Abstract
BACKGROUND
Recent studies have revealed that sustained ingestion of angiotensin converting enzymes inhibitors or angiotensin receptor blockers (ACEIs/ARBs) had no harmful effects on coronavirus disease 2019 (COVID-19) patients complicated with hypertension.
AIM
To investigate the impact on COVID-19 patients complicated with hypertension who discontinued using ACEIs/ARBs.
METHODS
All COVID-19 patients complicated with hypertension admitted to our isolated unit were consecutively recruited in this study. Some patients switched from ACEIs/ARBs to calcium channel blocker (CCBs) after admission, while others continued using non-ACEIs/ARBs. We compared characteristics and clinical outcomes between these two groups of patients.
RESULTS
A total of 53 patients were enrolled, 27 patients switched from ACEIs/ARBs to CCBs while 26 patients continued with non-ACEIs/ARBs. After controlling potential confounding factors using the Cox proportional hazards model, hospital stay was longer in patients who discontinued ACEIs/ARBs, with a hazard ratio of 0.424 (95% confidence interval: 0.187-0.962; P = 0.040), upon discharge than patients using other anti-hypertensive drugs. A sub-group analysis showed that the effect of discontinuing use of ACEIs/ARBs was stronger in moderate cases [hazard ratio = 0.224 (95% confidence interval: 0.005-0.998; P = 0.0497)].
CONCLUSION
Patients in the discontinued ACEIs/ARBs group had longer hospital stays. Our findings suggest that COVID-19 patients complicated with hypertension should continue to use ACEIs/ARBs.
Keywords: COVID-19, Hypertension, Angiotensin converting enzymes inhibitors, Angiotensin receptor blockers, Angiotensin-converting enzyme-2, Prognosis
Core Tip: Recent research revealed that use of angiotensin converting enzymes inhibitors or angiotensin receptor blockers (ACEIs/ARBs) had no significant harm on coronavirus disease 2019 (COVID-19) patients complicated with hypertension. However, the impact of discontinue using ACEIs/ARBs in those patients was still unclear. In the present study, we retrospectively collected the clinical data of patients with both COVID-19 and hypertension to explore whether any difference in disease outcomes existed between patients who discontinued using ACEIs/ARBs and those who continued using other anti-hypertensive drugs.
INTRODUCTION
Coronavirus disease 2019 (COVID-19) has become a critical global health issue. It was first reported in December 2019, and the epidemic of the disease spread extremely rapidly since its discovery[1].
Patients might suffer from mild, self-limiting upper airway respiratory infection, severe deteriorating pneumonia, or even fatal complications. Epidemiological surveys have shown that the proportion of patients with COVID-19 who have hypertension is between 10% and 35%, making hypertension the most common comorbidity[2-6]. Specifically, angiotensin converting enzyme (ACE) inhibitors (ACEIs) or angiotensin receptor blockers (ARBs) are taken by 25%-50% of patients with hypertension for blood pressure control[5-8]. As the first-line inhibitors of renin-angiotensin-aldosterone system (RAAS)[9], they always act as a cornerstone in treatment of cardiovascular disease.
The severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) that causes COVID-19 infects the human body in a similar pattern as SARS coronavirus (SARS-CoV) does. They bind ACE2 in the lung via S protein, and subsequently enter the host alveolar cells, where they replicate and activate the immune system. The release of inflammatory factors and cytokines causes lung injury and even fatal complications in critical cases[10-12]. Additionally, several studies[13-15] have shown that ACEIs or angiotensin receptor blockers (ACEIs/ARBs) might upregulate level of ACE2 expression. This may confer increased susceptibility and aggravation of COVID-19. Therefore, some researchers considered that ACEIs/ARBs should be discontinued in COVID-19 patients with hypertension[16]. However, the American Heart Association/ American College of Cardiologists and the European Society of Cardiology[17-19] pointed out that there was no clinical evidence on worse outcomes in COVID-19 patients with previous ACEIs/ARBs prescription, and they did not recommend discontinuing ACEIs/ARBs. Moreover, most research had revealed that sustained ingestion of ACEIs/ARBs had no significant harmful effects on COVID-19 patients complicated with hypertension during hospitalization. However, few studies investigated the effect of discontinuing ACEIs/ARBs in COVID-19 patients with hypertension. Therefore, we conducted a study to investigate whether the discontinuation of ACEIs/ARBs in COVID-19 patients with hypertension had a negative impact on patient prognosis.
In the present study, we aimed to collect retrospectively the clinical data of patients with both COVID-19 and hypertension and to explore the differences between patients who discontinued using ACEIs/ARBs and those who kept using other types of anti-hypertensive medications.
MATERIALS AND METHODS
Study subjects
This study was based on the COVID-19 cohort from a patient registry of all COVID-19 patients admitted to an isolated unit in a tertiary hospital from February 8, 2020 to February 24, 2020. All patients were diagnosed with COVID-19 pneumonia according to national guidelines[20].
The exclusion criteria were as follows: (1) Uncured malignant tumors; (2) Regular dialysis; (3) Immunosuppression therapy post transplantation; (4) Steroids or immunosuppressive agents for autoimmune diseases; (5) Chronic pulmonary diseases; (6) Active hepatitis or liver cirrhosis; (7) Pregnancy and lactation; (8) Sequelae of cerebrovascular disease; (9) Hematological diseases; and (10) Age < 18 years.
All patients were given standard care. However, due to limited resources during the initial stage of the epidemic, there was shortage of ACEIs/ARBs supply. Thus, all patients who had previously taken ACEIs/ARBs were prescribed other antihyper-tensive drugs, mostly calcium channel blocker (CCBs). There were 53 moderate or severe patients enrolled, 27 of which switched from ACEIs/ARBs to CCBs and 26 continued with their usual non-ACEIs/ARBs anti-hypertensive drugs.
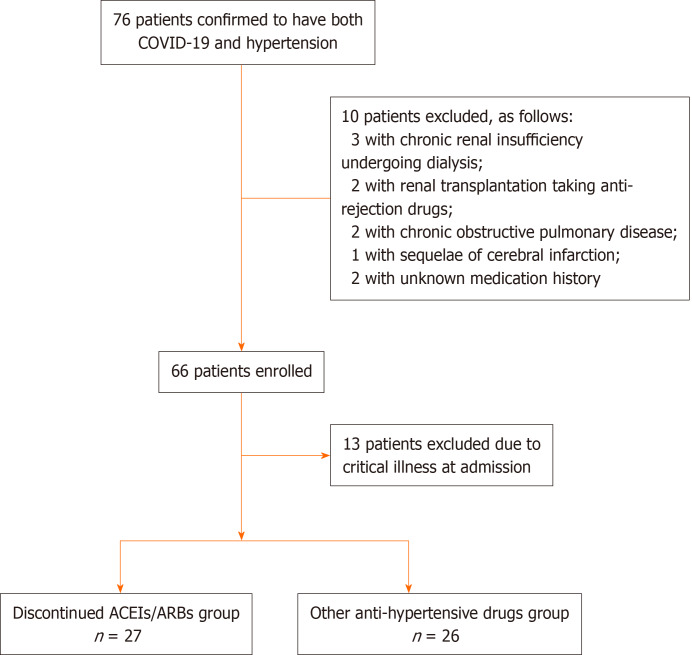
In the majority of critical patients with multi-organ dysfunction syndrome or hemodynamic instability, oral anti-hypertensive drugs could not be administered. The prognosis of those patients was profoundly affected by severity of illness. In order to avoid potential bias, 13 critical cases were excluded from the final analysis (see Figure 1).
Figure 1.
Enrollment and randomization of patients. COVID-19: Coronavirus disease 2019; ACEIs/ARBs: Angiotensin converting enzymes inhibitors or angiotensin receptor blockers.
Study methods
The present study described the baseline characteristics of patients with moderate and severe COVID-19 who were using anti-hypertensive drugs, including their gender, age, disease severity classification, quick sequential organ failure assessment (qSOFA) score, clinical and radiological manifestations, inflammatory factors, labs, and other disease-related characteristics. In addition, those patients switched from ACEIs/ARBs to CCBs after admission were assigned to the discontinued ACEIs/ARBs group. The remaining patients who have taken non-ACEIs/ARBs anti-hypertensive drugs who maintained the previous therapeutic regime were enrolled in other anti-hypertensive drugs group. When exploring the association between the discontinuation of ACEIs/ARBs and disease prognosis, we compared baseline characteristics between the two groups.
The blood pressure on the day of admission and the 2nd, 3rd, 5th, 8th, and 12th d after admission were recorded, evaluating the consistency of blood pressure control for both groups.
The follow-up study continued until March 24, 2020. Discharged or in-hospital death were also considered end-point of study. The discharge criteria were as follows: (1) Normal body temperature for more than 3 d; (2) Respiratory symptoms significantly improved; (3) Chest imaging remission; and (4) Negative nucleic acid tests of sputum, nasopharyngeal swabs, or other respiratory tract samples for two consecutive times sampled with at least 24-h interval. The deceased patients were recorded as right censored, as no discharge outcome had occurred.
The study was approved by the Institutional Review Board of Peking University Third Hospital (IRB00006761-M2020060), and a waiver of written informed consent was obtained.
Statistical analysis
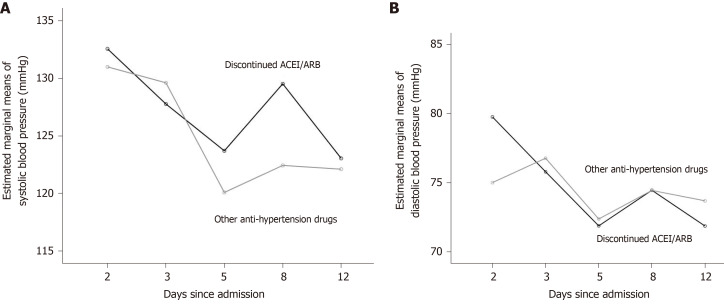
Continuous variables were described by median and quartile, and the Mann-Whitney U test was used for comparison between groups. For categorical variables, the chi-square test, continuity-corrected chi-square test, or Fisher's exact test was used. Patients receiving ACEIs/ARBs before hospitalization might have experienced blood pressure fluctuations after medication switching. We used repeated-measures analysis of variance (repeated-measures ANOVA) to compare the changes in blood pressure level after admission, as well as the overall blood pressure level, between the discontinued ACEIs/ARBs group and the other anti-hypertensive drugs group. In the repeated-measures ANOVA, the choice to use ACEIs/ARBs was defined as a between-subject factor, while blood pressure on the day of admission was defined as a covariant (see Figure 2). Cox proportional hazard model was used to estimate the hazard ratio (HR) and its 95% confidence interval (CI) for significant factor screening. Factors with a P value < 0.1 were selected as potential confounders in the comparison of baseline characteristics between the discontinued ACEIs/ARBs group and the other anti-hypertensive drugs group, as well as in the univariant Cox proportional hazards model. Cox risk ratio model was used to explore whether the previous use of ACEIs/ARBs, after deducting confounding factors, was related to the prognosis of the disease. The qSOFA score was closely related to COVID-19 disease severity, so it was included in the multi-factor Cox proportional hazard model to indicate severity of disease.
Figure 2.
Comparison of blood pressure level after admission between two groups.
To clarify further the impact of ACEIs/ARBs on prognosis in patients with different severities on admission, multivariate Cox proportional hazard models of moderate and severe patients were established to explore the association between the discontinuous usage of ACEIs/ARBs and disease severity. A two-sided α of less than 0.05 was considered statistically significant. The researchers used the SPSS version 24.0 program for statistical analysis (IBM Corp., Armonk, NY, United States).
RESULTS
Baseline characteristics
A total of 53 patients met the inclusion criteria, of whom 27 (51%) switched from ACEIs/ARBs to CCBs and 26 continued with their usual non-ACEIs/ARBs anti-hypertensive drugs. The patients’ median age was 67 years, and the median time from symptoms onset to hospital admission was 14 d. There were 24 moderate cases (45.3%) and 29 severe cases (54.7%) in the cohort. The initial symptom commonly experienced was fever in 35 cases (66%) and cough in 12 cases (22.6%). Bilateral ground-glass opacity was the most commonly seen chest imaging findings.
As shown in Table 1, age, qSOFA score, and the levels of albumin and interleukin-10 (IL-10) differed significantly between the discontinued ACEIs/ARBs group and the other anti-hypertensive drugs group (P < 0.05). There was no significant difference between the two groups in other labs or clinical management strategy (see Supplementary Table 1).
Table 1.
Patients’ characteristics and differences between discontinued angiotensin converting enzymes inhibitors or angiotensin receptor blockers group and other anti-hypertensive drugs group
|
Characteristic
|
|
Patients with both COVID-19 and hypertension
|
χ²
/z
|
P
value
|
|
|
Total (n = 53)
|
Discontinued ACEIs/ARBs (n = 27)
|
Other anti-hypertensive drugs (n = 26)
|
|||
| Male sex, n (%) | 26 (49.1) | 15 (57.7) | 11 (42.3) | 0.930 | 0.335 |
| Age, median yr (IQR) | 67 (59, 73) | 64 (55, 72) | 70 (66, 73) | -2.369 | 0.018 |
| Initial symptom, n (%) | |||||
| Fever (temperature ≥ 37.3 °C) | 35 (66) | 19 (54.3) | 16 (45.7) | 6.895 | 0.1861 |
| Cough | 12 (22.6) | 4 (33.3) | 8 (66.7) | ||
| Dyspnea | 1 (1.9) | 0 (0) | 1 (100) | ||
| Chest tension | 1 (1.9) | 0 (0) | 1 (100) | ||
| Exhausted | 1 (1.9) | 1 (100) | 0 (0) | ||
| Anorexia | 1 (1.9) | 1 (100) | 0 (0) | ||
| Diarrhea | 2 (3.8) | 2 (100) | 0 (0) | ||
| Severity of illness, n (%) | |||||
| Moderate | 24 (45.3) | 12 (50) | 12 (50) | 0.016 | 0.901 |
| Severe | 29 (54.7) | 15 (51.7) | 14 (48.3) | ||
| qSOFA score, n (%) | |||||
| 0 | 26 (49.1) | 17 (65.4) | 9 (34.6) | 4.259 | 0.039 |
| ≥ 1 | 27 (50.9) | 10 (37) | 17 (63) | ||
| CURB-65 score, n (%) | |||||
| 0 | 17 (32.1) | 12 (70.6) | 5 (29.4) | 3.874 | 0.1451 |
| 1 | 28 (52.8) | 12 (42.9) | 16 (57.1) | ||
| ≥ 2 | 8 (15.1) | 3 (37.5) | 5 (62.5) | ||
| Comorbidities, n (%) | |||||
| Diabetes | 21 (39.6) | 10 (47.6) | 11 (52.4) | 0.154 | 0.695 |
| Coronary heart disease- | 13 (24.5) | 8 (61.5) | 5 (38.5) | 0.774 | 0.379 |
| Duration of hypertension, median-yr (IQR) | 10 (5,16) | 10 (6, 20) | 10 (5, 10) | 1.811 | 0.707 |
| Chest CT results, n (%) | |||||
| Bilateral lesions | 48 (90.6) | 24 (50) | 24 (50) | 0 | 1 |
| Ground glass | 32 (60.4) | 15 (46.9) | 17 (53.1) | 0.535 | 0.465 |
| Consolidation | 6 (11.3) | 4 (66.7) | 2 (33.3) | 0.148 | 0.701 |
| Hydrothorax | 5 (9.4) | 2 (40) | 3 (60) | 0.002 | 0.965 |
| Patch shadow | 37 (69.8) | 19 (51.4) | 18 (48.6) | 0.008 | 0.928 |
| Laboratory tests, median (IQR) | |||||
| White blood cell count, × 109/L | 5.23 (4.56, 6.46) | 5.22 (4.28, 6.99) | 5.41 (4.76, 6.01) | -0.285 | 0.776 |
| Lymphocyte count, × 109/L | 0.99 (0.68, 1.49) | 0.81 (0.61, 1.5) | 1.05 (0.78, 1.49) | -1.228 | 0.219 |
| Platelet count, × 109/L | 247 (209, 285) | 250 (202, 319) | 247 (217, 280) | 1.087 | 0.852 |
| Hemoglobin, g/L | 122 (115, 130) | 122 (115, 129) | 122 (115, 132) | -0.142 | 0.887 |
| Alanine transaminase, U/L | 21 (15, 31) | 21 (14, 31) | 20 (15, 30) | -0.027 | 0.979 |
| Albumin, g/L | 34.9 (31.3, 38.3) | 33.4 (30.1, 35.9) | 36.4 (31.7, 39) | -2.127 | 0.033 |
| Total bilirubin, µmol/L | 9.5 (7, 13.4) | 9.1 (6.4, 13.4) | 10.2 (7.4, 14.1) | -0.881 | 0.378 |
| LDH, µmol/L | 265 (228 ,313) | 290 (228, 367) | 262 (224, 300) | 1.174 | 0.240 |
| BUN, mmol/L | 4.6 (3.4, 5.9) | 4.6, (3.9, 6) | 4.5 (3.2, 5.7) | 0.854 | 0.393 |
| Creatinine, µmol/L | 71 (58, 90) | 68 (59, 90) | 71.5 (58, 90) | 0.089 | 0.929 |
| Prothrombin time, s | 13.9 (13.2, 14.4) | 13.8 (13.2, 14.3) | 14 (13.2, 14.6) | -0.633 | 0.527 |
| APTT, s | 40.2 (35.6, 43.7) | 40.8 (36.8, 44.6) | 37.7 (35.3, 43.5) | 1.148 | 0.251 |
| Fibrous protein, g/L | 4.81 (4, 6.18) | 4.73 (4, 6.98) | 4.85 (3.72, 6) | 0.534 | 0.593 |
| D-dimer, µg/L | 0.93 (0.48, 1.77) | 0.75 (0.47, 1.69) | 1.11 (0.48, 1.81) | -0.409 | 0.682 |
| hs-CRP, mg/L | 21.2 (1.2, 81.4) | 27.2 (5, 97.5) | 16.6 (0.8, 57.9) | 1.130 | 0.258 |
| Procalcitonin, ng/mL | 0.04 (0.02, 0.08) | 0.04 (0.02, 0.06) | 0.04 (0.02, 0.09) | 0.290 | 0.772 |
| Serum ferritin, µg/L | 486 (328.35, 1023.85) | 601.15 (387, 1173.3) | 438.35 (321.4, 767.2) | 1.244 | 0.213 |
| IL-2R, U/mL | 669 (445.5, 1013.5) | 684.5 (488, 1172) | 669 (407, 1010) | 0.522 | 0.602 |
| IL-6, pg/mL | 8.76 (3.08, 37.12) | 10.23 (3.91, 40.54) | 7.66 (3.08, 34.24) | 0.439 | 0.660 |
| IL-8, pg/mL | 13.35 (5.75, 23.2) | 17.55 (7.4, 27.2) | 10.4 (5.3, 18.9) | 1.395 | 0.163 |
| IL-10, pg/mL | 5 (5, 5.45) | 5 (5, 7.6) | 5 (5, 5) | 2.463 | 0.0142 |
| hs-TnI, pg/mL | 3.6 (2.9, 5.3) | 4.5 (2.3, 7.6) | 3.6 (3.6, 3.6) | 1.090 | 0.267 |
| Myoglobin, ng/mL | 51.6 (37.8, 90) | 50.7 (40, 134.6) | 55.5 (31.8, 86.3) | 0.365 | 0.715 |
| CKMB, ng/mL | 1.1 (0.5, 1.7) | 1.2 (0.5, 1.9) | 0.8 (0.5, 1.5) | 0.508 | 0.611 |
| NT-ProBNP, pg/mL | 142.0 (71.0, 308.0) | 113.0 (57.0, 311.0) | 179.5 (75,0, 290.0) | -1.005 | 0.315 |
| Time from onset to admission, median day (IQR) | 14 (10, 18) | 15 (10, 18) | 12 (9, 18) | 0.919 | 0.358 |
Only one patient's organ failure assessment (QSOFA) was 2, QSOFA for all other patients was 0 or 1. Therefore, QSOFA was involved as a binary variable (0, ≥1) in univariate and multivariate Cox proportional hazard model. 1Chi-square test for continuity correction. 2Mean rank in the discontinued angiotensin converting enzymes inhibitors or angiotensin receptor blockers (ACEIs/ARBs) group = 30.73—slightly higher than that in the other anti-hypertensive drugs group (22.27). COVID-19: Coronavirus disease 2019; ACEIs/ARBs: Angiotensin converting enzymes inhibitors or angiotensin receptor blockers; qSOFA: Quick sequential organ failure assessment; LDH: Lactate dehydrogenase; BUN: Blood urea nitrogen; APTT: Activated partial thromboplastin time; hs-CRP: High-sensitive C-reactive protein; IL-2R: Interleukin-2 receptor; IL-6: Interleukin-6; IL-8: Interleukin-8; IL-10: Interleukin-10; hs-TnI: High sensitive cardiac troponin I; CKMB: Creatine phosphokinase isoenzyme; NT-ProBNP: N-terminal pro-brain natriuretic peptide; IQR: Interquartile range; CT: Computed tomography.
The results of repeated-measures ANOVA showed that the models of both systolic and diastolic blood pressure met the spherical test (systolic pressure: P = 0.287, diastolic pressure: P = 0.653). There was no significant difference between the groups in terms of the trends in systolic and diastolic blood pressure over time (Mauchly’s test of Sphericity; systolic: P = 0.533, diastolic: P = 0.308). The tests of between-subject effects showed that systolic and diastolic blood pressure were not significantly different between the two groups (P = 0.355 and 0.822, respectively). The blood pressure levels of the two groups after admission were generally similar.
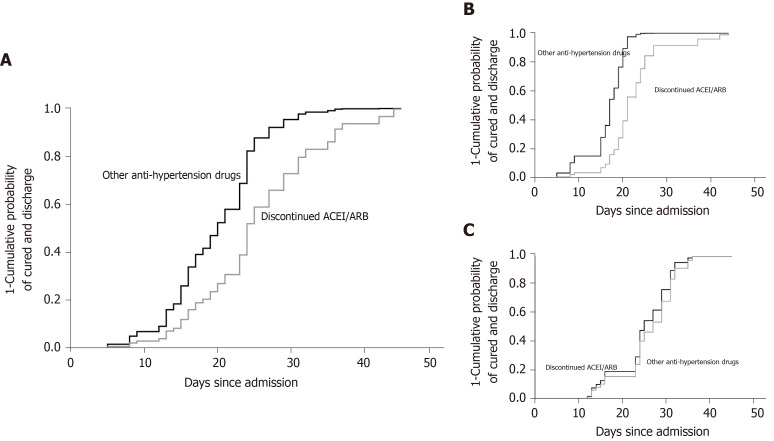
The univariate Cox proportional risk model showed that the hospital stay between those two groups was 23 d [interquartile range (IQR) 16.0-31.0] and 21.5 d (IQR 15.0-27.0), respectively. In subgroup analysis, the median time of hospital stay in moderate patients was 21 d (IQR 17.0-25.5) and 16.5 d (IQR 12.0-19.5), respectively. In severe patients, it was 23 d (IQR 15.0-32.0) and 24.5 d (IQR 23.0-31.0), respectively. Among patients who had not been discharged, those who had discontinued ACEIs/ARBs were 42.4% less likely to be discharged (HR = 0.424, 95%CI is 0.187-0.962, P = 0.040) compared to those who had been using other anti-hypertensive drugs. The results are shown in Table 2 and Figure 3A.
Table 2.
Univariate Cox proportional risk model associated with each indicator and prognosis
|
|
B
|
HR (95%CI)
|
Significance
|
| ACEIs/ARBs | -0.289 | 0.749 (0.426, 1.319) | 0.317 |
| Gender | -0.516 | 0.597 (0.333, 1.070) | 0.083 |
| Age | -0.007 | 0.993 (0.961, 1.025) | 0.650 |
| Initial symptom | 0.280 | ||
| Fever (temperature ≥ 37.3 °C) | -0.225 | 0.799 (0.393, 1.623) | 0.534 |
| Cough | 1.464 | 4.324 (0.547, 34.167) | 0.165 |
| Dyspnea | 0.012 | 1.012 (0.137, 7.496) | 0.991 |
| Chest tension | 1.811 | 6.115 (0.75, 49.843) | 0.091 |
| Exhaustion | 1.811 | 6.115 (0.75, 49.843) | 0.091 |
| Anorexia | 0.782 | 2.185 (0.286, 16.699) | 0.451 |
| Severity of illness | -0.541 | 0.582 (0.328, 1.032) | 0.064 |
| qSOFA score | -0.719 | 0.487 (0.272, 0.871) | 0.015 |
| CURB-65 score | 0.178 | ||
| CURB-65 (1) | -0.552 | 0.576 (0.305, 1.086) | 0.088 |
| CURB-65 (2) | -0.65 | 0.522 (0.211, 1.288) | 0.158 |
| Diabetes | -0.093 | 0.911 (0.512, 1.622) | 0.752 |
| Coronary heart disease | 0.62 | 1.860 (0.953, 3.630) | 0.069 |
| Chest CT results (single and bilateral) | 0.658 | 1.930 (0.681, 5.466) | 0.216 |
| Chest CT results (ground glass opacity) | -0.135 | 0.874 (0.487, 1.569) | 0.652 |
| Chest CT results (consolidation) | 0.127 | 1.136 (0.481, 2.681) | 0.772 |
| Chest CT results (pleural effusion) | -0.976 | 0.377 (0.134, 1.060) | 0.064 |
| Chest CT results (patch shadow) | 0.199 | 1.220 (0.651, 2.287) | 0.534 |
| Urine protein | -0.151 | 0.860 (0.466, 1.587) | 0.630 |
| Influenza antibody | 0.607 | ||
| Influenza A antibody | -0.305 | 0.737 (0.405, 1.341) | 0.318 |
| Influenza B antibody | -0.117 | 0.889 (0.305, 2.596) | 0.830 |
| Duration of hypertension | -0.008 | 0.992 (0.953, 1.032) | 0.678 |
| White blood cell count | -0.112 | 0.894 (0.749, 1.067) | 0.216 |
| Lymphocyte count | 0.322 | 1.380 (0.774, 2.461) | 0.275 |
| Platelet | 0.001 | 1.001 (0.997, 1.005) | 0.651 |
| Hemoglobin | -0.007 | 0.993 (0.969, 1.018) | 0.576 |
| Alanine transaminase | -0.015 | 0.985 (0.965, 1.005) | 0.131 |
| Albumin | 0.138 | 1.148 (1.058, 1.246) | 0.001 |
| Total bilirubin | -0.031 | 0.969 (0.918, 1.024) | 0.263 |
| LDH | -0.004 | 0.996 (0.992, 0.999) | 0.013 |
| BUN | -0.088 | 0.916 (0.806, 1.041) | 0.177 |
| Creatinine | -0.01 | 0.990 (0.979, 1.002) | 0.099 |
| Prothrombin time | 0.027 | 1.027 (0.895, 1.178) | 0.701 |
| APTT | -0.022 | 0.978 (0.944, 1.013) | 0.217 |
| Fibrous protein | -0.039 | 0.962 (0.794, 1.165) | 0.689 |
| BD-dimer | -0.056 | 0.945 (0.89, 1.004) | 0.065 |
| Bhs-CRP | -0.006 | 0.994 (0.989, 0.999) | 0.017 |
| B procalcitonin | -0.703 | 0.495 (0.069, 3.562) | 0.485 |
| B Serum ferritin | -0.001 | 0.999 (0.999, 1.000) | 0.027 |
| IL-1β | 0.098 | 1.103 (0.927, 1.311) | 0.268 |
| IL-2R | -0.001 | 0.999 (0.998, 1.000) | 0.007 |
| IL-6 | -0.002 | 0.998 (0.993, 1.003) | 0.370 |
| IL-10 | -0.093 | 0.911 (0.813, 1.021) | 0.108 |
| hs-TnI | -0.016 | 0.984 (0.924, 1.048) | 0.619 |
| Myoglobin | -0.004 | 0.996 (0.993, 1.000) | 0.051 |
| CKMB | -0.037 | 0.964 (0.879, 1.058) | 0.440 |
| NT-ProBNP | 0 | 1.000 (1.000, 1.000) | 0.938 |
ACEIs/ARBs: Angiotensin converting enzymes inhibitors or angiotensin receptor blockers; qSOFA: Quick sequential organ failure assessment; LDH: Lactate dehydrogenase; BUN: Blood urea nitrogen; APTT: Activated partial thromboplastin time; hs-CRP: High-sensitive C-reactive protein; IL-1β: Interleukin-1β; IL-2R: Interleukin-2 receptor; IL-6: Interleukin-6; IL-8: Interleukin-8; IL-10: Interleukin-10; hs-TnI: High sensitive cardiac troponin I; CKMB: Creatine phosphokinase isoenzyme; NT-ProBNP: N-terminal pro-brain natriuretic peptide; IQR: Interquartile range; CT: Computed tomography; HR: Hazard ratio; CI: Confidence interval.
Figure 3.
The cumulative probability of discharge in patients. A: The cumulative probability of discharge (all patients); B: The cumulative probability of discharge (moderate cases); C: The cumulative probability of discharge (severe cases).
After control for other potential confounding factors, the multivariate Cox proportional risk model showed that patients who discontinued ACEIs/ARBs underwent longer hospital stay; the difference was statistically significant (P < 0.05).
The effect of discontinue using ACEIs/ARBs was stronger in patients with moderate disease than among all patients [HR = 0.224 (0.005, 0.998); P = 0.0497]. The results are shown in Table 3 and Figure 3B.
Table 3.
Multivariate Cox proportional risk model to investigate the association between discontinuation of angiotensin converting enzymes inhibitors or angiotensin receptor blockers and prognosis
|
All the objects
|
Stratified by admission severity
|
|||||
|
|
Moderate
|
Severe
|
||||
|
HR (95%CI)
|
P
value
|
HR (95%CI)
|
P
value
|
HR (95%CI)
|
P
value
|
|
| ACEIs/ARBs | 0.424 (0.187, 0.962) | 0.040 | 0.224 (0.050, 0.998) | 0.0497 | 0.793 (0.215, 2.926) | 0.728 |
| qSOFA score | 0.455 (0.201, 1.026) | 0.058 | 0.685 (0.112, 4.199) | 0.682 | 0.576 (0.161, 2.053) | 0.395 |
| Gender | 1.116 (0.527, 2.363) | 0.774 | 0.733 (0.100, 5.368) | 0.760 | 0.850 (0.267, 2.698) | 0.782 |
| Coronary heart disease | 3.497 (1.475, 8.291) | 0.004 | 2.207 (0.569, 8.562) | 0.252 | 3.695 (0.933, 14.64) | 0.063 |
| Chest CT results pleural effusion | 0.577 (0.187, 1.778) | 0.338 | 0.348 (0.042, 2.871) | 0.327 | 0.294 (0.037, 2.303) | 0.244 |
| Albumin | 1.087 (0.969, 1.219) | 0.153 | 1.110 (0.860, 1.433) | 0.423 | 1.163 (0.970, 1.394) | 0.104 |
| LDH | 0.997 (0.992, 1.002) | 0.239 | 1.000 (0.988, 1.012) | 0.982 | 0.995 (0.988, 1.001) | 0.128 |
| Creatinine | 0.978 (0.959, 0.997) | 0.026 | 0.958 (0.920, 0.997) | 0.036 | 0.993 (0.964, 1.023) | 0.660 |
| D-dimer | 0.972 (0.907, 1.043) | 0.431 | 0.489 (0.147, 1.624) | 0.243 | 0.963 (0.889, 1.042) | 0.349 |
| hs-CRP | 0.999 (0.988, 1.009) | 0.793 | 0.993 (0.963, 1.025) | 0.670 | 1.000 (0.985, 1.016) | 0.953 |
| Serum ferritin | 1.000 (0.999, 1.001) | 0.998 | 1.001 (0.997, 1.006) | 0.600 | 1.001 (1.000, 1.002) | 0.172 |
| IL2R | 1.000 (0.999, 1.002) | 0.485 | 1.000 (0.998, 1.003) | 0.814 | 1.000 (0.999, 1.002) | 0.722 |
| Myoglobin | 1.002 (0.995, 1.010) | 0.489 | 1.014 (1.002, 1.026) | 0.026 | 0.993 (0.983, 1.003) | 0.153 |
ACEIs/ARBs: Angiotensin converting enzymes inhibitors or angiotensin receptor blockers; qSOFA: Quick sequential organ failure assessment; LDH: Lactate dehydrogenase; hs-CRP: High-sensitive C-reactive protein; IL-2R: Interleukin-2 receptor; HR: Hazard ratio; CI: Confidence interval.
In patients with severe disease, no association between discontinue using ACEIs/ARBs and disease prognosis was found [HR = 0.793 (0.215, 2.926); P = 0.728]. The results are shown in Table 3 and Figure 3C.
DISCUSSION
In the present study, we analyzed the clinical characteristics of 53 COVID-19 patients with hypertension and found that the median time of hospital stay was longer in the discontinued ACEIs/ARBs group than the other anti-hypertensive drugs. Moreover, this phenomenon was more significant in moderate cases, which revealed the significant impact of ACEIs/ARBs in COVID-19 patients with confirmed hypertension.
Concerns raised about using ACEIs/ARBs in hypertensive patients with COVID-19 because SARS-CoV-2 enters and infects human through ACE2. ACE and ACE2 are key regulatory enzymes in RAAS network. ACEIs/ARBs are RAAS blockers that control blood pressure by antagonizing ACE and angiotensin receptors[21-23]. ACE2 is a counterregulatory enzyme that degrades Ang II to angiotensin 1-7 (Ang1-7) and reverses the vasoconstrictive effect of ACE to maintain the balance of RAAS system. Additionally, studies have shown that ACEIs/ARBs might increase ACE2 expression, which might facilitate infection by SARS-CoV-2 and aggravate the disease, thus leading to potential longer hospital stay[16]. But solid evidence is still lacking for confirming the effects of ACEIs and ARBs on lung-specific expression of ACE2 in animal models and humans, especially in COVID-19 patients.
On the other hand, several studies have shown that ACEIs/ARBs may also have a protective effect. It was reported[24-26] that long-term ACEIs/ARBs treatment was associated with shorter length of hospital stay, lower rate of intubation/mechanical ventilation, and reduced 30-d mortality in patients with pneumonia. Further studies found that serum Ang II levels increased significantly in patients with acute lung injury, promoting the progress of acute lung injury through AT1R, which causes vasoconstriction, inflammation, fibrosis, and oxidation[27,28]. A study in COVID-19 patients published by Liu et al[29] confirmed that plasma Ang II level increased significantly in these patients and that it was linearly correlated with virus titer and severity of lung injury. A small-sample clinical study in patients with acute respiratory distress syndrome by Khan et al[30] showed that the level of Ang II decreased and the level of Ang 1-7 increased after an injection of human recombinant ACE2.
Evidence showing the effects of ACEIs and ARBs on lung-specific expression of ACE2 in humans and animal models are still controversial[31-35]. However, in animal models of acute lung injury, ACE activity and Ang II expression increased, but ACE2 activity and Ang-1-7 level decreased in animal models of acute lung injury, suggesting that ACE, Ang II, and AT1R are important factors in promoting acute lung injury, while the ACE2, Ang 1-7, and Mas receptor pathway might play protective roles. A study published by Imai et al[36] showed that ACE2 knock-out mice had significantly higher levels of Ang II than normal wild-type control mice and that pulmonary vascular permeability and lung injury were significantly higher in the knock-out mice. In the same study, recombinant human ACE2 and AT1R inhibitors improved the symptoms of acute lung injury in the ACE2 knock-out mice. In a mouse model of acute lung injury induced using the Spike-Fc protein of SARS-CoV, the level of Ang II also increased significantly, while the expression of ACE2 was down-regulated, and applying ARBs effectively attenuated pulmonary edema[11]. These studies indicated that ACE2 converts Ang II to Ang1-7, which finally binds to Mas receptor and mediates many beneficial actions, including vasodilation and anti-inflammatory, anti-oxidant, and anti-apoptotic effects[37-39]. Therefore, potential explanation for longer hospital stays in our patients who discontinued ACEIs/ARBs might be down-regulation of ACE2 and subsequent deteriorated lung injury[40,41].
Furthermore, according to a recent study by Huang et al[42] in hypertensive COVID-19 patients, there was no significant difference in length of hospital stay and clinical outcome between patients with both COVID-19 and hypertension who continued to take RAAS blockers and those who took non-RAAS blockers. Another study conducted by Zhang et al[43] showed that it was unlikely that inpatient ACEIs/ARBs would be associated with an increased risk of mortality.
As we all know, COVID-19 is particularly severe in patients with underlying cardiovascular diseases. There is no extra benefit to withdraw RAAS inhibitors in patients in otherwise stable condition. RAAS inhibitors show renal and myocardial protective effects, and discontinuation may cause damage in high-risk patients who show decompensation[44]. Monteil et al[45] show that SARS-CoV-2 can directly infect engineered human blood vessel organoids and human kidney organoids, which can be inhibited by ACE2. Combining the current study with other medical evidence[5,6,46], we would rather recommend not to change patients’ medication. However, the mechanism of drug interaction is complex, and the impact of drugs on patients may depend on the final equilibrium state. The effects of ACEIs/ARBs for COVID-19 patients complicated with hypertension requires further study.
There were several limitations of our study. First, our study was carried out in a single center, and sample size was limited by patient volume and strict recruitment criteria. Interpretation of our findings might be underestimated. However, our study was the first clinical study to explore the effect of ACEIs/ARBs withdrawal in COVID-19 patients. Second, we could not manage to enroll a third arm of patients who continued using ACEIs/ARBs after admission. Therefore, we could not objectively compare the differences among the patients who were using ACEIs/ARBs continuously, patients adjusted drugs after admission, and patients using non-ACEI/ARBs continuously.
CONCLUSION
Discontinuing ACEIs/ARBs in confirmed COVID-19 patients with hypertension resulted in a prolonged hospital stay. This phenomenon was more significant in moderate cases. Nonetheless, we believe that ACEIs/ARBs should be continued in patients with both COVID-19 and hypertension unless further evidence demonstrates adverse outcomes.
ARTICLE HIGHLIGHTS
Research background
During the coronavirus disease 2019 (COVID-19) pandemic, several studies have revealed that sustained ingestion of angiotensin converting enzymes inhibitors or angiotensin receptor blockers (ACEIs/ARBs) had no harmful effects on COVID-19 patients complicated with hypertension.
Research motivation
The role of angiotensin converting enzyme-2 (ACE2) receptor in COVID-19 pathophysiological process remains unclear. We expect to provide more important evidence for ACEIs/ARBs usage in clinical application.
Research objectives
To explore the impact of ACEIs/ARBs discontinued usage on COVID-19 patients complicated with hypertension.
Research methods
This study was based on a COVID-19 cohort from a patient registry of all COVID-19 patients admitted to an isolated unit in a tertiary hospital. All COVID-19 patients complicated with hypertension were recruited in our study and divided into discontinued ACEIs/ARBs group or other anti-hypertensive drugs group. We compared characteristics and clinical outcomes between those two different groups of patients.
Research results
A total of 53 patients were enrolled, 27 patients switched from ACEIs/ARBs to CCBs while 26 patients continued with non-ACEIs/ARBs. After controlling potential confounding factors using the Cox proportional hazards model, hospital stay was longer in patients who discontinued ACEIs/ARBs, with a hazard ratio (HR) of 0.424 [95% confidence interval (CI): 0.187-0.962; P = 0.040], than in patients using other anti-hypertensive drugs. A sub-group analysis showed that the effect of discontinuing ACEIs/ARBs was stronger in moderate cases [HR = 0.224 (95%CI: 0.005-0.998; P = 0.0497)].
Research conclusions
Our data revealed that discontinuing ACEIs/ARBs treatment after COVID-19 diagnosis results in a prolonged hospital stay. This phenomenon was more significant in hospitalized patients with moderate COVID-19. Our research suggested that ACEIs/ARBs should be continued in patients with both COVID-19 and hypertension unless further evidence demonstrates adverse outcomes.
Research perspectives
The mechanism of ACE2 in COVID-19 patients complicated with hypertension remains unclear and may be more complex, requiring further research to explore this area in the future.
Footnotes
Institutional review board statement: This study was approved by Institutional Review Board of Peking University Third Hospital, China (No. IRB00006761-M2020060).
Informed consent statement: The study was approved by Institutional Review Board of Peking University Third Hospital and got waiver of written informed consent.
Conflict-of-interest statement: The authors declare that there is no conflict of interest.
STROBE statement: The authors have read the STROBE Statement-checklist of items, and the manuscript was prepared and revised according to the STROBE Statement-checklist of items.
Manuscript source: Unsolicited manuscript
Peer-review started: October 9, 2020
First decision: November 3, 2020
Article in press: November 21, 2020
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Caca K, Nair K S-Editor: Gao CC L-Editor: Filipodia P-Editor: Xing YX
Contributor Information
Ci Tian, Department of Emergency Medicine, Peking University Third Hospital, Beijing 100191, China.
Nan Li, Research Center of Clinical Epidemiology, Peking University Third Hospital, Beijing 100191, China.
Yi Bai, Department of Emergency Medicine, Peking University Third Hospital, Beijing 100191, China.
Han Xiao, Department of Cardiology and Institute of Vascular Medicine, Peking University Third Hospital, Beijing 100191, China.
Shu Li, Department of Emergency Medicine, Peking University Third Hospital, Beijing 100191, China.
Qing-Gang Ge, Department of Critical Care Medicine, Peking University Third Hospital, Beijing 100191, China.
Ning Shen, Department of Pulmonary and Critical Care Medicine, Department of Medicine, Peking University Third Hospital, Beijing 100191, China.
Qing-Bian Ma, Department of Emergency Medicine, Peking University Third Hospital, Beijing 100191, China. maqingbian@bjmu.edu.cn.
Data sharing statement
The data used to support the findings of this study are available from the corresponding author upon request.
References
- 1.Wu Z, McGoogan JM. Characteristics of and Important Lessons From the Coronavirus Disease 2019 (COVID-19) Outbreak in China: Summary of a Report of 72 314 Cases From the Chinese Center for Disease Control and Prevention. JAMA. 2020;323:1239–1242. doi: 10.1001/jama.2020.2648. [DOI] [PubMed] [Google Scholar]
- 2.Li B, Yang J, Zhao F, Zhi L, Wang X, Liu L, Bi Z, Zhao Y. Prevalence and impact of cardiovascular metabolic diseases on COVID-19 in China. Clin Res Cardiol. 2020;109:531–538. doi: 10.1007/s00392-020-01626-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yang X, Yu Y, Xu J, Shu H, Xia J, Liu H, Wu Y, Zhang L, Yu Z, Fang M, Yu T, Wang Y, Pan S, Zou X, Yuan S, Shang Y. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir Med. 2020;8:475–481. doi: 10.1016/S2213-2600(20)30079-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhang JJ, Dong X, Cao YY, Yuan YD, Yang YB, Yan YQ, Akdis CA, Gao YD. Clinical characteristics of 140 patients infected with SARS-CoV-2 in Wuhan, China. Allergy. 2020;75:1730–1741. doi: 10.1111/all.14238. [DOI] [PubMed] [Google Scholar]
- 5.Mancia G, Rea F, Ludergnani M, Apolone G, Corrao G. Renin-Angiotensin-Aldosterone System Blockers and the Risk of Covid-19. N Engl J Med. 2020;382:2431–2440. doi: 10.1056/NEJMoa2006923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Reynolds HR, Adhikari S, Pulgarin C, Troxel AB, Iturrate E, Johnson SB, Hausvater A, Newman JD, Berger JS, Bangalore S, Katz SD, Fishman GI, Kunichoff D, Chen Y, Ogedegbe G, Hochman JS. Renin-Angiotensin-Aldosterone System Inhibitors and Risk of Covid-19. N Engl J Med. 2020;382:2441–2448. doi: 10.1056/NEJMoa2008975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lu J, Lu Y, Wang X, Li X, Linderman GC, Wu C, Cheng X, Mu L, Zhang H, Liu J, Su M, Zhao H, Spatz ES, Spertus JA, Masoudi FA, Krumholz HM, Jiang L. Prevalence, awareness, treatment, and control of hypertension in China: data from 1·7 million adults in a population-based screening study (China PEACE Million Persons Project) Lancet. 2017;390:2549–2558. doi: 10.1016/S0140-6736(17)32478-9. [DOI] [PubMed] [Google Scholar]
- 8.Wang Z, Chen Z, Zhang L, Wang X, Hao G, Zhang Z, Shao L, Tian Y, Dong Y, Zheng C, Wang J, Zhu M, Weintraub WS, Gao R China Hypertension Survey Investigators. Status of Hypertension in China: Results From the China Hypertension Survey, 2012-2015. Circulation. 2018;137:2344–2356. doi: 10.1161/CIRCULATIONAHA.117.032380. [DOI] [PubMed] [Google Scholar]
- 9.Patel VB, Zhong JC, Grant MB, Oudit GY. Role of the ACE2/Angiotensin 1-7 Axis of the Renin-Angiotensin System in Heart Failure. Circ Res. 2016;118:1313–1326. doi: 10.1161/CIRCRESAHA.116.307708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hoffmann M, Kleine-Weber H, Schroeder S, Krüger N, Herrler T, Erichsen S, Schiergens TS, Herrler G, Wu NH, Nitsche A, Müller MA, Drosten C, Pöhlmann S. SARS-CoV-2 Cell Entry Depends on ACE2 and TMPRSS2 and Is Blocked by a Clinically Proven Protease Inhibitor. Cell 2020; 181: 271-280. :e8. doi: 10.1016/j.cell.2020.02.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kuba K, Imai Y, Rao S, Gao H, Guo F, Guan B, Huan Y, Yang P, Zhang Y, Deng W, Bao L, Zhang B, Liu G, Wang Z, Chappell M, Liu Y, Zheng D, Leibbrandt A, Wada T, Slutsky AS, Liu D, Qin C, Jiang C, Penninger JM. A crucial role of angiotensin converting enzyme 2 (ACE2) in SARS coronavirus-induced lung injury. Nat Med. 2005;11:875–879. doi: 10.1038/nm1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang H, Penninger JM, Li Y, Zhong N, Slutsky AS. Angiotensin-converting enzyme 2 (ACE2) as a SARS-CoV-2 receptor: molecular mechanisms and potential therapeutic target. Intensive Care Med. 2020;46:586–590. doi: 10.1007/s00134-020-05985-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ferrario CM, Jessup J, Chappell MC, Averill DB, Brosnihan KB, Tallant EA, Diz DI, Gallagher PE. Effect of angiotensin-converting enzyme inhibition and angiotensin II receptor blockers on cardiac angiotensin-converting enzyme 2. Circulation. 2005;111:2605–2610. doi: 10.1161/CIRCULATIONAHA.104.510461. [DOI] [PubMed] [Google Scholar]
- 14.Jessup JA, Gallagher PE, Averill DB, Brosnihan KB, Tallant EA, Chappell MC, Ferrario CM. Effect of angiotensin II blockade on a new congenic model of hypertension derived from transgenic Ren-2 rats. Am J Physiol Heart Circ Physiol. 2006;291:H2166–H2172. doi: 10.1152/ajpheart.00061.2006. [DOI] [PubMed] [Google Scholar]
- 15.Li YQ, Wu JJ, Gao DF, Fan YM, Zhang Y, Qin XJ. [The impact of telmisartan on angiotensin converting enzyme 2 mRNA expression in monocyte-derived macrophages of diabetic hypertensive patients] Zhonghua Neike Zazhi. 2013;52:26–29. [PubMed] [Google Scholar]
- 16.Fang L, Karakiulakis G, Roth M. Are patients with hypertension and diabetes mellitus at increased risk for COVID-19 infection? Lancet Respir Med. 2020;8:e21. doi: 10.1016/S2213-2600(20)30116-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.American Heart Association. HFSA/ACC/AHA statement addresses concerns re using RAAS antagonists in COVID19. Available from: https://professional.heart.org/professional/ScienceNews/UCM_505836_HFSAACCAHA-statement-addresses-concerns-re-using-RAAS-antagonists-in-COVID-19.jsp .
- 18.European Society of Cardiology. Position Statement of the ESC Council on Hypertension on ACE-Inhibitors and Angiotensin Receptor Blockers. [Cited Mar 17, 2020]. Available from: https://www.escardio.org/Councils/Council-on-Hypertension (CHT)/News/position-statement-of-the-esc-council-on-hypertension-on-ace-inhibitors-and-ang .
- 19.Driggin E, Madhavan MV, Bikdeli B, Chuich T, Laracy J, Biondi-Zoccai G, Brown TS, Der Nigoghossian C, Zidar DA, Haythe J, Brodie D, Beckman JA, Kirtane AJ, Stone GW, Krumholz HM, Parikh SA. Cardiovascular Considerations for Patients, Health Care Workers, and Health Systems During the COVID-19 Pandemic. J Am Coll Cardiol. 2020;75:2352–2371. doi: 10.1016/j.jacc.2020.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.National Health Commission and National Administration of Traditional Chinese Medicine. Diagnosis and Treatment Protocol for Novel Coronavirus Pneumonia (Trial version 7) [EB/OL]. [cited 2020 Mar 29]. Available from: https://www.chinadaily.com.cn/pdf/2020/1.Clinical.Protocols.for.the.Diagnosis.and.Treatment.of.COVID-19.V7.pdf .
- 21.Santos RAS, Sampaio WO, Alzamora AC, Motta-Santos D, Alenina N, Bader M, Campagnole-Santos MJ. The ACE2/Angiotensin-(1-7)/MAS Axis of the Renin-Angiotensin System: Focus on Angiotensin-(1-7) Physiol Rev. 2018;98:505–553. doi: 10.1152/physrev.00023.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Simões E Silva AC, Teixeira MM. ACE inhibition, ACE2 and angiotensin-(1-7) axis in kidney and cardiac inflammation and fibrosis. Pharmacol Res. 2016;107:154–162. doi: 10.1016/j.phrs.2016.03.018. [DOI] [PubMed] [Google Scholar]
- 23.Vickers C, Hales P, Kaushik V, Dick L, Gavin J, Tang J, Godbout K, Parsons T, Baronas E, Hsieh F, Acton S, Patane M, Nichols A, Tummino P. Hydrolysis of biological peptides by human angiotensin-converting enzyme-related carboxypeptidase. J Biol Chem. 2002;277:14838–14843. doi: 10.1074/jbc.M200581200. [DOI] [PubMed] [Google Scholar]
- 24.Mortensen EM, Nakashima B, Cornell J, Copeland LA, Pugh MJ, Anzueto A, Good C, Restrepo MI, Downs JR, Frei CR, Fine MJ. Population-based study of statins, angiotensin II receptor blockers, and angiotensin-converting enzyme inhibitors on pneumonia-related outcomes. Clin Infect Dis. 2012;55:1466–1473. doi: 10.1093/cid/cis733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wu A, Good C, Downs JR, Fine MJ, Pugh MJ, Anzueto A, Mortensen EM. The association of cardioprotective medications with pneumonia-related outcomes. PLoS One. 2014;9:e85797. doi: 10.1371/journal.pone.0085797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Henry C, Zaizafoun M, Stock E, Ghamande S, Arroliga AC, White HD. Impact of angiotensin-converting enzyme inhibitors and statins on viral pneumonia. Proc (Bayl Univ Med Cent) 2018;31:419–423. doi: 10.1080/08998280.2018.1499293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Balakumar P, Jagadeesh G. A century old renin-angiotensin system still grows with endless possibilities: AT1 receptor signaling cascades in cardiovascular physiopathology. Cell Signal. 2014;26:2147–2160. doi: 10.1016/j.cellsig.2014.06.011. [DOI] [PubMed] [Google Scholar]
- 28.Kaparianos A, Argyropoulou E. Local renin-angiotensin II systems, angiotensin-converting enzyme and its homologue ACE2: their potential role in the pathogenesis of chronic obstructive pulmonary diseases, pulmonary hypertension and acute respiratory distress syndrome. Curr Med Chem. 2011;18:3506–3515. doi: 10.2174/092986711796642562. [DOI] [PubMed] [Google Scholar]
- 29.Liu Y, Yang Y, Zhang C, Huang F, Wang F, Yuan J, Wang Z, Li J, Li J, Feng C, Zhang Z, Wang L, Peng L, Chen L, Qin Y, Zhao D, Tan S, Yin L, Xu J, Zhou C, Jiang C, Liu L. Clinical and biochemical indexes from 2019-nCoV infected patients linked to viral loads and lung injury. Sci China Life Sci. 2020;63:364–374. doi: 10.1007/s11427-020-1643-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Khan A, Benthin C, Zeno B, Albertson TE, Boyd J, Christie JD, Hall R, Poirier G, Ronco JJ, Tidswell M, Hardes K, Powley WM, Wright TJ, Siederer SK, Fairman DA, Lipson DA, Bayliffe AI, Lazaar AL. A pilot clinical trial of recombinant human angiotensin-converting enzyme 2 in acute respiratory distress syndrome. Crit Care. 2017;21:234. doi: 10.1186/s13054-017-1823-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Burchill LJ, Velkoska E, Dean RG, Griggs K, Patel SK, Burrell LM. Combination renin-angiotensin system blockade and angiotensin-converting enzyme 2 in experimental myocardial infarction: implications for future therapeutic directions. Clin Sci (Lond) 2012;123:649–658. doi: 10.1042/CS20120162. [DOI] [PubMed] [Google Scholar]
- 32.Walters TE, Kalman JM, Patel SK, Mearns M, Velkoska E, Burrell LM. Angiotensin converting enzyme 2 activity and human atrial fibrillation: increased plasma angiotensin converting enzyme 2 activity is associated with atrial fibrillation and more advanced left atrial structural remodelling. Europace. 2017;19:1280–1287. doi: 10.1093/europace/euw246. [DOI] [PubMed] [Google Scholar]
- 33.Ramchand J, Patel SK, Kearney LG, Matalanis G, Farouque O, Srivastava PM, Burrell LM. Plasma ACE2 Activity Predicts Mortality in Aortic Stenosis and Is Associated With Severe Myocardial Fibrosis. JACC Cardiovasc Imaging. 2020;13:655–664. doi: 10.1016/j.jcmg.2019.09.005. [DOI] [PubMed] [Google Scholar]
- 34.Ramchand J, Patel SK, Srivastava PM, Farouque O, Burrell LM. Elevated plasma angiotensin converting enzyme 2 activity is an independent predictor of major adverse cardiac events in patients with obstructive coronary artery disease. PLoS One. 2018;13:e0198144. doi: 10.1371/journal.pone.0198144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Epelman S, Shrestha K, Troughton RW, Francis GS, Sen S, Klein AL, Tang WH. Soluble angiotensin-converting enzyme 2 in human heart failure: relation with myocardial function and clinical outcomes. J Card Fail. 2009;15:565–571. doi: 10.1016/j.cardfail.2009.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Imai Y, Kuba K, Rao S, Huan Y, Guo F, Guan B, Yang P, Sarao R, Wada T, Leong-Poi H, Crackower MA, Fukamizu A, Hui CC, Hein L, Uhlig S, Slutsky AS, Jiang C, Penninger JM. Angiotensin-converting enzyme 2 protects from severe acute lung failure. Nature. 2005;436:112–116. doi: 10.1038/nature03712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Klein N, Gembardt F, Supé S, Kaestle SM, Nickles H, Erfinanda L, Lei X, Yin J, Wang L, Mertens M, Szaszi K, Walther T, Kuebler WM. Angiotensin-(1-7) protects from experimental acute lung injury. Crit Care Med. 2013;41:e334–e343. doi: 10.1097/CCM.0b013e31828a6688. [DOI] [PubMed] [Google Scholar]
- 38.Meng Y, Yu CH, Li W, Li T, Luo W, Huang S, Wu PS, Cai SX, Li X. Angiotensin-converting enzyme 2/angiotensin-(1-7)/Mas axis protects against lung fibrosis by inhibiting the MAPK/NF-κB pathway. Am J Respir Cell Mol Biol. 2014;50:723–736. doi: 10.1165/rcmb.2012-0451OC. [DOI] [PubMed] [Google Scholar]
- 39.Sodhi CP, Wohlford-Lenane C, Yamaguchi Y, Prindle T, Fulton WB, Wang S, McCray PB Jr, Chappell M, Hackam DJ, Jia H. Attenuation of pulmonary ACE2 activity impairs inactivation of des-Arg9 bradykinin/BKB1R axis and facilitates LPS-induced neutrophil infiltration. Am J Physiol Lung Cell Mol Physiol. 2018;314:L17–L31. doi: 10.1152/ajplung.00498.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tan WSD, Liao W, Zhou S, Mei D, Wong WF. Targeting the renin-angiotensin system as novel therapeutic strategy for pulmonary diseases. Curr Opin Pharmacol. 2018;40:9–17. doi: 10.1016/j.coph.2017.12.002. [DOI] [PubMed] [Google Scholar]
- 41.Sanchis-Gomar F, Lavie CJ, Perez-Quilis C, Henry BM, Lippi G. Angiotensin-Converting Enzyme 2 and Antihypertensives (Angiotensin Receptor Blockers and Angiotensin-Converting Enzyme Inhibitors) in Coronavirus Disease 2019. Mayo Clin Proc. 2020;95:1222–1230. doi: 10.1016/j.mayocp.2020.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Huang Z, Cao J, Yao Y, Jin X, Luo Z, Xue Y, Zhu C, Song Y, Wang Y, Zou Y, Qian J, Yu K, Gong H, Ge J. The effect of RAS blockers on the clinical characteristics of COVID-19 patients with hypertension. Ann Transl Med. 2020;8:430. doi: 10.21037/atm.2020.03.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhang P, Zhu L, Cai J, Lei F, Qin JJ, Xie J, Liu YM, Zhao YC, Huang X, Lin L, Xia M, Chen MM, Cheng X, Zhang X, Guo D, Peng Y, Ji YX, Chen J, She ZG, Wang Y, Xu Q, Tan R, Wang H, Lin J, Luo P, Fu S, Cai H, Ye P, Xiao B, Mao W, Liu L, Yan Y, Liu M, Chen M, Zhang XJ, Wang X, Touyz RM, Xia J, Zhang BH, Huang X, Yuan Y, Loomba R, Liu PP, Li H. Association of Inpatient Use of Angiotensin-Converting Enzyme Inhibitors and Angiotensin II Receptor Blockers With Mortality Among Patients With Hypertension Hospitalized With COVID-19. Circ Res. 2020;126:1671–1681. doi: 10.1161/CIRCRESAHA.120.317134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Vaduganathan M, Vardeny O, Michel T, McMurray JJV, Pfeffer MA, Solomon SD. Renin-Angiotensin-Aldosterone System Inhibitors in Patients with Covid-19. N Engl J Med. 2020;382:1653–1659. doi: 10.1056/NEJMsr2005760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Monteil V, Kwon H, Prado P, Hagelkrüys A, Wimmer RA, Stahl M, Leopoldi A, Garreta E, Hurtado Del Pozo C, Prosper F, Romero JP, Wirnsberger G, Zhang H, Slutsky AS, Conder R, Montserrat N, Mirazimi A, Penninger JM. Inhibition of SARS-CoV-2 Infections in Engineered Human Tissues Using Clinical-Grade Soluble Human ACE2. Cell 2020; 181: 905-913. :e7. doi: 10.1016/j.cell.2020.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mehra MR, Desai SS, Kuy S, Henry TD, Patel AN. Retraction: Cardiovascular Disease, Drug Therapy, and Mortality in Covid-19. N Engl J Med. DOI: 10.1056/NEJMoa2007621. N Engl J Med. 2020;382:2582. doi: 10.1056/NEJMoa2007621. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data used to support the findings of this study are available from the corresponding author upon request.