Abstract
This cohort study describes the number of patients undergoing cancer screening tests and of ensuing cancer diagnoses during the COVID-19 pandemic in 1 health care system in the northeastern United States.
Oncology patient care may be disrupted secondary to coronavirus disease 2019 (COVID-19) through delays in diagnostic investigations and surgical procedures, as well as delayed cancer diagnoses because of reduced cancer screening. This study assesses the number of patients undergoing cancer screening tests and of ensuing cancer diagnoses during the COVID-19 pandemic in the largest health care system in the northeastern United States, Massachusetts General Brigham.
Methods
This study comprised four 3-month periods. One period, during the first peak of the pandemic in the New England area of the United States (from March 2 to June 2, 2020),1 was compared with 3 control periods before and after the main study period (the preceding 3 months from December 1, 2019, to March 2, 2020; the same 3 months in the preceding year from March 2 to June 2, 2019; and the 3 months after the main study period from June 3 to September 3, 2020). The percentage decrease in screening tests and in diagnoses during the pandemic period compared with each of the control periods was computed as percentage decrease = (Npandemic − Ncontrol)/Ncontrol. The 95% CIs were computed using the Clopper-Pearson method using the DescTools package in R. All analyses were performed using R, version 3.6.1 (R Foundation for Statistical Computing) (eMethods in the Supplement). Ethical approval for the study was provided by Brigham and Women’s Hospital prior to commencement of data analysis, including a waiver of the requirement for individual patient consent given the retrospective and noninterventional nature of the research.
Results
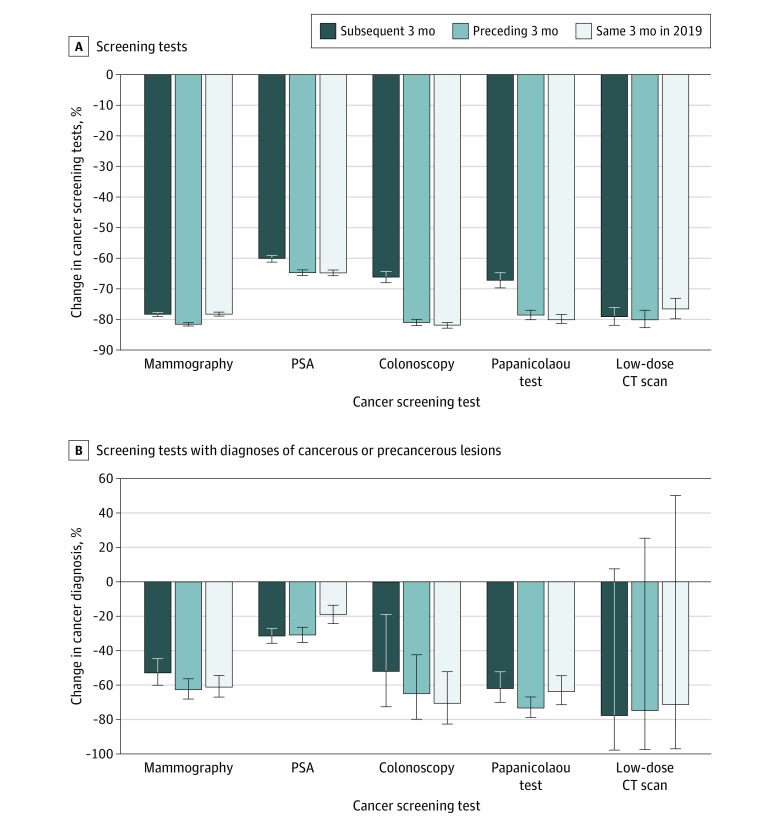
A total of 192 060 patients underwent screening during the 4 screening periods. The overall mean (SD) age was 59.6 (12.2) years, 58.6% of all patients were female, and 80.1% were non-Hispanic White. Overall, 15 453 patients (with 1985 ensuing diagnoses) had undergone 1 of the 5 cancer screening examinations (low-dose computed tomography, Papanicolaou test, colonoscopy, prostate-specific antigen screening, or mammography) during the 3-month pandemic study period, compared with 51 944 patients (3190 diagnoses) during the subsequent 3 months, 64 269 patients (3423 diagnoses) in the preceding 3 months, and 60 344 patients (2961 diagnoses) during the same 3 months of the preceding year (2019). The decrease in screening tests was accompanied by decreases in ensuing diagnoses and was found across the 5 screening tests (Figure 1). The percentage of positivity of screening tests appeared to be higher during the primary pandemic period compared with the 3 control periods for mammographies (4.1% vs 1.9%-2.3%), prostate-specific antigen screenings (22.7% vs 9.9%-13.2%), colonoscopies (1.3% vs 0.7%-0.9%), and Papanicolaou tests (11.6% vs 6.5%-10.0%), but not for low-dose computed tomography scans (0.8% vs 0.7%-0.8%). The percentage decreases in screening were pronounced across all screening tests, compared with all 3 control periods, and ranged from –60% to –82% (Figure 2A). The percentage decreases in diagnoses resulting from the cancer screening tests, compared with all 3 control periods, were also pronounced (–19% to –78%; Figure 2B). Assuming the same number of patients (64 269) would have otherwise been screened during the pandemic period as in the previous 3 months, approximately 1438 cancerous and precancerous lesion diagnoses (1985 vs 3423 diagnoses) were “missed” during the primary pandemic period.
Figure 1. Changes in the Numbers of Cancer Screening Tests and Ensuing Diagnoses.
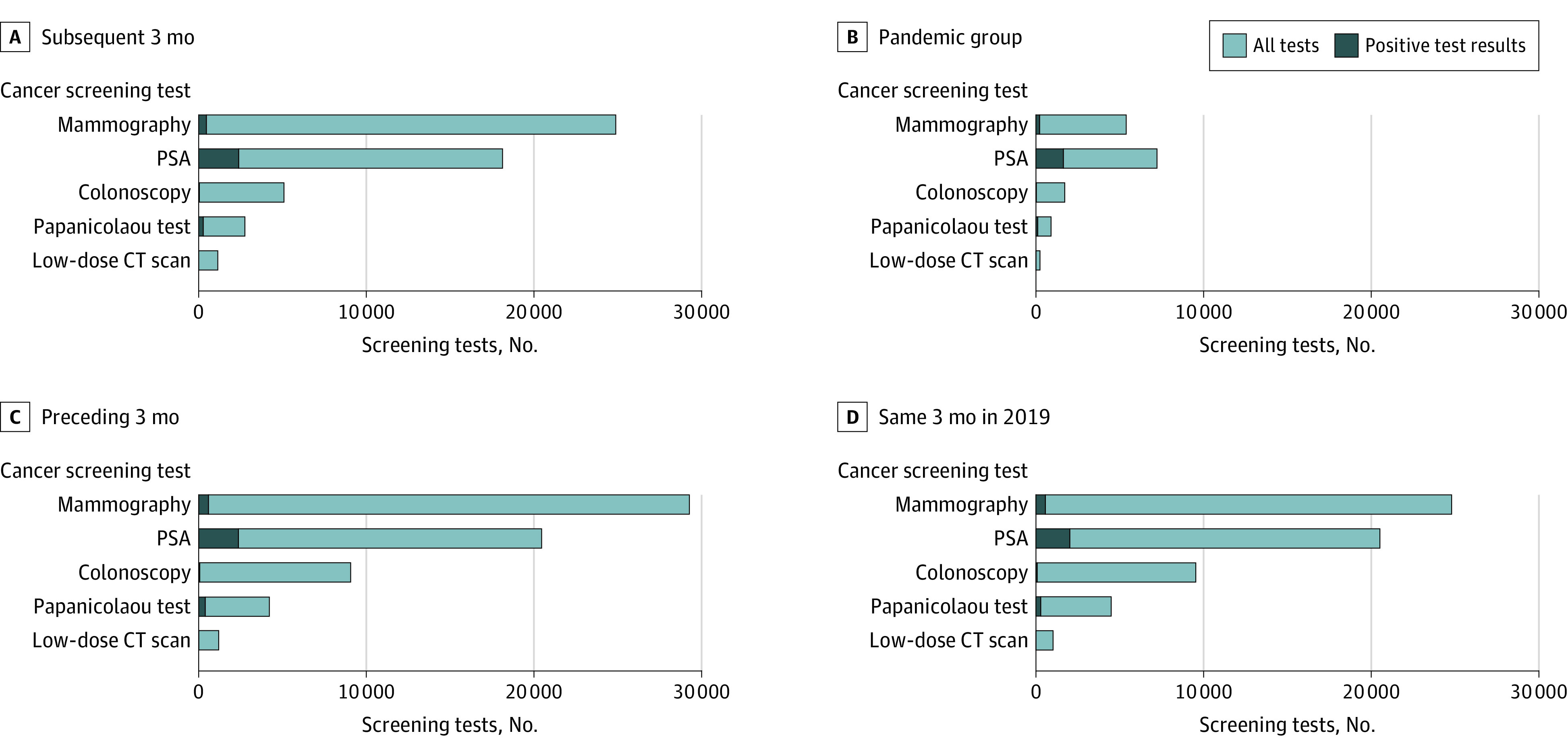
Changes in the numbers of cancer screening tests and ensuing diagnoses by cancer screening test and screening period during the primary pandemic study period compared with 3 control periods (subsequent 3 months, preceding 3 months, and same 3 months in the preceding year). CT indicates computed tomography; PSA, prostate-specific antigen.
Figure 2. Percentage Decreases in the Numbers of Screening Tests and Ensuing Diagnoses.
Percentage decreases during the primary pandemic study period compared with 3 control periods (subsequent 3 months, preceding 3 months, and same 3 months in the preceding year) in the number of screening tests (A) and in the number of screening tests leading to diagnoses of cancerous or precancerous lesions (B). Error bars indicate 95% CIs. CT indicates computed tomography; PSA, prostate-specific antigen.
Discussion
This study reports a significant decrease in the number of patients undergoing screening tests for cancer and in the number of ensuing diagnoses of cancerous and precancerous lesions during the COVID-19 pandemic in 1 health care system in the Northeastern United States. We found that, from June to September 2020, there was a significant recovery in the number of screening tests and ensuing diagnoses, to almost prepandemic levels. Moreover, we report that the number of potential “missed” diagnoses during the primary pandemic period were likely lower than would have been expected because the percentage of screening tests leading to a diagnosis of a cancerous or precancerous lesion was higher during the primary pandemic period, which may reflect the prioritization of high-risk patients for cancer screening during the pandemic. The limitations of this study include the incomplete capture of the population of Massachusetts and not accounting for patients who may have transitioned their screening procedures closer to home during the pandemic to a clinician not captured in the network.
eMethods.
eReferences.
Reference
- 1.Mass.gov. Archive of COVID-19 cases in Massachusetts. Accessed October 25, 2020. https://www.mass.gov/info-details/archive-of-covid-19-cases-in-massachusetts
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eMethods.
eReferences.