Key Points
Question
What is the utility of cardiac magnetic resonance imaging (MRI) as a part of a comprehensive screening program to evaluate student athletes recovering from coronavirus disease 2019 (COVID-19)?
Findings
In this study of 145 student athletes with COVID-19 who had mild to moderate symptoms or no symptoms during acute infection, cardiac MRI findings (at a median of 15 days after a positive test result for COVID-19) were consistent with myocarditis in only 2 patients (1.4%), based on updated Lake Louise criteria.
Meaning
Based on a low prevalence of COVID-19–associated findings consistent with myocarditis in 145 competitive student athletes with mild to moderate or no COVID-19 symptoms and normal serum study results, the utility of cardiac MRI as a screening tool for myocarditis was low and concordant with normal laboratory serum assays.
This case series describes the prevalence and severity of cardiac magnetic resonance imaging findings of myocarditis in a population of competitive student athletes recovering from coronavirus disease 2019.
Abstract
Importance
The utility of cardiac magnetic resonance imaging (MRI) as a screening tool for myocarditis in competitive student athletes returning to training after recovering from coronavirus disease 2019 (COVID-19) infection is unknown.
Objective
To describe the prevalence and severity of cardiac MRI findings of myocarditis in a population of competitive student athletes recovering from COVID-19.
Design, Setting, and Participants
In this case series, an electronic health record search was performed at our institution (University of Wisconsin) to identify all competitive athletes (a consecutive sample) recovering from COVID-19, who underwent gadolinium-enhanced cardiac MRI between January 1, 2020, and November 29, 2020. The MRI findings were reviewed by 2 radiologists experienced in cardiac imaging, using the updated Lake Louise criteria. Serum markers of myocardial injury and inflammation (troponin-I, B-type natriuretic peptide, C-reactive protein, and erythrocyte sedimentation rate), an electrocardiogram, transthoracic echocardiography, and relevant clinical data were obtained.
Exposures
COVID-19 infection, confirmed using reverse transcription–polymerase chain reaction testing.
Main Outcomes and Measures
Prevalence and severity of MRI findings consistent with myocarditis among young competitive athletes recovering from COVID-19.
Results
A total of 145 competitive student athletes (108 male and 37 female individuals; mean age, 20 years; range, 17-23 years) recovering from COVID-19 were included. Most patients had mild (71 [49.0%]) or moderate (40 [27.6%]) symptoms during the acute infection or were asymptomatic (24 [16.6%]). Symptoms were not specified or documented in 10 patients (6.9%). No patients required hospitalization. Cardiac MRIs were performed a median of 15 days (range, 11-194 days) after patients tested positive for COVID-19. Two patients had MRI findings consistent with myocarditis (1.4% [95% CI, 0.4%-4.9%]). Of these, 1 patient had marked nonischemic late gadolinium enhancement and T2-weighted signal abnormalities over multiple segments, along with an abnormal serum troponin-I level; the second patient had 1-cm nonischemic mild late gadolinium enhancement and mild T2-weighted signal abnormalities, with normal laboratory values.
Conclusions and Relevance
In this case series study, based on MRI findings, there was a low prevalence of myocarditis (1.4%) among student athletes recovering from COVID-19 with no or mild to moderate symptoms. Thus, the utility of cardiac MRI as a screening tool for myocarditis in this patient population is questionable.
Introduction
After outbreaks of the novel coronavirus disease 2019 (COVID-19) in late 2019 and 2020, complications of this disease1,2 including myocarditis were reported.3,4,5,6 Myocarditis is an inflammatory condition associated with viral infections.7,8 Manifestations of myocarditis are varied, ranging from mild disease to serious complications, including heart failure and sudden cardiac death.8 Recommended diagnostic testing includes serum troponin levels, electrocardiogram (ECG), transthoracic echocardiography (TTE), and cardiac magnetic resonance imaging (MRI).9
Myocarditis is a known cause of sudden cardiac death in athletes,10 even occurring in patients with preserved systolic left ventricular (LV) function.11 Given emerging reports of COVID-19–associated MRI findings consistent with myocarditis, the question arises as to whether athletes recovering from COVID-19 should be evaluated with MRI.6 While myocarditis is an uncommon finding in COVID-19 autopsy reports (1%-7%),12 a recent case series6 reported MRI findings consistent with myocarditis in 4 of 26 athletes (15%) recovering from COVID-19.
Our institution, University of Wisconsin, is a large Midwestern university with a Division I National Collegiate Athletic Association athletics program. With the return of students to campus, spikes in the number of COVID-19 cases occurred, including among student athletes. Given growing reports of COVID-19–associated complications,3,4,5 our institution, in concert with the Big Ten conference, initiated a screening program to evaluate all student athletes who had tested positive for COVID-19. Evaluation included serum markers of myocardial injury, ECG, TTE, and cardiac MRI. This work describes our institutional experience, including the prevalence, severity, and pattern of MRI findings consistent with myocarditis in a population of student athletes recovering from COVID-19.
Methods
Patients
This retrospective, Health Insurance Portability and Accountability Act–compliant study was performed with University of Wisconsin–Madison Health Sciences Institutional Review Board approval, including a waiver of informed consent. Electronic health records at the University of Wisconsin were searched for cardiac MRI examinations performed between January 1, 2020, and November 29, 2020, in all athletes recovering from COVID-19.
MRI Protocol
A standardized cardiac MRI myocarditis protocol was performed in all participants on 1.5-T or 3-T MRI systems (GE Healthcare). The MRI and image analysis protocol are described in the eMethods section and eTable 1 in the Supplement.
Data Collection
Images were initially reviewed and interpreted by experienced cardiovascular imaging faculty members (D.A.B., W.S.B., T.M.G., J.E.K., M.L.S., and S.B.R., plus several nonauthors; 6-30 years of experience) as part of clinical care, and subsequently reviewed by 2 cardiovascular imaging professionals (with 6 and 16 years of experience; J.S. and S.B.R.). Evaluation for myocarditis was based on the updated Lake Louise criteria,9 which were developed for patients with a high pretest probability of myocarditis. In the context of a presumed low pretest probability in this cohort, MRI findings may not provide a definitive diagnosis of myocarditis. Furthermore, there is a high prevalence of late gadolinium enhancement (LGE) at the inferior right ventricular (RV) insertion site in athletes,13,14 so that we did not consider isolated LGE at this site as a positive criteria for myocarditis. A COVID-19 diagnosis was confirmed using reverse transcription–polymerase chain reaction in all patients. Detailed data collection and statistical analysis are in the eMethods section of the Supplement. Evaluations of the LV and RV were performed with cvi42 software version 5.11 (Circle Cardiovascular Imaging).
Results
A total of 145 competitive athletes (37 female and 108 male individuals; mean age, 20 years; age range, 17-23 years; Table) competing in 12 sports were included. Initially, 118 patients (81.4%) were symptomatic (eTable 2 in the Supplement), with mild symptoms in 71 (49.0%), moderate symptoms in 40 (27.6%), and severity not specified in 7 (4.8%). Symptoms were not documented in 3 patients (2.1%); 24 (16.6%) were asymptomatic. No patient experienced severe symptoms, and none required chest radiography or hospitalization.
Table. Demographic and Clinical Information and Quantitative Cardiac Magnetic Resonance Imaging (MRI) Measurements From All Patients Included in This Study (N = 145).
| Clinical parameter | Mean (SD) | ||
|---|---|---|---|
| All athletes (n = 145) | Male athletes (n = 108) | Female athletes (n = 37) | |
| Age, y | 19.6 (1.3) | 19.7 (1.3) | 19.3 (1.1) |
| Weight, kg | 92 (25) | 100 (23) | 70 (11) |
| Height, m | 1.84 (0.11) | 1.88 (0.82) | 1.72 (0.89) |
| BMI | 27.0 (5.2) | 27.9 (5.3) | 23.3 (3.1) |
| Body surface area, m2 | 2.1 (0.3) | 2.3 (0.3) | 1.8 (0.2) |
| Blood pressure at rest, mm Hg | |||
| Systolic | 122 (12) | 125 (13) | 115 (9) |
| Diastolic | 72 (9) | 72 (9) | 70 (10) |
| Blood parametersa | |||
| Troponin-I, ng/mL | 0.02 (0.05) | 0.02 (0.06) | 0.02 (0.03) |
| B-type natriuretic peptide, pg/mL | 14 (7) | 12 (6) | 17 (10) |
| Erythrocyte sedimentation rate, mm/h | 3.6 (5.9) | 2.5 (3.9) | 6.8 (9.0) |
| C-reactive protein, mg/dL | 0.2 (0.2) | 0.2 (0.2) | 0.2 (0.2) |
| Cardiac MRI measurements | |||
| Left ventricular | |||
| Ejection fraction, % | 58 (5) | 58 (5) | 59 (5) |
| Mass index, g/m2 | 64 (13) | 67 (12) | 53 (9) |
| Cardiac index, L/min/m2 | 3.7 (0.8) | 3.8 (0.7) | 3.6 (0.8) |
| End-diastolic volume index, mL/m2 | 104 (26) | 108 (27) | 93 (16) |
| End-systolic volume index, mL/m2 | 45 (10) | 47 (10) | 39 (8) |
| Stroke volume index, mL/m2 | 60 (12) | 62 (12) | 54 (10) |
| Right ventricular | |||
| Ejection fraction, % | 54 (6) | 54 (6) | 55 (6) |
| Cardiac index, L/min/m2 | 3.6 (0.8) | 3.7 (0.8) | 3.6 (0.8) |
| End-diastolic volume index, mL/m2 | 110 (22) | 113 (22) | 101 (19) |
| End-systolic volume index, mL/m2 | 51 (13) | 53 (12) | 47 (14) |
| Stroke volume index, mL/m2 | 59 (12) | 60 (13) | 53 (10) |
| Native T1 value septum, ms | |||
| At 1.5 T | 978 (40) | 973 (37) | 1000 (48) |
| At 3 T | 1129 (84) | 1097 (84) | 1190 (38) |
| Native T2 value septum, ms | |||
| At 1.5 T | 48 (4) | 48 (4) | 48 (5) |
| At 3 T | 49 (5) | 49 (5) | 48 (4) |
| Late gadolinium enhancement present, No. | 42 | 35 | 7 |
| Time from positive COVID-19 test result to clinical test, d | |||
| Troponin-Ib | 16 (17) | 15 (17) | 20 (15) |
| Median (range) | 13 (9-184) | 11 (9-184) | 15 (9-82) |
| MRIb | 20 (18) | 19 (18) | 25 (16) |
| Median (range) | 15 (11-194) | 15 (11-194) | 19 (12-82) |
| Time from COVID-19 symptoms to MRI, dc | 21 (24) | 20 (27) | 24 (12) |
| Median (range) | 16 (7-194) | 15 (7-194) | 20 (13-59) |
Abbreviations: BMI, body mass index (calculated as weight in kilograms divided by height in meters squared); COVID-19, coronavirus disease 2019.
SI conversion factors: To convert B-type natriuretic peptide to ng/L, multiply by 1.0; C-reactive protein to mg/L, multiply by 10.0; erythrocyte sedimentation rate to mm/h, multiply by 1.0; troponin-I to μg/L, multiply by 1.0.
Reference values: B-type natriuretic peptide, 99 pg/mL or less; C-reactive protein, 1.0 mg/dL or less; erythrocyte sedimentation rate: male patients, less than 15 mm/h (for ages 0-50 years); female patients, less than 20 mm/h (for ages 0-50 years); troponin-I, 0.03 ng/mL or less.
Time between a patient testing positive for COVID-19 and a cardiac MRI or serum troponin-I level being obtained.
Time between onset of symptoms in a patient with COVID-19 and cardiac MRI.
Seventy-nine patients were imaged at 1.5 T and 66 at 3 T. We performed T2-weighted and T1-weighted mapping in 115 and 145 patients, respectively, and these data were analyzable in 102 of the 115 patients (88.7%) and 141 of the 145 patients (97.2%), respectively. At the time of the electronic health records search, complete laboratory studies were available in 138 patients and troponin-I levels in 143 patients. We completed TTEs in 137 patients and ECGs in all patients.
We performed MRIs a median of 15 days (range, 11-194 days) after a positive COVID-19 test result. Per institutional protocol, at least a 10-day quarantine and a minimum 72-hour asymptomatic period was required prior to an MRI. One patient had recovered from COVID-19 earlier in 2020, but because of persistent symptoms, evaluation for myocarditis was performed.
Two of the 145 patients (1.4% [95% CI, 0.4%-4.9%]) had abnormal MRI findings consistent with updated Lake Louise criteria for myocarditis (eTable 3 in the Supplement). These patients were reimaged 1 month after the initial MRIs.
In 1 patient, marked findings of myopericarditis were present with patchy midmyocardial and subepicardial LGE, predominantly in the apical inferolateral LV wall (Figure 1), with corresponding T2-weighted signal abnormalities and pericardial enhancement. Imaged at 3 T, native T1-weighted and T2-weighted times in areas demonstrating LGE were 1223 milliseconds and 57 milliseconds, respectively. The LV ejection fraction was within normal limits (51%). Importantly, the troponin-I level was normal 1 day prior to MRI (0.03 ng/mL; to convert to micrograms per liter, multiply by 1.0) but increased 2 days later (0.04 ng/mL) and peaked 4 days (0.09 ng/mL) after MRI. The troponin-I level normalized 18 days after the positive reverse transcription–polymerase chain reaction test was completed. Other serum marker levels were normal. Nonspecific ST–T-wave ECG abnormalities were detected 1 day before the MRI (eTable 3 in the Supplement). This patient was initially asymptomatic and tested for COVID-19 as a part of routine screening. At 1-month MRI re-evaluation, the LGE findings were unchanged, but T2-weighted signal abnormalities had improved and the troponin-I level was normal. Clinical follow-up 1 month after the MRI revealed 1 episode of mild dyspnea, but the patient was otherwise asymptomatic.
Figure 1. Magnetic Resonance Imaging (MRI) Findings Consistent With Acute Myopericarditis in a Patient Recovering From Initially Asymptomatic Coronavirus Disease 2019.
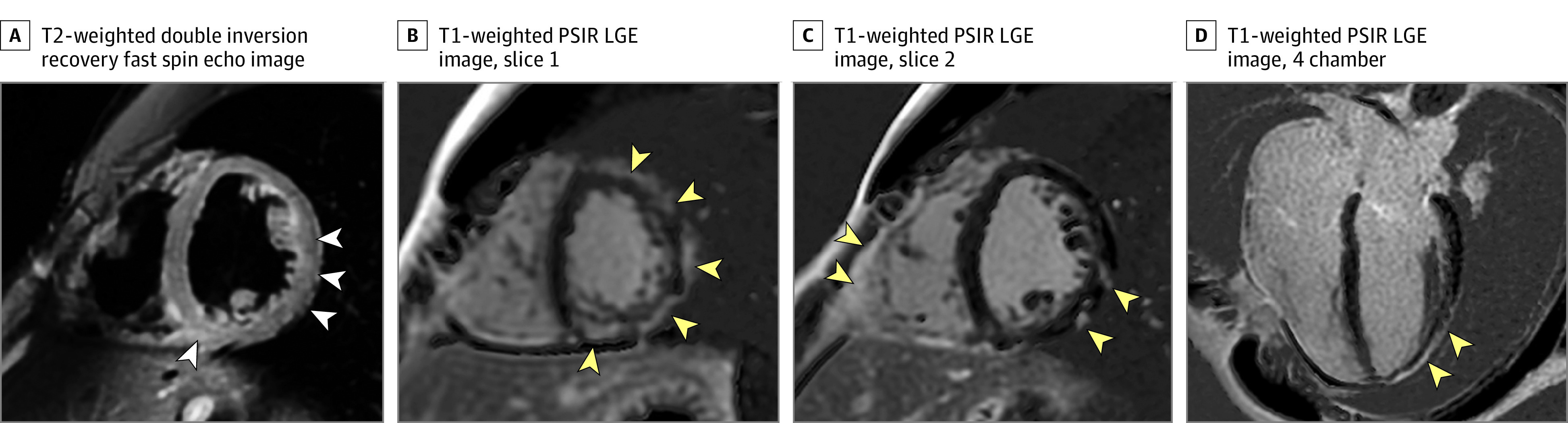
Shown are MRI findings consistent with acute inflammatory changes, with patchy midmyocardial and epicardial late gadolinium enhancement as well as pericardial enhancement (yellow arrowheads) on T1-weighted phase-sensitive inversion recovery late gadolinium–enhanced (PSIR LGE) short-axis images (B and C) and 4-chamber image (D), and corresponding elevated T2-weighted signal on fat-saturated double inversion recovery fast spin echo image (A) (white arrowheads). The troponin-I level was normal 1 day prior to the cardiac MRI (0.03 ng/mL; to convert to micrograms per liter, multiply by 1.0) but increased to 0.04 ng/mL and 0.09 ng/mL 2 and 4 days after the cardiac MRI examination, respectively.
The MRI in the second patient revealed an approximately 1-cm focus of mild epicardial LGE at the inferior basal LV wall and a corresponding mild T2-weighted signal abnormality (Figure 2). Imaged at 3 T, native T1-weighted time in areas demonstrating LGE was 1250 milliseconds. We did not perform T2 mapping in this patient. Additionally, a small nonspecific LGE focus at the inferior RV insertion was observed. This patient had mild to moderate symptoms (eTable 3 in the Supplement) lasting at least 3 days but was asymptomatic at the time of MRI. Serum marker levels were normal. At 1-month MRI follow-up, the T2-weighted signal abnormalities and LGE had resolved. The focus of RV insertion LGE was unchanged.
Figure 2. Mild Magnetic Resonance Imaging Findings Consistent With Lake Louise Criteria for Myocarditis in an Athlete Recovering From Coronavirus Disease 2019.
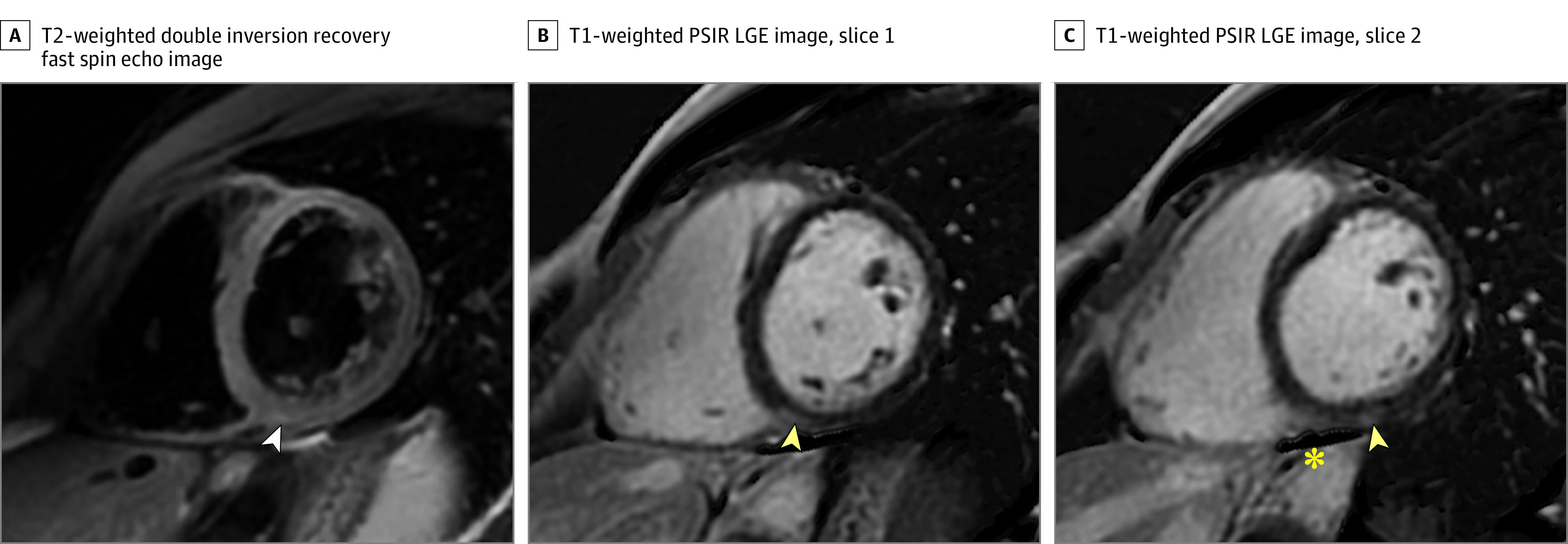
A, T2-weighted fat-saturated double inversion recovery fast spin echo short-axis image. B and C, T1-weighted phase-sensitive inversion recovery late gadolinium–enhanced (PSIR LGE) short-axis images. Yellow arrowheads demonstrate a 1-cm focus of epicardial LGE in the inferior wall at the base and the inferior right ventricular insertion point. A mildly elevated T2-weighted signal was also noted (white arrowhead), as well as a small amount of adjacent pericardial fluid (asterisk). Serum troponin levels were normal in this patient.
A small nonspecific focus of LGE was observed in the LV septum at the inferior RV insertion point in 38 of 145 patients (26.2%; eFigure in the Supplement). In 2 of 145 patients (1.4%) mild, nonspecific, midmyocardial LGE in the inferior basal LV wall and midseptum, without corresponding T2-weighted signal abnormalities, was seen. These abnormalities did not meet updated Lake Louise MRI criteria for myocarditis.
Four patients with negative MRI results had elevated troponin-I levels. In 2 patients, troponin-I levels were elevated in a single test result (0.08 and 0.18 ng/mL) and these were thus considered false-positive results. In 2 others, troponin-I levels were repeatedly elevated (maximum, 0.04 and 0.72 ng/mL) over 2 and 14 days, respectively. The results of ECGs were normal in 3 of these patients, and 1 patient had minor, nonspecific abnormalities; TTE results were negative in all 4 patients. The ECG and TTE results were normal in the others or had minor, nonspecific abnormalities not indicative of myocarditis.
Discussion
This report documents our experience with cardiac MRI testing performed to evaluate student athletes for myocarditis during recovery from COVID-19 infection. Only 2 patients had MRI findings consistent with myocarditis. The overall positive yield for MRI evidence of myocarditis was low, raising doubt regarding its utility to evaluate athletes without a clinical presentation or abnormal ancillary tests to support the diagnosis of myocarditis. We conclude that further studies are needed to determine the utility of MRI in screening athletes recovering from COVID-19 who have normal ancillary diagnostic tests, such as an ECG and troponin-I level.
A strength of this study is in a relatively well-defined population, relevant to many university campuses. These patients constitute a unique population of highly trained athletes, as part of a subpopulation of a larger undergraduate population. All athletes who tested positive for COVID-19 at our institution have undergone MRI. In this way, the study constitutes a census study, avoiding bias that can occur with selective enrollment, providing a comprehensive cross-sectional evaluation.
Limitations
There are several study limitations. As this was a retrospective study, control groups including athletes who have tested negative for COVID-19 or athletes infected with other respiratory viruses were not included. Future prospective studies that include case-controls and serum laboratory tests, such as troponin levels, obtained contemporaneously with symptoms and positive reverse transcription–polymerase chain reaction testing are needed.
A second limitation is that extension of these results from a cohort of trained competitive athletes to other patient groups may not be possible. Furthermore, troponin-I levels and MRI were obtained a median of 13 and 15 days after a positive COVID-19 test result. Thus, detection of mild myocardial injury may have been missed. The time course of COVID-19–associated myocarditis is not understood, and the optimal time to perform MRI appears to be unknown at this time.
Nonspecific LGE at the inferior RV insertion point is a potential confounder and was present in 26% of our cohort. Because this MRI finding has been described in association with athletic training,13,14 we did not consider this finding in isolation to be consistent with myocarditis.
Finally, this is a cross-sectional study based on a case series without long-term clinical follow-up. Long-term follow-up may be helpful to assess the clinical relevance of MRI findings suggestive of myocarditis.
Conclusions
In summary, we have reported the prevalence, findings, and pattern of cardiac MRI findings in a cohort of student athletes from University of Wisconsin–Madison recovering from COVID-19. Our results demonstrated a low prevalence of MRI, ancillary laboratory, and ECG findings consistent with myocarditis. We conclude that the utility of cardiac MRI in competitive athletes who are asymptomatic or mildly to moderately symptomatic with normal serum laboratory and ECG findings is low. Further studies are needed to confirm this conclusion.
eMethods.
eTable 1. Cardiac MRI Protocol.
eTable 2. Clinical and quantitative cardiac MRI parameters of two athletes recovering from COVID-19 with MRI findings consistent with myocarditis.
eTable 3. Number of athletes who had COVID-19, and documented specific symptoms (n=145; 37 female/108 male) during the course of their disease.
eFigure. Nonspecific inferior-septal right ventricular insertion point late gadolinium enhancement.
eReferences.
References
- 1.Puntmann VO, Carerj ML, Wieters I, et al. Outcomes of cardiovascular magnetic resonance imaging in patients recently recovered from coronavirus disease 2019 (COVID-19). JAMA Cardiol. 2020;5(11):1265-1273. doi: 10.1001/jamacardio.2020.3557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Knight DS, Kotecha T, Razvi Y, et al. COVID-19: myocardial injury in survivors. circulation. 2020;142(11):1120-1122. doi: 10.1161/CIRCULATIONAHA.120.049252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Beşler MS, Arslan H. Acute myocarditis associated with COVID-19 infection. Am J Emerg Med. 2020;38(11):2489.e1-2489.e2. doi: 10.1016/j.ajem.2020.05.100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Trogen B, Gonzalez FJ, Shust GF. COVID-19-associated myocarditis in an adolescent. Pediatr Infect Dis J. 2020;39(8):e204-e205. doi: 10.1097/INF.0000000000002788 [DOI] [PubMed] [Google Scholar]
- 5.Ho JS, Sia C-H, Chan MY, Lin W, Wong RC. Coronavirus-induced myocarditis: a meta-summary of cases. Heart Lung. 2020;49(6):681-685. doi: 10.1016/j.hrtlng.2020.08.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rajpal S, Tong MS, Borchers J, et al. Cardiovascular magnetic resonance findings in competitive athletes recovering from COVID-19 infection. JAMA Cardiol. Published online September 11, 2020. doi: 10.1001/jamacardio.2020.4916 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Andréoletti L, Lévêque N, Boulagnon C, Brasselet C, Fornes P. Viral causes of human myocarditis. Arch Cardiovasc Dis. 2009;102(6-7):559-568. doi: 10.1016/j.acvd.2009.04.010 [DOI] [PubMed] [Google Scholar]
- 8.Caforio ALP, Pankuweit S, Arbustini E, et al. ; European Society of Cardiology Working Group on Myocardial and Pericardial Diseases . Current state of knowledge on aetiology, diagnosis, management, and therapy of myocarditis: a position statement of the European Society of Cardiology Working Group on Myocardial and Pericardial Diseases. Eur Heart J. 2013;34(33):2636-2648, 2648a-2648d. doi: 10.1093/eurheartj/eht210 [DOI] [PubMed] [Google Scholar]
- 9.Ferreira VM, Schulz-Menger J, Holmvang G, et al. Cardiovascular magnetic resonance in nonischemic myocardial inflammation: expert recommendations. J Am Coll Cardiol. 2018;72(24):3158-3176. doi: 10.1016/j.jacc.2018.09.072 [DOI] [PubMed] [Google Scholar]
- 10.Maron BJ, Haas TS, Ahluwalia A, Murphy CJ, Garberich RF. Demographics and epidemiology of sudden deaths in young competitive athletes: from the United States National Registry. Am J Med. 2016;129(11):1170-1177. doi: 10.1016/j.amjmed.2016.02.031 [DOI] [PubMed] [Google Scholar]
- 11.Schumm J, Greulich S, Wagner A, et al. Cardiovascular magnetic resonance risk stratification in patients with clinically suspected myocarditis. J Cardiovasc Magn Reson. 2014;16(1):14. doi: 10.1186/1532-429X-16-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Halushka MK, Vander Heide RS. Myocarditis is rare in COVID-19 autopsies: cardiovascular findings across 277 postmortem examinations. Cardiovasc Pathol. 2021;50:107300. doi: 10.1016/j.carpath.2020.107300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Domenech-Ximenos B, Sanz-de la Garza M, Prat-González S, et al. Prevalence and pattern of cardiovascular magnetic resonance late gadolinium enhancement in highly trained endurance athletes. J Cardiovasc Magn Reson. 2020;22(1):62. doi: 10.1186/s12968-020-00660-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wilson M, O’Hanlon R, Prasad S, et al. Diverse patterns of myocardial fibrosis in lifelong, veteran endurance athletes. J Appl Physiol (1985). 2011;110(6):1622-1626. doi: 10.1152/japplphysiol.01280.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eMethods.
eTable 1. Cardiac MRI Protocol.
eTable 2. Clinical and quantitative cardiac MRI parameters of two athletes recovering from COVID-19 with MRI findings consistent with myocarditis.
eTable 3. Number of athletes who had COVID-19, and documented specific symptoms (n=145; 37 female/108 male) during the course of their disease.
eFigure. Nonspecific inferior-septal right ventricular insertion point late gadolinium enhancement.
eReferences.


