Abstract
OBJECTIVE
We compared the performance of the FreeStyle Libre Pro continuous glucose monitoring (CGM) and point-of-care capillary glucose testing (POC) among insulin-treated hospitalized patients with type 2 diabetes (T2D).
RESEARCH DESIGN AND METHODS
This was a prospective study in adult patients with T2D admitted to general medicine and surgery wards. Patients were monitored with POC before meals and bedtime and with CGM during the hospital stay. Study end points included differences between POC and CGM in mean daily blood glucose (BG), hypoglycemia <70 and <54 mg/dL, and nocturnal hypoglycemia. We also calculated the mean absolute relative difference (MARD), ±15%/15 mg/dL, ±20%/20 mg/dL, and ±30%/30 mg/dL and error grid analysis between matched glucose pairs.
RESULTS
Mean daily glucose was significantly higher by POC (188.9 ± 37.3 vs. 176.1 ± 46.9 mg/dL) with an estimated mean difference of 12.8 mg/dL (95% CI 8.3–17.2 mg/dL), and proportions of patients with glucose readings <70 mg/dL (14% vs. 56%) and <54 mg/dL (4.1% vs. 36%) detected by POC BG were significantly lower compared with CGM (all P < 0.001). Nocturnal and prolonged CGM hypoglycemia <54 mg/dL were 26% and 12%, respectively. The overall MARD was 14.8%, ranging between 11.4% and 16.7% for glucose values between 70 and 250 mg/dL and higher for 51–69 mg/dL (MARD 28.0%). The percentages of glucose readings within ±15%/15 mg/dL, ±20%/20 mg/dL, and ±30%/30 mg/dL were 62%, 76%, and 91%, respectively. Error grid analysis showed 98.8% of glucose pairs within zones A and B.
CONCLUSIONS
Compared with POC, FreeStyle Libre CGM showed lower mean daily glucose and higher detection of hypoglycemic events, particularly nocturnal and prolonged hypoglycemia in hospitalized patients with T2D. CGM’s accuracy was lower in the hypoglycemic range.
Introduction
Extensive evidence has associated inpatient hyperglycemia with poor clinical outcomes in patients with and without diabetes (1–3). Several randomized controlled trials and meta-analyses have demonstrated that basal-bolus insulin therapy improves glycemic control and clinical outcomes in non–critically ill hospitalized patients with type 2 diabetes (T2D) (4). Consequently, clinical practice guidelines recommend basal-bolus insulin therapy as the preferred approach for the management of hyperglycemia in hospitalized non–critically ill patients with T2D (5,6).
Bedside point-of-care capillary glucose testing (POC) is the current standard of care to assess glycemic control in the hospital and to adjust insulin therapy (5,6). As recommended by national guidelines, bedside POC is performed before meals and at bedtime, matching meal intake and insulin administration, or every 4–6 h for patients not eating or on continuous enteral feeding (5,6). However, this approach fails to provide a complete 24-h glycemic profile assessment and particularly does not properly detect asymptomatic and/or nocturnal hypoglycemia (7,8), both common complications of inpatient insulin therapy (9).
Continuous glucose monitoring (CGM) provides the advantage of measuring interstitial glucose every 5–15 min, thus providing a comprehensive 24-h glycemic profile, with better assessment of nocturnal and/or asymptomatic hypoglycemia and pattern recognition after each treatment intervention. Several studies using CGM have shown improved glycemic control in insulin-treated ambulatory patients with type 1 diabetes (T1D) and T2D (10,11). Fewer studies with small sample sizes have also reported on the use of CGM in hospitalized non–critically ill patients (12–14). However, no prospective studies have compared the newer factory-calibrated CGM for glucose monitoring in non–critically ill hospitalized patients. Accordingly, we compared the performance and efficacy of the FreeStyle Libre Pro CGM to POC (standard of care) in non–critically ill hospitalized patients with T2D treated with basal-bolus insulin regimen.
Research Design and Methods
This was an ancillary study to the Glargine U300 Hospital Trial (NCT03013985, ClinicalTrials.gov) aiming to evaluate the feasibility and performance of CGM use in non–intensive care unit hospital settings and to obtain preliminary estimates of the role of CGM in non–critically ill hospitalized patients. This study was performed at Grady Memorial Hospital and Emory University Midtown Hospital in Atlanta, GA, and at Hennepin County Medical Center in Minneapolis, MN. We used the FreeStyle Libre CGM Pro, a commercially available factory-calibrated sensor; thus, there was no need for POC blood glucose (BG) testing for sensor calibration. This device measures interstitial glucose in the range of ≥40 mg/dL to 500 mg/dL (15).
Study Design
Patients with T2D were treated with basal-bolus regimen with glargine U300 and U100 plus glulisine insulin before meals. We included adult patients >18 years of age admitted to general medicine or surgical services previously treated with diet alone, oral monotherapy, or a combination of oral agents, glucagon-like peptide 1 agonists, or insulin therapy and with admission BG >140–400 mg/dL, without evidence of ketoacidosis. We excluded patients with type 1 diabetes and pregnant patients, those with an inability to provide informed consent, or patients with severe liver, kidney, or pancreas disorders or undergoing treatment with steroids. We also aimed to enroll patients with an anticipated length of stay of at least 3 days, as estimated by the primary treating team. The study was approved by each hospital’s institutional review board.
Procedures
The study team approached patients shortly after hospital admission to explain the design and potential risks of the study. After obtaining informed consent, sensors were placed by the study team in the posterior upper arm following manufacturer recommendations and under an aseptic technique. The study team reviewed each sensor daily to ensure proper placement and functional status for up to 10 days or until discharge. CGM sensors were removed before surgery, computed tomography scan, or MRI.
Patients and the research team were blinded to the CGM glucose results during the hospital stay. All CGM sensor data were downloaded after hospital discharge using LibreView software. All glucose values were exported into standard Excel data files for further statistical analysis by the study statistician. Each POC BG was paired with the corresponding CGM value within 5 min and was used for accuracy analysis.
Diabetes was managed by the research team, targeting glucose readings between 70 and 180 mg/dL. Glucose monitoring was performed before meals and at bedtime or as clinically required. Insulin dosage was adjusted daily based on POC BG values obtained by hospital-calibrated Nova StatStrip (Grady Memorial Hospital and Hennepin County Medical Center) and Accu-Chek Inform II glucose meters (Emory University Midtown Hospital).
Outcomes Measures
For clinical efficacy, we adapted CGM metrics defined by an international consensus meeting for ambulatory patients (16,17). Our primary end point was the comparison between POC and CGM on glycemic control metrics in the hospital, including mean daily glucose, time in range (TIR) (defined as percentage of glucose readings and percentage of TIR between 70 and 180 mg/dL), time below range (defined as percentage of glucose and time <70 mg/day, <54 mg/dL, and ≤40 mg/dL), and time above range (defined as percentage of glucose readings and percentage of time >180 mg/dL and >250 mg/dL).
We also calculated the number of episodes and proportion of subjects with episodes of hypoglycemia <70 mg/dL and <54 mg/dL for at least 15 min detected by CGM. As previously reported, hypoglycemia was defined as glucose <70 mg/dL or <54 mg/day as measured by hospital-calibrated glucose meters. We calculated and defined nocturnal hypoglycemia occurring from 2200 to 0600 h (only collected by CGM). We also calculated episodes of prolonged hypoglycemia, defined as CGM values recording glucose <54 mg/dL for at least 120 consecutive minutes. Glucose variability was calculated as percentage coefficient of variation = SD/mean glucose × 100%.
For performance evaluation, the major outcome was the mean absolute relative difference (MARD) between matched glucose pairs obtained from POC and CGM (within 5 min). Secondary aims included MARD for glucose readings <70 mg/dL, 70–180 mg/dL, >180 mg/dL, and >250 mg/dL. We also analyzed the overall percentage of CGM values within ±15%/15 mg/dL of POC glucose values, the ±20%/20 mg/dL and ±30%/30 mg/dL, and among different glucose ranges (hyperglycemia and hypoglycemia ranges) (16,17).
We further assessed the clinical safety using the error grid analysis. Additionally, we recorded the number of sensor failures and/or malfunctions, sensors lost, and sensors removed for procedures/surgery.
Statistical Analysis
Data are presented as mean ± SD for continuous variables and count (percentage) for categorical variables. We defined CGM variables, ranges, and cutoffs (hypoglycemia, hyperglycemia, percentage of glucose in range, and glycemic variability) as detailed by the International Consensus Conference (16,17). MARD was determined as the average relative difference between the CGM and POC glucose–matched pairs and expressed as a percentage. For CGM performance evaluation, we followed statistical recommendations as provided by Clarke and Kovatchev (18). The numbers of glucose pairs in various risk zones of error grid analyses were determined with the R package “ega,” which is designed for Clarke or Parkes error grid analysis (https://cran.r-project.org/web/packages/ega/ega.pdf).
Results
Population
A total of 134 patients were enrolled in this ancillary study, with 72.4% (n = 97) having adequate data for our analyses. Causes for excluding data from the analysis included screen failure (n = 4), early study termination/withdrawal (n = 9), <24 h of sensor data/<4 matched glucose pairs (n = 8), lost sensor data/removal for imaging/procedures (n = 6), sensor loss/unplanned removal by staff (n = 8), and sensor malfunction/not data recorded (n = 2).
Overall, the mean age was 54.5 ± 11 years, and 63 patients (66%) were male. The mean admission hemoglobin A1c (HbA1c) was 10.2 ± 2% (88 mmol/mol), BMI was 33.8 ± 9 kg/m2, and mean duration of diabetes was 11.5 ± 9 years. The clinical characteristics and outcomes of the study population are displayed in Table 1.
Table 1.
Study participant characteristics
| Characteristic | |
|---|---|
| Age, years | 54.5 ± 11 |
| Sex, male, n (%) | 63 (66) |
| Race: White/Black/other/Hispanic (%) | 16/78/3.1/2.1 |
| BMI, kg/m2 | 33.8 ± 9 |
| Duration of diabetes, years | 11.5 ± 9 |
| Admission HbA1c, % | 10.2 ± 2 |
| Length of stay (median [minimum–maximum]), days | 7.5 (2–30) |
| Admission causes, n (%) | |
| Cardiovascular | 23 (28) |
| Pulmonary | 2 (2.4) |
| Cancer | 1 (1.2) |
| Renal | 1 (1.2) |
| Infectious | 34 (41) |
| Gastrointestinal | 3 (3.7) |
| Neurological | 6 (7.3) |
Data are means ± SD unless otherwise indicated.
Glycemic Patterns During Hospitalization
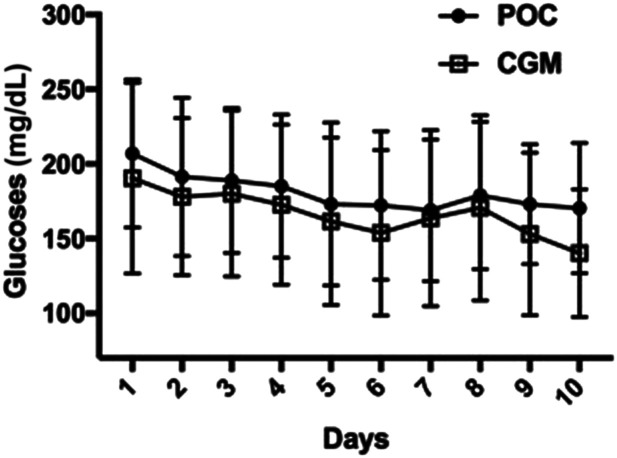
The mean daily glucose (Fig. 1) during the hospital stay was significantly higher by POC BG compared with CGM (188.9 ± 37.3 vs. 176.1 ± 46.9 mg/dL) (P < 0.001), with an estimated mean glucose difference of 12.8 mg/dL (95% CI 8.3–17.2).
Figure 1.
Mean hospital daily glucose as measured by POC (circles) and CGM (squares). The overall mean daily glucose was significantly higher as measured by POC BG compared with CGM (188.9 ±37.3 vs. 176.1 ± 46.9 mg/dL), with an estimated mean glucose difference of 12.8 mg/dL (CI 8.3–17.2 mg/dL) (P < 0.001).
The mean percentage of readings/TIR for POC and CGM glucose values between 70 and 180 mg/dL was 48.4 ± 22.9% and 53.5 ± 25.8% (P = 0.001), respectively. The percentage of TIR for POC and CGM glucose readings between 70 and 140 mg/dL was 24.5 ± 18.6% and 32.8 ± 21.7%, respectively (P < 0.001). The mean percentage time above range (>180 mg/dL) was 50.5 ± 23.2% by POC and 42.2 ± 27.7% by CGM (P < 0.001) and for severe hyperglycemia (>250 mg/dL) was 17.8 ± 19.1% and 16.1 ± 20.2%, respectively (P = 0.17). The mean percentage time below range for glucose readings <70 mg/dL, <54 mg/dL, and ≤40 mg/dL was 1.1 ± 3.9%, 0.20 ± 1.2%, and 0% by POC and 4.5 ± 6.9%, 1.58 ± 3.3%, and 0.54 ± 1.8% by CGM, respectively (P < 0.01).
Glycemic variability was stable, as demonstrated by an overall percentage coefficient of variation of 30 ± 1% as measured by POC and of 32 ± 1% based on CGM.
Hypoglycemia Detection
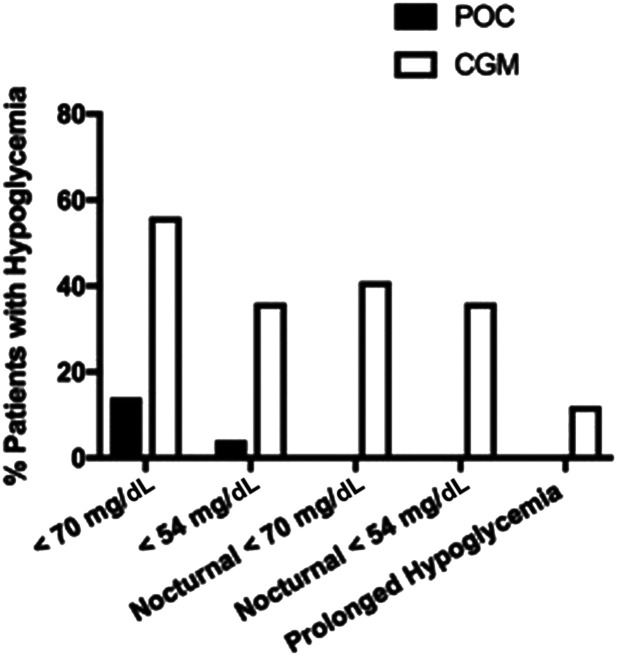
Overall, the proportion of patients with hypoglycemia <70 mg/dL (14% [n = 14] vs. 56% [n = 54]; P < 0.001) and <54 mg/dL (4.1% [n = 4] vs. 36% [n = 35]; P < 0.001) detected by POC BG was significantly lower compared with CGM, respectively (Fig. 2). Among those patients with hypoglycemia, the average number of episodes of glucose values <70 mg/dL (1.86 ± 0.95 vs. 5.14 ± 3.88; P < 0.001) and of glucose values <54 mg/dL was also significantly lower (1 ± 0 vs. 4.5 ± 6.4; P < 0.001) by POC compared with CGM. There were no episodes of severe hypoglycemia ≤40 mg/dL detected by either method.
Figure 2.
Hypoglycemia detection by POC (filled bars) and CGM (open bars).
The prevalence of nocturnal CGM hypoglycemia (from 2200 to 0600 h) <70 mg/dL and <54 mg/dL was 41% (n = 40) and 26% (n = 25), respectively, as shown in Fig. 2. The prevalence of prolonged CGM hypoglycemia (<54 mg/dL) was 12.3% (n = 12).
CGM Performance and Clarke Error Grid Analysis
Among 1,829 POC-CGM–matched glucose pairs, the overall MARD was 14.8% (as shown in Table 2). MARD ranged from 11.4% to 16.7% for glucose readings >70 to 250 mg/dL and was higher for glucose readings 51–69 mg/dL (MARD 28.0%). However, there were limited matched glucose pairs (n = 13) in hypoglycemic range.
Table 2.
Comparison of the FreeStyle Libre Pro CGM and POC among non–critically ill hospitalized patients with T2D treated with basal-bolus insulin therapy
| Glucose range (mg/dL) | Matched pairs (n) | MARD (%) | ±15%/15 mg/dL (%) | ±20%/20 mg/dL (%) | ±30%/30 mg/dL (%) |
|---|---|---|---|---|---|
| Overall | 1,829 | 14.8 | 61.5 | 75.8 | 90.4 |
| 51–69* | 13 | 27.9 | 53.8 | 53.8 | 76.9 |
| 70–180 | 829 | 16.7 | 54.9 | 70.2 | 87.8 |
| >180 | 731 | 12.1 | 69.2 | 82.6 | 94.4 |
| >250 | 253 | 11.4 | 71.5 | 88.1 | 95.3 |
There were only three paired matched glucose values for glucose readings <51 mg/dL.
The overall percentages of glucose values within ±15%/15 mg/dL, ±20%/20 mg/dL, and ±30%/30 mg/dL of the POC reference value were 61.5%, 75.8%, and 90.4%, respectively. The percentages ±15%/15 mg/dL, ±20%/20 mg/dL, and ±30%/30 mg/dL for glucose values >70 to 250 mg/dL ranged from 54.9 to 71.5, 70.2 to 88.1, and 87.8 to 95.3, respectively. For glucose readings 51–69 mg/dL, the ±15%/15 mg/dL, ±20%/20 mg/dL, and ±30%/30 mg/dL were 53.8%, 53.8%, and 76.9%, respectively. We performed additional comparisons for the above-described accuracy outcomes (data not shown), including from day 2 to day 10 (excluding day 1), different age groups (<40, 41–65, and >65 years of age), and BMI categories (<30 and >30 kg/m2), and found no relevant differences.
As shown in Supplementary Fig. 1, the error grid analysis showed acceptable clinical accuracy with 98.8% of glucose values falling into zones A (75.1%; n = 1,184) and B (23.7%; n = 374), based on established evaluation tools (17,19). Accuracy was lower for glucose readings <70 mg/dL; however, there were limited matched glucose pairs in hypoglycemic range (n = 13).
Conclusions
In this prospective study, we compared the performance of FreeStyle Libre CGM and POC in non–critically ill insulin-treated hospitalized patients with diabetes. Compared with the current POC (standard of care), our results showed a tendency for lower glucose concentration with CGM measurements, with an estimated mean daily glucose difference of 12.8 mg/dL (95% CI 8.3–17.2 mg/dL). Based on established CGM performance evaluation tools (15–18), the clinical accuracy was acceptable, with an overall MARD of 14.4% and with 98.8% of glucose readings falling into zones A and B. MARD ranged between 11% and 16% for glucose values between 70 and 250 mg/dL. The CGM accuracy was lower for glucose values <70 mg/dL; however, there were lower numbers of matched POC-CGM pairs in hypoglycemia range. As expected, CGM detected more nocturnal and prolonged hypoglycemic episodes compared with limited daily testing with POC.
Bedside POC is the standard of care to assess glycemic control in the hospital. Diabetes guidelines recommend bedside POC before meals and at bedtime to assess glycemic control and to adjust insulin therapy in the hospital (5,6). This approach has been shown to fail to detect nocturnal hypoglycemia and asymptomatic hypoglycemia, a common scenario in the hospital setting (9). In addition, the POC approach is labor intensive, costly, and prone to errors and mismatched measurements (20). Hence, there is a need for an improved method to monitor glycemic control in the hospital setting.
CGM utilization has expanded significantly in recent years, with several studies showing improved outcomes in ambulatory patients (10,11,16,21). CGM provides the advantage of measuring interstitial glucose every 5–15 min, thus providing a comprehensive 24-h glycemic profile, with better assessment of nocturnal and/or asymptomatic hypoglycemia and pattern recognition after each treatment intervention. Large studies using CGM have been shown to facilitate and improve diabetes care in insulin-treated ambulatory patients with T1D and T2D (10,11). Few studies, however, have reported on the use of CGM in hospitalized patients, particularly in non–critically ill patients (12–14). These studies have shown good correlation between CGM and capillary and/or laboratory glucose values (7,22,23).
In our study, we found an overall MARD percentage of 14.4%, ranging between 11% and 16% for glucose values between 70 and 250 mg/dL. These values agree with those reported in previous studies, in which the MARD percentage ranged from 11.4% to 14.3% in patients with T1D and T2D. In addition, in the pivotal studies of the FreeStyle Libre Pro CGM, an overall 66.5%, 79.4%, and 93.4% of values were within ±15%/15 mg/dL, ±20%/20 mg/dL, or ±30%/30 mg/dL of the capillary glucose reference (FreeStyle Precision Meter), respectively. In agreement with these studies, we found overall percentages of glucose values within ±15%/15 mg/dL, ±20%/20 mg/dL, or ±30%/30 mg/dL of the POC reference value were 63%, 78%, and 92%, respectively (15,24–27). The FreeStyle Libre CGM is a commercially available, factory-calibrated sensor system; thus, there is no need for POC BG testing for sensor calibration. Prior studies in the non–critically ill hospitalized population were performed with previous CGM technology (7,22,23,28) requiring capillary glucose for calibration, including Medtronic MiniMed Gold and Medtronic iPro2, reporting up to 91.9–99% (7,22) of values falling into zones A and B of the error grid analysis, respectively. Our error grid analyses showed acceptable clinical accuracy, with 98.8% of glucose readings falling into zones A (75.1%; n = 1,184) and B (23.7%; n = 374).
Potential explanations for our differences in performance from the pivotal studies of the FreeStyle Libre CGM could be explained by the real-world design in the hospital setting and the use of hospital-calibrated, hand-held glucose meters (6). Future studies should compare glucose values obtained from CGM to more sophisticated references, such as the Yellow Springs Instrument or by standard laboratory assays. By using glucose meters with validated accuracy in our study (29), we provide good correlation estimates. FreeStyle Libre and other CGM systems have been reported to have lower accuracy in the hypoglycemic range (26,27,30). Newer data presented at the recent American Diabetes Association Scientific Sessions in 2019 on FreeStyle Libre 2 suggest improved accuracy results (31).
A recent panel of experts on inpatient diabetes care reported that CGM could more effectively identify trends toward hypoglycemia and hyperglycemia, allowing for better and safer management of patients with inpatient hyperglycemia (14). In a recent study, Gómez et al. (7) compared the efficacy of a blinded/professional CGM (iPro Medtronic) versus POC in non–critically ill hospitalized patients with T2D treated with basal-bolus insulin. CGM detected a higher number of hypoglycemic events, with 60% of events occurring at night or early morning between dinner and 0600 h. Moreover, CGM was able to detect up to 86% of the asymptomatic hypoglycemic events. In agreement with these studies, we found a higher proportion of patients with hypoglycemia <70 mg/dL detected by the factory-calibrated CGM compared with POC.
The assessment of nocturnal hypoglycemia and the duration of hypoglycemia events are poorly assessed by POC before meals and bedtime, the current standard of care. In our study, CGM detected nocturnal CGM hypoglycemia (from 2200 to 0600 h) <70 mg/dL in 41% of participants and <54 mg/dL in 26%. Prolonged CGM hypoglycemia <54 mg/dL for >120 consecutive minutes was detected in 12%. In a recent prospective observational study, we reported that ∼45% of insulin-treated hospitalized patients with glucose <70 mg/dL had asymptomatic hypoglycemia (9). These findings are clinically relevant and underlie the potential impact of CGM in hospitalized patients, because improved detection of hypoglycemia in hospitalized patients may facilitate better insulin adjustments and potentially reduce hypoglycemia-related morbidity and mortality. To fill this gap, we are currently testing the use of CGM technology integrated with a telemetry system to alert nurses and providers about downtrending glucose levels (<80 mg/dL) to intervene early and prevent hypoglycemia.
Our study has several limitations, including a small sample size and the low number of hypoglycemic episodes observed. Subsequent studies should aim for larger sample sizes with power to detect differences in low glucose ranges and compare glucose values obtained from CGM to more sophisticated references, such as the Yellow Springs Instrument or by standard laboratory assays. Because the exact time of collection and sample processing of venous glucose is not correctly documented in the medical records, we did not pair CGM values with those of venous samples. These studies are in process and will be needed to confirm the accuracy of CGM in the hospital under diverse clinical situations (medicine vs. surgery, hypoxia, edema, etc.). Current prospective randomized studies (NCT03877068 and NCT03508934) are evaluating whether the use of real-time CGM, with an alarm system transmitting glucose values from the patient’s room to the nursing station, can reduce the frequency of clinically significant and severe hypoglycemia events. Another limitation is that patients received different basal insulin formulations; however, because we evaluated the accuracy between matched glucose pairs, it is unlikely that treatment assignment would have influenced our results. Despite these limitations, we believe that our study provides significant evidence on methodology and implementation for further interventional studies using CGM systems among hospitalized patients. With increased use of CGM among ambulatory patients and high rates of patient satisfaction and improved quality of life, one can expect an increasing number of patients choosing to continue using CGM while hospitalized. Moreover, CGM provides a more comprehensive evaluation of glycemic patterns (i.e., TIR) that may facilitate the study of new glucose control metrics and their impact on clinical outcomes in the hospital, as recently demonstrated by studies in ambulatory settings (32,33).
In conclusion, compared with POC glucose meter measurements, FreeStyle Libre CGM showed a tendency toward lower mean glucose, with an estimated mean glucose difference of 12.8 mg/dL (95% CI 8.3–17.2 mg/dL). As expected, compared with limited measurements with POC BG before each meal and bedtime, CGM provided a higher detection of hypoglycemic events, particularly nocturnal hypoglycemia, and prolonged hypoglycemia. The clinical implications of higher detection of hypoglycemia with these devices need further investigations given the known tendency to overread hypoglycemia at lower glucose levels. Ongoing studies are testing the accuracy of other devices and the effectiveness of glucose management based on a glucose telemetry system using CGM technology. Based on established performance evaluation tools, the CGM performance in this study appears to be acceptable for glycemic monitoring among hospitalized non–critically ill patients with T2D. Larger studies are needed to determine the performance of the CGM in hypoglycemia range.
Article Information
Funding. R.J.G. is supported in part by a grant from the National Institute of Diabetes and Digestive and Kidney Diseases of the National Institutes of Health under awards P30-DK-11102 and 1K23-DK-123384-01. P.V. is supported in part by National Institutes of Health grant 1K23-DK-113241. F.J.P. is supported in part by National Institutes of Health grant 1K23-GM-128221-01A1. G.E.U. is partly supported by research grants from the National Center for Advancing Translational Sciences of the National Institutes of Health under award UL1-TR-002378 from the Clinical and Translational Science Award program and National Institutes of Health grant U30, P30-DK-11102.
Duality of Interest. This work was supported by an investigator-initiated grant, with unrestricted research support provided by Sanofi to Emory University. R.J.G. received research support (to Emory University) for investigator-initiated studies from Novo Nordisk and consulting fees from Abbott Diabetes Care, Sanofi, Novo Nordisk, and Valeritas Holdings Inc. P.V. has received consulting fees from Merck and Boehringer Ingelheim. F.J.P. has received research support from Merck and Dexcom and consulting fees from Merck, Boehringer Ingelheim, Sanofi, Eli Lilly and Company, and AstraZeneca. G.E.U. has received research grant support to Emory University for investigator-initiated studies from Sanofi, Novo Nordisk, and Dexcom. No other potential conflicts of interest relevant to this article were reported.
The funding source had no input to the design of the study, interpretation of the results, or manuscript preparation.
Author Contributions. R.J.G. and G.E.U. wrote the initial research proposal and the initial draft of the manuscript. A.L.M., G.M.D., M.A.U., B.A., C.Z., P.V., F.J.P., M.F., and L.P. conducted the study, reviewed and edited the manuscript, and contributed to the discussion. L.P. performed the statistical analysis. R.J.G. and G.E.U. are the guarantors of this work and, as such, had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Prior Presentation. Parts of this study were presented in abstract form at the 79th Scientific Sessions of the American Diabetes Association, San Francisco, CA, 7–11 June 2019.
Footnotes
This article contains supplementary material online at https://doi.org/10.2337/figshare.12302660.
This article is featured in a podcast available at https://www.diabetesjournals.org/content/diabetes-core-update-podcasts.
References
- 1.Galindo RJ, Davis GM, Fayfman M, et al. Comparison of efficacy and safety of glargine and detemir insulin in the management of inpatient hyperglycemia and diabetes. Endocr Pract 2017;23:1059–1066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pasquel FJ, Spiegelman R, McCauley M, et al. Hyperglycemia during total parenteral nutrition: an important marker of poor outcome and mortality in hospitalized patients. Diabetes Care 2010;33:739–741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Umpierrez GE, Isaacs SD, Bazargan N, You X, Thaler LM, Kitabchi AE. Hyperglycemia: an independent marker of in-hospital mortality in patients with undiagnosed diabetes. J Clin Endocrinol Metab 2002;87:978–982 [DOI] [PubMed] [Google Scholar]
- 4.Murad MH, Coburn JA, Coto-Yglesias F, et al. Glycemic control in non-critically ill hospitalized patients: a systematic review and meta-analysis. J Clin Endocrinol Metab 2012;97:49–58 [DOI] [PubMed] [Google Scholar]
- 5.Moghissi ES, Korytkowski MT, DiNardo M, et al.; American Association of Clinical Endocrinologists; American Diabetes Association . American Association of Clinical Endocrinologists and American Diabetes Association consensus statement on inpatient glycemic control. Diabetes Care 2009;32:1119–1131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Umpierrez GE, Hellman R, Korytkowski MT, et al.; Endocrine Society . Management of hyperglycemia in hospitalized patients in non-critical care setting: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab 2012;97:16–38 [DOI] [PubMed] [Google Scholar]
- 7.Gómez AM, Umpierrez GE, Muñoz OM, et al. Continuous glucose monitoring versus capillary point-of-care testing for inpatient glycemic control in type 2 diabetes patients hospitalized in the general ward and treated with a basal bolus insulin regimen. J Diabetes Sci Technol 2015;10:325–329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Davis GM, Galindo RJ, Migdal AL, Umpierrez GE. Diabetes technology in the inpatient setting for management of hyperglycemia. Endocrinol Metab Clin North Am 2020;49:79–93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cardona S, Gomez PC, Vellanki P, et al. Clinical characteristics and outcomes of symptomatic and asymptomatic hypoglycemia in hospitalized patients with diabetes. BMJ Open Diabetes Res Care 2018;6:e000607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Beck RW, Riddlesworth T, Ruedy K, et al.; DIAMOND Study Group . Effect of continuous glucose monitoring on glycemic control in adults with type 1 diabetes using insulin injections: the DIAMOND randomized clinical trial. JAMA 2017;317:371–378 [DOI] [PubMed] [Google Scholar]
- 11.Beck RW, Riddlesworth TD, Ruedy K, et al.; DIAMOND Study Group . Continuous glucose monitoring versus usual care in patients with type 2 diabetes receiving multiple daily insulin injections: a randomized trial. Ann Intern Med 2017;167:365–374 [DOI] [PubMed] [Google Scholar]
- 12.Gomez AM, Umpierrez GE. Continuous glucose monitoring in insulin-treated patients in non-ICU settings. J Diabetes Sci Technol 2014;8:930–936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Umpierrez GE, Klonoff DC. Diabetes technology update: use of insulin pumps and continuous glucose monitoring in the hospital. Diabetes Care 2018;41:1579–1589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wallia A, Umpierrez GE, Rushakoff RJ, et al.; DTS Continuous Glucose Monitoring in the Hospital Panel . Consensus statement on inpatient use of continuous glucose monitoring. J Diabetes Sci Technol 2017;11:1036–1044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bailey T, Bode BW, Christiansen MP, Klaff LJ, Alva S. The performance and usability of a factory-calibrated flash glucose monitoring system. Diabetes Technol Ther 2015;17:787–794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Battelino T, Danne T, Bergenstal RM, et al. Clinical targets for continuous glucose monitoring data interpretation: recommendations from the international consensus on time in range. Diabetes Care 2019;42:1593–1603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Danne T, Nimri R, Battelino T, et al. International consensus on use of continuous glucose monitoring. Diabetes Care 2017;40:1631–1640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Clarke W, Kovatchev B. Statistical tools to analyze continuous glucose monitor data. Diabetes Technol Ther 2009;11(Suppl. 1):S45–S54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Klonoff DC, Lias C, Vigersky R, et al.; Error Grid Panel . The surveillance error grid. J Diabetes Sci Technol 2014;8:658–672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Aragon D. Evaluation of nursing work effort and perceptions about blood glucose testing in tight glycemic control. Am J Crit Care 2006;15:370–377 [PubMed] [Google Scholar]
- 21.Galindo RJ, Migdal AL, Umpierrez GE. Are we ready to move beyond capillary glucose testing and insulin injections? Am J Med Sci 2019;358:315–316 [DOI] [PubMed] [Google Scholar]
- 22.Schaupp L, Donsa K, Neubauer KM, et al. Taking a closer look--continuous glucose monitoring in non-critically ill hospitalized patients with type 2 diabetes mellitus under basal-bolus insulin therapy. Diabetes Technol Ther 2015;17:611–618 [DOI] [PubMed] [Google Scholar]
- 23.Burt MG, Roberts GW, Aguilar-Loza NR, Stranks SN. Brief report: comparison of continuous glucose monitoring and finger-prick blood glucose levels in hospitalized patients administered basal-bolus insulin. Diabetes Technol Ther 2013;15:241–245 [DOI] [PubMed] [Google Scholar]
- 24.Abbott Diabetes Care FreeStyle Libre User’s Manual [Internet], 2019. Available from https://freestyleserver.com/Payloads/IFU/2017_dec/ART34745-107_rev-A-WEB.pdf. Accessed 15 October 2019 [Google Scholar]
- 25.Ólafsdóttir AF, Attvall S, Sandgren U, et al. A clinical trial of the accuracy and treatment experience of the flash glucose monitor FreeStyle Libre in adults with type 1 diabetes. Diabetes Technol Ther 2017;19:164–172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Aberer F, Hajnsek M, Rumpler M, et al. Evaluation of subcutaneous glucose monitoring systems under routine environmental conditions in patients with type 1 diabetes. Diabetes Obes Metab 2017;19:1051–1055 [DOI] [PubMed] [Google Scholar]
- 27.Moser O, Eckstein ML, McCarthy O, et al. Performance of the Freestyle Libre flash glucose monitoring (flash GM) system in individuals with type 1 diabetes: a secondary outcome analysis of a randomized crossover trial. Diabetes Obes Metab 2019;21:2505–2512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Burt MG, Roberts GW, Aguilar-Loza NR, Frith P, Stranks SN. Continuous monitoring of circadian glycemic patterns in patients receiving prednisolone for COPD. J Clin Endocrinol Metab 2011;96:1789–1796 [DOI] [PubMed] [Google Scholar]
- 29.Karon BS, Blanshan CT, Deobald GR, Wockenfus AM. Retrospective evaluation of the accuracy of Roche AccuChek Inform and Nova StatStrip glucose meters when used on critically ill patients. Diabetes Technol Ther 2014;16:828–832 [DOI] [PubMed] [Google Scholar]
- 30.Pleus S, Heinemann L, Freckmann G. Blood glucose monitoring data should be reported in detail when studies about efficacy of continuous glucose monitoring systems are published. J Diabetes Sci Technol 2018;12:1061–1063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Karinka SA, Bailey TS, Brazg RL, et al. Improved accuracy of 14-day factory-calibrated FreeStyle Libre system with new glucose algorithm (Abstract). Diabetes 2019;68(Suppl. 1):910-P [Google Scholar]
- 32.Beck RW, Bergenstal RM, Riddlesworth TD, et al. Validation of time in range as an outcome measure for diabetes clinical trials. Diabetes Care 2019;42:400–405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lu J, Ma X, Zhou J, et al. Association of time in range, as assessed by continuous glucose monitoring, with diabetic retinopathy in type 2 diabetes. Diabetes Care 2018;41:2370–2376 [DOI] [PubMed] [Google Scholar]