This randomized clinical trial evaluates the clinical benefits of sequential chemoradiation and concurrent chemoradiation compared with radiation alone as a postoperative adjuvant treatment in early-stage cervical cancer.
Key Points
Question
When compared with radiation alone, does sequential chemoradiation or concurrent chemoradiation improve disease-free survival (DFS) in patients with early-stage cervical cancer after radical hysterectomy?
Findings
In this phase 3 randomized clinical trial of 1048 eligible women, sequential chemoradiation improved 3-year DFS and distant DFS compared with concurrent chemoradiation or radiation alone, with tolerable toxic effects, especially in patients with high-risk factors. However, no substantial improvements in survival were seen among patients receiving concurrent chemoradiation compared with radiation alone.
Meaning
Sequential chemoradiation may be an optimal adjuvant treatment after radical hysterectomy for early-stage cervical cancer.
Abstract
Importance
There is no current consensus on the role of chemotherapy in addition to radiation for postoperative adjuvant treatment of patients with early-stage cervical cancer with adverse pathological factors.
Objective
To evaluate the clinical benefits of sequential chemoradiation (SCRT) and concurrent chemoradiation (CCRT) compared with radiation alone (RT) as a postoperative adjuvant treatment in early-stage cervical cancer.
Design, Setting, and Participants
After radical hysterectomy at 1 of 8 participating hospitals in China, patients with FIGO (International Federation of Gynecology and Obstetrics) stage IB to IIA cervical cancer with adverse pathological factors were randomized 1:1:1 to receive adjuvant RT, CCRT, or SCRT. Data were collected from February 2008 to December 2018.
Interventions
Patients received adjuvant RT (total dose, 45-50 Gy), CCRT (weekly cisplatin, 30-40 mg/m2), or SCRT (cisplatin, 60-75 mg/m2, plus paclitaxel, 135-175 mg/m2) in a 21-day cycle, given 2 cycles before and 2 cycles after radiotherapy, respectively.
Main Outcomes and Measures
The primary end point was the rate of disease-free survival (DFS) at 3 years.
Results
A total of 1048 women (median [range] age, 48 [23-65] years) were included in the analysis (350 in the RT group, 345 in the CCRT group, and 353 in the SCRT group). Baseline demographic and disease characteristics were balanced among the treatment groups except that the rate of lymph node involvement was lowest in the RT group (18.3%). In the intention-to-treat population, SCRT was associated with a higher rate of DFS than RT (3-year rate, 90.0% vs 82.0%; hazard ratio [HR], 0.52; 95% CI, 0.35-0.76) and CCRT (90.0% vs 85.0%; HR, 0.65; 95% CI, 0.44-0.96). Treatment with SCRT also decreased cancer death risk compared with RT (5-year rate, 92.0% vs 88.0%; HR, 0.58; 95% CI, 0.35-0.95) after adjustment for lymph node involvement. However, neither DFS nor cancer death risk was different among patients treated with CCRT or RT.
Conclusions and Relevance
In this randomized clinical trial, conducted in a postoperative adjuvant treatment setting, SCRT, rather than CCRT, resulted in a higher DFS and lower risk of cancer death than RT among women with early-stage cervical cancer.
Trial Registration
ClinicalTrials.gov Identifier: NCT00806117
Introduction
Cervical cancer is a life-threatening disease that affects more than 560 000 women worldwide, with the majority of these women living in resource-limited countries.1 Radical surgery remains the preferred treatment for early-stage cervical cancer in most countries. After radical surgery, patients with pathological high-risk factors, such as parametrial invasion, positive surgical margins, and lymph node metastasis, should receive adjuvant radiotherapy with concurrent cisplatin or cisplatin-based chemotherapy.2,3,4,5,6 Even then, approximately 30% of patients will relapse, which raises the need for more effective treatments.7 Additionally, for patients presenting with intermediate-risk factors, such as large tumor size, deep stromal invasion, or lymphatic vascular space involvement, there is no current consensus on the role of chemotherapy in addition to adjuvant radiotherapy. Thus, the current modality for risk-adapted postoperative treatment needs further investigation.3,8,9,10,11
Paclitaxel combined with cisplatin is a well-tolerated regimen with favorable response in both neoadjuvant and palliative treatment settings for cervical cancer. Sehouli and colleagues12 reported that combined chemotherapy and radiation sequentially administered in adjuvant treatment showed noninferiority in efficacy with fewer hematological toxic effects than concurrent chemoradiation. The integration of chemotherapy in the adjuvant radiation setting deserves further investigation.
In this prospective randomized clinical trial (Comparison of Different Subsequent Treatments After Radical Surgery, or STARS), we propose a new modality of adjuvant treatment used in some areas in China with limited radiation resources. This modality can be shortly administered after surgery, including sequential systemic chemotherapy and radiotherapy. We present herein the results of the study investigating 3 modalities for adjuvant therapy after radical hysterectomy.
Methods
Study Design and Participants
This is an open-label, phase 3, multicenter, randomized clinical trial aiming to assess the efficacy and safety of the addition of concurrent or sequential chemotherapy to radiation as postoperative adjuvant therapy for patients with cervical cancer with pathological adverse factors. The study was conducted in compliance with the Declaration of Helsinki13 and Good Clinical Practice guidelines. The protocol was approved by the Scientific Committee of the Department of Medical Sciences, Sun Yat-sen University and the Ethics Committee of Sun Yat-sen University Cancer Center (Supplement 1). All patients provided written informed consent before enrollment.
Patients were eligible if they had squamous cell, adenocarcinoma, or adenosquamous carcinoma of the uterine cervix, FIGO (International Federation of Gynecology and Obstetrics) stage IB1, IB2, IIA1, or IIA2; underwent type II or III radical hysterectomy (Piver classification) and pelvic lymphadenectomy; and had at least 1 of the following adverse factors confirmed by postoperative pathological examination: lymph node metastasis, positive parametrium or margins, lymphatic vascular space involvement, or deep stromal invasion. Exclusion criteria included unresectable tumor and peritoneal metastases identified in surgery. The trial was conducted at 8 hospitals in China (Sun Yat-sen University Cancer Center, Guangdong Provincial People's Hospital, Guangzhou Panyu Central Hospital, Hunan Cancer Hospital, Shenzhen People's Hospital, Yunnan Cancer Hospital, Guangxi Medical University Affiliated Tumor Hospital, The First Affiliated Hospital of Sun Yat-sen University).
Randomization and Masking
Random assignment was completed at the Clinical Trial Center of Sun Yat-sen University Cancer Center by a computer-generated random number code. Details of the group allocations were maintained in sequentially numbered, opaque, sealed envelopes prepared by a statistician with no clinical involvement in the trial. Patients were randomly assigned in a 1:1:1 ratio to receive adjuvant radiation alone (RT), concurrent chemoradiation (CCRT), or sequential chemoradiation (SCRT). After acquisition of a postoperative pathology report, randomization was performed centrally with stratification according to tumor size (≤4 cm vs >4 cm) and participating centers based on the random permuted blocks method. Treatment allocation was not blinded. After informed consent was obtained from eligible patients, a clinical research coordinator in Sun Yat-sen University Cancer Center opened the envelopes sequentially, assigned the patients to interventions, and told the investigators at each center by telephone.
Treatments
Patients in the RT and CCRT groups started radiation within 6 weeks after surgery. The CCRT group was given weekly cisplatin, 30 to 40 mg/m2, for a maximum of 6 doses during radiation. The SCRT group began chemotherapy at 5 to 14 days after surgery, followed by radiation. Chemotherapy in the SCRT group was given 2 cycles prior to and 2 cycles after radiotherapy, respectively, consisting of cisplatin, 60 to 75 mg/m2, on day 1 and 2 plus paclitaxel, 135 to 175 mg/m2, on day 1 in a 21-day cycle. Additional dosing details are described in eProcedures in Supplement 2. After completion of treatments, patients were followed up every 3 months during the first 2 years and every 6 months thereafter or as clinically indicated.
Outcomes
The primary end point was disease-free survival (DFS), which was defined as the time from randomization to disease recurrence. Patients who were alive without recurrence or lost to follow-up were censored at last available assessment. Patients who died from other causes without prior recurrence were censored at the date of death. Secondary end points included overall survival (OS), distant DFS, and toxic effects assessed by the National Cancer Institute Common Terminology Criteria for Adverse Events, version 4.0.14 Exploratory analysis included the patients’ quality of life and modified DFS, which was defined as the time from the end of treatment to disease recurrence. We defined OS as the time from randomization to death. Quality of life is not presented in this article because of limited space.
Statistical Analysis
The primary hypothesis of this study was that CCRT or SCRT would lead to better survival in comparison with RT. A recruitment of 990 patients over 5 years and a 3-year additional follow-up would provide the trial with 85% power to detect a 10% improvement in 3-year DFS in either the CCRT (80%) or SCRT (80%) groups compared with the RT (70%) group at a 2-sided significance level (P = .05). The primary DFS analysis was performed using log-rank test, with hazard ratios (HRs) and 95% CIs estimated using a Cox proportional hazards model with a 2-sided P < .05 considered statistically significant. A further exploratory analysis for comparison between the 2 groups of SCRT and CCRT was also performed with the same methods (P < .05 was required). Comparisons of cumulative incidences of recurrence by the Fine-Gray competing risk model across treatment groups were performed using R software, version 4.0.2 (R Foundation).
Efficacy was analyzed in the intention-to-treat population and per-protocol population. The per-protocol population was defined as those who underwent at least 45 Gy radiation in each group, with 5 doses of weekly cisplatin in the CCRT group and 4 cycles of the combination of paclitaxel and cisplatin in the SCRT group. Continuous variables were compared with parametric methods if normal distribution was confirmed. Non-normally distributed variables and categorical data were compared with nonparametric tests. Survival curves were generated by Kaplan-Meier method. A Cox proportional hazards model was used to perform univariate and multivariate analyses. Unless otherwise stated, all analyses were performed with a 2-sided significance level of P = .05 using SPSS statistical software, version 23.0 (IBM). In subgroup analyses, high-risk factors were defined as lymph node metastasis or positive parametrium or margins, and intermediate-risk factors as lymphatic vascular space involvement or deep stromal invasion.
Results
Patients and Treatments
Between February 2008 and August 2015, a total of 1080 women were enrolled from 8 hospitals in China. Of the total, 28 patients withdrew their consent and 4 did not meet the inclusion criteria (ineligible stage of disease). Thus, a total of 1048 patients were randomly assigned to the RT group (n = 350), CCRT group (n = 345), and SCRT group (n = 353), then finally included in the intention-to-treat population (Figure 1). There were 15 patients in the RT group, 13 in the CCRT group, and 15 in the SCRT group lost to follow-up. The data of efficacy and safety were updated in December 2018.
Figure 1. Randomization and Intervention of the Cohort.

The baseline demographic and clinical characteristics were well balanced among the 3 groups except for lymph node metastasis. The RT group presented a lower rate (18.3%) of lymph node metastasis than the CCRT group (30.1%) and SCRT group (29.7%; P = .01; eTable 1 in Supplement 2). The majority of patients underwent open surgery, and only 81 of the 1048 (7.7%) underwent laparoscopic surgery. The 221 of 262 (84.4%) patients with tumor larger than 4 cm at diagnosis received neoadjuvant chemotherapy. The detailed regimens and cycles of neoadjuvant chemotherapy are provided in eTable 2 in Supplement 2. These 2 factors were evenly distributed among the 3 groups.
A total of 324 of 328 (98.8%) patients in the RT group, 190 of 305 (62.3%) patients in the CCRT group, and 235 of 320 (73.4%) patients in the SCRT group completed the assigned treatments according to protocol (P < .001). The major reasons for discontinuation of treatment were grade 3 or 4 toxic effects or intolerableness to treatment (Figure 1). The mean (SD) overall dose of radiation was 47.83 (4.66) Gy, and neither dose intensity nor duration of radiation was significantly different among the 3 groups (eTable 3 in Supplement 2). The median (range) intervals between surgery and adjuvant treatment were 32 (13-70) days in the RT group, 33 (12-70) days in the CCRT group, and 8 (4-36) days in the SCRT group (P = .005).
Efficacy
After a median follow-up of 56 (interquartile range, 42-80) months, 172 of 1048 (16.4%) patients experienced disease recurrences or disease-specific deaths. In the intention-to-treat population, 68 of 350 (19.4%) patients in the RT group, 60 of 345 (17.4%) patients in the CCRT group, and 44 of 353 (12.5%) patients in the SCRT group developed recurrences (eTable 4 in Supplement 2). Adjuvant treatment with SCRT was associated with a higher rate of DFS at 3 years than treatment with RT (90.0% vs 82.0%; HR, 0.61 [95% CI, 0.42-0.89]; P = .01) and with CCRT (90.0% vs 85.0%; HR, 0.67 [95% CI, 0.46-0.99]; P = .04). After adjustment for the status of lymph node, the differences of risk for recurrence remained more significant among the treatments (SCRT vs RT: HR, 0.52 [95% CI, 0.35-0.76]); and SCRT vs CCRT: HR, 0.65 [95% CI, 0.44-0.96]; Figure 2A and Table). Moreover, the SCRT group had fewer distant metastases (23 of 350 [6.5%] patients) than the RT group (37 of 345 [10.6%] patients; P = .05) and the CCRT group (38 of 353 [11.0%] patients; P = .04). Unless otherwise indicated, all HRs were estimated with adjustment of lymph node status in each of analyses. In the Fine-Gray competing risk model, the deaths from other causes were considered competing events. The patients receiving SCRT appeared to have lower cumulative incidence of recurrence compared with those receiving RT or CCRT by this model (eFigure 6 and eTable 8 in Supplement 2), which were consistent with the above results.
Figure 2. Disease-Free Survival (DFS) and Overall Survival (OS) in Intention-to-Treat (ITT) and Per-Protocol (PP) Populations.

CCRT indicates concurrent chemoradiation; HR, hazard ratio; RT, radiation alone; SCRT, sequential chemoradiation.
Table. Survival Rates by Treatment Groups in Intention-to-Treat and Per-Protocol Populations.
| Treatment group | Disease-free survival | Overall survival | ||
|---|---|---|---|---|
| 3 y, % (95% CI) | Events/patients, No. (%) | 5 y, % (95% CI) | Events/patients, No. (%) | |
| Intention-to-treat population | ||||
| Radiation alone | 82 (78-86) | 68/350 (19.4) | 88 (84-92) | 39/350 (11.1) |
| Concurrent chemoradiation | 85 (81-89) | 60/345 (17.4) | 89 (85-93) | 36/345 (10.4) |
| Sequential chemoradiation | 90 (86-94) | 44/353 (12.5) | 92 (88-96) | 27/353 (7.6) |
| Per-protocol population | ||||
| Radiation alone | 82 (78-86) | 63/324 (19.4) | 88 (84-92) | 35/324 (10.8) |
| Concurrent chemoradiation | 86 (80-92) | 27/190 (14.2) | 90 (85-94) | 18/190 (9.5) |
| Sequential chemoradiation | 91 (87-95) | 26/235 (11.1) | 93 (89-97) | 14/235 (6.0) |
By December 31, 2018, a total of 100 disease-specified deaths had been reported, with 39 of 350 (11.1%) patient deaths in the RT group, 34 of 345 (9.9%) patient deaths in the CCRT group, and 27 of 353 (7.6%) patient deaths in the SCRT group (eTable 4 in Supplement 2). Sequential chemoradiation also decreased the risk of cancer-specific death compared with RT (HR, 0.58 [95% CI, 0.35-0.95]; P = .03) but did not show significant reduction in death risk compared with CCRT (HR, 0.74 [95% CI, 0.45-1.23]; P = .25; Figure 2B). The differences in DFS and OS were not statistically significant between the CCRT and RT groups (Figure 2A and B, and eFigure 1 in Supplement 2).
The per-protocol analyses showed similar results in terms of DFS and OS. In comparison with the RT group, the SCRT group had a significantly higher DFS rate and a decreased risk for cancer deaths. Again, no significant differences in DFS or OS were seen between the CCRT and RT groups in the per-protocol population (Figure 2C and D).
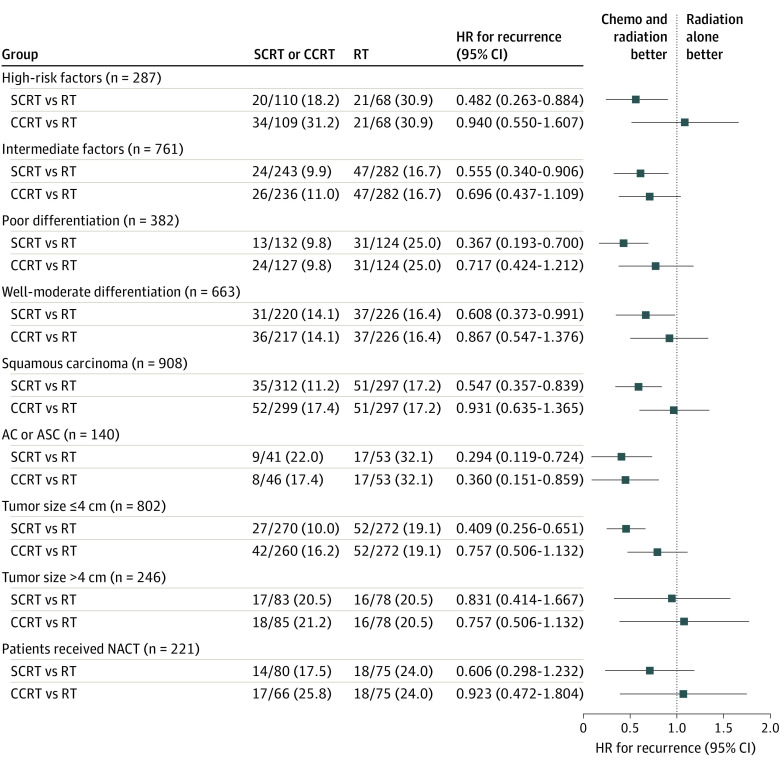
In subgroup analyses, SCRT improved DFS compared with CCRT and RT for patients with high-risk factors, and also showed DFS benefit compared with radiation alone among the intermediate-risk subgroup (eFigures 2 and 3 in Supplement 2). In most subgroups stratified by histological type, tumor grade, and tumor size, SCRT had a higher likelihood of DFS benefit compared with RT; however, CCRT did not show a superiority in DFS compared with radiation alone in subgroup analyses (Figure 3 and eFigure 4 in Supplement 2). In patients who had a cervical tumor larger than 4 cm and in those who received neoadjuvant chemotherapy, there were no statistically significant differences in DFS among the 3 treatment groups (Figure 3). The clinicopathological factors, including FIGO stage II, adenocarcinoma and adenosquamous histology, lymph node metastasis, deep stromal invasion, and lymphatic vascular space involvement, were adversely associated with DFS in multivariate analyses; however, the treatment with SCRT was correlated with better DFS (eTables 5 and 6 in Supplement 2).
Figure 3. Hazard Ratios for Recurrence in Subgroup Analysis.
AC indicates adenocarcinoma; ASC, adenosquamous carcinoma; CCRT, concurrent chemoradiation; chemo, chemotherapy; HR, hazard ratio; NACT, neoadjuvant chemotherapy; RT, radiation alone; SCRT, sequential chemoradiation.
Among patients with high-risk factors, the SCRT group presented a higher distant recurrence-free survival rate at 3 years (92% [95% CI, 86%-98%]) than the CCRT group (77% [95% CI, 69%-85%]; HR, 0.34 [95% CI, 0.16-0.73]; P = .006) and RT group (77% [95% CI, 65%-89%]; HR, 0.34 [95% CI, 0.15-0.78]; P = .01). However, the local recurrence-free survival was not statistically different between the 3 arms in either high-risk or intermediate-risk subgroups (eFigure 5 in Supplement 2).
Safety
In total, 921 patients were assessable for toxic effects (eTable 7 in Supplement 2). There were no treatment-related deaths recorded. According to the National Cancer Institute Common Terminology Criteria for Adverse Events, version 4.0,14 271 of 303 (89.4%) patients in the RT group, 291 of 298 (97.7%) patients in the CCRT group, and 311 of 320 (97.2%) patients in the SCRT group had at least 1 adverse event of any grade (P < .001). The RT group presented with the lowest rate (12.9%) of grade 3 or 4 adverse events compared with the CCRT (28.5%) and SCRT groups (25.3%) (P < .001). Between the CCRT and SCRT groups, no statistically significant differences were noted in the incidence of grade 3 or 4 hematological toxic effects (18.8% vs 19.1%; P = .93). However, the CCRT group, when compared with the SCRT group, had higher rates of grade 3 or 4 gastrointestinal toxic effects, such as nausea (6.4% vs 2.5%) and vomiting (5.4% vs 1.9%) (P = .02). In contrast, higher proportions of lymphocele and peripheral sensory neuropathy were observed in the SCRT group.
Discussion
In this prospective randomized clinical trial, adjuvant SCRT was associated with improved DFS in patients with early-stage cervical cancer who had pathological risk factors for recurrence, resulting in 48% or 35% risk reductions in recurrence compared with RT or CCRT, respectively. Moreover, the risk of death was decreased by 42% in the SCRT group compared with the RT group. In patients with high-risk factors, the distant recurrence risk was also substantially decreased in the SCRT group as compared with the RT or CCRT groups. In addition, the superiority of SCRT remained in modified DFS (period from the end of treatment to disease recurrence; eFigure 1 in Supplement 2), indicating that the DFS benefits were not at the cost of a prolonged treatment process. All of the findings together suggest that SCRT could be the most effective method among current adjuvant treatments for early-stage cervical cancer.
In Gynecologic Oncology Group Study 109,5 patients with high-risk factors were randomized to receive radiation alone or chemoradiation with 5-fluorouracil and cisplatin. The results demonstrated that both DFS and OS were substantially improved in the chemoradiation group. However, the combined regimen of 5-fluorouracil and cisplatin was not widely applied for concurrent chemoradiation in clinical practice because of toxic effects and administration inconvenience (4-day infusion per cycle).15 The combination of platinum and taxane has been widely used in cervical cancer with favorable response and acceptable toxic effects.16,17,18 In patients with advanced or recurrent cervical cancer, the regimen of platinum plus taxane revealed superior efficacy than single cisplatin.16 In clinical practice, chemoradiation with weekly cisplatin has been more widely adopted for primary treatment of locally advanced cervical cancer.19,20,21,22 However, the survival outcome of treatment with weekly cisplatin or combined regimen in addition to radiation has not been previously investigated in any randomized clinical trials for adjuvant treatment in patients with early-stage disease. Cisplatin was believed to augment the effect of radiation majorly by radiosensitization in primary treatment of cervical cancer, though there were no advantages for distant control.19
In the present study, postoperative adjuvant CCRT with single cisplatin did not meaningfully improve DFS or distant recurrence-free survival in patients with adverse factors compared with RT, whereas SCRT with combination of cisplatin and taxane did improve the prognosis, especially for the patients with high-risk factors. According to previous reports,23,24,25 patients with lymph node metastasis had a high risk for recurrence and distant failure. It is thus necessary for postoperative adjuvant therapy not only to control local recurrence, but also to prevent distant metastasis. The present study showed that the SCRT group had a statistically significantly higher distant recurrence-free survival than the other 2 groups, which indicated an important role in distant control by adding the combination of paclitaxel and cisplatin. Another study26 compared standard CCRT with single cisplatin vs SCRT with cisplatin plus gemcitabine in patients with stage IIB to IVA cervical carcinoma. The progression-free survival and OS were meaningfully improved in the SCRT arm. The distant failure rate was obviously lower in the SCRT arm (8.1%) than in the CCRT arm (16.4%). This is consistent with the findings seen in the present study.
In the subgroup with tumor size larger than 4 cm, SCRT did not show DFS superiority when compared with the other 2 groups (Figure 3). This is probably because of the small population in this subgroup. Nevertheless, the majority of patients received 2 to 3 cycles of combined neoadjuvant chemotherapy, which may decrease the risk of distant metastases.
Radiotherapy is generally not administered soon after surgery given the concerns about wound healing and complications. In the current study, median interval between surgery and adjuvant treatment in the SCRT group (8 days) was much shorter than the other 2 groups (32 days for RT and 33 days for CCRT). We believed that early initiation of adjuvant treatment after surgery would be beneficial for oncologic outcomes, as previously reported.27 Sequential chemoradiation could be an alternative and more applicable modality for adjuvant treatment in resource-limited countries where shortages of radiation resources are severe because chemotherapy can be easily administered while waiting for radiation.
In addition, chemotherapy and radiotherapy were sequentially administrated in the SCRT arm in a separate period rather than simultaneously in the same period as in the CCRT arm. Therefore, acute toxic effects caused by chemotherapy or radiotherapy were not overlapping in a short period in the sequential arm, which made SCRT more tolerable than CCRT. Cetina and colleagues28 also reported that only 67% of patients were able to complete the planned cycles in CCRT treatment. In the present study, although there were no significant differences regarding grade 3 or 4 toxic effects between the CCRT and SCRT groups, the CCRT arm had a higher rate of grade 3 or 4 gastrointestinal toxic effects and grade 1 or 2 fatigue than the SCRT arm, which may lead to intolerableness to treatment.
Limitations
Surgery approach (laparotomy or laparoscopy) was not stratified for randomization, which could be a limitation of the present study. Laparoscopic surgery has been now reported to be associated with higher recurrence and worse prognosis.29 When the present trial initiated, laparoscopic surgery was considered equally as safe as laparotomy for treatment of cervical cancer.30 Fortunately, the majority of patients (92.3%) in the present study underwent laparotomy, and the number of patients who underwent laparoscopic surgery was evenly distributed among the 3 study groups.
Lymph node metastasis is the strongest adverse factor that affects survival in cervical cancer. Because the study was not designed to stratify patients according to each risk factor separately, it happened that fewer patients with lymph node metastasis were assigned to the RT group (18.3%) than the CCRT group (30.1%) and SCRT group (29.7%). This was another limitation of this trial. Even so, SCRT still revealed a better DFS than RT, further emphasizing its strengths in treating patients with high-risk features.
Conclusions
In summary, compared with RT or CCRT, SCRT improved the DFS and decreased the distant recurrence, as well as the risk of death, which supports that SCRT should be considered a preferred adjuvant treatment after radical hysterectomy for patients with early-stage cervical cancer.
Trial Protocol
eTable 1. Characteristics of the patients at baseline
eTable 2. Patients received neo-adjuvant chemotherapy
eTable 3. Dose intensities and duration among the three treatment arms
eTable 4. Recurrence and death in ITT population
eTable 5. Cox Proportional Hazard analysis for DFS in ITT population
eTable 6. Cox proportional hazard analysis for DFS in PP population
eTable 7. Summary of adverse events
eTable 8. The comparisons of cumulative incidences of recurrence by Fine-Gray competing risk model
eFigure 1. Modified disease-free survival (MDFS) and modified overall survival (MOS)
eFigure 2. DFS and OS in intention-to-treat patients according to high or intermediate risk factor
eFigure 3. Disease-free survival (DFS) and overall survival (OS) for patients with high or intermediate risk factors by treatments
eFigure 4. Hazard ratios for recurrence in subgroup analysis (SCRT compared to CCRT)
eFigure 5. Distant and local recurrence free survival (DRFS and LRFS) for patients with high or intermediate risk factors by treatments
eFigure 6. Estimated cumulative incidence curves for disease recurrence (coded as 1) and died from other causes (coded as 2) across the 3 treatment groups in ITT (Panel A) and PP population (Panel B)
Data Sharing Statement
References
- 1.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394-424. doi: 10.3322/caac.21492 [DOI] [PubMed] [Google Scholar]
- 2.Estape RE, Angioli R, Madrigal M, et al. Close vaginal margins as a prognostic factor after radical hysterectomy. Gynecol Oncol. 1998;68(3):229-232. doi: 10.1006/gyno.1998.4960 [DOI] [PubMed] [Google Scholar]
- 3.Monk BJ, Wang J, Im S, et al. ; Gynecologic Oncology Group; Southwest Oncology Group; Radiation Therapy Oncology Group . Rethinking the use of radiation and chemotherapy after radical hysterectomy: a clinical-pathologic analysis of a Gynecologic Oncology Group/Southwest Oncology Group/Radiation Therapy Oncology Group trial. Gynecol Oncol. 2005;96(3):721-728. doi: 10.1016/j.ygyno.2004.11.007 [DOI] [PubMed] [Google Scholar]
- 4.Diaz ES, Aoyama C, Baquing MA, et al. Predictors of residual carcinoma or carcinoma-in-situ at hysterectomy following cervical conization with positive margins. Gynecol Oncol. 2014;132(1):76-80. doi: 10.1016/j.ygyno.2013.11.019 [DOI] [PubMed] [Google Scholar]
- 5.Peters WA III, Liu PY, Barrett RJ II, et al. Concurrent chemotherapy and pelvic radiation therapy compared with pelvic radiation therapy alone as adjuvant therapy after radical surgery in high-risk early-stage cancer of the cervix. J Clin Oncol. 2000;18(8):1606-1613. doi: 10.1200/JCO.2000.18.8.1606 [DOI] [PubMed] [Google Scholar]
- 6.Trifiletti DM, Swisher-McClure S, Showalter TN, Hegarty SE, Grover S. Postoperative chemoradiation therapy in high-risk cervical cancer: re-evaluating the findings of Gynecologic Oncology Group Study 109 in a large, population-based cohort. Int J Radiat Oncol Biol Phys. 2015;93(5):1032-1044. doi: 10.1016/j.ijrobp.2015.09.001 [DOI] [PubMed] [Google Scholar]
- 7.Uno T, Isobe K, Yamamoto S, Kawata T, Ito H. Postoperative radiation therapy for carcinoma of the uterine cervix. Radiat Med. 2006;24(2):91-97. doi: 10.1007/BF02493274 [DOI] [PubMed] [Google Scholar]
- 8.Rotman M, Sedlis A, Piedmonte MR, et al. A phase III randomized trial of postoperative pelvic irradiation in stage IB cervical carcinoma with poor prognostic features: follow-up of a gynecologic oncology group study. Int J Radiat Oncol Biol Phys. 2006;65(1):169-176. doi: 10.1016/j.ijrobp.2005.10.019 [DOI] [PubMed] [Google Scholar]
- 9.Sedlis A, Bundy BN, Rotman MZ, Lentz SS, Muderspach LI, Zaino RJ. A randomized trial of pelvic radiation therapy versus no further therapy in selected patients with stage IB carcinoma of the cervix after radical hysterectomy and pelvic lymphadenectomy: a Gynecologic Oncology Group Study. Gynecol Oncol. 1999;73(2):177-183. doi: 10.1006/gyno.1999.5387 [DOI] [PubMed] [Google Scholar]
- 10.Chernofsky MR, Felix JC, Muderspach LI, et al. Influence of quantity of lymph vascular space invasion on time to recurrence in women with early-stage squamous cancer of the cervix. Gynecol Oncol. 2006;100(2):288-293. doi: 10.1016/j.ygyno.2005.08.019 [DOI] [PubMed] [Google Scholar]
- 11.Marchiolé P, Buénerd A, Benchaib M, Nezhat K, Dargent D, Mathevet P. Clinical significance of lympho vascular space involvement and lymph node micrometastases in early-stage cervical cancer: a retrospective case-control surgico-pathological study. Gynecol Oncol. 2005;97(3):727-732. doi: 10.1016/j.ygyno.2005.01.004 [DOI] [PubMed] [Google Scholar]
- 12.Sehouli J, Runnebaum IB, Fotopoulou C, et al. A randomized phase III adjuvant study in high-risk cervical cancer: simultaneous radiochemotherapy with cisplatin (S-RC) versus systemic paclitaxel and carboplatin followed by percutaneous radiation (PC-R): a NOGGO-AGO Intergroup Study. Ann Oncol. 2012;23(9):2259-2264. doi: 10.1093/annonc/mdr628 [DOI] [PubMed] [Google Scholar]
- 13.World Medical Association . World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA. 2013;310(20):2191-2194. doi: 10.1001/jama.2013.281053. [DOI] [PubMed] [Google Scholar]
- 14.Common Terminology Criteria for Adverse Events (CTCAE). National Cancer Institute, Division of Cancer Treatment and Diagnosis. 2010. Accessed December 10, 2020. https://ctep.cancer.gov/protocolDevelopment/electronic_applications/ctc.htm#ctc_40
- 15.Takekuma M, Kasamatsu Y, Kado N, et al. Reconsideration of postoperative concurrent chemoradiotherapy with fluorouracil and cisplatin for uterine cervical cancer. J Obstet Gynaecol Res. 2015;41(10):1638-1643. doi: 10.1111/jog.12754 [DOI] [PubMed] [Google Scholar]
- 16.Moore DH, Blessing JA, McQuellon RP, et al. Phase III study of cisplatin with or without paclitaxel in stage IVB, recurrent, or persistent squamous cell carcinoma of the cervix: a gynecologic oncology group study. J Clin Oncol. 2004;22(15):3113-3119. doi: 10.1200/JCO.2004.04.170 [DOI] [PubMed] [Google Scholar]
- 17.Moore DH. Chemotherapy for advanced, recurrent, and metastatic cervical cancer. J Natl Compr Canc Netw. 2008;6(1):53-57. doi: 10.6004/jnccn.2008.0006 [DOI] [PubMed] [Google Scholar]
- 18.Monk BJ, Sill MW, McMeekin DS, et al. Phase III trial of four cisplatin-containing doublet combinations in stage IVB, recurrent, or persistent cervical carcinoma: a Gynecologic Oncology Group study. J Clin Oncol. 2009;27(28):4649-4655. doi: 10.1200/JCO.2009.21.8909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rose PG, Bundy BN, Watkins EB, et al. Concurrent cisplatin-based radiotherapy and chemotherapy for locally advanced cervical cancer. N Engl J Med. 1999;340(15):1144-1153. doi: 10.1056/NEJM199904153401502 [DOI] [PubMed] [Google Scholar]
- 20.Keys HM, Bundy BN, Stehman FB, et al. Cisplatin, radiation, and adjuvant hysterectomy compared with radiation and adjuvant hysterectomy for bulky stage IB cervical carcinoma. N Engl J Med. 1999;340(15):1154-1161. doi: 10.1056/NEJM199904153401503 [DOI] [PubMed] [Google Scholar]
- 21.Morris M, Eifel PJ, Lu J, et al. Pelvic radiation with concurrent chemotherapy compared with pelvic and para-aortic radiation for high-risk cervical cancer. N Engl J Med. 1999;340(15):1137-1143. doi: 10.1056/NEJM199904153401501 [DOI] [PubMed] [Google Scholar]
- 22.Monk BJ, Tewari KS, Koh WJ. Multimodality therapy for locally advanced cervical carcinoma: state of the art and future directions. J Clin Oncol. 2007;25(20):2952-2965. doi: 10.1200/JCO.2007.10.8324 [DOI] [PubMed] [Google Scholar]
- 23.Lahousen M, Haas J, Pickel H, et al. Chemotherapy versus radiotherapy versus observation for high-risk cervical carcinoma after radical hysterectomy: a randomized, prospective, multicenter trial. Gynecol Oncol. 1999;73(2):196-201. doi: 10.1006/gyno.1999.5343 [DOI] [PubMed] [Google Scholar]
- 24.Rose PG. Advances in the management of cervical cancer. J Reprod Med. 2000;45(12):971-978. [PubMed] [Google Scholar]
- 25.Pearcey R, Dundas G, Schepansky A, et al. ‘Is there a role for adjuvant pelvic radiotherapy after radical hysterectomy in early stage cervical cancer?’. Int J Gynecol Cancer. 1992;2(1):56. doi: 10.1046/j.1525-1438.1992.02010056.x [DOI] [PubMed] [Google Scholar]
- 26.Dueñas-González A, Zarbá JJ, Patel F, et al. Phase III, open-label, randomized study comparing concurrent gemcitabine plus cisplatin and radiation followed by adjuvant gemcitabine and cisplatin versus concurrent cisplatin and radiation in patients with stage IIB to IVA carcinoma of the cervix. J Clin Oncol. 2011;29(13):1678-1685. doi: 10.1200/JCO.2009.25.9663 [DOI] [PubMed] [Google Scholar]
- 27.Jhawar S, Hathout L, Elshaikh MA, Beriwal S, Small W Jr, Mahmoud O. Adjuvant chemoradiation therapy for cervical cancer and effect of timing and duration on treatment outcome. Int J Radiat Oncol Biol Phys. 2017;98(5):1132-1141. doi: 10.1016/j.ijrobp.2017.03.045 [DOI] [PubMed] [Google Scholar]
- 28.Cetina L, Rivera L, Hinojosa J, et al. Routine management of locally advanced cervical cancer with concurrent radiation and cisplatin. Five-year results. BMC Womens Health. 2006;6:3. doi: 10.1186/1472-6874-6-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ramirez PT, Frumovitz M, Pareja R, et al. Minimally invasive versus abdominal radical hysterectomy for cervical cancer. N Engl J Med. 2018;379(20):1895-1904. doi: 10.1056/NEJMoa1806395 [DOI] [PubMed] [Google Scholar]
- 30.Ramirez PT, Soliman PT, Schmeler KM, dos Reis R, Frumovitz M. Laparoscopic and robotic techniques for radical hysterectomy in patients with early-stage cervical cancer. Gynecol Oncol. 2008;110(3)(suppl 2):S21-S24. doi: 10.1016/j.ygyno.2008.03.013 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial Protocol
eTable 1. Characteristics of the patients at baseline
eTable 2. Patients received neo-adjuvant chemotherapy
eTable 3. Dose intensities and duration among the three treatment arms
eTable 4. Recurrence and death in ITT population
eTable 5. Cox Proportional Hazard analysis for DFS in ITT population
eTable 6. Cox proportional hazard analysis for DFS in PP population
eTable 7. Summary of adverse events
eTable 8. The comparisons of cumulative incidences of recurrence by Fine-Gray competing risk model
eFigure 1. Modified disease-free survival (MDFS) and modified overall survival (MOS)
eFigure 2. DFS and OS in intention-to-treat patients according to high or intermediate risk factor
eFigure 3. Disease-free survival (DFS) and overall survival (OS) for patients with high or intermediate risk factors by treatments
eFigure 4. Hazard ratios for recurrence in subgroup analysis (SCRT compared to CCRT)
eFigure 5. Distant and local recurrence free survival (DRFS and LRFS) for patients with high or intermediate risk factors by treatments
eFigure 6. Estimated cumulative incidence curves for disease recurrence (coded as 1) and died from other causes (coded as 2) across the 3 treatment groups in ITT (Panel A) and PP population (Panel B)
Data Sharing Statement