-free survival | 52 |
| TITAN12,41 | Trial registration: NCT02489318; 2015-000735-32; regulatory document: FDA drug label (09/2019) | 23 (Asia, Europe, North America, South America, Oceania) | (1) Prostate adenocarcinoma with bone metastasis, (2) ADT <6 mo without disease progression, (3) ECOG 0-1, (4) previous chemotherapy ≤6 cycles without disease progression allowed, (5) no previous hormone therapy in metastatic setting | Apalutamide vs placebo | 1052 | OS, radiographic progression-free survival | 23 |
| ARCHES11 | Trial registration: NCT02677896; 2015-003869-28; regulatory document: NA | 24 (Asia, Europe, North America, South America, Oceania) | (1) Prostate adenocarcinoma with metastasis, (2) ADT <3 mo without disease progression, (3) ECOG 0-1, (4) previous chemotherapy ≤6 cycles without disease progression allowed, (5) previous hormone therapy in metastatic setting within the past 3 mo allowed | Enzalutamide vs placebo | 1150 | OS, radiographic progression-free survival | 14 |
| ENZAMET35 | Trial registration: NCT02446405, regulatory document: NA | 6 (Asia, Europe, North America, Oceania) | (1) Prostate adenocarcinoma with metastasis, (2) ADT <3 mo without disease progression, (3) ECOG 0-2, (4) previous chemotherapy ≤2 cycles without disease progression allowed, (5) previous hormone therapy in metastatic setting within the past 3 mo allowed | Enzalutamide vs standard nonsteroidal antiandrogene | 1125 | OS, clinical progression-free survival | 34 |
Abbreviations: ADT, androgen-deprivation therapy; CHAARTED, ChemoHormonal Therapy vs Androgen Ablation Randomized Trial for Extensive Disease in Prostate Cancer; ECOG, Eastern Cooperative Oncology Group; ENZAMET, Enzalutamide in First Line Androgen Deprivation Therapy for Metastatic Prostate Cancer; FDA, US Food and Drug Administration; mCSPC, metastatic castration-sensitive prostate cancer; NA, not applicable; OS, overall survival; STAMPEDE, Systemic Therapy in Advancing or Metastatic Prostate Cancer: Evaluation of Drug Efficacy; TITAN, Targeted Investigational Treatment Analysis of Novel Anti-androgen; WHO, World Health Organization.
Treatment added to ADT.
Study outcomes are listed in eTable 2 in the Supplement.
The STAMPEDE trial used a multiarm, multistage platform design in which active treatments were tested sharing the same control arm; therefore, the number of patients within the control arm was counted only once, and the larger number was used in the present analysis.
LATITUDE high-risk factors: Gleason score greater than or equal to 8, presence of 3 or more lesions on bone scan, and presence of measurable visceral (excluding lymph node disease) metastasis on computed tomography or magnetic resonance imaging.
Nonsteroidal antiandrogen agents included bicalutamide, nilutamide, or flutamide.
Treatment and Assessment of Outcomes
Treatments were given until disease progression or prohibitive toxic effects occurred. Docetaxel, 75 mg/m2, was given every 3 weeks for 6 cycles with premedication or concurrent use of corticosteroids, with the exception of the GETUG-AFU15 trial, in which patients received a median of 8 cycles of docetaxel.34 Abiraterone acetate, 1000 mg/d, was given with concurrent corticosteroids; the other treatments were apalutamide, 240 mg/d, and enzalutamide, 160 mg/d.
All included trials assessed OS and SAEs, 4 trials assessed rPFS, and 3 trials assessed a modified version of rPFS. Specifically, the STAMPEDE trial33 examined progression-free survival, defined as the time from randomization to radiographic progression or death from prostate cancer, whichever occurred first. The ChemoHormonal Therapy Versus Androgen Ablation Randomized Trial for Extensive Disease in Prostate Cancer (CHAARTED) and Enzalutamide in First Line Androgen Deprivation Therapy for Metastatic Prostate Cancer (ENZAMET) trials5,35 assessed clinical progression-free survival, comprising radiographic progression, symptomatic progression/initiation of new anticancer treatment, or death as the failure events. We combined rPFS and modified rPFS, assuming that radiographic progression occurs earlier than symptomatic progression or initiation of new anticancer treatment and death from other causes. Trials also assessed prostate-specific antigen progression-free survival, time to pain progression, and other outcomes (eTable 2 in the Supplement). However, the inconsistency in outcome measures across trials precluded treatment comparison based on these outcomes.
Risk of Bias
For rPFS, all 7 trials raised some concerns regarding the overall risk of bias. For OS, the overall risk of bias was low in 2 trials (CHAARTED and ENZAMET), but the remaining 5 trials raised some concerns (Table 2). Specifically, missing outcome data raised concerns of bias in 3 trials for both outcomes.7,11,33 In these trials, the number of participants with missing data was more than 10% of the observed number of events and distributed unevenly between treatment groups, yet no analysis was done to correct for bias due to missing data and sensitivity analysis was not performed to determine whether the results were little changed under a range of plausible assumptions about the association between missing data and the true value of those data. Selection of the reported results raised concerns of bias in 2 trials12,34 for both outcomes. In these trials, the trial protocol and, when available, the statistical analysis plan, was finalized after the unblinded outcome data may have been made available to the data analysts. Measurement of the outcome raised concerns of bias in 4 trials for rPFS5,33,34,35; in these trials, the outcome assessors were aware of the intervention received by the study participants; thus, the open-label assessment of the outcome could have been influenced by the knowledge of intervention received.
Table 2. Risk of Bias Within Trials.
| Trial | Added to ADT | Randomization process | Deviation from intended intervention | Missing outcome dataa | Measurement of outcomeb | Selection of reported resultc | Overall biasd | |
|---|---|---|---|---|---|---|---|---|
| Experimental | Comparator | |||||||
| Overall survival | ||||||||
| GETUG-AFU1534,38 | Docetaxel | No treatment | Low | Low | Low | Low | Some concerns | Some concerns |
| CHAARTED5,36,37 | Docetaxel | No treatment | Low | Low | Low | Low | Low | Low |
| STAMPEDE6,8,10,33,42 | Arm 1, docetaxel; arm 2, abiraterone | No treatment | Low | Low | Some concerns | Low | Low | Some concerns |
| LATITUDE7,9,39,40 | Abiraterone | Placebo | Low | Low | Some concerns | Low | Low | Some concerns |
| TITAN12,41 | Apalutamide | Placebo | Low | Low | Low | Low | Some concerns | Some concerns |
| ARCHES11 | Enzalutamide | Placebo | Low | Low | Some concerns | Low | Low | Some concerns |
| ENZAMET35 | Enzalutamide | Standard nonsteroidal antiandrogene | Low | Low | Low | Low | Low | Low |
| Radiographic progression-free survivalf | ||||||||
| GETUG-AFU1534,38 | Docetaxel | No treatment | Low | Low | Low | Some concerns | Some concerns | Some concerns |
| CHAARTED5,36,37 | Docetaxel | No treatment | Low | Low | Low | Some concerns | Low | Some concerns |
| STAMPEDE6,8,10,33,42 | Arm 1 docetaxel; arm 2 abiraterone | No treatment | Low | Low | Some concerns | Some concerns | Low | Some concerns |
| LATITUDE7,9,39,40 | Abiraterone | Placebo | Low | Low | Some concerns | Low | Low | Some concerns |
| TITAN12,41 | Apalutamide | Placebo | Low | Low | Low | Low | Some concerns | Some concerns |
| ARCHES11 | Enzalutamide | Placebo | Low | Low | Some concerns | Low | Low | Some concerns |
| ENZAMET35 | Enzalutamide | Standard nonsteroidal antiandrogen | Low | Low | Low | Some concerns | Low | Some concerns |
Abbreviation expansions appear in footnotes to Table 1.
Concerns raised for missing outcome data when (1) number of patients with missing data was more than 10% the number of events and distributed unevenly between treatment groups, (2) missing data may relate to outcome and treatment effect, (3) no analysis to correct for bias due to missing data, and (4) no sensitivity analysis to show that results were little changed under different assumptions about the association between missing data and their true value.
Concerns raised for measurement of the outcome when the outcome assessors were unmasked and the assessment of the outcome could have been influenced.
Concerns raised for selection of the reported results when the trial protocol (statistical analysis plan) was finalized after the data cutoff date, the trial was open-label, or the trial was double-blind but the protocol specified unblinding for the analysis leading to the reported results.
Overall bias: low if all domains were low, high if at least 1 domain was high and there were some concerns in multiple domains, and some concerns otherwise.
Nonsteroidal antiandrogen agents included bicalutamide, nilutamide, or flutamide.
Radiographic progression-free survival included progression-free survival in the STAMPEDE trial and clinical progression-free survival in the CHAARTED and ENZAMET trials.
Syntheses of Results
The main results of individual trials are summarized in eTable 3 in the Supplement. Network meta-analyses included all 7 trials for effectiveness outcomes and 6 trials for safety outcomes; the STAMPEDE trial did not report safety outcomes separately for patients with mCSPC.
Efficacy Outcomes
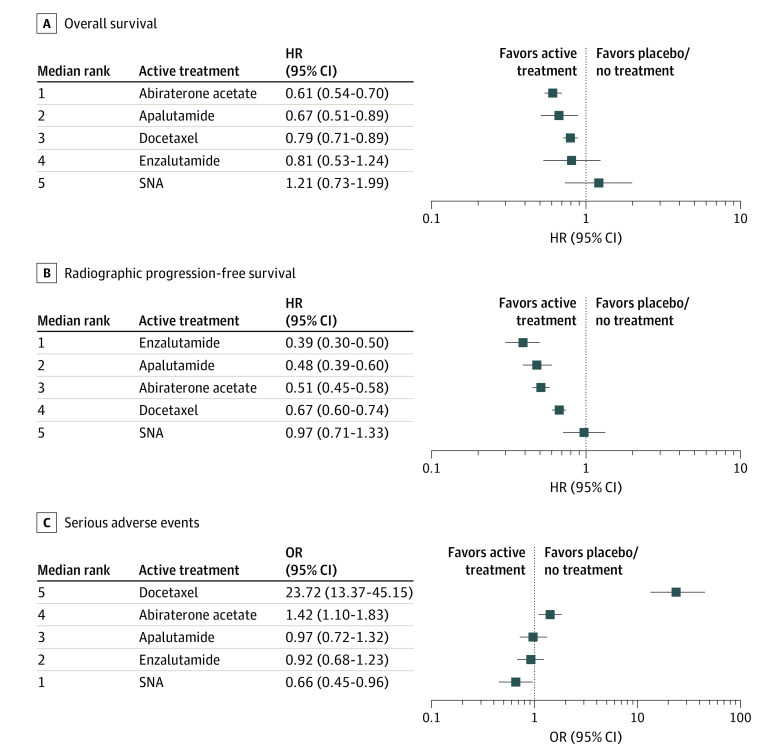
Ordered from the most to the least effective, treatments with significantly improved OS in randomized clinical trials when added to ADT included abiraterone acetate (HR, 0.61; 95% CI, 0.54-0.70), apalutamide (HR, 0.67; 95% CI, 0.51-0.89), docetaxel (HR, 0.79; 95% CI, 0.71-0.89), with nonsignificant findings for enzalutamide (HR, 0.81; 95% CI, 0.53-1.24), and standard nonsteroidal antiandrogen (HR, 1.21; 95% CI, 0.73-1.99); treatments associated with significantly improved rPFS when added to ADT included enzalutamide (HR, 0.39; 95% CI, 0.30-0.50), apalutamide (HR, 0.48; 95% CI, 0.39-0.60), abiraterone acetate (HR, 0.51; 95% CI, 0.45-0.58), docetaxel (HR, 0.67; 95% CI, 0.60-0.74), with nonsignificant findings for standard nonsteroidal antiandrogen (HR, 0.97; 95% CI, 0.71-1.33) (Figure 2). A league table presenting the overall time-invariant HR for all possible pairwise comparisons between treatments is available in eTable 4 in the Supplement. Treatment ranking probabilities suggested that abiraterone acetate had the highest probability of being the best treatment regarding OS (64%, ie, based on the available RCT evidence, there is a 64% probability that abiraterone acetate is the best treatment for patients with mCSPC regarding OS) and enzalutamide had the highest probability of being the best treatment regarding rPFS (88%) (eFigure 2 in the Supplement).
Figure 2. Treatment Ranking and Relative Effect.
CI indicates credible interval; HR, hazard ratio; OR, odds ratio.
All included trials reported time-invariant HR of rPFS for patient subgroups based on disease volume, high vs low stratified by the CHAARTED trial criteria.5 High-volume disease was defined as the presence of visceral metastases or 4 or more bone metastases, with at least 1 metastasis outside the vertebral column or pelvis. Subgroup analysis based on disease volume provided consistent results with the primary analysis. Ordered from the most to the least effective, for high-volume prostate cancers, treatments associated with significantly improved rPFS in randomized clinical trials when added to ADT included enzalutamide (HR, 0.43; 95% CI, 0.33-0.56), abiraterone acetate (HR, 0.46; 95% CI, 0.40-0.53), apalutamide (HR, 0.53; 95% CI, 0.41-0.68), docetaxel (HR, 0.57; 95% CI, 0.49-0.66), with nonsignificant findings for standard nonsteroidal antiandrogen (HR, 0.96; 95% CI, 0.67-1.36); for low-volume prostate cancers, treatments associated with significantly improved rPFS when added to ADT included enzalutamide (HR, 0.25; 95% CI, 0.14-0.45), apalutamide (HR, 0.36; 95% CI, 0.22-0.58), abiraterone acetate (HR, 0.48; 95% CI, 0.37-0.63), docetaxel (HR, 0.74; 95% CI, 0.61-0.91), with nonsignificant findings for standard nonsteroidal antiandrogen (HR, 0.83; 95% CI, 0.42-1.64) (eFigure 3 in the Supplement). Subgroup analysis based on disease volume was not feasible for OS, because the ARCHES trial did not report OS results based on disease volume.11 Subgroup analyses based on other baseline characteristics (age, Eastern Cooperative Oncology Group performance, Gleason score, prostate-specific antigen level) were not feasible because not all trials examined these subgroups and, for those that did, the cutoff points were inconsistent across trials.
Allowing the HR to change over time, parametric survival network meta-analysis provided consistent treatment ranking. Specifically, the first-order fractional polynomial model fit the OS data best when power parameter = −0.5 and fit rPFS data best when power parameter = 0 (equivalent to Weibull distribution). Figure 3 shows the expected OS and rPFS curves up to 48 months post randomization for each treatment, which are based on the estimated time-varying HRs of each treatment relative to placebo/no treatment and subsequently applied to a parametric reference curve with no treatment obtained from the STAMPEDE trial. According to the survival curves, abiraterone acetate appeared to be associated with the highest OS probability and enzalutamide appeared to be associated with the highest rPFS probability. The treatment ranking and the HR of each treatment relative to placebo/no treatment over time are presented in eFigure 4 and eFigure 5 in the Supplement.
Figure 3. Overall Survival and Radiographic Progression-Free Survival Based on Relative Treatment Effect Estimates.
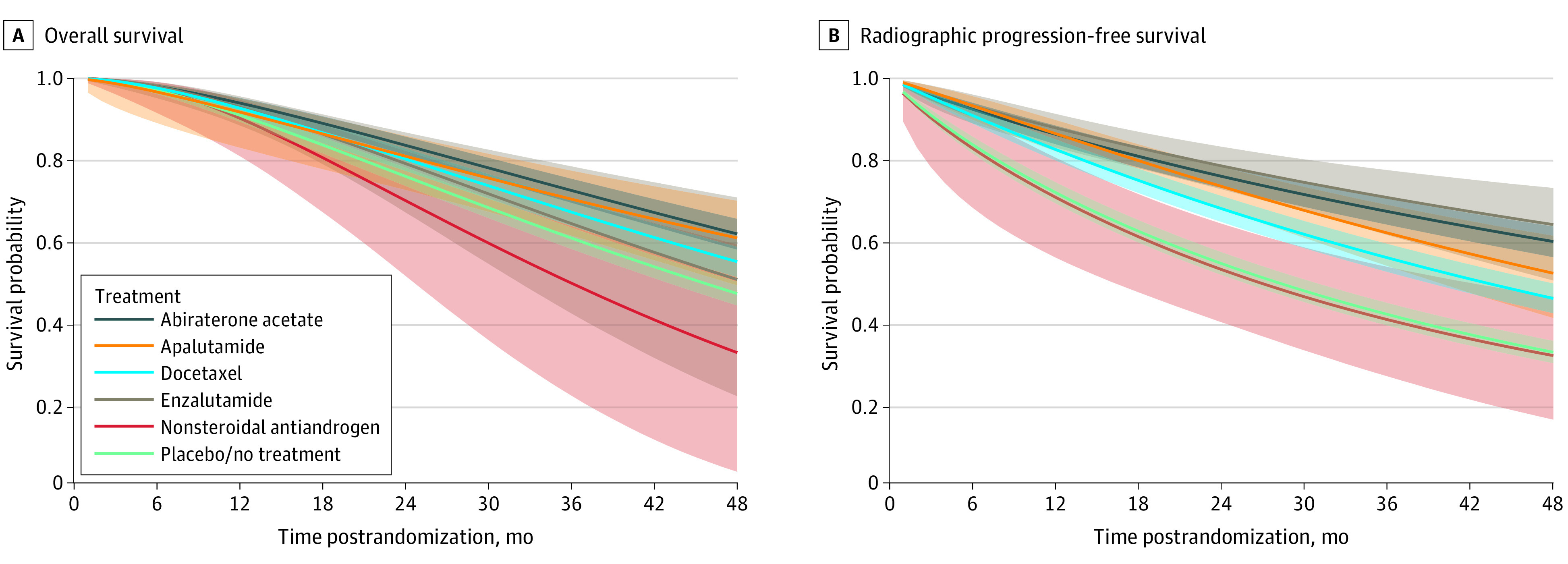
Overall (A) and progression-free survival (B). Median indicated by solid lines; 95% credible intervals indicated by shaded areas.
Safety Outcomes
According to the overall ORs compared with placebo/no treatment and median ranks, treatments ordered from the safest to the least safe regarding their associations with SAE risks were standard nonsteroidal antiandrogen (OR, 0.66; 95% CI, 0.45-0.96), enzalutamide (OR, 0.92; 95% CI, 0.68-1.23), apalutamide (0.97; 95% CI, 0.72-1.32), abiraterone acetate (1.42; 95% CI, 1.10-1.83), and docetaxel (23.72; 95% CI, 13.37-45.15) (Figure 2). A league table presenting the ORs for all possible treatment comparisons is available in eTable 4 in the Supplement. Treatment ranking probabilities suggested that standard nonsteroidal antiandrogen agents have the highest probability of being the safest (94%) regarding SAEs and docetaxel has the highest probability of being the least safe treatment (100%) (eFigure 2 in the Supplement).
Exploration for Inconsistency
According to the treatment network (Figure 1), effect estimates in the triangular loop formed by docetaxel, abiraterone acetate, and placebo/no treatment were informed by both direct and indirect evidence. We found no evidence of statistical inconsistency for any outcomes (P = .82 for OS; P = .14 for rPFS).
Discussion
This study compared systemic treatments for mCSPC to inform decision-making. We conducted a comprehensive search for eligible RCTs, critically appraised trial quality, synthesized trial data, and ranked treatments by effectiveness and safety shown in randomized clinical trials. We identified 7 eligible trials constructing a scarce network in which most treatments have not been compared in head-to-head trials, which highlights the importance of the study. Three drugs were associated with significantly improved OS when added to ADT. Among them, abiraterone acetate was associated with significantly larger OS benefit than docetaxel, and similar OS benefit to apalutamide. In terms of safety, docetaxel was associated with substantially increased SAEs, abiraterone acetate with slightly increased SAEs, and apalutamide with no increase in SAEs.
Our study provides several insights. First, we evaluated comparative drug safety, which was not evaluated in previous reviews. Second, we modeled the time-varying HR, which has not been addressed by previous reviews. We closely modeled the observed Kaplan-Meier curves and validated the robustness of results against different assumptions of HRs (time invariant vs time varying). This analysis is necessary given that nonproportional hazards were detected in the STAMPEDE trial.8,42 Third, we confirmed and updated findings of previous reviews. Our results are consistent with those of a previous review that compared abiraterone acetate, docetaxel, zoledronic acid, and celecoxib for mCSPC and suggested that abiraterone acetate may be the most effective treatment followed by docetaxel.43 Our findings are different from those of a review that suggested enzalutamide to be the most effective treatment; relative rankings of other drugs were similar to our estimates.44 However, the most recent ARCHES trial was not included in that review, which showed no OS benefit when comparing enzalutamide with placebo.11
This study is important for patients, clinicians, and payers given the uncertainty about the optimal treatment for mCSPC, which causes significant morbidity and mortality among older men. The findings of this study may be applicable to patients with mCSPC in different countries because most included trials were multinational, recruiting patients from America, Europe, Asia, and Oceania. Future cost-effectiveness research based on our findings may inform value-based decision-making.
Limitations
Our study has limitations, many of which reflect opportunities for improving the design and data sharing of the underlying RCTs that we relied on for our analysis. First, the inconsistency in outcome measures across trials precluded treatment comparison for many outcomes. Although we examined rPFS, its inconsistent definition required us to assume that radiographic progression occurs earlier than symptomatic progression and initiation of new anticancer treatment and death from other causes. This assumption is supported by observations in the LATITUDE trial in which the median rPFS was 14.7 months compared with a median time to pain progression of 16.6 months and a median time to subsequent prostate cancer therapy of 21.2 months in the placebo plus ADT arm.9 Second, subgroup analyses were not feasible for many baseline characteristics because of different cutoff levels across trials. Third, the follow-up durations were different across trials. The relatively short follow-up periods for some treatments may bias against their long-term effectiveness estimation. Specifically, trials for enzalutamide and apalutamide had 14- and 23-month follow-ups, respectively. The immature OS data may bias against these treatments, especially for enzalutamide. Fourth, although hormonal therapies are estimated to be safer than docetaxel in terms of SAEs, the results were based on RCTs with 14- to 84-month duration. In addition, although docetaxel is typically used for 6 cycles (18 weeks), hormonal therapies are administered until disease progression occurs (approximately 2 years).9 Long-term adverse effects of hormonal therapies need continuous monitoring and further assessment. Fifth, a network meta-analysis based on individual patient data, which would be more informative, was attempted but infeasible owing to suboptimal data sharing.45
Conclusions
As add-on treatments to ADT, abiraterone acetate and apalutamide may provide the largest OS benefits with relatively low SAE risks among patients with mCSPC in RCTs. Although enzalutamide may improve rPFS to the greatest extent, longer follow-up is needed to examine its OS benefits.
eMethods 1. Search Strategies
eMethods 2. Models and WINBUGS Code
eTable 1. Data Items Extracted
eTable 2. Outcomes Assessed in Included Trials
eTable 3. Results of Included for Overall Survival, Radiographic Progression Free Survival, and Serious Adverse Events
eTable 4. Relative Effect Estimates for All Possible Pairwise Treatment Comparisons
eFigure 1. Flowchart of Study Selection Process
eFigure 2. Treatment Ranking Probabilities for Overall Survival, Radiographic Progression-Free Survival, and Serious Adverse Events
eFigure 3. Network Meta-analysis Results for Subgroups Based on Disease Volume
eFigure 4. Treatment Ranking Over Time Derived From Parametric Survival Network Meta-analysis
eFigure 5. Hazard Ratios Over Time Derived From Parametric Survival Network Meta-analysis
eReferences
References
- 1.US Cancer Society. Prostate at a glance. Estimated new cases, 2020. Accessed May 28, 2020. https://cancerstatisticscenter.cancer.org/#!/cancer-site/Prostate
- 2.Cancer Stat Facts SEER. Prostate cancer. National Cancer Institute. Accessed May 28, 2020. https://seer.cancer.gov/statfacts/html/prost.html
- 3.Alpajaro SIR, Harris JAK, Evans CP. Non-metastatic castration resistant prostate cancer: a review of current and emerging medical therapies. Prostate Cancer Prostatic Dis. 2019;22(1):16-23. doi: 10.1038/s41391-018-0078-1 [DOI] [PubMed] [Google Scholar]
- 4.Sartor O, de Bono JS. Metastatic prostate cancer. N Engl J Med. 2018;378(7):645-657. doi: 10.1056/NEJMra1701695 [DOI] [PubMed] [Google Scholar]
- 5.Sweeney CJ, Chen YH, Carducci M, et al. Chemohormonal therapy in metastatic hormone–sensitive prostate cancer. N Engl J Med. 2015;373(8):737-746. doi: 10.1056/NEJMoa1503747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.James ND, Sydes MR, Clarke NW, et al. ; STAMPEDE investigators . Addition of docetaxel, zoledronic acid, or both to first-line long-term hormone therapy in prostate cancer (STAMPEDE): survival results from an adaptive, multiarm, multistage, platform randomised controlled trial. Lancet. 2016;387(10024):1163-1177. doi: 10.1016/S0140-6736(15)01037-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fizazi K, Tran N, Fein L, et al. ; LATITUDE Investigators . Abiraterone plus prednisone in metastatic, castration-sensitive prostate cancer. N Engl J Med. 2017;377(4):352-360. doi: 10.1056/NEJMoa1704174 [DOI] [PubMed] [Google Scholar]
- 8.James ND, de Bono JS, Spears MR, et al. ; STAMPEDE Investigators . Abiraterone for prostate cancer not previously treated with hormone therapy. N Engl J Med. 2017;377(4):338-351. doi: 10.1056/NEJMoa1702900 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fizazi K, Tran N, Fein L, et al. Abiraterone acetate plus prednisone in patients with newly diagnosed high-risk metastatic castration-sensitive prostate cancer (LATITUDE): final overall survival analysis of a randomised, double-blind, phase 3 trial. Lancet Oncol. 2019;20(5):686-700. doi: 10.1016/S1470-2045(19)30082-8 [DOI] [PubMed] [Google Scholar]
- 10.Hoyle AP, Ali A, James ND, et al. ; STAMPEDE Investigators . Abiraterone in “high-” and “low-risk” metastatic hormone-sensitive prostate cancer. Eur Urol. 2019;76():719-728. doi: 10.1016/j.eururo.2019.08.006 [DOI] [PubMed] [Google Scholar]
- 11.Armstrong AJ, Szmulewitz RZ, Petrylak DP, et al. ARCHES: a randomized, phase III study of androgen deprivation therapy with enzalutamide or placebo in men with metastatic hormone-sensitive prostate cancer. J Clin Oncol. 2019;37(32):2974-2986. doi: 10.1200/JCO.19.00799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chi KN, Agarwal N, Bjartell A, et al. ; TITAN Investigators . Apalutamide for metastatic, castration-sensitive prostate cancer. N Engl J Med. 2019;381(1):13-24. doi: 10.1056/NEJMoa1903307 [DOI] [PubMed] [Google Scholar]
- 13.European Association of Oncology. Guidelines on prostate cancer. Full-text guidelines. Published 2020.Accessed May 28, 2020 https://uroweb.org/guidelin.e/prostate-cancer/
- 14.National Comprehensive Cancer Network. Prostate cancer: version 2.2020. Accessed May 28, 2020. https://www.nccn.org/professionals/physician_gls/pdf/prostate.pdf
- 15.US Department of Veterans Affairs National Acquisition Center (CCST) . Published 2019. Accessed October 27, 2019. https://www.vendorportal.ecms.va.gov/nac/Pharma/List
- 16.Moher D, Liberati A, Tetzlaff J, Altman DG; PRISMA Group . Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6(7):e1000097. doi: 10.1371/journal.pmed.1000097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hutton B, Salanti G, Caldwell DM, et al. The PRISMA extension statement for reporting of systematic reviews incorporating network meta-analyses of health care interventions: checklist and explanations. Ann Intern Med. 2015;162(11):777-784. doi: 10.7326/M14-2385 [DOI] [PubMed] [Google Scholar]
- 18.Sterne JAC, Savović J, Page MJ, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. 2019;366:l4898. doi: 10.1136/bmj.l4898 [DOI] [PubMed] [Google Scholar]
- 19.Sofia Dias AEA, Welton NJ, Jansen JP, Sutton AJ. Network Meta-Analysis for Decision-Making (Statistics in Practice). Wiley; 2018. [Google Scholar]
- 20.Franchini AJ, Dias S, Ades AE, Jansen JP, Welton NJ. Accounting for correlation in network meta-analysis with multi-arm trials. Res Synth Methods. 2012;3(2):142-160. doi: 10.1002/jrsm.1049 [DOI] [PubMed] [Google Scholar]
- 21.Rohatigi A. WebPlotDigitizer, version 4.3. Published 2020. Accessed October 23, 2020. https://automeris.io/WebPlotDigitizer
- 22.Guyot P, Ades AE, Ouwens MJ, Welton NJ. Enhanced secondary analysis of survival data: reconstructing the data from published Kaplan-Meier survival curves. BMC Med Res Methodol. 2012;12:9. doi: 10.1186/1471-2288-12-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wei Y, Royston P. Reconstructing time-to-event data from published Kaplan-Meier curves. Stata J. 2017;17(4):786-802. doi: 10.1177/1536867X1801700402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jansen JP. Network meta-analysis of survival data with fractional polynomials. BMC Med Res Methodol. 2011;11:61. doi: 10.1186/1471-2288-11-61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Spiegelhalter DJ, Best N, Carlin BP, Linde A. Bayesian measures of model complexity and fit (with discussion). J Royal Stat Soc. 2002;64:583-639. doi: 10.1111/1467-9868.00353 [DOI] [Google Scholar]
- 26.Van Valkenhoef G, Kuiper J. gemtc: network meta-analysis using bayesian methods. R package, version 0.8-4. Updated August 10, 2020. Accessed May 28, 2020. https://CRAN.R-project.org/package=gemtc
- 27.R Foundation for Statistical Computing. R: a language and environment for statistical computing. Published 2019. Accessed May 28, 2020. https://www.R-project.org/
- 28.Lunn DJ, Thomas A, Best N, Spiegelhalter D. WinBUGS: a bayesian modelling framework: concepts, structure, and extensibility. Stat Comput. 2000;10:325-337. doi: 10.1023/A:1008929526011 [DOI] [Google Scholar]
- 29.Dias S, Welton NJ, Caldwell DM, Ades AE. Checking consistency in mixed treatment comparison meta-analysis. Stat Med. 2010;29(7-8):932-944. doi: 10.1002/sim.3767 [DOI] [PubMed] [Google Scholar]
- 30.Van Valkenhoef G, Dias S, Ades AE, Welton NJ. Automated generation of node-splitting models for assessment of inconsistency in network meta-analysis. Res Synth Methods. 2016;7(1):80-93. doi: 10.1002/jrsm.1167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gelman A. Inference and monitoring convergence. In: Markov Chain Monte Carlo in Practice. London: Chapman & Hall; 1996. [Google Scholar]
- 32.Brooks S, Gelman A. General methods for monitoring convergence of iterative simulations. J Comput Graph Stat. 1998;7:434-455. [Google Scholar]
- 33.Sydes MR, Spears MR, Mason MD, et al. ; STAMPEDE Investigators . Adding abiraterone or docetaxel to long-term hormone therapy for prostate cancer: directly randomised data from the STAMPEDE multi-arm, multi-stage platform protocol. Ann Oncol. 2018;29(5):1235-1248. doi: 10.1093/annonc/mdy072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gravis G, Fizazi K, Joly F, et al. Androgen-deprivation therapy alone or with docetaxel in non-castrate metastatic prostate cancer (GETUG-AFU 15): a randomised, open-label, phase 3 trial. Lancet Oncol. 2013;14(2):149-158. doi: 10.1016/S1470-2045(12)70560-0 [DOI] [PubMed] [Google Scholar]
- 35.Davis ID, Martin AJ, Stockler MR, et al. ; ENZAMET Trial Investigators and the Australian and New Zealand Urogenital and Prostate Cancer Trials Group . Enzalutamide with standard first-line therapy in metastatic prostate cancer. N Engl J Med. 2019;381(2):121-131. doi: 10.1056/NEJMoa1903835 [DOI] [PubMed] [Google Scholar]
- 36.Kyriakopoulos CE, Chen YH, Carducci MA, et al. Chemohormonal therapy in metastatic hormone-sensitive prostate cancer: long-term survival analysis of the randomized phase III E3805 CHAARTED trial. J Clin Oncol. 2018;36(11):1080-1087. doi: 10.1200/JCO.2017.75.3657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Morgans AK, Chen YH, Sweeney CJ, et al. Quality of life during treatment with chemohormonal therapy: analysis of E3805 chemohormonal androgen ablation randomized trial in prostate cancer. J Clin Oncol. 2018;36(11):1088-1095. doi: 10.1200/JCO.2017.75.3335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gravis G, Boher JM, Joly F, et al. ; GETUG . Androgen deprivation therapy (ADT) plus docetaxel versus ADT alone in metastatic non castrate prostate cancer: impact of metastatic burden and long-term survival analysis of the randomized phase 3 GETUG-AFU15 Trial. Eur Urol. 2016;70(2):256-262. doi: 10.1016/j.eururo.2015.11.005 [DOI] [PubMed] [Google Scholar]
- 39.Chi KN, Protheroe A, Rodríguez-Antolín A, et al. Patient-reported outcomes following abiraterone acetate plus prednisone added to androgen deprivation therapy in patients with newly diagnosed metastatic castration-naive prostate cancer (LATITUDE): an international, randomised phase 3 trial. Lancet Oncol. 2018;19(2):194-206. doi: 10.1016/S1470-2045(17)30911-7 [DOI] [PubMed] [Google Scholar]
- 40.Fukasawa S, Suzuki H, Kawaguchi K, et al. Efficacy and safety of abiraterone acetate plus prednisone in Japanese patients with newly diagnosed, metastatic hormone-naïve prostate cancer: a subgroup analysis of LATITUDE, a randomized, double-blind, placebo-controlled, Phase 3 study. Jpn J Clin Oncol. 2018;48(11):1012-1021. doi: 10.1093/jjco/hyy129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Agarwal N, McQuarrie K, Bjartell A, et al. ; TITAN investigators . Health-related quality of life after apalutamide treatment in patients with metastatic castration-sensitive prostate cancer (TITAN): a randomised, placebo-controlled, phase 3 study. Lancet Oncol. 2019;20(11):1518-1530. doi: 10.1016/S1470-2045(19)30620-5 [DOI] [PubMed] [Google Scholar]
- 42.Clarke NW, Ali A, Ingleby FC, et al. Addition of docetaxel to hormonal therapy in low- and high-burden metastatic hormone sensitive prostate cancer: long-term survival results from the STAMPEDE trial. Ann Oncol. 2019;30(12):1992-2003. doi: 10.1093/annonc/mdz396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Vale CL, Fisher DJ, White IR, et al. What is the optimal systemic treatment of men with metastatic, hormone-naive prostate cancer? a STOPCAP systematic review and network meta-analysis. Ann Oncol. 2018;29(5):1249-1257. doi: 10.1093/annonc/mdy071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sathianathen NJ, Koschel S, Thangasamy IA, et al. Indirect comparisons of efficacy between combination approaches in metastatic hormone-sensitive prostate cancer: a systematic review and network meta-analysis. Eur Urol. 2020;77(3):365-372. doi: 10.1016/j.eururo.2019.09.004 [DOI] [PubMed] [Google Scholar]
- 45.Wang L, Hong H, Paller C, Brawley O, Li T. Is current trial data sharing status conducive for evidence generation for personalized medicine: a failed attempt to conduct an individual pattient trial data network meta-analysis. Drugs Generics Org Pract. 2020;23(suppl 1):S139-S140. doi: 10.1016/j.jval.2020.04.345 [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eMethods 1. Search Strategies
eMethods 2. Models and WINBUGS Code
eTable 1. Data Items Extracted
eTable 2. Outcomes Assessed in Included Trials
eTable 3. Results of Included for Overall Survival, Radiographic Progression Free Survival, and Serious Adverse Events
eTable 4. Relative Effect Estimates for All Possible Pairwise Treatment Comparisons
eFigure 1. Flowchart of Study Selection Process
eFigure 2. Treatment Ranking Probabilities for Overall Survival, Radiographic Progression-Free Survival, and Serious Adverse Events
eFigure 3. Network Meta-analysis Results for Subgroups Based on Disease Volume
eFigure 4. Treatment Ranking Over Time Derived From Parametric Survival Network Meta-analysis
eFigure 5. Hazard Ratios Over Time Derived From Parametric Survival Network Meta-analysis
eReferences