Abstract
OBJECTIVE
To determine the incidence of and factors associated with an estimated glomerular filtration rate (eGFR) <60 mL/min/1.73 m2 in people with diabetes.
RESEARCH DESIGN AND METHODS
We identified people with diabetes in the EXamining ouTcomEs in chroNic Disease in the 45 and Up Study (EXTEND45), a population-based cohort study (2006–2014) that linked the Sax Institute’s 45 and Up Study cohort to community laboratory and administrative data in New South Wales, Australia. The study outcome was the first eGFR measurement <60 mL/min/1.73 m2 recorded during the follow-up period. Participants with eGFR < 60 mL/min/1.73 m2 at baseline were excluded. We used Poisson regression to estimate the incidence of eGFR <60 mL/min/1.73 m2 and multivariable Cox regression to examine factors associated with the study outcome.
RESULTS
Of 9,313 participants with diabetes, 2,106 (22.6%) developed incident eGFR <60 mL/min/1.73 m2 over a median follow-up time of 5.7 years (interquartile range, 3.0–5.9 years). The eGFR <60 mL/min/1.73 m2 incidence rate per 100 person-years was 6.0 (95% CI 5.7–6.3) overall, 1.5 (1.3–1.9) in participants aged 45–54 years, 3.7 (3.4–4.0) for 55–64 year olds, 7.6 (7.1–8.1) for 65–74 year olds, 15.0 (13.0–16.0) for 75–84 year olds, and 26.0 (22.0–32.0) for those aged 85 years and over. In a fully adjusted multivariable model incidence was independently associated with age (hazard ratio 1.23 per 5-year increase; 95% CI 1.19–1.26), geography (outer regional and remote versus major city: 1.36; 1.17–1.58), obesity (obese class III versus normal: 1.44; 1.16–1.80), and the presence of hypertension (1.52; 1.33–1.73), coronary heart disease (1.13; 1.02–1.24), cancer (1.30; 1.14–1.50), and depression/anxiety (1.14; 1.01–1.27).
CONCLUSIONS
In participants with diabetes, the incidence of an eGFR <60 mL/min/1.73 m2 was high. Older age, remoteness of residence, and the presence of various comorbid conditions were associated with higher incidence.
Introduction
Diabetes is the leading cause of end-stage kidney disease worldwide (1,2). An estimated 425 million adults (20–79 years) are affected, with projections indicating substantial growth to over 629 million by 2045 (3). The comorbid burden of diabetes and chronic kidney disease (CKD) leads to an increased risk of cardiovascular disease and death (4–7), as well as an increased rate of depression (8), poorer quality of life, and decreased productivity (9,10). Identifying people with diabetes who are at increased risk of developing CKD is a key step to developing preventative strategies for improving health outcomes in this high-risk population.
Traditionally, incidence and prevalence estimates of chronic health conditions, such as CKD, in patients with diabetes have been derived from cross-sectional or repeated cross-sectional studies. While these studies yield valuable information, all have important design limitations, such as vulnerability to substantial healthy volunteer bias (11,12). Alternative methods that can monitor the burden of the health complications of diabetes are needed to assess changing epidemiology and the efficacy of health service interventions. Large-scale routinely collected administrative and clinical data offer the opportunity to efficiently monitor the incidence and prevalence of comorbidities over time.
In this study, we use a large linked data set comprising multiple routinely collected data sources to 1) determine the incidence rate of an estimated glomerular filtration rate (eGFR) <60 mL/min/1.73 m2 in a population-based cohort of people with diabetes and 2) assess the sociodemographic, lifestyle, and clinical factors associated with eGFR <60 mL/min/1.73 m2.
Research Design and Methods
Study Design and Data Sources
EXamining ouTcomEs in chroNic Disease in the 45 and Up Study (EXTEND45) is a large population-based cohort, built on the Sax Institute’s 45 and Up Study, a prospective cohort of residents aged 45 years or older in the state of New South Wales (NSW), Australia. The 45 and Up Study participants and their baseline questionnaire responses have been described in detail elsewhere (13). In brief, between 2006 and 2009, potential participants were randomly selected from the Department of Human Services (DHS) enrollment database, invited to join the study and complete a detailed baseline questionnaire containing information on their health and socioeconomic characteristics, and provided informed consent to long-term follow-up, including having their data linked to other information sources.
In the EXTEND45 study, participants of the 45 and Up Study together with their baseline questionnaire responses were linked to routinely collected health and administrative databases (between 2006 and 2014), including the following: 1) community laboratory testing services; 2) the Pharmaceutical Benefits Scheme (PBS) (14) and 3) the Medicare Benefits Schedule (MBS) (15), both provided by the DHS; 4) the NSW Admitted Patient Data Collection; and 5) the Registry of Births, Deaths and Marriages.
Community laboratory testing services included test results for serum creatinine, serum glucose, and HbA1c results that were ordered as part of routine care. The PBS and MBS are part of Medicare, Australia’s universal public health insurance scheme, established to provide free or subsidized access to a range of medical and allied health services to all Australians within both government (public) and private organizations. The PBS data include claims for all subsidized pharmaceutical products nationally, while the MBS data include all claims for subsidized medical and diagnostic services provided by medical and other health service providers. The NSW Admitted Patient Data Collection captures inpatient separations from all public, private, and repatriation hospitals as well as day procedure centers and aged care facilities, with diagnostic information coded according to the ICD-10 Australian Modification (16).
MBS and PBS data were linked deterministically by the Sax Institute using a unique identifier that was provided to the DHS. All other data linkage was performed probabilistically by the NSW Centre for Health Record Linkage (https://www.cherel.org.au) covering the period 2005–2014. Probabilistic linkage takes into account a wider range of potential identifiers and seeks to link them based on computed weights in order to determine the probability of a match (17). Participants were excluded if they had inconsistent records suggesting incorrect linkages (e.g., death before date of study entry).
Ethical approval for the EXTEND45 Study was obtained from the NSW Population and Health Services Research Ethics Committee (study reference number HREC/13/CIPHS/69). The 45 and Up Study received ethics approval from the University of New South Wales Human Research Ethics Committee.
Identification of Study Cohort and Inclusion Criteria
Participants were included in the current study if they had one or more linked serum creatinine measurements in the 3-year period prior to their enrollment into the 45 and Up Study (hereafter referred to as the prebaseline kidney function ascertainment period 2003–2006). This was to ensure we could adequately determine prevalent eGFR <60 mL/min/1.73 m2. Prevalent eGFR <60 mL/min/1.73 m2 was defined as an eGFR or an imputed eGFR of <60 mL/min/1.73 m2 on or before the enrollment date. The use of routine clinical data meant that an eGFR was not available at the time of enrollment for most participants. We therefore imputed from the most recent available eGFR prior to enrollment.
Among individuals with a linked serum creatinine measurement, we identified participants with diabetes, defined as the presence of at least one of the following prespecified criteria: 1) a community laboratory record of fasting serum glucose >7.0 mmol/L, 2) a random serum glucose >11.1 mmol/L, 3) an HbA1c result ≥6.5% (all in line with society guidelines [18]), 4) a dispensation record of an oral glucose-lowering agent or insulin analog as documented in the PBS, or 5) self-reported diabetes on the 45 and Up Study baseline questionnaire (“Has a doctor EVER told you that you have diabetes?” = Yes) (Supplementary Fig. 1). Both prevalent and incident cases of diabetes were included, with prevalent cases defined as individuals who had diabetes at their time of enrollment into the 45 and Up Study and incident cases as those who developed diabetes at any point throughout the study period (2006–2014). Outcome ascertainment was only determined once a participant met criteria for incident or prevalent diabetes (Supplementary Fig. 1).
Study Outcome
The outcome of interest was the development of incident eGFR <60 mL/min/1.73 m2, recorded from the time a participant met the criteria for diabetes until death or end of linked data (June 2014), whichever came first. GFR was estimated using the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation (19). Prevalent eGFR <60 mL/min/1.73 m2 status was ascertained during the prebaseline kidney function ascertainment period whereby we assumed a rate of decline of 2 mL/min/1.73 m2/year. Individuals identified as having prevalent CKD at enrollment were excluded from the analysis.
To minimize selection and survivor bias, only one eGFR measurement prior to enrollment was required for inclusion into the cohort and only one eGFR measurement after enrollment was required for incident eGFR <60 mL/min/1.73 m2. Participants who did not have an eGFR <60 mL/min/1.73 m2 after enrollment were conservatively deemed to not have incident disease.
Covariates
Baseline sociodemographic and lifestyle variables were derived from self-reported responses of the 45 and Up Study baseline questionnaire and included the following: age, sex, country of birth, region of residence/remoteness from major city, highest qualification, annual household pretax income, partner status, area-level quintile of disadvantage (defined by an index of disadvantage using the Australian Bureau of Statistics index, where 1 is most disadvantaged and 5 is least disadvantaged) (20), smoking status, alcohol consumption, and BMI (<18.5 kg/m2 underweight, 18.5 to <25 kg/m2 normal, 25 to <30 kg/m2 overweight, 30 to <35 kg/m2 class I obesity, 35 to <40 kg/m2 class II obesity, and ≥40 kg/m2 class III obesity).
Baseline clinical variables included baseline comorbidities (hypertension, hyperlipidemia, coronary heart disease [CHD], previous stroke, cancer, and depression or anxiety), diabetes-specific variables, and baseline eGFR. Baseline comorbidities were defined using both self-reported answers to the 45 and Up Study baseline questionnaire and available linked data up to 3 years prior to the 45 and Up Study enrollment date. Hypertension, hyperlipidemia, CHD, previous stroke, cancer, and depression or anxiety were defined by self-reported answers to the 45 and Up Study baseline questionnaire. Hypertension, hyperlipidemia, and depression were also defined by dispensing of antihypertensive, lipid-lowering therapy, or antidepressant medication, respectively, in the PBS database. Additionally, ICD-10 Australian Modification codes were used to identify hospitalizations involving diagnoses for hypertension, hyperlipidemia, as well as acute myocardial infarction and coronary artery bypass grafting (indicating CHD) and stroke (Supplementary Table 1).
Diabetes-specific variables included diabetes duration calculated from the date of diagnosis until the end of the study period for incident participants and self-report for participants with prevalent diabetes. The baseline eGFR up to 1 month prior to meeting criteria for the diagnosis of diabetes was also examined.
Statistical Analysis
The incidence rate of eGFR <60 mL/min/1.73 m2 was determined using Poisson regression and presented as incidence rate per 100 person-years (95% CIs). This was calculated for the entire cohort and stratified by age using the following age brackets: 45–54, 55–64, 65–74, 75–84, and ≥85 years. Rates were adjusted for sociodemographic, lifestyle, and clinical variables as well as baseline eGFR.
The covariates eGFR at study enrollment and age were treated as continuous variables. All other covariates were categorical variables, which were analyzed in the smallest available unit to allow for the assessment of trends. All comorbidity variables were dichotomous variables (presence or absence of disease).
Missing data were imputed using chained equations with linear regression for log-transformed continuous variables (e.g., age, eGFR at baseline, and time to incidence/censoring) and discriminant analysis for categorical variables (including censoring status) (21). This procedure method assumes that data are missing at random, i.e., with missingness predicted by conditioning on all the variables included in the imputation model. Forty imputed versions of the data set were created, and the resulting parameter estimates were combined using Rubin’s rules (22). No outcome data were missing in our cohort, and only missing baseline characteristics were imputed.
Cox regression was used to determine associations between baseline demographic, socioeconomic, and lifestyle factors; comorbidities; and the outcome of incident eGFR <60 mL/min/1.73 m2 over the follow-up period. Results were combined using Rubin’s rules (22). The hazard ratio (HR) (with 95% CI) was estimated for each variable with adjustment for age and sex in a minimally adjusted model. All variables were included in a fully adjusted model.
Median follow-up time was estimated with the Kaplan-Meier method (23). The proportional hazards assumption and linearity of the continuous covariates was checked visually on the first imputed data set using cumulative sums of residuals (24). We checked the proportional hazards assumption and the linearity of continuous variables by examining the cumulative sums of the martingale-based residuals and found no evidence of violations of proportional hazards or nonlinearity (24).
All data management and analyses were completed in SAS Enterprise Guide 7.1 with SAS/STAT 14.1 (SAS Institute, Cary, NC).
Results
Cohort Characteristics
Among 9,313 participants with diabetes at risk for developing eGFR <60 mL/min/1.73 m2 (Supplementary Fig. 1), at baseline, 5,105 (54.8%) had prevalent diabetes, and 4,208 (45.2%) developed incident diabetes during follow-up. Mean age was 65.4 years (SD 9.7), and 55% of participants were male. The cardiovascular burden of disease was high with hypertension present in 73.4% and hypercholesterolemia in 65.8% of individuals. Participants living in a major city represented 65.4% of the cohort, while 25.4% resided in inner regional and 8.7% in outer regional and remote areas (Table 1).
Table 1.
Baseline characteristics of EXTEND45 participants who were at risk of developing incident eGFR <60 mL/min/1.73 m2
| Baseline characteristics | Diabetes with no incident eGFR <60 mL/min/1.73 m2 (n = 7,207) | Diabetes with incident eGFR <60 mL/min/1.73 m2 (n = 2,106) | All (n = 9,313) |
|---|---|---|---|
| Age (years), mean (SD) | 63.9 (9.3) | 70.5 (9.4) | 65.4 (9.7) |
| Male | 3,976/7,207 (55.2) | 1,165/2,106 (55.3) | 5,141/9,313 (55.2) |
| Diabetes status at 45 and Up enrollment | |||
| Prevalent | 3,584/7,207 (49.7) | 1,521/2,106 (72.2) | 5,105/9,313 (54.8) |
| Incident (during follow-up) | 3,623/7,207 (50.3) | 585/2,106 (27.8) | 4,208/9,313 (45.2) |
| Diabetes duration (years), mean (SD) | 1.53 (2.310) | 2.63 (2.647) | 1.78 (2.434) |
| Diabetes duration >5 years | 931/7,207 (12.9) | 508/2,106 (24.1) | 1,439/9,313 (15.5) |
| Baseline eGFR (mL/min/1.73 m2), mean (SD) | 83.55 (16.014) | 72.12 (9.758) | 80.96 (15.583) |
| Baseline eGFR >90 mL/min/1.73 m2 | 2,232/7,207 (31.0) | 136/2,106 (6.5) | 2,368/9,313 (25.4) |
| Baseline eGFR 60–89 mL/min/1.73 m2 | 4,975/7,207 (69.0) | 1,970/2,106 (93.5) | 6,945/9,313 (74.6) |
| Hypertension, present | 5,019/7,207 (69.6) | 1,817/2,106 (86.3) | 6,836/9,313 (73.4) |
| Hypercholesterolemia, present | 4,587/7,207 (63.6) | 1,539/2,106 (73.1) | 6,126/9,313 (65.8) |
| CHD, present | 1,361/7,207 (18.9) | 652/2,106 (31.0) | 2,013/9,313 (21.6) |
| Previous stroke | 301/7,207 (4.2) | 149/2,106 (7.1) | 450/9,313 (4.8) |
| Prior or current cancer | 503/7,207 (7.0) | 242/2,106 (11.5) | 745/9,313 (8.0) |
| Prior or current depression | 1,390/7,207 (19.3) | 404/2,106 (19.2) | 1,794/9,313 (19.3) |
| Country of birth | |||
| Europe | 1,229/7,207 (17.1) | 353/2,106 (16.8) | 1,582/9,313 (17.0) |
| Australia | 4,957/7,207 (68.8) | 1,567/2,106 (74.4) | 6,524/9,313 (70.1) |
| New Zealand and Polynesia | 164/7,207 (2.3) | 27/2,106 (1.3) | 191/9,313 (2.1) |
| Africa and the Middle East | 199/7,207 (2.8) | 41/2,106 (1.9) | 240/9,313 (2.6) |
| Asia | 482/7,207 (6.7) | 76/2,106 (3.6) | 558/9,313 (6.0) |
| Americas | 103/7,207 (1.4) | 19/2,106 (0.9) | 122/9,313 (1.3) |
| Inadequately described or missing | 73/7,207 (1.0) | 23/2,106 (1.1) | 96/9,313 (1.0) |
| Area of residence | |||
| Major city | 4,806/7,119 (67.5) | 1,247/2,074 (60.1) | 6,053/9,193 (65.8) |
| Inner regional | 1,744/7,119 (24.5) | 594/2,074 (28.6) | 2,338/9,193 (25.4) |
| Outer regional and remote | 569/7,119 (8.0) | 233/2,074 (11.2) | 802/9,193 (8.7) |
| Highest qualification | |||
| No school certificate | 1,051/7,088 (14.8) | 382/2,054 (18.6) | 1,433/9,142 (15.7) |
| School or intermediate certificate | 1,585/7,088 (22.4) | 549/2,054 (26.7) | 2,134/9,142 (23.3) |
| Higher School Certificate | 727/7,088 (10.3) | 210/2,054 (10.2) | 937/9,142 (10.2) |
| Trade or apprenticeship | 840/7,088 (11.9) | 266/2,054 (13.0) | 1,106/9,142 (12.1) |
| Certificate or diploma | 1,374/7,088 (19.4) | 344/2,054 (16.7) | 1,718/9,142 (18.8) |
| University degree or higher | 1,511/7,088 (21.3) | 303/2,054 (14.8) | 1,814/9,142 (19.8) |
| Household income | |||
| Less than $20,000 | 1,581/6,833 (22.2) | 651/1,957 (33.2) | 2,232/8,790 (25.4) |
| $20,000 to <50,000 | 1,697/6,833 (24.8) | 552/1,957 (28.2) | 2,249/8,790 (25.6) |
| $50,000 to <70,000 | 748/6,833 (10.9) | 151/1,957 (7.7) | 899/8,790 (10.2) |
| $70,000 and over | 1,552/6,833 (22.7) | 222/1,957 (11.3) | 1,774/8,790 (20.2) |
| Partner status | |||
| Partner | 5,458/7,154 (76.3) | 1,450/2,089 (69.4) | 6,908/9,243 (74.7) |
| No partner | 1,696/7,154 (23.7) | 639/2,089 (30.6) | 2,335/9,243 (25.3) |
| Level of disadvantage | |||
| Q1 (most disadvantaged) | 1,526/7,064 (21.6) | 524/2,056 (25.5) | 2,050/9,120 (22.5) |
| Q2 | 1,300/7,064 (18.4) | 421/2,056 (20.5) | 1,721/9,120 (18.9) |
| Q3 | 1,297/7,064 (18.4) | 371/2,056 (18.0) | 1,668/9,120 (18.3) |
| Q4 | 1,196/7,064 (16.9) | 320/2,056 (15.6) | 1,516/9,120 (16.6) |
| Q5 (least disadvantaged) | 1,745/7,064 (24.7) | 420/2,056 (20.4) | 2,165/9,120 (23.7) |
| Smoking status | 636/7,181 (8.9) | 101/2,097 (4.8) | 737/9,278 (7.9) |
| Current | 2,884/7,181 (40.2) | 920/2,097 (43.9) | 3,804/9,278 (41.0) |
| Previous | 3,661/7,181 (51.0) | 1,076/2,097 (51.3) | 4,737/9,278 (51.1) |
| Never | 636/7,181 (8.9) | 101/2,097 (4.8) | 737/9,278 (7.9) |
| Alcohol consumption (number of standard drinks/week) | |||
| 0 | 2,885/7,021 (41.1) | 965/2,032 (47.5) | 3,850/9,053 (42.5) |
| 1–6 | 1,925/7,021 (27.4) | 507/2,032 (25.0) | 2,432/9,053 (26.9) |
| 7–13 | 1,030/7,021 (14.7) | 254/2,032 (12.5) | 1,284/9,053 (14.2) |
| 14–20 | 635/7,021 (9.0) | 160/2,032 (7.9) | 795/9,053 (8.8) |
| Over 21 | 546/7,021 (7.8) | 146/2,032 (7.2) | 692/9,053 (7.6) |
| BMI | |||
| <18.5 kg/m2 | 40/6,698 (0.6) | 11/1,927 (0.6) | 51/8,625 (0.6) |
| 18.5 to <25 kg/m2 | 1,267/6,698 (18.9) | 329/1,927 (17.1) | 1,596/8,625 (18.5) |
| 25 to <30 kg/m2 | 2,497/6,698 (37.3) | 741/1,927 (38.5) | 3,238/8,625 (37.5) |
| 30 to <35 kg/m2 | 1,746/6,698 (26.1) | 513/1,927 (26.6) | 2,259/8,625 (26.2) |
| 35 to <40 kg/m2 | 707/6,698 (10.6) | 216/1,927 (11.2) | 923/8,625 (10.7) |
| ≥40 kg/m2 | 441/6,698 (6.6) | 117/1,927 (6.1) | 558/8,625 (6.5) |
Data are n/n (%) unless otherwise noted.
Overall and Age-Stratified eGFR <60 mL/min/1.73 m2 Incidence Rate in Diabetes
Of 9,313 participants at risk, 2,106 (22.6%) developed incident eGFR <60 mL/min/1.73 m2 over a median follow-up time of 5.7 years (interquartile range [IQR], 3.0–5.9), corresponding to an incidence rate of 6.0 (95% CI 5.7–6.3) per 100 person-years.
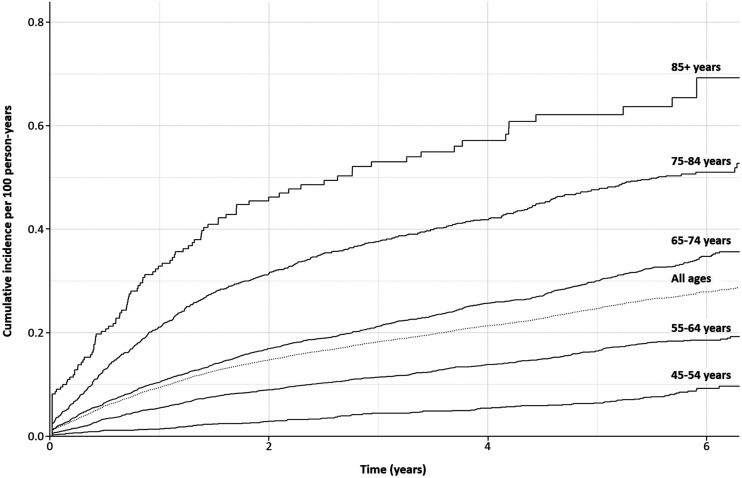
The incidence rate was higher in older age groups, increasing from 1.5 (IQR 1.3–1.9) per 100 person-years in participants aged 45–54 years to 26.0 (22.0–32.0) per 100 person-years in those aged 85 years and over (Table 2). The incidence rate remained constant over time except in those aged 85 years and over (Fig. 1).
Table 2.
Incidence rates of eGFR <60 mL/min/1.73 m2 in those at risk stratified by age group
| incident eGFR <60 mL/min/1.73 m2 | |||
|---|---|---|---|
| Age (years) | Number at risk | Number | Rate per 100 person-years (95% CI) |
| 45–54 | 1,820 | 121 | 1.5 (1.3–1.9) |
| 55–64 | 3,367 | 517 | 3.7 (3.4–4.0) |
| 65–74 | 2,756 | 830 | 7.6 (7.1–8.1) |
| 75–84 | 1,230 | 558 | 15 (13.0–16.0) |
| 85 and over | 140 | 80 | 26 (22.0–32.0) |
Incidence rates calculated using Poisson regression incorporate follow-up time and will differ from manual calculation using numbers at risk and developing incident eGFR <60 mL/min/1.73 m2.
Figure 1.
Kaplan-Meier curves of the time to incident eGFR <60 mL/min/1.73 m2 in the overall cohort as well as stratified by age. Kaplan-Meier curves do not start at 0 because some participants meet the criteria for diabetes and CKD at the same time or at very close time intervals.
Associations of Incident eGFR <60 mL/min/1.73 m2 in Diabetes
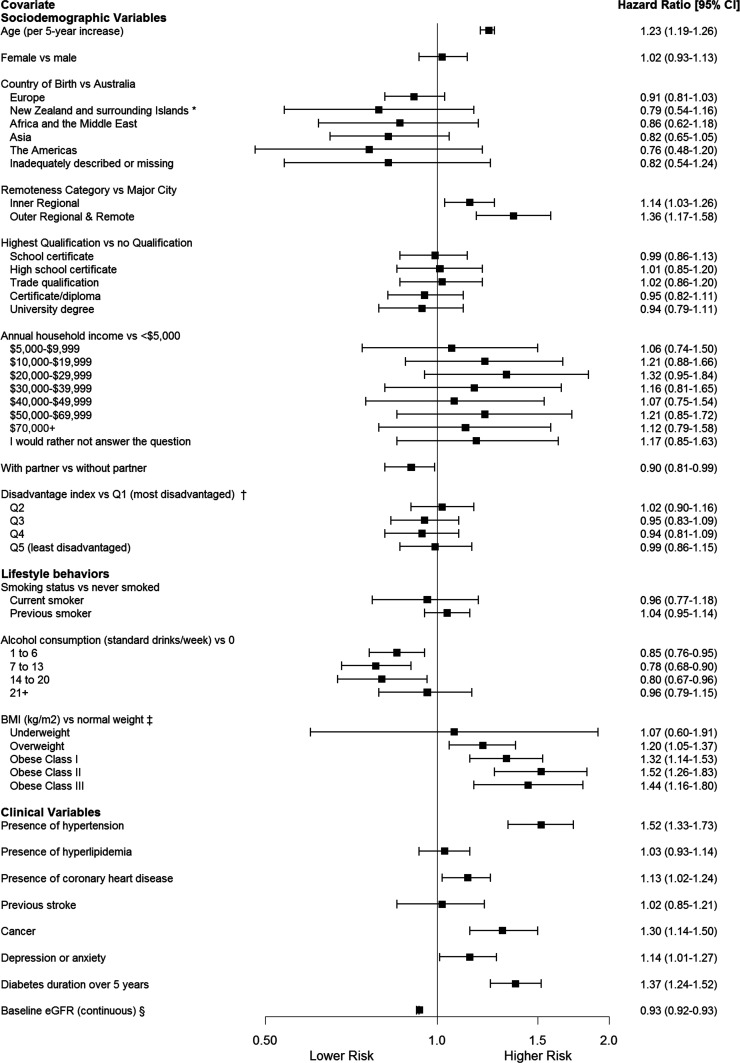
In both sex-adjusted (Supplementary Table 2) and fully adjusted multivariable models, age was independently associated with incident eGFR <60 mL/min/1.73 m2 (HR 1.23 per 5-year increase; 95% CI 1.19–1.26). In fully adjusted analyses, geographical remoteness of residence was also predictive of eGFR <60 mL/min/1.73 m2; compared with living in a major city, living in an inner regional city was associated with a higher incidence (1.14; 1.03–1.26) as was living in an outer regional city (1.36; 1.17–1.58).
Compared with a normal-range BMI (18.0–24.9 kg/m2), BMI values in the overweight class (HR 1.20; 95% CI 1.05–1.37), obese class I (1.32; 1.14–1.53), obese class II (1.52; 1.26–1.83), and obese class III (1.44; 1.16–1.80) ranges were all predictive in a graded fashion (Fig. 2).
Figure 2.
Multivariable Cox regression analyses for baseline variables as associations for the development of incident eGFR <60 mL/min/1.73 m2 among those with diabetes. *Surrounding Islands: New Caledonia, Papua New Guinea, Solomon Islands, Vanuatu, Cook Islands, Fiji, Niue, Samoa, Tokelau, Tonga, Tuvalu. †As defined by the Australian Bureau of Statistics. ‡Normal weight = BMI 18.5 to <25 kg/m2, underweight = BMI <18.5 kg/m2, overweight = BMI 25 to <30 kg/m2, obese class I = BMI 30 to <35 kg/m2, obese class II = BMI 35 to <40 kg/m2, obese class III = BMI ≥40 kg/m2. §Baseline eGFR (continuous), eGFR (mL/min/1.73 m2).
In regard to comorbidities at baseline, the presence of hypertension (HR 1.52; 95% CI 1.33–1.73), coronary heart disease (1.13; 1.02–1.24), cancer (1.30; 1.14–1.50), and depression or anxiety (1.14; 1.01–1.27) also predicted incident eGFR <60 mL/min/1.73 m2. The presence of diabetes for more than 5 years was also predictive (1.37; 1.24–1.52) (Fig. 2).
Factors that were associated with a lower incidence of eGFR <60 mL/min/1.73 m2 included a higher baseline eGFR (HR 0.93; 95% CI 0.92–0.93), having a partner (0.90; 0.81–0.99), and alcohol consumption of <20 standard drinks per week (Fig. 2).
Several demographic and socioeconomic variables were not predictive, including sex, annual household income, highest qualification, area-level quintile of disadvantage, and smoking status.
Conclusions
In our Australian population-based cohort of participants with diabetes aged 45 years and over, the age-adjusted incidence rate of eGFR <60 mL/min/1.73 m2 was 6.0 new cases per 100 person-years between 2006 and 2014. This represents an updated real-world estimate of the burden of CKD in NSW, Australia. In a fully adjusted multivariable model, eGFR <60 mL/min/1.73 m2 was independently associated with sociodemographic variables of increasing age and geographical remoteness, and the clinical variables of elevated BMI, hypertension, coronary heart disease, depression, cancer, and a diabetes duration over 5 years.
The incidence rate of 6.0 (95% CI 5.7–6.3) new cases in 100 person-years in our diabetes cohort is higher than that reported in other population-based studies of adults in regions such as North America (11,12,25), Australia (26), Scandinavia (27), and Europe (28,29). In these studies, estimates of incident CKD ranged between 2.0 and 3.0 new cases per 100 person-years, despite considerable variations in terms of data sources and study design (e.g., national surveys, registries, and randomized controlled trials). In contrast to our study that used linked clinical data and ensured complete follow-up of our entire cohort of participants with diabetes, the National Health and Nutrition Survey (NHANES), which used repeated cross-sectional cohorts of adults over a 20-year period (11,12,25), and the Australian Diabetes, Obesity and Lifestyle Study (AusDiab), which followed a longitudinal cohort of adults over 25 years (26), were limited by steadily declining response rates, which limit their validity and representativeness. Similarly, the Swedish National Diabetes Registry of 3,667 people over the age of 30 years with diabetes recruited from around 95% of hospital-based outpatient clinics and 60% of primary health care centers (27) required participants to be alive at 5 years of follow-up, essentially selecting out a healthier diabetes cohort.
There are several possible contributors to the higher incidence rate observed in our study compared with the published estimates. First, our study uses real-world clinical data from multiple data sources to ascertain eGFR <60 mL/min/1.73 m2 status. This approach may more comprehensively capture incident CKD than methodologies that rely on self-report or repeated biochemical testing limited to those attending follow-up study visits. Moreover, the use of routinely collected real-world data reduces the amount of healthy volunteer bias, which could otherwise lead to an underestimate of disease burden. Second, in contrast to other studies, our cohort was 5–10 years older than other cohorts (25–27), which is a factor that has been continually shown to be associated with a higher incidence of CKD (28–32). Third, in contrast to studies (28,30,33) that investigate CKD incidence among incident cases of diabetes, our study cohort consisted of participants with both incident and prevalent diabetes, thus including people with more advanced disease. Finally, previous published estimates of incidence and prevalence of CKD in diabetes have used a number of definitions and various equations, each with their limitations. We defined CKD as an eGFR of <60 mL/min/1.73 m2 using the CKD-EPI equation. The CKD-EPI equation has been shown to provide a better estimate of early CKD and may, thus, result in a higher estimate of CKD incidence (34).
Our study confirmed some previously identified associations of CKD in diabetes suggesting irreversible factors or factors where effective mitigation strategies are yet to be identified and implemented. Advancing age continues to be an established association of CKD in diabetes (28–32). The incidence rate of CKD increased almost fivefold in NSW adults with diabetes aged 85 years and over compared with those in younger age categories. However, it is unclear whether this is a reflection of the high competing risk of mortality in this group, leading to an inflation of the incidence rate estimate, or whether other factors are at play. Other factors that continue to predict CKD in people with diabetes, despite being potentially amenable to mitigation, include obesity (31,35) and high blood pressure (31).
Some factors do not universally predict CKD in all diabetes cohorts. Socioeconomic markers, such as income, educational level (31), and social disadvantage (36), have predicted CKD in diabetes in other settings but not in our setting of contemporary NSW, Australia. Living remotely compared with living in a major city was independently associated in our study. Australia is serviced by a government-funded universal healthcare system, which may mitigate the impact of many socioeconomic factors. Nevertheless, it is important to note that around 90% of the Australian population is urbanized (Australian Bureau of Statistics, 2016) and situated on the coastal fringes of the continent, while most of the remaining land mass is relatively arid and sparsely populated. As a result, the higher risk of eGFR <60 mL/min/1.73 m2 in those living remotely might be due to poorer access to health care and preventative programs.
Our study found no sex differences in the risk of CKD in those with diabetes, whereas previous studies have found both an increased risk (27,31,37,38) and a reduced risk (30) in both sexes. Possible reasons for these disparate results might lie in the inherent health behaviors that are influenced by the societal roles ascribed to different sexes across cultures and countries. These factors are harder to adjust for in multivariable modeling and raise the possibility of unmeasured confounding.
Our study found that a history of cancer at baseline predicted incident eGFR <60 mL/min/1.73 m2. The epidemiology of cancer and CKD is not well understood. Cancer patients may be exposed to various pathways through which they might accumulate kidney damage. There are direct nephrotoxic effects of anticancer treatments and radiological contrast material for imaging studies for cancer diagnosis, staging, and monitoring as well as direct effects of tumors involving the kidneys either extrinsically through compression or intrinsically through primary or metastatic disease. CKD has significant implications for cancer patients. Most notably, it may confer an increased cancer-specific mortality independent of age and cancer type (39). Taken together, these results suggest that those with diabetes who are receiving treatment for cancer warrant closer monitoring for the development of CKD.
We found that the presence of baseline depression and anxiety predicted incident disease. A recent prospective cohort study in U.S. veterans with diabetes found a similar association (8), despite our study cohort being younger with a lower comorbid burden. It is unclear whether this association is entirely due to residual confounding owing to disease severity and comorbid burden or whether a causal relationship exists, such as adverse changes in health behaviors due to depression.
Our study has many strengths. It is a large, prospective, longitudinal population-based cohort using real-world clinical data to assess CKD incidence and risk factors. Clinical data contained within the linked data sources are comprehensive and continuously collected. We defined eGFR <60 mL/min/1.73 m2 using routinely collected laboratory data, which is more sensitive than relying on administrative codes (40). Similarly, comorbidities were defined by multiple data sources, including medication dispensation and ICD-10 codes, a more robust method than self-report alone. Our methodology ensured complete follow-up without face-to-face visits, reducing participant burden and susceptibility to healthy volunteer bias seen in more traditional longitudinal cohort studies.
Our study has important limitations. The reliance on routinely collected serum creatinine results for the identification and classification of CKD could have introduced an indication bias. We explored this further by examining the baseline characteristics of the diabetes cohort who had a serum creatinine measure and compared this with the baseline characteristics of those who did not. We found that the groups were well matched for all baseline characteristics, including age, sex, geography, markers of socioeconomic status, and comorbidities (Supplementary Table 3). The only difference between those with a serum creatinine and those without was that there was a greater proportion with incident diabetes and a diabetes duration >5 years, which is likely reflective of appropriate testing rather than a selection bias toward a healthier or more advantaged population. Nonetheless, the possibility of some residual indication bias remains.
Our cohort is limited to those who present for serum creatinine testing who may represent as few as 50% annually of individuals with diabetes despite annual CKD screening being stipulated by national and international guidelines (41). Hence, the incidence of CKD found in our study may be an overestimation of the incidence in the general population. Our inclusion criteria only required one creatinine measurement in the 3 years prior to recruitment to the 45 and Up Study because we did not wish to further select our cohort. As a result, we were unable to account for fluctuations in eGFR measurements over the follow-up period, which may have led to misclassification of CKD. Furthermore, only limited linked albuminuria or proteinuria assessments were available, preventing meaningful incorporation of these values in our model. This may have resulted in an underestimation of our incidence rate because we may have missed participants who met the criteria for CKD due to the presence of albuminuria. Our cohort was limited to adults over the age of 45 years, making our results less generalizable to younger age groups. However, given the association of age with hospitalizations, our results are very relevant to health service planning. Our definition of diabetes using routinely collected data precluded the ability to distinguish between type 1 and type 2 diabetes that may have confounded our estimate. Finally, our study was conducted in the context of a universal health care system that may not be generalizable to other settings.
Conclusion and Future Research
In a population-based study of participants aged over 45 years in Australia, the incident rate of eGFR <60 mL/min/1.73 m2 in diabetes is high and increases with advancing age. In a universal health care setting, the presence of the comorbidities of hypertension, CHD, cancer, and depression or anxiety were all found to be associated with a higher risk of developing CKD, while socioeconomic markers of disadvantage were not. This study demonstrates the role that routine clinical data can have for monitoring disease incidence over time.
Future research should investigate the implications of this high incidence rate on individual health outcomes, such as disease progression, as well as on future health service planning.
Supplementary Material
Article Information
Acknowledgments. This research was completed using data collected through the 45 and Up Study (www.saxinstitute.org.au). The 45 and Up Study is managed by the Sax Institute in collaboration with major partner Cancer Council NSW, and partners, the National Heart Foundation of Australia (NSW Division), NSW Ministry of Health, NSW Government Family & Community Services-Ageing, Carers and the Disability Council NSW, and the Australian Red Cross Blood Service. The authors thank the many thousands of people participating in the 45 and Up Study.
Funding and Duality of Interest. The EXTEND45 Study is in part funded through peer-reviewed, unconditional grants, including a NSW Cardiovascular Research Network Development Project Grant from the National Heart Foundation of Australia (100720) and a Rebecca L. Cooper grant (REB002). The Study was also supported in part by a seeding GENESIS grant supported by Roche Products Pty Ltd. (FR-MIR-0095), by major unconditional research grants from Merck Sharp & Dohme (Australia) Pty Ltd., and unconditional research grants from Eli Lilly (Australia) Pty Ltd. (PO4100294024) and Amgen (Australia) Pty Ltd. (Amgen01). C.P. is the current chair of Kidney Health Australia, NSW Bureau Health Information, and the NSW Cardiovascular Research Network, Clinical Trial Steering Committee for CHINA (Baxter); is a principal international investigator for the DELIGHT Study, funded by AstraZeneca Sweden); is a member of the International Advisory Board for AstraZeneca; is a member of Local Advisory Boards for Vifor, Merck Sharpe & Dohme, Boehringer Ingelheim, and Otsuka; serves on the Scientific Advisory Board of Pharmaxis; has received travel and accommodation support from Amgen, AstraZeneca, and Roche; and has received speaker support from Amgen, AstraZeneca, Novartis, and Vifor. D.S. has received research support from Amgen, Sanofi, Pfizer, Regeneron, Amrin, AstraZeneca, and Novartis and provided consultancy services to Amgen, AstraZeneca, and Sanofi. J.K. has received a grant from Baxter for the Dialysis Outcomes in India Study. M.G. has received honoraria from Shire Pharmaceuticals and Amgen Pty Ltd. M.Ju. has received unrestricted grant support from VentureWise (a wholly own commercial subsidiary of National Prescribing Service MedicineWise) to conduct a commissioned project funded by AstraZeneca. M.Ja. is supported by a Medical Research Future Fund Next Generation Clinical Researchers Program Career Development Fellowship; is responsible for research projects that have received unrestricted funding from Gambro, Baxter, Commonwealth Serum Laboratories, Amgen, Eli Lilly, and Merck; has served on advisory boards sponsored by Akebia, Baxter, Boehringer Ingelheim, and Vifor; and has spoken at scientific meetings sponsored by Janssen, Amgen, and Roche, with any consultancy, honoraria, or travel support paid to her institution. No other potential conflicts of interest relevant to this article were reported.
The study sponsors were not involved in the content development of EXTEND45. None of the funding bodies were involved in the design of the study and analysis and interpretation of the study. The opinions expressed in this article are those of the authors and do not necessarily represent those of Eli Lilly (Australia) Pty Ltd., Merck Sharp & Dohme (Australia) Pty Ltd., or Amgen (Australia) Pty Ltd.
Author Contributions. L.S., A.K., M.Ju., and M.Ja. were responsible for the study concept and design. L.S. drafted the manuscript. All authors gave critical revision of the manuscript for important intellectual content and interpreted the data. C.H. and K.R. provided statistical analysis. C.H. and M.Ja. supervised the manuscript. L.S. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Prior Presentation. The study was accepted as an abstract to the Annual Scientific Meeting of the Australian and New Zealand Society of Nephrology, Sydney, NSW, Australia, 2018, and the annual scientific meeting of the American Society of Nephrology, San Diego, CA, 2018.
Footnotes
This article contains Supplementary Data online at https://care.diabetesjournals.org/lookup/suppl/doi:10.2337/dc19-1803/-/DC1.
This article is featured in a podcast available at https://www.diabetesjournals.org/content/diabetes-core-update-podcasts.
Contributor Information
Collaborators: Louisa Sukkar, Amy Kang, Carinna Hockham, Tamara Young, Min Jun, Celine Foote, Roberto Pecoits-Filho, Brendon Neuen, Kris Rogers, Carol Pollock, Alan Cass, David Sullivan, Germaine Wong, John Knight, David Peiris, Martin Gallagher, and Meg Jardine
References
- 1.Zimmet P, Alberti KG, Shaw J. Global and societal implications of the diabetes epidemic. Nature 2001;414:782–787 [DOI] [PubMed] [Google Scholar]
- 2.Atkins RC, Zimmet P. World Kidney Day 2010: diabetic kidney disease--act now or pay later. Am J Kidney Dis 2010;55:205–208 [DOI] [PubMed] [Google Scholar]
- 3.International Diabetes Federation IDF Diabetes Atlas. 8th ed. Cho NH, Ed. Brussels, Belgium, International Diabetes Federation, 2017 [Google Scholar]
- 4.Svensson MK, Cederholm J, Eliasson B, Zethelius B, Gudbjörnsdottir S; Swedish National Diabetes Register . Albuminuria and renal function as predictors of cardiovascular events and mortality in a general population of patients with type 2 diabetes: a nationwide observational study from the Swedish National Diabetes Register. Diab Vasc Dis Res 2013;10:520–529 [DOI] [PubMed] [Google Scholar]
- 5.Ninomiya T, Perkovic V, de Galan BE, et al.; ADVANCE Collaborative Group . Albuminuria and kidney function independently predict cardiovascular and renal outcomes in diabetes. J Am Soc Nephrol 2009;20:1813–1821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Go AS, Chertow GM, Fan D, McCulloch CE, Hsu CY. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization [published correction appears in N Engl J Med 2008;18:4] N Engl J Med 2004;351:1296–1305 [DOI] [PubMed] [Google Scholar]
- 7.Toyama T, Furuichi K, Ninomiya T, et al. The impacts of albuminuria and low eGFR on the risk of cardiovascular death, all-cause mortality, and renal events in diabetic patients: meta-analysis. PLoS One 2013;8:e71810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Novak M, Mucsi I, Rhee CM, et al. Increased risk of incident chronic kidney disease, cardiovascular disease, and mortality in patients with diabetes with comorbid depression. Diabetes Care 2016;39:1940–1947 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zimbudzi E, Lo C, Ranasinha S, et al. Predictors of health-related quality of life in patients with co-morbid diabetes and chronic kidney disease. PLoS One 2016;11:e0168491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mujais SK, Story K, Brouillette J, et al. Health-related quality of life in CKD patients: correlates and evolution over time. Clin J Am Soc Nephrol 2009;4:1293–1301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bailey RA, Wang Y, Zhu V, Rupnow MFT. Chronic kidney disease in US adults with type 2 diabetes: an updated national estimate of prevalence based on Kidney Disease: Improving Global Outcomes (KDIGO) staging. BMC Res Notes 2014;7:415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wu B, Bell K, Stanford A, et al. Understanding CKD among patients with T2DM: prevalence, temporal trends, and treatment patterns—NHANES 2007–2012. BMJ Open Diab Res Care 2016;4:e000154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Banks E, Redman S, Jorm L, et al.; 45 and Up Study Collaborators . Cohort profile: the 45 and Up Study. Int J Epidemiol 2008;37:941–947 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Australian Government, Department of Health. Pharmaceutical benefits scheme [Internet], 2016. Available from https://www.health.gov.au/pbs. Accessed 14 January 2016.
- 15. Australian Government, Department of Health. Medicare benefits schedule [Internet], 2017. Available from https://www.mbsonline.gov.au/internet/mbsonline/publishing.nsf/Content/Home. Accessed 12 January 2016.
- 16.Australian Consortium for Classification Development The International Statistical Classification of Diseases and Related Health Problems, Tenth Revision, Australian Modification. Darlinghurst, NSW, Australia, Independant Hospital Pricing Authority, 2017 [Google Scholar]
- 17.Sayers A, Ben-Shlomo Y, Blom AW, Steele F. Probabilistic record linkage. Int J Epidemiol 2016;45:954–964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.American Diabetes Association 2. Classification and diagnosis of diabetes. Diabetes Care 2015;38:S8–S16 [DOI] [PubMed] [Google Scholar]
- 19.Levey AS, Stevens LA, Schmid CH, et al.; CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration) . A new equation to estimate glomerular filtration rate. Ann Intern Med 2009;150:604–612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Australian Bureau of Statistics. Census of Population and Housing: Socio-Economic Indexes for Areas (SEIFA), Australia, 2016. cat no. 2033.0.55.001 [Internet], 2016. Available from https://www.abs.gov.au/ausstats/abs@.nsf/mf/2033.0.55.001. Accessed 1 October 2018
- 21.van Buuren S. Multiple imputation of discrete and continuous data by fully conditional specification. Stat Methods Med Res 2007;16:219–242 [DOI] [PubMed] [Google Scholar]
- 22.Rubin DB. Multiple Imputation for Non-response in Surveys. New York, John Wiley, 1987 [Google Scholar]
- 23.Schemper M, Smith TL. A note on quantifying follow-up in studies of failure time. Control Clin Trials 1996;17:343–346 [DOI] [PubMed] [Google Scholar]
- 24.Lin DY, Wei LJ, Ying Z. Checking the Cox model with cumulative sums of martingale-based residuals. Biometrika 1993;80:557–572 [Google Scholar]
- 25.de Boer IH, Rue TC, Hall YN, Heagerty PJ, Weiss NS, Himmelfarb J. Temporal trends in the prevalence of diabetic kidney disease in the United States. JAMA 2011;305:2532–2539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tanamas SK, Magliano D, Lynch B, et al. The Australian Diabetes, Obesity and Lifestyle Study. Melbourne, Australia, Baker IDI Heart and Diabetes Institute, 2012 [Google Scholar]
- 27.Afghahi H, Cederholm J, Eliasson B, et al. Risk factors for the development of albuminuria and renal impairment in type 2 diabetes--the Swedish National Diabetes Register (NDR). Nephrol Dial Transplant 2011;26:1236–1243 [DOI] [PubMed] [Google Scholar]
- 28.Adler AI, Stevens RJ, Manley SE, Bilous RW, Cull CA, Holman RR; UKPDS GROUP . Development and progression of nephropathy in type 2 diabetes: the United Kingdom Prospective Diabetes Study (UKPDS 64). Kidney Int 2003;63:225–232 [DOI] [PubMed] [Google Scholar]
- 29.Salinero-Fort MA, San Andrés-Rebollo FJ, de Burgos-Lunar C, et al.; MADIABETES Group . Five-year incidence of chronic kidney disease (stage 3-5) and associated risk factors in a Spanish cohort: the MADIABETES Study. PLoS One 2015;10:e0122030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Retnakaran R, Cull CA, Thorne KI, Adler AI, Holman RR; UKPDS Study Group . Risk factors for renal dysfunction in type 2 diabetes: U.K. Prospective Diabetes Study 74. Diabetes 2006;55:1832–1839 [DOI] [PubMed] [Google Scholar]
- 31.Jardine MJ, Hata J, Woodward M, et al.; ADVANCE Collaborative Group . Prediction of kidney-related outcomes in patients with type 2 diabetes. Am J Kidney Dis 2012;60:770–778 [DOI] [PubMed] [Google Scholar]
- 32.Dunkler D, Gao P, Lee SF, et al.; ONTARGET and ORIGIN Investigators . Risk prediction for early CKD in type 2 diabetes. Clin J Am Soc Nephrol 2015;10:1371–1379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gatwood J, Chisholm-Burns M, Davis R, et al. Evidence of chronic kidney disease in veterans with incident diabetes mellitus. PLoS One 2018;13:e0192712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Matsushita K, Mahmoodi BK, Woodward M, et al.; Chronic Kidney Disease Prognosis Consortium . Comparison of risk prediction using the CKD-EPI equation and the MDRD study equation for estimated glomerular filtration rate. JAMA 2012;307:1941–1951 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hsu CY, McCulloch CE, Iribarren C, Darbinian J, Go AS. Body mass index and risk for end-stage renal disease. Ann Intern Med 2006;144:21–28 [DOI] [PubMed] [Google Scholar]
- 36.Nicholas SB, Kalantar-Zadeh K, Norris KC. Socioeconomic disparities in chronic kidney disease. Adv Chronic Kidney Dis 2015;22:6–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yu MK, Katon W, Young BA. Associations between sex and incident chronic kidney disease in a prospective diabetic cohort. Nephrology (Carlton) 2015;20:451–458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Luk AO, So WY, Ma RC, et al.; Hong Kong Diabetes Registry . Metabolic syndrome predicts new onset of chronic kidney disease in 5,829 patients with type 2 diabetes: a 5-year prospective analysis of the Hong Kong Diabetes Registry. Diabetes Care 2008;31:2357–2361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Launay-Vacher V, Janus N, Spano J, et al. Impact of renal insufficiency on cancer survival: results of the IRMA-2 study. J Clin Oncol 2009;27(Suppl. 15):9585 [Google Scholar]
- 40.Vlasschaert ME, Bejaimal SA, Hackam DG, et al. Validity of administrative database coding for kidney disease: a systematic review. Am J Kidney Dis 2011;57:29–43 [DOI] [PubMed] [Google Scholar]
- 41.Manns L, Scott-Douglas N, Tonelli M, et al. A population-based analysis of quality indicators in CKD. Clin J Am Soc Nephrol 2017;12:727–733 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.