Abstract
BACKGROUND
Large inter-individual and inter-population differences in the susceptibility to and outcome of severe acute respiratory syndrome coronavirus 2 or coronavirus disease 2019 (COVID-19) have been noted. Understanding these differences and how they influence vulnerability to infection and disease severity is critical to public health intervention.
AIM
To analyze and compare the profile of COVID-19 cases between China and North America as two regions that differ in many environmental, host and healthcare factors related to disease risk.
METHODS
We conducted a meta-analysis to examine and compare demographic information, clinical symptoms, comorbidities, disease severity and levels of disease biomarkers of COVID-19 cases from clinical studies and data from China (105 studies) and North America (19 studies).
RESULTS
COVID-19 patients from North America were older than their Chinese counterparts and with higher male: Female ratio. Fever, cough, fatigue and dyspnea were the most common clinical symptoms in both study regions (present in about 30% to 75% of the cases in both regions). Meta-analysis for the prevalence of comorbidities (such as obesity, hypertension, diabetes, cardiovascular diseases, chronic obstructive pulmonary disease, cancer, and chronic kidney diseases) in COVID-19 patients were all significantly more prevalent in North America compared to China. Comorbidities were positively correlated with age but at a significantly younger age range in China compared to North American. The most prevalent infection outcome was acute respiratory distress syndrome which was 2-fold more frequent in North America than in China. Levels of C-reactive protein were 4.5-fold higher in the North American cases than in cases from China.
CONCLUSION
The differences in the profile of COVID-19 cases from China and North America may relate to differences in environmental-, host- and healthcare-related factors between the two regions. Such inter-population differences-together with intra-population variability-underline the need to characterize the effect of health inequities and inequalities on public health response to COVID-19 and can assist in preparing for the re-emergence of the epidemic.
Keywords: COVID-19, Symptoms, Comorbidities, China, North America, Adults
Core Tip: The study evaluates the inter-population differences in the susceptibility to acute respiratory syndrome coronavirus-2 or coronavirus disease 2019 (COVID-19) between China and North America. Fever, cough, fatigue and dyspnea were the most common clinical symptoms of COVID-19 in both study regions. Hypertension, diabetes and cancer were the most prevalent comorbidities in COVID-19 cases from China whereas obesity, hypertension and diabetes were the most prevalent in North America. Prevalence of comorbidities in COVID-19 cases increased with age but was at younger ages in cases from China compared to those from North America. Inflammatory markers such as C-reactive protein were 4.5-fold higher in the North American cases than in cases from China. The differences in COVID-19 profile between China and North America reflects the differences in environmental-, host- and healthcare-related factors between the two regions.
INTRODUCTION
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) or coronavirus disease 2019 (COVID-19) is a novel, zoonotic, positive-sense, single-stranded RNA b-coronavirus that belongs-together with SARS-CoV and Middle Eastern respiratory syndrome (MERS)-CoV-to the Coronaviridae family, sub-family Orthocoronaviridae[1]. In December 2019, COVID-19 infection began to spread from Wuhan, Hubei, China where it was declared on January 30, 2020 as a public health emergency of international concern by the World Health Organization (WHO)[2], a status that was raised to its highest level on February 28 and the disease was declared a global pandemic on March 11, 2020[2]. That pandemic status was declared when the number of cases was increased 13 times (outside China) than the initial number of cases, reporting > 118000 infections with triple the number of countries primarily involved (114 countries) and with > 4000 deaths[2]. Presently, i.e., within less than 6 mo, the number of COVID-19 cases has increased by approximately 200-fold with a similar increase in the number of deaths[3].
The clinical manifestations of the disease severity have shown great inter-individual variation in response to infection[4] with 81%, 14% and 5% of the cases were, respectively manifested with mild, severe and critical outcomes[4-6]. The rapid global spread of the disease, together with the inter-individual variation in response to infection, underlines the need for systematic analysis of global datasets from clinical research to better characterize the potential risk factors that play a role in population vulnerability to development of severe disease.
As of mid-August 2020, there are over 6 million cases and 235000 deaths in North America (United States, Mexico and Canada) and approximately 90000 cases and 5000 deaths in China[3]. Taken together, at that time, over 30% of the world’s confirmed cases and deaths were reported in North America (mainly the United States) and China. Given this high prevalence, it is unsurprising that most of the epidemiological, clinical, etiological, and immunological studies on COVID-19 have so far emerged from clinical research in these two regions. However, East Asia and North America have significant differences in lifestyle and sociodemographic factors, and population characteristics which may differently affect response to infection. Understanding these inter-population differences may highlight factors that influence population vulnerability and facilitate a better understanding of the disease etiology. Therefore, the present study was undertaken to systematically analyze, summarize and compare findings from China and North America as two highly affected populations with COVID-19. The outcome of this study may permit designing population-based prevention and control measures for COVID-19 and other infectious diseases caused by coronaviruses.
MATERIALS AND METHODS
Study selections
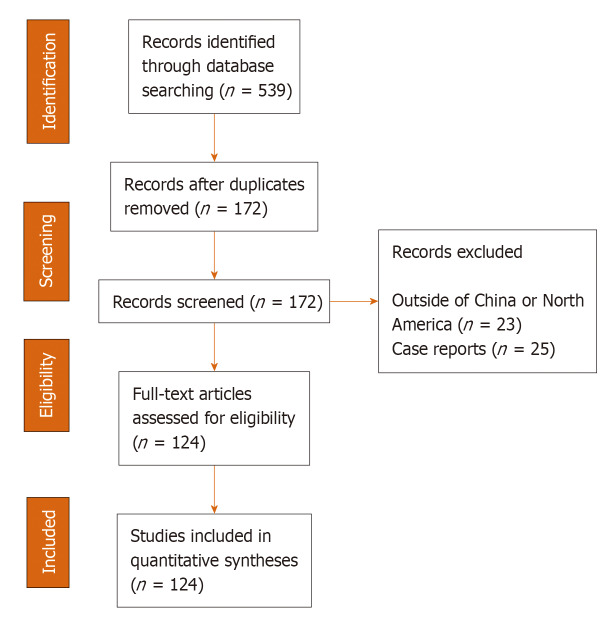
A systematic literature search was conducted in conformity with the Preferred Reporting Items for Systematic reviews and Meta-Analysis (PRISMA) guidelines (Supplementary Table 1). The studies examined here were selected from the Aggregated Dataset of Clinical Outcomes for COVID-19 Patients – a publicly accessible dataset which aggregates data from published clinical studies and preprints released between December 2019 and April 2020[7]. This dataset is largely derived from studies conducted in hospitals in the countries affected with COVID-19. Reports in this aggregated dataset were collected from 17 countries (Australia, Chile, China, France, Germany, Iceland, Italy, Japan, Korea, Mexico, Netherlands, Scotland, Singapore, South Korea, United Kingdom and United States) representing the regions of East Asia, Europe, and the Americas. The studies were observational, randomized clinical trials and case reports. For each paper in the aggregated dataset, an MIT researcher reads the paper and gathers the relevant numerical data from the tables and text into a standard format. In the present study, we excluded case reports and studies with patients from countries other than China and North America. The inclusion of case studies would have added more heterogeneity to the meta-analysis findings (see below). Only reports with adult cases were included. As shown in Figure 1, we included 124 studies and reports – 105 from China and 19 from North America (Supplementary Table 2).
Figure 1.
Flowchart of study selection and systematic literature review process. The flow diagram describes the systematic review of literature evaluating the prevalence of comparative profile for coronavirus disease 2019 (COVID-19) cases from China and North America from studies selected from Aggregated Dataset of Clinical Outcomes for COVID-19 Patients[7]. Quantitative analysis is developed from 124 studies from China (n = 105) and North America (n = 19).
Data extracted for the purpose of this study included demographic information (number of patients, age, gender, and ethnicity), frequency of clinical symptoms (fever, cough, dyspnea, headache, expectoration, myalgia, fatigue, diarrhea, vomiting or nausea, sore throat and chills), prevalence of comorbidities [obesity, hypertension, diabetes, cardiovascular diseases, chronic obstructive pulmonary disease (COPD), cancer, liver diseases and chronic kidney diseases], infection outcomes [acute respiratory distress syndrome (ARDS), heart failure, septic shock, acute kidney injury and secondary infection] and levels of standard disease biomarkers [white blood cell count (109/L), lymphocyte count (109/L), aspartate aminotransferase (U/L), total bilirubin (μmoL/L), creatine kinase (U/L), C-reactive protein (CRP) (mg/L) and procalcitonin (ng/mL)].
Statistical analysis and meta-analysis
Weighted average and 95% confidence intervals (CI) were used to calculate the mean age and percentages of males, ethnicity, disease severity, the prevalence of infection outcome and average levels of biomarkers in COVID-19 cases from the selected studies. Meta-analysis tests were conducted-by Comprehensive Meta-Analysis software (CMA), version 3.9 (Englewood, NJ, United States)[8] – on the comorbidities prevalence and clinical symptoms frequency. Pooling of the variance in proportions or percentages (raw) were based on a binary random effects model[9]. This was due to the heterogeneity of the examined populations and the possible across-population variability in the relationships between COVID-19 and the evaluated factors (e.g., comorbidities or clinical symptoms). Comparisons between the assessed factors in the two evaluated populations was carried out using the Chi-squared test as previously recommended[10],[11]. Comparisons of the average age and levels of disease markers were carried out by Student’s t-test. Pearson's correlation coefficient (r) was used to measure of the association between the proportion of the examined comorbidities and age in the examined regions. We used SPSS Statistics, Version 21.0 (SPSS Inc., Chicago, IL, United States) for all statistical tests (two-sided).
Heterogeneity among the selected studies was assessed by the Q test[12]. Information provided by the Q test conveys if heterogeneity is present or absent. However, this test does not inform on the heterogeneity level besides it has insufficient power to detect heterogeneity, especially when there is small number of assessed studies (e.g., for some comorbidities). Consequently, I2index was calculated as a complement to the Q test and to define the extent of between-study heterogeneity[13]. I2index values are typically categorized as considerable (> 90%), substantial (60%-90%), moderate (30%-60%) and low (< 30%)[14]. True heterogeneity was also examined by assessing τ2 that informs on the within-study variance in the used random-effects model[15]. Publication bias (Supplementary Table 3) was assessed as described earlier[15] by Egger’s test and to examine the tendency for an effect estimated from studies with a small sample size to be different from that obtained from large studies. Kendall’s tau with continuity correction test (Begg and Mazumdar rank correlation test) was however used to test the correlation between the ranks of effect sizes and the ranks of their variances across the selected studies.
RESULTS
In the present study we examined the data of 72025 COVID-19 cases from 124 reports from China (105 studies) and North America (19 studies). The number of entries from the reports from North America was 1.28-fold higher than those from China. Data collected from these studies were obtained from the “Aggregated Dataset of Clinical Outcomes for COVID-19 Patients” and were compared between patients from the two regions for demographic information, frequency of clinical symptoms, prevalence of comorbidities and levels of standard disease biomarkers. As shown in Table 1, COVID-19 cases from North America were older than their Chinese counterparts and with higher male: Female ratio. Reports from North America examined Hispanics (approximately 50%), Whites (approximately 30%), and African Americans and Asians (approximately 20%) whereas reports from China, as expected, examined only Chinese (100%) patients (data not shown). As for the infection outcomes, significantly (P < 0.001) higher rates of cases from North America were found to develop ARDS, septic shock and acute kidney injury compared to their Chinese counterparts but had lowers rates of heart failure and secondary infection. In China, the most prevalent infection outcomes were ARDS and secondary infection which occurred in about 15% of the cases (95%CI: 14.1%-14.9% and 14.7%-15.9%; respectively). Similarly, in North America, ARDS was the chief infection complication (34.9%, 95%CI: 33.9-36.1), followed by septic shock (28.4%; 95%CI: 27.3-29.5).
Table 1.
Characteristics of the study populations with coronavirus disease 2019 from China and North America
| Characteristic |
China
|
North America
|
P
3
value
|
||
|
Number of cases (n)1
|
Mean2 (95%CI)
|
Number of cases (n)1
|
Mean2 (95%CI)
|
||
| Age | 23088 | 50.6 (50.4-50.8) | 37293 | 55.5 (55.4-55.6) | < 0.001 |
| Male (%) | 29755 | 52.6 (52.5-52.7) | 36298 | 54.4 (54.2-54.5) | < 0.001 |
| Severity (%) | 26810 | 18.7 (12.8-24.6) | 7824 | 25.1 (19.1-31.3) | < 0.001 |
| Infection outcome (%) | |||||
| ARDS | 13524 | 14.5 (14.1-14.9) | 1849 | 34.9 (33.9-36.1) | < 0.001 |
| Heart failure | 4847 | 10.6 (10.2-11.1) | 2654 | 2.5 (2.4-2.6) | < 0.001 |
| Septic shock | 10886 | 4.0 (3.8-4.2) | 2266 | 28.4 (27.3-29.5) | < 0.001 |
| Acute kidney injury | 12767 | 5.5 (5.4-5.7) | 4761 | 26.7 (26.4-27.1) | < 0.001 |
| Secondary infection | 4617 | 15.3 (14.7-15.9) | 807 | 5.3 (5.1-5.6) | < 0.001 |
n is the number of subjects assessed for each characteristic as per the source database[7].
Weighted mean.
Student’s t-test or Chi Square test. ARDS: Acute respiratory distress syndrome.
Meta-analysis for the frequency of clinical symptoms associated with COVID-19 in both study populations is shown in Table 2. Fever, cough, fatigue and dyspnea were the most common clinical symptoms in COVID-19 cases, but with varying frequencies between the studies regions. These symptoms were present in about 30% to 75% of the cases in both regions. Also, about 10% to 30% of the cases in both regions had expectoration, myalgia, diarrhea, headache, sore throat, chills, vomiting and/or nausea. Significantly lower rates of fever and expectoration were noted in North America compared to China (P < 0.001) whereas similar frequencies of cough, headache, fatigue and sore throat were observed in both study areas. From the selected reports, the mortality rates of COVID-19 (± SD) was 5.9 ± 13.4% in China, not significantly different from rates of 13.9 ± 14.8% in North America. Substantial to considerable I2 values (60%-> 90%) were obtained for the combined frequency of the majority of clinical symptoms from the evaluated reports, indicating a high degree of heterogeneity among studies. On the other hand, the combined frequency from studies reporting fatigue, sore throat and chills have shown low among-studies level of heterogenicity (I2: 18%-51%).
Table 2.
Meta-analyses for the frequency of clinical symptoms in coronavirus disease 2019 cases from China and North America
| Symptoms |
China
|
North America
|
P
2
value
|
||||||||||
| Number of cases (n)1 |
Meta-analysis
|
Heterogenicity
|
Number of cases (n) |
Meta-analysis
|
Heterogenicity
|
||||||||
|
Frequency (%)
|
95%CI
|
τ
2
|
Q
|
I
2
|
Frequency (%)
|
95%CI
|
τ
2
|
Q
|
I
2
|
||||
| Fever | 25223 | 77.5 | 75.8-79.2 | 0.015 | 3591 | 93 | 4,502 | 43.3 | 30.8-56.1 | 0.099 | 5023 | 96 | < 0.001 |
| Cough | 24105 | 59.6 | 56.9-62.4 | 0.041 | 4843 | 95 | 3766 | 58.3 | 44.7-71.9 | 0.951 | 1997 | 98 | 0.131 |
| Dyspnea | 20558 | 29.4 | 26.7-32.1 | 0.028 | 9566 | 98 | 3711 | 38.9 | 24.3-53.5 | 0.103 | 4636 | 99 | < 0.001 |
| Headache | 13627 | 9.8 | 8.6-11.1 | 0.003 | 726 | 85 | 2483 | 8.9 | 6.4-11.4 | 0.001 | 36 | 64 | 0.163 |
| Expectoration | 14426 | 28.1 | 25.3-30.7 | 0.018 | 1719 | 93 | 2024 | 8.2 | 5.5-10.9 | 0.002 | 14 | 72 | < 0.001 |
| Myalgia | 16.184 | 19.1 | 17.1-21.2 | 0.012 | 3596 | 96 | 1224 | 22.5 | 12.8-32.3 | 0.019 | 159 | 94 | < 0.001 |
| Fatigue | 19561 | 36.3 | 33.2-39.4 | 0.038 | 5004 | 97 | 21 | 48.3 | 20.1-76.5 | 0.032 | 4 | 51 | 0.253 |
| Diarrhea | 20535 | 10.4 | 9.3-11.6 | 0.004 | 2210 | 92 | 3719 | 12.9 | 7.6-18.2 | 0.012 | 538 | 96 | < 0.001 |
| Vomiting and nausea | 14974 | 7.2 | 6.1-8.3 | 0.003 | 1485 | 91 | 2198 | 11.6 | 6.1-17.1 | 0.006 | 108 | 91 | < 0.001 |
| Sore throat | 11379 | 9.1 | 7.7-10.4 | 0.002 | 476 | 84 | 2542 | 8.3 | 6.8-9.8 | 0.001 | 16 | 23 | 0.202 |
| Chills | 5949 | 7.5 | 5.2-9.8 | 0.003 | 386 | 94 | 2108 | 17.7 | 16.1-19.3 | 0.001 | 5 | 18 | < 0.001 |
n is the number of subjects assessed for each clinical symptom as per the source database[7].
Chi Square test for the difference between symptom frequency in China and North America.
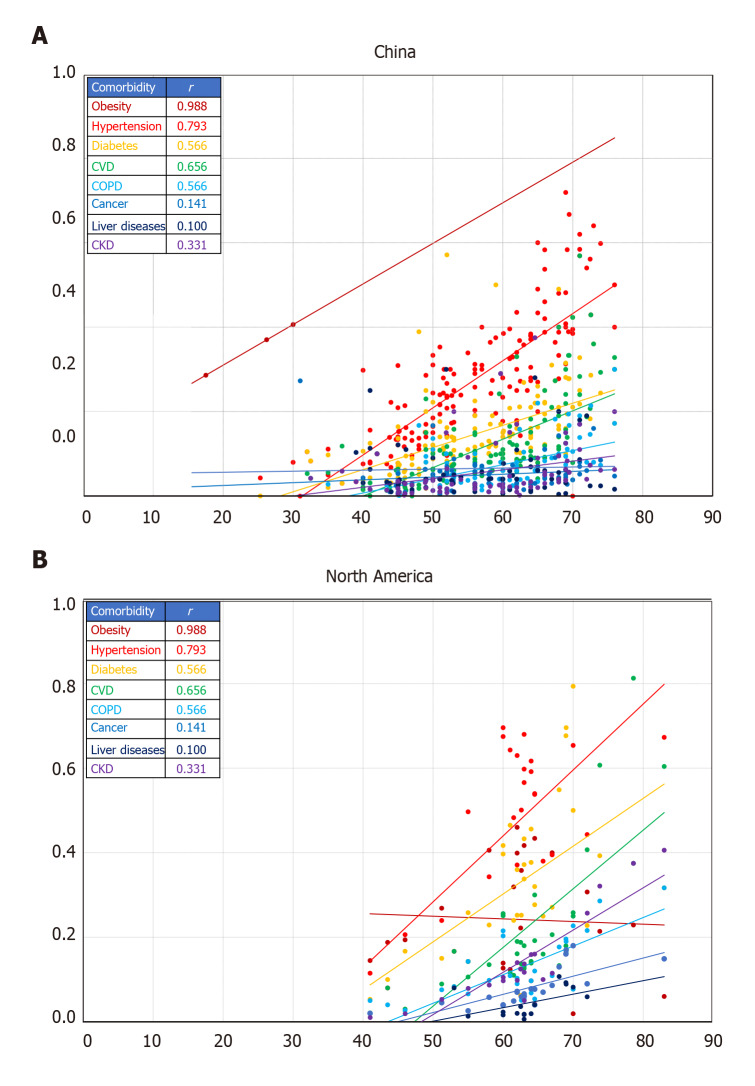
Meta-analysis for the prevalence of comorbidities in COVID-19 cases from studies in China and North America is shown in Table 3. Except for liver diseases and cancer, the prevalence of all comorbidities was significantly higher in North America compared to China (P < 0.001). The prevalence of liver diseases (any, e.g., alcohol-related liver disease, hepatitis, cirrhosis, hemochromatosis, liver Cysts, non-alcoholic fatty liver disease) was similar in both regions whereas that of cancer was 1.5-fold higher in China than in North America (P < 0001). Hypertension followed by diabetes were the most prevalent comorbidities in both regions with the North American cases having on average 2.5-fold higher prevalence on the two diseases than cases from China. Although obesity was the third most prevalent co-existing medical condition in COVID-19 cases from North America (approximately 35% of the cases), it was the least prevalent comorbidity in China (only 2% of the cases). Similar to hypertension and diabetes, cardiovascular diseases were 2.5-fold higher in cases from North America (21%) than in those from China (8%). Chronic kidney diseases and COPD were, respectively 5.6- and 3.5-fold significantly (P < 0.001) more prevalent in COVID-19 cases from North America than China. Substantial to considerable I2 values (60%-> 90%) were obtained for the combined prevalence of each comorbidity from the examined reports, indicating high among-studies heterogeneity in the evaluated conditions in reports from both regions. Analysis for the correlation between comorbidities and age in COVID-19 cases from China and North America is shown in Figure 2. All comorbidities were positively correlated with age in both study regions except for rates of obesity in North America that were steady along the examined age range. Although the overall average age was comparable between cases from the two regions, comorbidities were reported at a significantly younger age range in China compared to North American.
Table 3.
Meta-analyses for the prevalence of comorbidities in the study populations of coronavirus disease 2019 cases from China and North America
| Comorbidities |
China
|
North America
|
P
2
value
|
||||||||||
| Number of cases (n)1 |
Meta-analysis
|
Heterogenicity
|
Number of cases (n) |
Meta-analysis
|
Heterogenicity
|
||||||||
|
Frequency (%)
|
95%CI
|
τ
2
|
Q
|
I
2
|
Frequency (%)
|
95%CI
|
τ
2
|
Q
|
I
2
|
||||
| Obesity | 666 | 2.1 | 0.1-4.3 | 0.001 | 54 | 91 | 26895 | 22.9 | 18.1-27.9 | 0.016 | 2130 | 99 | < 0.001 |
| Hypertension | 22650 | 25.6 | 23.8-27.4 | 0.013 | 2154 | 91 | 34442 | 47.6 | 41.0-54.2 | 0.034 | 5347 | 99 | < 0.001 |
| Diabetes | 23770 | 11.8 | 10.9-12.6 | 0.002 | 980 | 79 | 35384 | 34.1 | 30.1-38.2 | 0.015 | 3169 | 98 | < 0.001 |
| Cardiovascular diseases | 22439 | 7.9 | 7.1-8.7 | 0.002 | 1339 | 86 | 33459 | 20.8 | 18.4-23.3 | 0.005 | 4379 | 99 | < 0.001 |
| COPD | 17263 | 3.3 | 2.8-3.7 | 0.001 | 540 | 78 | 29697 | 11.6 | 10.1-13.1 | 0.001 | 716 | 94 | < 0.001 |
| Cancer | 16527 | 9.7 | 7.1-12.2 | 0.017 | 19185 | 99 | 24564 | 6.6 | 5.5-7.6 | 0.001 | 254 | 89 | < 0.001 |
| Liver diseases (any) | 11087 | 3.3 | 2.8-3.9 | 0.001 | 276 | 68 | 9706 | 3.1 | 2.1-4.2 | 0.001 | 98 | 83 | 0.414 |
| Chronic kidney diseases | 16394 | 2.1 | 1.7-2.4 | 0.001 | 236 | 59 | 30761 | 11.8 | 10.0-13.6 | 0.002 | 1082 | 86 | < 0.001 |
n is the number of subjects assessed for each comorbidity as per the source database[7].
Chi Square test for the difference between prevalence of comorbidity in China and North America. COPD: Chronic obstructive pulmonary disease.
Figure 2.
Comparative profile for the correlation between comorbidities (proportion) and age (years) in coronavirus disease 2019 cases from China and North America. A: China; B: North America. Lines represent linear regression between the proportion of comorbidity and age. Colors correspond to the comorbidity in the insert table with the related Pearson correlation coefficient (r). Each data marker represents one entry. All r values are significant at P < 0.05 for the corresponding number of entries shown. Number of entries is per the source database[7].
The average levels of biomarkers in COVID-19 cases from China and North America is shown in Table 4. Studies from both regions showed levels within the reference clinical range for white blood cell count, lymphocyte count and total bilirubin. In both regions, however, levels significantly higher than the reference range were reported in aspartate aminotransferase, creatine kinase, CRP, and procalcitonin and were higher in cases from North America compared to China. For example, aspartate amino-transferase and creatine kinase were approximately 1.4-fold significantly higher in North American cases than Chinese cases whereas CRP levels were 4.5-fold higher (P < 0.001).
Table 4.
Average levels of biomarkers in coronavirus disease 2019 cases from China and North America
| Biomarker |
China
|
North America
|
P 3 value | Reference range | ||
|
Number of cases (n)1
|
Mean2 (95%CI)
|
Number of cases (n)1
|
Mean2 (95%CI)
|
|||
| White blood cell count (109/L) | 21703 | 5.47 (5.4-5.6) | 9094 | 7.53 (7.51-7.55) | < 0.0001 | 3.4-9.6 |
| Lymphocyte count (109/L) | 22053 | 1.04 (1.0-1.3) | 8653 | 0.88 (0.87-0.89) | < 0.0001 | 0.95-3.07 |
| Aspartate aminotransferase (U/L) | 15495 | 30.8 (30.5-31.3) | 8627 | 45.2 (45.1-45.3) | < 0.0001 | 8-20 |
| Total bilirubin (μmoL/L) | 10824 | 10.7 (10.6-10.8) | 281 | 1.4 (1.1-1.7) | < 0.0001 | 2-17 |
| Creatine kinase (U/L) | 11382 | 85.3 (84.7-85.8) | 9033 | 115 (113-117) | < 0.0001 | M: 25-90 F: 10-70 |
| C-reactive protein (mg/L) | 16144 | 25.9 (25.5-26.4) | 12059 | 116 (115-117) | < 0.0001 | 0-8 |
| Procalcitonin (ng/mL) | 12050 | 2.44 (2.2-2.6) | 11661 | 0.70 (0.68-0.72) | < 0.0001 | 0-0.15 |
n is the number of subjects assessed for a given characteristic as per the source database[7].
Weighted mean; 3Student’s t-test.
DISCUSSION
The present study provides an insight into population-related differences in the clinical features of COVID-19 between China and North America. On the first of April 2020, the case fatality rate (CFR) of the disease was 4.02% in China, 1.74% in the United States, 1.15% in Canada and 2.56% in Mexico (averaging 1.7% for the three North American countries)[2,16]. These rates, however, changed over time as the pandemic evolved with the CFRs in North America superseding those in China. As of the end of August 2020, the CFR has increased in both world regions to 5.23% in China, 3.14% in the United States, 7.31% in Canada and 10.87% in Mexico (averaging 7.1% in North America)[2,16]. In general, these CFRs of COVID-19 are still lower than that of the other two coronavirus epidemics, namely, SARS (10%)[17-19] and MERS-CoV (38%)[20]. The regional changes and differences in COVID-19 CFRs are thought to be due to the varying rates of disease severity and the different diagnostic tools and detection protocols between the two populations[21]. Moreover, climate (e.g., temperature and humidity), immune status, age, gender, genetic profile, prevalence of comorbidities, and the healthcare services and facilities can be also important factors affecting the differences in COVID-19 profile[22-26]. These factors differ markedly not only between but also within the studied regions[27]. Furthermore, research has shown that the expression of angiotensin I converting enzyme 2 (ACE2) receptors may play a role in the severity of coronavirus infection in different populations[28]. Variations in ACE2 gene expression have been proposed to be associated with different ACE2 activity and population-related differences in COVID-19 risk[28]. For example, East Asian populations have been shown to have high allele frequency of the eQTL variants (expression quantitative trait loci, the genomic loci that explain disparity in mRNA expression) associated with higher ACE2 tissue expression[29] and the possible subsequent population-related differences in susceptibility (or response) to COVID-19[28].
As a newly emerging infectious disease, the entire population is expected to be vulnerable to COVID-19. However, studies have shown that the median age of patients infected with COVID-19 has been between 42 to 59 years[30-33]. The average age range of 50 (China) to 55 (North America) years reported here falls within that most susceptible age interval. It seems however that COVID-19 cases from North America tend to be slightly – but significantly – older than their counterparts from China. Older patients (> 60 years) with underlying diseases were found to be more vulnerable to severe illness that progresses to death[34,35]. This observation was apparent in the cases from North America where patients with co-existing medical conditions were, on average, older (40-80 years) than their counterparts from China (18-75 years). In the present study, the incidence of the disease was higher in men than in women in both study regions. Although sex-related difference in disease incidence were not statistically significant, confirming earlier findings[32], a study by Wei et al[36] reported that males have higher odds ratio (OR) of disease-related morbidity (OR: 1.12), severity (1.63), and mortality (1.71) than females.
Based on symptoms, disease indicators and imaging results, the clinical manifestations of the disease severity has been divided into mild (non- or mild-pneumonia; occurred in 81% of the cases); severe (dyspnea, ≥ 30/min respiratory frequency, ≤ 93% blood oxygen saturation, and/or more than 50% lung infiltrates within 1-2 d; in 14% of the cases) and critical (respiratory failure, septic shock, and/or multiple organ dysfunction or failure; in 5% of the cases)[5]. Typical clinical symptoms of COVID-19 include fever, fatigue, and dry cough while expectoration, headache, nausea, vomiting, and diarrhea are among the atypical symptoms[21]. Although some confirmed cases are asymptomatic or have low typical clinical symptoms and are recovered within one week without presenting with pneumonia[32,37,38], many cases have shown some signs of bilateral lung injury[39]. In severe cases, however, the infection can progress rapidly to ARDS, septic shock, metabolic acidosis, coagulopathy, acute myocardial injury, injury to the kidney and even multiple organ failure[31,33,40] that collectively may eventually be fatal. In the present study the frequency of case severity was significantly higher in North America than China. This was apparently due to the higher rates of ARDS, septic shock and acute kidney injury that were all in higher rates in cases from North America compared to those from China. This may be due to the higher rates of clinical symptoms such as dyspnea, myalgia, diarrhea and chills noted in the North American cases. Dyspnea is associated with severe pneumonia or ARDS whereas symptoms such as diarrhea may indicate an involvement in gastrointestinal tract, particularly in patients with non-severe cases. Currently, there is no firm evidence to suggest that severity of digestive symptoms corresponds to severity of COVID-19 clinical course. These symptoms are known to be associated with inflammatory syndrome, pneumonia and/or respiratory infections[41]. In a retrospective cohort study, critically ill COVID-19 patients were presented with high levels of inflammation associated with fever, coughing, dyspnea, diarrhea together with lymphopenia and hypoalbuminemia[41]. In this respect, we noted that levels of CRP, the acute phase reactant and the downstream factor in the innate immunity-related inflammatory pathway[42], were 4.5-fold higher in the cases from North America than in those from China. Therefore, subsequent to infection, and in response to the individual’s immune status, high levels of innate immunity-related inflammation may emerge to combat the disease leading to a range of clinical symptoms that may manifest into an array of disease complications and severe outcomes. In line with this proposition, early stages of COVID-19 are characterized by a decrease in the absolute number of peripheral blood lymphocytes that progressively decline as the disease develops into its severe outcome[43-46]. This state is suggested to result in inflammatory lesions in the lungs and extensive inflammatory reactions and necrosis in the spleen, lymph nodes and blood vessels that may lead to fatal complications[6].
Of the populations vulnerable to increased risk of infection and severe outcomes of COVID-19 are those with pre-existing medical conditions such as hypertension, diabetes, cardiovascular disease and other underlying conditions[30,31,40]. In a meta-analysis of 11 reports with 521 mild COVID-19 cases and 704 severe cases[47-57], we found severe COVID-19 cases to have a 3-fold higher prevalence of cardiovascular diseases, hypertension and diabetes than mild cases (unpublished data). These comorbidities were all significantly higher in the cases from North America compared to those from China. Interestingly, in both study regions, the most common comorbidities were hypertension followed by diabetes and cardiovascular diseases. This was also noted in a number of studies reported in a recent meta-analysis of 656 COVID-19 patients from 19 studies[39]. Hypertension, diabetes and cardiovascular diseases are all metabolic syndrome-related conditions known to be at higher prevalence in North America than in East Asia. For example, the 2019 trends of age-specific incidence of diagnosed diabetes have shown a lower diseases prevalence in China[58] compared to United States[59] at the same age group (≥ 65 years). In general, the metabolic syndrome related conditions are usually linked to endothelial dysfunction, attenuation of anti-inflammatory responses and generation of a pro-inflammatory state; features that are also common in many infectious disorders[60,61]. Overproduction of cytokines and acute-phase reactants[6], such as CRP, related to chronic comorbidities may lead to endothelial dysfunction and subsequent complications including pericardial effusion, vascular leakage, allergy and ascites[62]. Furthermore, inadequate secretion of insulin and hyperglycemia can deteriorate the functions of lymphocyte and macrophage causing a state of diminished acquired immunity[63]. The latter was associated with 60% higher risk of complications related to pneumonia[64]. Indeed, the etiological link between severity of COVID-19 and chronic diseases has not been fully elucidated. However, it is rational to propose that in infected patients, chronic comorbidities can synergistically (with the infection) downregulate both the individual’s innate and adaptive immune responses[65,66]. Such a synergism between infection and chronic diseases may also impair other critical components of immunity such as the activity of neutrophils, phagocytosis and chemotaxis and attenuate the functions of neutrophils and T cells[65,66] to aggravate the severity of infectious diseases outcome.
Several limitations can be identified in the present study. Across the selected studies, sub-cohort divisions may have followed different criteria which may be of independent interest[7]. These include severity of disease (e.g., severe vs mild), death (e.g., survivors vs non-survivors) and comorbidity (e.g., diabetic vs non-diabetic). This subgrouping was not considered here as we were attentive only to COVID-19 cases and comparing the profile of the disease in the two study regions. Moreover, studies in this dataset are designed with different objectives which may have affected data reporting. For example, some studies will only report data from non-survivors, some studies will focus on the profile of the disease clinical symptoms with little information on its outcome and some reports will vary in their definition to disease severity. Therefore, data points may exhibit a high proportion of missing or re-reported features and hence the different number of examined subjects for the assessed disease characteristics[7]. Additionally, the diverse methodologies used to define the disease characteristics may have contributed to the large among-studies variation in each region. Furthermore, selected papers were not consistent in reporting the mortality or discharge rates[7]. Some only report mortality whereas others report discharges rates while many patients remain in the hospital at the conclusion of the study. Additionally, the studies from the examined database aggregates data from different locations and hospitals within each of the two regions. This may have led to different reporting standards even within the same study. Furthermore, the large difference between the number of studies from China and North America may have influenced the degree of among-studies heterogeneity in the combined frequency of findings within the studied regions. Sources of heterogeneity may also relate to the large variation among studies in the sample size and the different study designs, objectives, and outcome measures.
CONCLUSION
In conclusion, marked differences in the profile of COVID-19 have been observed between cases from China and North America in terms of disease severity, clinical symptoms, prevalence of comorbidities and levels of disease markers. These differences may relate to both environmental- and host-related factors as well as differences in the diagnostic and detection protocols between the two study regions. Furthermore, the varied prevalence of comorbidities and status of healthcare services between China and North America may be other important factors affecting the COVID-19 profile difference[67]. Indeed, a possible convergence between chronic and infectious diareses may influence the severity of COVID-19 in subjects with pre-existing medical conditions[68]. These observations underline the necessity to conduct more systematic population-based epidemiological assessments to elucidate both environmental-, socioeconomic- and host-related factors in disease etiology. The distinct disease profile among different world regions underlines the need to characterize the effect of health inequities and inequalities on public health response to COVID-19 and address the influence of inter- and intra-population differences on health.
ARTICLE HIGHLIGHTS
Research background
Large inter-individual and inter-population differences in the susceptibility to and outcome of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) or coronavirus disease 2019 (COVID-19) have been noted. Understanding these differences and how they influence vulnerability to infection and disease severity is critical to public health intervention.
Research motivation
To evaluates the inter-population differences in the SARS-CoV-2 or COVID-19 between China and North America.
Research objectives
The objective of this systematic review and meta-analysis is to analyze and compare the profile of COVID-19 cases between China and North America as two regions that differ in many environmental, host and healthcare factors related to disease risk.
Research methods
We conducted a meta-analysis to examine and compare demographic information, clinical symptoms, comorbidities, disease severity and levels of disease biomarkers of COVID-19 cases from clinical studies and data from China (105 studies) and North America (19 studies).
Research results
Fever, cough, fatigue and dyspnea were the most common clinical symptoms of COVID-19 in both study regions. Hypertension, diabetes and cancer were the most prevalent comorbidities in COVID-19 cases from China whereas obesity, hypertension and diabetes were the most prevalent in North America. Prevalence of comorbidities in COVID-19 cases increased with age but was at younger ages in cases from China compared to those from North America. Inflammatory markers such as C-reactive protein were 4.5-fold higher in the North American cases than in cases from China.
Research conclusions
The differences in COVID-19 profile between China and North America reflects the differences in environmental-, host- and healthcare-related factors between the two regions.
Research perspectives
Inter-population differences – together with intra-population variability – underline the need to characterize the effect of health inequities and inequalities on public health response to COVID-19 and can assist in preparing for the re-emergence of the epidemic.
ACKNOWLEDGEMENTS
This work was supported by the Public Health Agency of Canada (AB).
Footnotes
Conflict-of-interest statement: The authors declare no conflict of interest.
PRISMA 2009 Checklist statement: The authors have read the PRISMA 2009 Checklist, and the manuscript was prepared and revised according to the PRISMA 2009 Checklist.
Manuscript source: Invited manuscript
Peer-review started: September 15, 2020
First decision: October 18, 2020
Article in press: November 21, 2020
Specialty type: Medicine, research and experimental
Country/Territory of origin: Canada
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Xie Y S-Editor: Fan JR L-Editor: A P-Editor: Wang LYT
Contributor Information
Alaa Badawi, Public Health Risk Sciences Division, Public Health Agency of Canada, Toronto M5V3L7, ON, Canada; Department of Nutritional Sciences, Faculty of Medicine, University of Toronto, Toronto M5S1A8, ON, Canada. alaa.badawi@canada.ca.
Denitsa Vasileva, Center for Heart Lung Innovation, University of British Columbia, Vancouver V6Z1Y6, BC, Canada.
References
- 1.Zhou P, Yang XL, Wang XG, Hu B, Zhang L, Zhang W, Si HR, Zhu Y, Li B, Huang CL, Chen HD, Chen J, Luo Y, Guo H, Jiang RD, Liu MQ, Chen Y, Shen XR, Wang X, Zheng XS, Zhao K, Chen QJ, Deng F, Liu LL, Yan B, Zhan FX, Wang YY, Xiao GF, Shi ZL. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579:270–273. doi: 10.1038/s41586-020-2012-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.World Health Organization (WHO) Coronavirus disease (COVID-2019) situation reports. WHO, 2020. Accessed August 18, 2020. Available from: https://www.who.int/emergencies/diseases/novel-coronavirus-2019/situation-reports .
- 3.Dong E, Du H, Gardner L. An interactive web-based dashboard to track COVID-19 in real time. Lancet Infect Dis. 2020;20:533–534. doi: 10.1016/S1473-3099(20)30120-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hassan SA, Sheikh FN, Jamal S, Ezeh JK, Akhtar A. Coronavirus (COVID-19): A Review of Clinical Features, Diagnosis, and Treatment. Cureus. 2020;12:e7355. doi: 10.7759/cureus.7355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Read JM, Bridgen JR, Cummings DA, Ho A, Jewell CP. Novel coronavirus 2019-nCoV: early estimation of epidemiological parameters and epidemic predictions. medRxiv. 2020 doi: 10.1098/rstb.2020.0265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Badawi A. Hypercytokinemia and Pathogen-Host Interaction in COVID-19. J Inflamm Res. 2020;13:255–261. doi: 10.2147/JIR.S259096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bertsimas D, Bandi H, Boussioux L, Cory-Wright R, Delarue A, Digalakis V, Gilmour S, Graham J, Kim A, Lahlou Kitane D, Lin Z, Lukin G, Li M, Mingardi L, Na L, Orfanoudaki A, Papalexopoulos T, Paskov I, Pauphilet J, Omar SL, Sobiesk M, Stellato B, Carballo K, Wang Y, Wiberg H, Zeng C. An Aggregated Dataset of Clinical Outcomes for COVID-19 Patients. 2020. Accessed July 15, 2020. Available from: http://www.covidanalytics.io/dataset_documentation .
- 8.Borenstein M. Software for publication bias. In: Rothstein HR, Sutton AJ, Borenstein M, eds. Publication Bias in Meta-Analysis - Prevention, Assessment and Adjustments. Hoboken, United States: John Wiley & Sons, Ltd, 2005: 193-220. [Google Scholar]
- 9.Tosuntas SB, Danişman S. Introduction to meta-analysis. In: Karadag E, ed. Leadership and Organizational Outcomes: Meta-Analysis of Empirical Studies. Switzerland: Springer International Publishing, 2015: 19-28. [Google Scholar]
- 10.Campbell I. Chi-squared and Fisher-Irwin tests of two-by-two tables with small sample recommendations. Stat Med. 2007;26:3661–3675. doi: 10.1002/sim.2832. [DOI] [PubMed] [Google Scholar]
- 11.Richardson JT. The analysis of 2 × 2 contingency tables--yet again. Stat Med. 2011;30:890; author reply 891–892. doi: 10.1002/sim.4116. [DOI] [PubMed] [Google Scholar]
- 12.Cochran WG. The Combination of estimates from different experiments. Biometrics . 1954;10:101–129. [Google Scholar]
- 13.Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21:1539–1558. doi: 10.1002/sim.1186. [DOI] [PubMed] [Google Scholar]
- 14.Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JP, Clarke M, Devereaux PJ, Kleijnen J, Moher D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ. 2009;339:b2700. doi: 10.1136/bmj.b2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Badawi A, Velummailum R, Ryoo SG, Senthinathan A, Yaghoubi S, Vasileva D, Ostermeier E, Plishka M, Soosaipillai M, Arora P. Prevalence of chronic comorbidities in dengue fever and West Nile virus: A systematic review and meta-analysis. PLoS One. 2018;13:e0200200. doi: 10.1371/journal.pone.0200200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.European Centre for Disease Prevention and Control. The Case fatality rate of COVID-19. 2020. Accessed August 21, 2020. Available from: https://ourworldindata.org/coronavirus .
- 17.Kuiken T, Fouchier RA, Schutten M, Rimmelzwaan GF, van Amerongen G, van Riel D, Laman JD, de Jong T, van Doornum G, Lim W, Ling AE, Chan PK, Tam JS, Zambon MC, Gopal R, Drosten C, van der Werf S, Escriou N, Manuguerra JC, Stöhr K, Peiris JS, Osterhaus AD. Newly discovered coronavirus as the primary cause of severe acute respiratory syndrome. Lancet. 2003;362:263–270. doi: 10.1016/S0140-6736(03)13967-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ksiazek TG, Erdman D, Goldsmith CS, Zaki SR, Peret T, Emery S, Tong S, Urbani C, Comer JA, Lim W, Rollin PE, Dowell SF, Ling AE, Humphrey CD, Shieh WJ, Guarner J, Paddock CD, Rota P, Fields B, DeRisi J, Yang JY, Cox N, Hughes JM, LeDuc JW, Bellini WJ, Anderson LJ SARS Working Group. A novel coronavirus associated with severe acute respiratory syndrome. N Engl J Med. 2003;348:1953–1966. doi: 10.1056/NEJMoa030781. [DOI] [PubMed] [Google Scholar]
- 19.Lee N, Hui D, Wu A, Chan P, Cameron P, Joynt GM, Ahuja A, Yung MY, Leung CB, To KF, Lui SF, Szeto CC, Chung S, Sung JJ. A major outbreak of severe acute respiratory syndrome in Hong Kong. N Engl J Med. 2003;348:1986–1994. doi: 10.1056/NEJMoa030685. [DOI] [PubMed] [Google Scholar]
- 20.Ahmed AE. The predictors of 3- and 30-day mortality in 660 MERS-CoV patients. BMC Infect Dis. 2017;17:615. doi: 10.1186/s12879-017-2712-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Guo G, Ye L, Pan K, Chen Y, Xing D, Yan K, Chen Z, Ding N, Li W, Huang H, Zhang L, Li X, Xue X. New insights of emerging SARS-CoV-2: Epidemiology, etiology, clinical features, clinical treatment, and prevention. Front Cell Dev Biol. 2020;8:410. doi: 10.3389/fcell.2020.00410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Deshmukh V, Tripathi SC, Pandey A, Deshmukh V, Vykoukal J, Patil A, Sontakke B. COVID-19: a conundrum to decipher. Eur Rev Med Pharmacol Sci. 2020;24(10):5830–5841. doi: 10.26355/eurrev_202005_21378. [DOI] [PubMed] [Google Scholar]
- 23.Ma Y, Zhao Y, Liu J, He X, Wang B, Fu S, Yan J, Niu J, Luo B. Effects of temperature variation and humidity on the mortality of COVID-19 in Wuhan. medRxiv 2020; 2003.2015. 20036426 doi: 10.1016/j.scitotenv.2020.138226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li H, Wang S, Zhong F, Bao W, Li Y, Liu L, Wang H, He Y. Age-Dependent Risks of Incidence and Mortality of COVID-19 in Hubei Province and Other Parts of China. Front Med (Lausanne) 2020;7:190. doi: 10.3389/fmed.2020.00190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Caramelo F, Ferreira N, Oliveiros B. Estimation of risk factors for COVID-19 mortality – preliminary results. medRxiv 2020; 2002.2024. 20027268 [Google Scholar]
- 26.Zeng Q, Li Y, Huang G, Wu W, Dong S, Xu Y. Mortality of COVID-19 is associated with cellular immune function compared to immune function in Chinese Han population. medRxiv 2020; 2003.2008. 20031229 [Google Scholar]
- 27.Link BG, Phelan JC. Understanding sociodemographic differences in health--the role of fundamental social causes. Am J Public Health. 1996;86:471–473. doi: 10.2105/ajph.86.4.471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cao Y, Li L, Feng Z, Wan S, Huang P, Sun X, Wen F, Huang X, Ning G, Wang W. Comparative genetic analysis of the novel coronavirus (2019-nCoV/SARS-CoV-2) receptor ACE2 in different populations. Cell Discov. 2020;6:11. doi: 10.1038/s41421-020-0147-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.GTEx Consortium. Human genomics. The Genotype-Tissue Expression (GTEx) pilot analysis: multitissue gene regulation in humans. Science. 2015;348:648–660. doi: 10.1126/science.1262110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Guan WJ, Ni ZY, Hu Y, Liang WH, Ou CQ, He JX, Liu L, Shan H, Lei CL, Hui DSC, Du B, Li LJ, Zeng G, Yuen KY, Chen RC, Tang CL, Wang T, Chen PY, Xiang J, Li SY, Wang JL, Liang ZJ, Peng YX, Wei L, Liu Y, Hu YH, Peng P, Wang JM, Liu JY, Chen Z, Li G, Zheng ZJ, Qiu SQ, Luo J, Ye CJ, Zhu SY, Zhong NS China Medical Treatment Expert Group for Covid-19. Clinical Characteristics of Coronavirus Disease 2019 in China. N Engl J Med. 2020;382:1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, Zhang L, Fan G, Xu J, Gu X, Cheng Z, Yu T, Xia J, Wei Y, Wu W, Xie X, Yin W, Li H, Liu M, Xiao Y, Gao H, Guo L, Xie J, Wang G, Jiang R, Gao Z, Jin Q, Wang J, Cao B. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ki M Task Force for 2019-nCoV. Epidemiologic characteristics of early cases with 2019 novel coronavirus (2019-nCoV) disease in Korea. Epidemiol Health. 2020;42:e2020007. doi: 10.4178/epih.e2020007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Li Q, Guan X, Wu P, Wang X, Zhou L, Tong Y, Ren R, Leung KSM, Lau EHY, Wong JY, Xing X, Xiang N, Wu Y, Li C, Chen Q, Li D, Liu T, Zhao J, Liu M, Tu W, Chen C, Jin L, Yang R, Wang Q, Zhou S, Wang R, Liu H, Luo Y, Liu Y, Shao G, Li H, Tao Z, Yang Y, Deng Z, Liu B, Ma Z, Zhang Y, Shi G, Lam TTY, Wu JT, Gao GF, Cowling BJ, Yang B, Leung GM, Feng Z. Early Transmission Dynamics in Wuhan, China, of Novel Coronavirus-Infected Pneumonia. N Engl J Med. 2020;382:1199–1207. doi: 10.1056/NEJMoa2001316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wu Z, McGoogan JM. Characteristics of and Important Lessons From the Coronavirus Disease 2019 (COVID-19) Outbreak in China: Summary of a Report of 72 314 Cases From the Chinese Center for Disease Control and Prevention. JAMA. 2020;323:1239–1242. doi: 10.1001/jama.2020.2648. [DOI] [PubMed] [Google Scholar]
- 35.Yang Y, Lu Q, Liu M, Wang Y, Zhang A, Jalali N, Dean N, Longini I, Halloran ME, Xu B, Zhang Z, Wang L, Liu W, Fang L. Epidemiological and clinical features of the 2019 novel coronavirus outbreak in China. medRxiv 2020; 2002.2010. 20021675 [Google Scholar]
- 36.Wei X, Xiao Y-T, Wang J, Chen R, Zhang W, Yang Y, Lv D, Qin C, Gu D, Zhang B, Chen W, Hou J, Song N, Zeng G, Ren S. Sex differences in severity and mortality among patients with COVID-19: evidence from pooled literature analysis and insights from integrated bioinformatic analysis; 2020. Preprint. Available from: arXiv: 2003.13547v13541.
- 37.Chang , Lin M, Wei L, Xie L, Zhu G, Dela Cruz CS, Sharma L. Epidemiologic and Clinical Characteristics of Novel Coronavirus Infections Involving 13 Patients Outside Wuhan, China. JAMA. 2020;323:1092–1093. doi: 10.1001/jama.2020.1623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rothe C, Schunk M, Sothmann P, Bretzel G, Froeschl G, Wallrauch C, Zimmer T, Thiel V, Janke C, Guggemos W, Seilmaier M, Drosten C, Vollmar P, Zwirglmaier K, Zange S, Wölfel R, Hoelscher M. Transmission of 2019-nCoV Infection from an Asymptomatic Contact in Germany. N Engl J Med. 2020;382:970–971. doi: 10.1056/NEJMc2001468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rodriguez-Morales AJ, Cardona-Ospina JA, Gutiérrez-Ocampo E, Villamizar-Peña R, Holguin-Rivera Y, Escalera-Antezana JP, Alvarado-Arnez LE, Bonilla-Aldana DK, Franco-Paredes C, Henao-Martinez AF, Paniz-Mondolfi A, Lagos-Grisales GJ, Ramírez-Vallejo E, Suárez JA, Zambrano LI, Villamil-Gómez WE, Balbin-Ramon GJ, Rabaan AA, Harapan H, Dhama K, Nishiura H, Kataoka H, Ahmad T, Sah R Latin American Network of Coronavirus Disease 2019-COVID-19 Research (LANCOVID-19) Electronic address: https://www.lancovid.org. Clinical, laboratory and imaging features of COVID-19: A systematic review and meta-analysis. Travel Med Infect Dis. 2020;34:101623. doi: 10.1016/j.tmaid.2020.101623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chen N, Zhou M, Dong X, Qu J, Gong F, Han Y, Qiu Y, Wang J, Liu Y, Wei Y, Xia J, Yu T, Zhang X, Zhang L. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395:507–513. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.de la Rica R, Borges M, Aranda M, Del Castillo A, Socias A, Payeras A, Rialp G, Socias L, Masmiquel L, Gonzalez-Freire M. Low Albumin Levels Are Associated with Poorer Outcomes in a Case Series of COVID-19 Patients in Spain: A Retrospective Cohort Study. Microorganisms. 2020;8:1106. doi: 10.3390/microorganisms8081106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Vasileva D, Badawi A. C-reactive protein as a biomarker of severe H1N1 influenza. Inflamm Res. 2019;68:39–46. doi: 10.1007/s00011-018-1188-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.He L, Ding Y, Zhang Q, Che X, He Y, Shen H, Wang H, Li Z, Zhao L, Geng J, Deng Y, Yang L, Li J, Cai J, Qiu L, Wen K, Xu X, Jiang S. Expression of elevated levels of pro-inflammatory cytokines in SARS-CoV-infected ACE2+ cells in SARS patients: relation to the acute lung injury and pathogenesis of SARS. J Pathol. 2006;210:288–297. doi: 10.1002/path.2067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ding Y, He L, Zhang Q, Huang Z, Che X, Hou J, Wang H, Shen H, Qiu L, Li Z, Geng J, Cai J, Han H, Li X, Kang W, Weng D, Liang P, Jiang S. Organ distribution of severe acute respiratory syndrome (SARS) associated coronavirus (SARS-CoV) in SARS patients: implications for pathogenesis and virus transmission pathways. J Pathol. 2004;203:622–630. doi: 10.1002/path.1560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ying T, Li W, Dimitrov DS. Discovery of T-Cell Infection and Apoptosis by Middle East Respiratory Syndrome Coronavirus. J Infect Dis. 2016;213:877–879. doi: 10.1093/infdis/jiv381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lo AW, Tang NL, To KF. How the SARS coronavirus causes disease: host or organism? J Pathol. 2006;208:142–151. doi: 10.1002/path.1897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wei XS, Wang X, Niu YR, Ye LL, Peng WB, Wang ZH, Yang WB, Yang BH, Zhang JC, Ma WL, Wang XR, Zhou Q. Diarrhea Is Associated With Prolonged Symptoms and Viral Carriage in Corona Virus Disease 2019. Clin Gastroenterol Hepatol 2020; 18: 1753-1759. :e2. doi: 10.1016/j.cgh.2020.04.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Qin C, Zhou L, Hu Z, Zhang S, Yang S, Tao Y, Xie C, Ma K, Shang K, Wang W, Tian DS. Dysregulation of Immune Response in Patients With Coronavirus 2019 (COVID-19) in Wuhan, China. Clin Infect Dis. 2020;71:762–768. doi: 10.1093/cid/ciaa248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Xu B, Fan CY, Wang AL, Zou YL, Yu YH, He C, Xia WG, Zhang JX, Miao Q. Suppressed T cell-mediated immunity in patients with COVID-19: A clinical retrospective study in Wuhan, China. J Infect. 2020;81:e51–e60. doi: 10.1016/j.jinf.2020.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Liu J, Li S, Liu J, Liang B, Wang X, Wang H, Li W, Tong Q, Yi J, Zhao L, Xiong L, Guo C, Tian J, Luo J, Yao J, Pang R, Shen H, Peng C, Liu T, Zhang Q, Wu J, Xu L, Lu S, Wang B, Weng Z, Han C, Zhu H, Zhou R, Zhou H, Chen X, Ye P, Zhu B, Wang L, Zhou W, He S, He Y, Jie S, Wei P, Zhang J, Lu Y, Wang W, Zhang L, Li L, Zhou F, Wang J, Dittmer U, Lu M, Hu Y, Yang D, Zheng X. Longitudinal characteristics of lymphocyte responses and cytokine profiles in the peripheral blood of SARS-CoV-2 infected patients. EBioMedicine. 2020;55:102763. doi: 10.1016/j.ebiom.2020.102763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chen G, Wu D, Guo W, Cao Y, Huang D, Wang H, Wang T, Zhang X, Chen H, Yu H, Zhang X, Zhang M, Wu S, Song J, Chen T, Han M, Li S, Luo X, Zhao J, Ning Q. Clinical and immunological features of severe and moderate coronavirus disease 2019. J Clin Invest. 2020;130:2620–2629. doi: 10.1172/JCI137244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Song CY, Xu J, He J-Q, Lu Y-Q. COVID-19 early warning score: a multi-parameter screening tool to identify highly suspected patients. medRxiv. 2020 [Google Scholar]
- 53.Zhang H, Wang X, Fu Z, Luo M, Zhang Z, Zhang Z, He Y, Wan D, Zhang L, Wang J, Yan X, Han M, Chen Y. Potential factors for prediction of disease severity of COVID-19 patients. medRxiv. 2020 [Google Scholar]
- 54.Zhang B, Zhou X, Zhu C, Song Y, Feng F, Qiu Y, Feng J, Jia Q, Song Q, Zhu B, Wang J. Immune Phenotyping Based on the Neutrophil-to-Lymphocyte Ratio and IgG Level Predicts Disease Severity and Outcome for Patients With COVID-19. Front Mol Biosci. 2020;7:157. doi: 10.3389/fmolb.2020.00157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wan S, Yi Q, Fan S, Lv J, Zhang X, Guo L, Lang C, Xiao Q, Xiao K, Yi Z, Qiang M, Xiang J, Zhang B, Chen Y, Gao C. Relationships among lymphocyte subsets, cytokines, and the pulmonary inflammation index in coronavirus (COVID-19) infected patients. Br J Haematol. 2020;189:428–437. doi: 10.1111/bjh.16659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wang Z, Yang B, Li Q, Wen L, Zhang R. Clinical Features of 69 Cases With Coronavirus Disease 2019 in Wuhan, China. Clin Infect Dis. 2020;71:769–777. doi: 10.1093/cid/ciaa272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ouyang Y, Yin J, Wang W, Shi H, Shi Y, Xu B, Qiao L, Feng Y, Pang L, Wei F, Guo X, Jin R, Chen D. Down-regulated gene expression spectrum and immune responses changed during the disease progression in COVID-19 patients. Clin Infect Dis. 2020:ciaa46. doi: 10.1093/cid/ciaa462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Liu X, Yu C, Wang Y, Bi Y, Liu Y, Zhang ZJ. Trends in the incidence and mortality of diabetes in China from 1990 to 2017: A joinpoint and age-period-cohort analysis. Int J Environ Res Public Health. 2019;16:158. doi: 10.3390/ijerph16010158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. 59 Center for Disease Control and Prevention (CDC) National Diabetes Statistics Report, 2020: Estimates of Diabetes and Its Burden in the United States. CDC 2020. Available from: https://www.cdc.gov/diabetes .
- 60.Dharmashankar K, Widlansky ME. Vascular endothelial function and hypertension: insights and directions. Curr Hypertens Rep. 2010;12:448–455. doi: 10.1007/s11906-010-0150-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lin SZ, Cheng LT, Zhang AH, Zheng DX, Han QF, Mao W, Wang T. The effect of decreased residual renal function on endothelial function in CAPD patients. Perit Dial Int. 2010;30:467–470. doi: 10.3747/pdi.2009.00028. [DOI] [PubMed] [Google Scholar]
- 62.Lee IK, Hsieh CJ, Chen RF, Yang ZS, Wang L, Chen CM, Liu CF, Huang CH, Lin CY, Chen YH, Yang KD, Liu JW. Increased production of interleukin-4, interleukin-10, and granulocyte-macrophage colony-stimulating factor by type 2 diabetes' mononuclear cells infected with dengue virus, but not increased intracellular viral multiplication. Biomed Res Int. 2013;2013:965853. doi: 10.1155/2013/965853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Dooley KE, Chaisson RE. Tuberculosis and diabetes mellitus: convergence of two epidemics. Lancet Infect Dis. 2009;9:737–746. doi: 10.1016/S1473-3099(09)70282-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kesavadev J, Misra A, Das AK, Saboo B, Basu D, Thomas N, Joshi SR, Unnikrishnan AG, Shankar A, Krishnan G, Unnikrishnan R, Mohan V. Suggested use of vaccines in diabetes. Indian J Endocrinol Metab. 2012;16:886–893. doi: 10.4103/2230-8210.102982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Casqueiro J, Casqueiro J, Alves C. Infections in patients with diabetes mellitus: A review of pathogenesis. Indian J Endocrinol Metab. 2012;16 Suppl 1:S27–S36. doi: 10.4103/2230-8210.94253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Benfield T, Jensen JS, Nordestgaard BG. Influence of diabetes and hyperglycaemia on infectious disease hospitalisation and outcome. Diabetologia. 2007;50:549–554. doi: 10.1007/s00125-006-0570-3. [DOI] [PubMed] [Google Scholar]
- 67.Gong X. The health care gap between China and America. Ann Transl Med. 2014;2:39. doi: 10.3978/j.issn.2305-5839.2014.04.04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Badawi A, Drebot M, Ogden NH. Convergence of chronic and infectious diseases: a new direction in public health policy. Can J Public Health. 2019;110:523–524. doi: 10.17269/s41997-019-00228-x. [DOI] [PMC free article] [PubMed] [Google Scholar]