Abstract
Background
Movement disorders are a group of heterogeneous neurological diseases including hyperkinetic disorders with unwanted excess movements and hypokinetic disorders with reduction in the degree of movements. The objective of our study is to investigate the genetic etiology of a cohort of paediatric patients with movement disorders by whole exome sequencing and to review the potential treatment implications after a genetic diagnosis.
Results
We studied a cohort of 31 patients who have paediatric-onset movement disorders with unrevealing etiologies. Whole exome sequencing was performed and rare variants were interrogated for pathogenicity. Genetic diagnoses have been confirmed in 10 patients with disease-causing variants in CTNNB1, SPAST, ATP1A3, PURA, SLC2A1, KMT2B, ACTB, GNAO1 and SPG11. 80% (8/10) of patients with genetic diagnosis have potential treatment implications and treatments have been offered to them. One patient with KMT2B dystonia showed clinical improvement with decrease in dystonia after receiving globus pallidus interna deep brain stimulation.
Conclusions
A diagnostic yield of 32% (10/31) was reported in our cohort and this allows a better prediction of prognosis and contributes to a more effective clinical management. The study highlights the potential of implementing precision medicine in the patients.
Keywords: Movement disorders, Whole exome sequencing, Genetic diagnosis, Treatment
Background
Paediatric movement disorders (MDs) are a group of complex and heterogeneous neurological diseases including both hyperkinetic [1] and hypokinetic disorders [2]. They are presented with overlapping phenotypes and with a wide spectrum of genetic mutations causing defects in various pathophysiological pathways [3–5].
Diagnosis of childhood MDs is not straightforward. Phenotypic diagnosis only has limitations as many symptoms may have more than one underlying etiology and any particular pathophysiology can result in a complex combination of symptoms [6]]. Genetic diagnosis allows a comprehensive understanding of the underlying pathophysiology and provides specific treatment options [7–12].
Conventionally, genetic testing is done by sequential single gene Sanger sequencing. This is an ineffective method in diagnosing diseases like MDs due to its genetic heterogeneity. With the advent of next-generation sequencing (NGS), diagnosing strategies has changed to gene-panel based NGS or whole exome sequencing (WES). Neveling et al. performed a retrospective study comparing the diagnostic yield by Sanger sequencing and NGS in five different cohorts. For patients with movement disorders, the diagnostic yield is increase from 5% by Sanger sequencing only to 20% by WES with target gene panel analysis. This shows that NGS is a more superior diagnostic tool when compare to conventional Sanger sequencing [13].
The effectiveness by gene-panel based NGS study has been shown in subsequent studies. Van Egmond et al. performed a study in 61 dystonia patients with a panel of 94 genes, reaching a diagnostic yield of 14.8% [14]. Reale et al., Montaut et al. and Graziola et al. conducted three separated studies using panels with 65, 127 and 102 genes, giving a diagnostic yield of 11.3%, 22%, and 28% [15–17]. Although these three studies started in the same year (2015), the number of genes included in the analysis differs. Another study by Cordeiro et al.’s performed the study used targeted direct sequencing, targeted panel of dystonia, of epilepsy, and of cellular energetic NGS or WES. The diagnostic yield was 51%. Although they did not mention the number of genes included in each panel, from the result, the diagnoses were made majority in epilepsy panel or WES. Six diagnoses (CAMTA1, CTNNB1, KCNA2, SLC13A5, SLC9A6, mitochondrial ND3) would be missed as these genes were not included in other movement studies. This demonstrated WES is superior to targeted sequencing which is limited by the pre-selected gene panels that have to be frequently updated owing to discovery of new disease-associated genes [18].
In the present study, we performed WES in a cohort of 31 patients with paediatric-onset MDs to review the genetic causes and potential treatment implications. We aim to highlight the importance of genetic diagnosis in guiding a more effective clinical management of these disorders.
Results
Cohort description
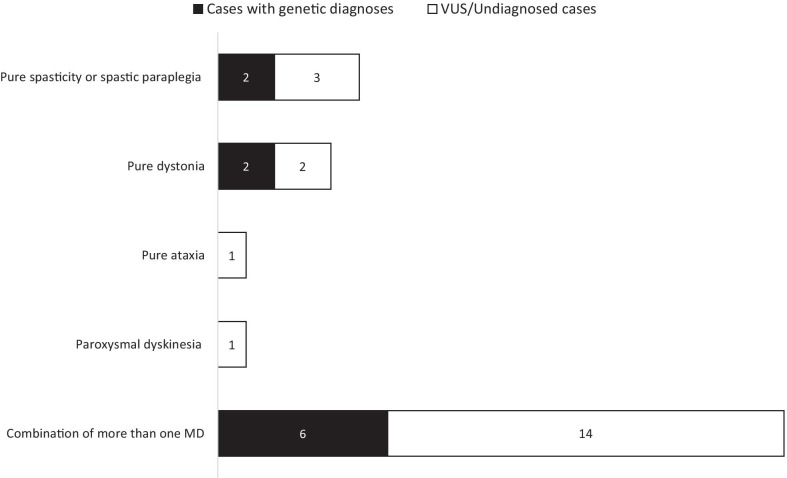
Clinical features of patients are summarized in Fig. 1, Table 1 and Additional file 1: Table 3. A total of 31 MDs patients were included in this study, in which 21 were males (68%) and ten were females (32%). Twenty-seven patients are Chinese (87%), while three patients (Patient 21, 22 and 30) are Pakistani and one patient (Patient 2) is African Chinese. Age of onset ranged from birth to 13 years of age. Five patients (16%) have pure spasticity or spastic paraplegia (SPG), four patients (13%) have pure dystonia, one patient (3%) has pure cerebellar ataxia, one patient (3%) has paroxysmal dyskinesia and 20 patients (65%) have a combination of more than one MDs. Dysmorphic features including microcephaly, and congenital anomalies including left atrophy kidney, duodenal atresia, pulmonary stenosis were seen in twelve patients (39%), and they are more common in patients with dystonia (9/18, 50%) when compared with other MDs patients (3/13, 23%). Abnormality in the Magnetic Resonance Imaging (MRI) of the brain were identified in fourteen patients (45%) (Table 1).
Fig. 1.
Graphical presentations of clinical and genetic outcome of patients
Table 1.
Clinical features and genetic diagnosis of patients with movement disorders
| Patient | Sex | Ethnicity | Movement disorders | Location | Clinical course | Onset | Dysmorphic features | Congenital anomalies | Other clinical features | Brain MRI features | aCGH findings | Variants found | Inherit- ance |
ACMG classification |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cases with pathogenic or likely pathogenic variants found | ||||||||||||||
| 12 | M | Chinese | Dystonia | Generalized |
Non- progressive |
Infancy | + | – | Mild ID, blue sclera, easy fracture | Normal | Not done | Heterozygous CTNNB1: c.367C > T, p.(Q123*); Heterozygous COL1A1: c.343G > T, p.(G115*) (incidental finding) | De novo | Pathogenic |
| 14 | M | Chinese | Spastic paraplegia with both upper limbs involvement | Generalized | Progressive | Infancy | – | – | – | Periventricular white matter change | Not done | Heterozygous SPAST: c.1253_1255delAAG, p.(Glu418del) | De novo | Pathogenic |
| 17 | M | Chinese | Dystonia, Choreoathetosis with status dystonicus | Generalized | Progressive | Infancy | – | – | Mild to moderate ID, Rhabdomyolysis | Normal | Not done | Heterozygous GNAO1: c.625C > T, p.(Arg209Cys) | Parents’ DNA not available | Likely pathogenic |
| 19 | M | Chinese | Spastic paraplegia | Lower limbs | Progressive | Early child- hood | – | – | Attention deficit hyperactivity disorder | Periventricular white matter changes with corpus callosum thinning | Not done | Compound heterozygous SPG11: c.4462_4463del, p.(V1488fs) & c.1569G > A, p.(W523*) | Paternal & Maternal | Likely pathogenic |
| 20 | M | Chinese | Rigidity with parkinsonism features, Spasticity, Paroxysmal worsening of parkinsonism | Generalized |
Non- progressive |
Birth | – | Pulmonary stenosis | Obstructive Sleep Apnoea Syndrome, Severe ID, laryngomalacia | Progressive cerebellar atrophy | Not done | Heterozygous ATP1A3: c.954C > G, p.(Ile318Met) | De novo | Pathogenic |
| 23 | M | Chinese | Dystonia | Generalized |
Non- progressive |
Infancy | + | – | Severe ID, Epilepsy | Normal | Normal | Heterozygous PURA: c.783C > G, p.(Tyr261*) | De novo | Pathogenic |
| 27 | M | Chinese | Cerebellar ataxia, spasticity | Generalized |
Non- progressive |
Infancy | – | – | Reactive hypoglycaemia, Mild ID | Normal | Not done | Heterozygous SLC2A1: c.388G > C, p.(Gly130Arg) # | De novo | Pathogenic |
| 29 | M | Chinese | Dystonia, Spasticity | Generalized | Progressive | 6y | – | Left atrophic kidney | Fatty liver, Recurrent patellar dislocation, prominent capillaries | Normal | Not done | Heterozygous KMT2B: c.2425C > T, p.(Gln809*) | De novo | Pathogenic |
| 30 | F | Pakistani | Cerebellar ataxia, spasticity, rigidity | Generalized | Progressive | 13y | – | – | Neuromuscular weakness | Periventricular white matter changes with corpus callosum thinning | Not done | Homozygous SPG11: c.5399_5402delinsTGGAGGAG:p.(Gln1800fs) | Paternal & Maternal | Likely pathogenic |
| 31 | M | Chinese | Dystonia, spasticity | Generalized | Progressive | Infancy | – | – | Bilateral hearing impairment, autism spectrum disorder, learning problem (formal IQ not available) | Normal | Not done |
Heterozygous ACTB: c.547C > T:p.(Arg183Trp) |
De novo | Pathogenic |
| Cases with variants of unknown significance (VUS) found | ||||||||||||||
| 1 | M | Chinese | Cerebellar ataxia | Generalized | Non-progressive | 6y | – | – | Limited intelligence with dementia, bipolar affective disorder | Stable cerebellar atrophy | Not done | Heterozygous KCND3: c.1917C > A, p.(Asn639Lys) | Maternal | VUS |
| 6 | M | Chinese | Dystonia, Spasticity | Generalized | Non-progressive | Birth | + | – | Mild ID, severe intrauterine growth retardation, autism spectrum disorder | Right parietal lobe developmental venous anomaly | Normal | Compound heterozygous VPS13D: c.5300C > T, p.(Thr1767Ile) & c.8213A > C, p.(Gln2738Pro) | Paternal & Maternal | VUS |
| 7 | F | Chinese | Dystonia, Spasticity | Generalized | Non-progressive | Birth | + | – | Mild ID to limited intelligence, intrauterine growth retardation | Dysgenesis of corpus callosum | Not done | Compound heterozygous VPS13D: c.5300C > T, p.(Thr1767Ile) & c.8213A > C, p.(Gln2738Pro) | Paternal & Maternal | VUS |
| 8 | F | Chinese | Cerebellar ataxia, Spasticity | Generalized | Non-progressive | Infancy | – | – | Mild ID with dementia, Ichthyosis | Progressive cerebellar atrophy | Not done | Heterozygous KCNC3: c.2105G > T, p.(Ser702Ile) | Paternal | VUS |
MRI Magnetic resonance imaging; ACMG American College of Medical Genetics, VUS variant of unknown significance, y years, ID intellectual disability; IQ intelligence quotient
#The family declined lumbar puncture
Diagnostic yield and genetic variants found
Singleton exome was performed in 19 subjects and trio exome was performed in 10 families. Patient 17 was initially recruited for WES, unfortunately his DNA was insufficient to proceed and he passed away suddenly. Sanger sequencing of the exons and splice junctions of the GNAO1 gene was performed for this patient due to a strong clinical suspicion for GNAO1 defect.
Genetic diagnoses were made in 10 patients (32%), among eight were by virtual gene panels analysis and two by open-exome analysis. Disease-causing variants were found in two patients with SPG (2/5, 40%) in SPAST and SPG11, six patients with have a combination of more than one MDs (6/20, 30%) in GNAO1, SLC2A1, KMT2B, SPG11, ATP1A3 and ACTB. The diagnosis made in two patients with pure dystonia (2/4, 50%) were by open exome analysis, one with PURA and one with CTNNB1 and COL1A1 representing one man two diseases. Four patients have variants of uncertain significance (VUS) associated with the phenotypes (Table 1, Fig. 1). Interestingly, patient 27 with a SLC2A1 mutation causing glucose transporter type 1 (GLUT1) deficiency syndrome, was initially suspected to have a neurometabolic disorder due to the presence of systemic hypoglycaemia, cerebellar ataxia, spasticity and mild intellectual disability. Subsequent endocrinological workup confirmed reactive hypoglycaemia. As the family refused lumbar puncture, GLUT1 deficiency was only diagnosed after WES.
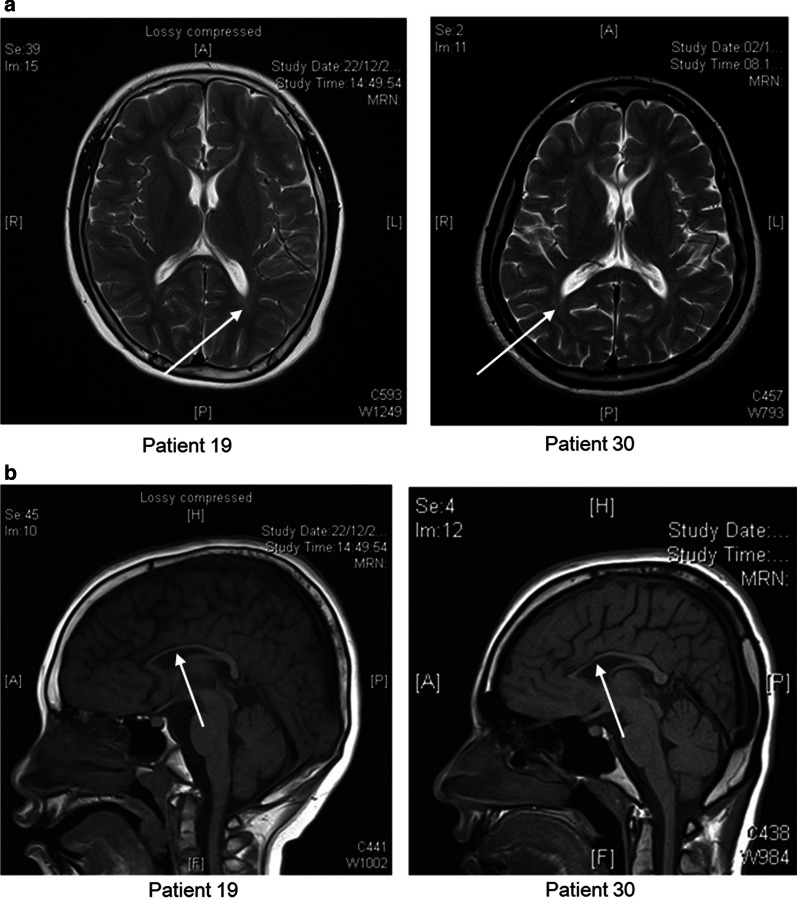
Among the 14 patients with abnormalities in neuroimaging, 4 (29%) had genetic causes identified. Neuroimaging for patients with SPG11 variants showed periventricular white matter changes and thinning of corpus callosum indicating that these features could be typical for SPG11 deficiency (Fig. 2).
Fig. 2.
Brain Magnetic Resonance Imaging (MRI) of 2 patients with variants identified in SPG11. a Brain MRI of Patient 19 and 30 with periventricular white matter changes; b brain MRI of Patient 19 and 30 with thinning of corpus callosum. Arrows indicated the area with periventricular white matter changes and thinning of corpus callosum
Clinical follow up with treatment implications
Among the 10 genetically diagnosed patients, 8 patients (80%) have potential treatment implications (Table 2). Patient 29 with KMT2B mutation received globus pallidus interna deep brain stimulation (GPi-DBS) with mild improvement in dystonia a few months after the surgery and more definitive effectiveness will be evaluated in the future. Patient 17 with GNAO1 mutation prescribed tetrabenazine and has been useful in controlling the significant dyskinesia before he passed away suddenly. Patient 30 with SPG11 did not show any response to L-dopa. For patient 19 with SPG11 and patient 31 ACTB mutation, Dopa and GPi-DBS was just started and planned, monitoring is required for treatment response. For patient 12 with CTNNB1 and COL1A1 mutation, Dopa was just started, and he has been referred to the endocrinologist for further management. Patient 20 and 27 decline the treatment offers.
Table 2.
Clinical management and Genotype-targeted treatment implications of patients with variants identified
| Patient | Movement disorders | Gene with variants found | Genotype-targeted treatment implication in literature | Treatment offered and clinical outcome/ follow up |
|---|---|---|---|---|
| 12 | Dystonia | CTNNB1 and COL1A1 |
(1) CTNNB1: L-dopa treatment [12] (2) COL1A1: Treatment to prevent bone fracture resulted from osteogenesis imperfecta |
Dopa treatment was offered but has not started yet Genetic finding explained the phenotypes of blue sclera and bone fractures in that patient who has been referred to the endocrinologist for further management |
| 17 | Dystonia, Choreoathetosis with status dystonicus | GNAO1 | Tetrabenazine, GPi-DBS [9] | Tetrabenazine was used with improvement before the patient passed away with sudden death |
| 19 | Spastic paraplegia | SPG11 | L-dopa and sapropterin treatment [8] | The patient had just commenced on Dopa treatment |
| 20 | Rigidity with parkinsonism features, Spasticity, Paroxysmal worsening of parkinsonism | ATP1A3 | Calcium channel blockers, ATP supplementation [26] | The treatment has been offered but declined by the patient |
| 27 | Cerebellar ataxia, spasticity | SLC2A1 | Ketogenic diets [27, 28] | The diet has been offered but the patient refused due to anticipated poor compliance |
| 29 | Dystonia, Spasticity | KMT2B | GPi-DBS [10, 30] | The patient has received GPi-DBS with mild improvement in dystonia a few months after the surgery and more definitive effectiveness will be evaluated in the future |
| 30 | Cerebellar ataxia, spasticity, rigidity | SPG11 | L-dopa and sapropterin treatment [8] | The patient has been checked to have low HVA in CSF and received Dopa replacement therapy without any clinical response |
| 31 | Dystonia, spasticity | ACTB | GPi-DBS [31–33] | Plan has been made for the patient to be evaluated for GPi-DBS |
GPi-DBS Globus pallidus interna deep brain stimulation, HVA homovanillic acid, CSF cerebrospinal fluid
Discussion
Our study includes paediatric-onset movement disorders with unrevealing etiologies after comprehensive investigations. Previous studies have investigated the genetic landscapes in cohorts with both adult and paediatric patients, or paediatric only by targeted NGS and/or WES [13–17, 19]. Diagnostic rates were relatively higher in cohorts with only paediatric patients and with the use of WES. Comparing with studies using targeted NGS approach with a diagnostic rate range from 11 to 28%, our study and Cordeiro et al.[19] with WES has a higher diagnostic rate of 32% and 51% respectively. Further looking into the diagnoses, two (2/10, 20%) and six (6/26, 23%) in our cohort and Cordeiro et al.’s cohort can be made by WES only. This shows WES is useful in making additional diagnoses in MDs patients (Table 3). Moreover, the inclusion criteria in this study are more stringent than the previous paediatric studies as patients with a clear neurometabolic phenotype which are subsequently confirmed with targeted gene sequencing were excluded. This illustrates that the diagnostic yield through WES is still considerable (32% in our study) after an initial comprehensive neurometabolic investigations.
Table 3.
Previous studies investigating underlying genetic causes in patient cohorts with movement disorders
| Neveling et al. [13] | Van Egmond et al. [14] | Reale et al. [17] | Montaut et al. [16] | Cordeiro et al. [19] | Graziola et al. [15] | The present study | |
|---|---|---|---|---|---|---|---|
| Country of patient recruitment | The Netherlands | The Netherlands | Italy | France, Luxembourg and Algeria | Canada | Italy | Hong Kong SAR, China |
| No. of patients with MDs | 50 | 61 (all with dystonia) | 221 | 378 | 51 | 148 | 31 |
| Onset age | Adult and paediatric | Adult and paediatric | Adult and paediatric | Adult and paediatric | Paediatric | Paediatric | Paediatric |
| No. of young-onset MDs | Not specified | 44 | Not specified | Not specified | 51 | 148 | 31 |
| Sequencing methods | Whole exome sequencing and target data analysis | Next generation sequencing and gene panel analysis | Targeted next generation sequencing | Targeted next generation sequencing | Targeted direct, targeted next generation or whole exome sequencing | Targeted next generation sequencing | Whole exome sequencing with both targeted and exome wide analysis. One variant was identified by Sanger sequencing |
| No. of genes in panel | 151 | 94 | 65 | 127 | – | 102 | – |
| Diagnostic yield | 20% (10/50) | 14.8% (9/61) | 11.31% (25/221) | 22% (83/378) | 51% (26/51) | 28% (42/148) | 32% (10/31) |
| Treatment implications | – | – | – | – | 38% of patients with a genetic diagnosis | – | 80% of patients with genetic diagnosis |
Better prediction of clinical courses
Genetic diagnoses allow the prediction of the subsequent clinical course. There is no definite difference in the clinical phenotype between the molecular positive and negative cases. Patient 14 and 17 have been initially diagnosed as cerebral palsy which is a static condition with non-progressive damage to the brain. Their neurological signs could have been overlooked if the clinical follow up was not over a prolonged period of time in terms of years. Patient 14 gradually developed progressive functional deterioration since 3 years of age from walking independently to requiring aids over 5 years with prominent spasticity over both lower limbs but minimally at the upper limbs. The neuroimaging was misleading due to the presence of periventricular leukomalacia which could be mistaken as perinatal insult. Patient 17 had an initially static course with mild generalized dystonia which evolved into recurrent status dystonicus and sudden death at 15 years old. Identification of the genetic etiologies in these patients directed more accurate predictions of the clinical course and prognosis including progressive lower limb spasticity for SPAST mutations and progressive MDs for GNAO1 encephalopathy with potential treatment implication according to previous case studies [20–24].
Potential genotype-targeted treatment implications
Treatments targeting specific genetic etiologies are significantly beneficial to patients’ prognosis. In our exome-positive cases, 80% (8/10) have potential treatment implications.
Five patients (with variants in SPG11, CTNNB1, GNAO1, ATP1A3 and SLC2A1) might be managed by conventional medical and / or surgical treatments. Previous studies in patients with spastic paraplegia 11 (SPG11) demonstrated neurotransmitter abnormalities in dopamine and tetrahydrobiopterin pathways [8]. In that study, all patients responded partially to L-dopa/carbidopa and sapropterin treatment and they suggested a trial of L-dopa/carbidopa and sapropterin treatment for extrapyramidal signs and symptoms of SPG11 even with normal neurotransmitter levels [8]. However, Patient 30 with SPG11 did not show any response to L-dopa despite the presence of secondary neurotransmitter deficiency in homovanillic acid before the molecular diagnosis was made. For CTNNB1 mutation, a recent case study reported a significant response to L-dopa treatment in a dystonic patient with a normal CSF neurotransmitter profile [12]. This response was possibly related to synaptic dopamine increase as a previous study suggested the role of beta-catenin in dopamine neurons development [25]. Treatment will be started for Patient 12. For GNAO1 encephalopathy, tetrabenazine was demonstrated to be the most effective drug for the management of involuntary movements [9]. This drug was useful in controlling the significant dyskinesia of Patient 17 before the molecular diagnosis was confirmed and he passed away suddenly. For ATP1A3-associated disorders, apart from the effective symptomatic treatment by calcium channel blockers, a recent study showed that adenosine-5′-triphosphate (ATP) supplementation in an alternating hemiplegia of childhood (AHC) patient had marked improvement in AHC episodes and psychomotor development [26]. Treatment was declined by Patient 20 with paroxysmal worsening of his parkinsonism features. In addition to medical management, ketogenic diet (KD) was proved to be a very effective therapy as first line treatment for GLUT1 deficiency syndrome, which is associated with SLC2A1 mutation, and should be started in early disease stage [27, 28]. It is a high-fat diet that produces ketone bodies serving as an alternative energy source for brain metabolism and bypassing the GLUT defect [28, 29]. It was reported that KD could help in development and restore mental decline [29]. Unfortunately, KD was declined by Patient 27 due to anticipated poor compliance to the diet.
Three out of 10 patients (with GNAO1, ACTB and KMT2B variants) could be considered for surgical interventions when medical therapies for dystonia fail. Patients with KMT2B-dystonia in previous studies showed good responses clinically after undergoing GPi-DBS especially for paediatric and adolescent patients [10, 30]. Patient 29 with KMT2B mutation, being medically intractable with 4 anti-dystonic medications (gabapentin, clonazepam, carbamazepine, trihexyphenidyl), received GPi-DBS after confirming the molecular diagnosis with mild improvement in dystonia a few months after the surgery and more definitive effectiveness will be further evaluated in the future. GPi-DBS was also demonstrated to have beneficial effect in ACTB-associated dystonia-deafness syndrome. In previous studies, four patients with the same mutation (p.Arg183Trp) showed substantial clinical improvement after GPi-DBS [31–33]. Therefore, patient 31 with ACTB mutation will be planned for GPi-DBS as the dystonia fails to improve on 5 medications (baclofen, trihexyphenidyl, L-dopa, clonazepam, gabapentin). For GNAO1 encephalopathy, although tetrabenazine was demonstrated to be the most effective drug, emergency GPi-DBS was shown to be helpful for those patients with hyperkinetic exacerbations [9]. Furthermore, dissection of phenotype-genotype correlation suggested that different GNAO1 mutations affect the G protein function for the signaling loop in distinct ways that implicated different treatment options [11]. This suggested possible application of precision medicine for different GNAO1 variants identified in the patients.
Most of the previous studies in MDs patient cohorts had not investigated the therapeutic potentials based on the genetic diagnosis. Only the study of Cordeiro et al. [19] suggested that 38% of their patients with genetic diagnoses had treatment implications (Table 3), while our study has a much higher percentage of 80%. With the growth of new treatment strategies emerging in recent years, genetic testing becomes even more crucial to direct genotype-targeted therapies in MDs.
Conclusions
Given the diagnostic yield of 32% in our patient cohort and clinical treatment implications in 80% of the molecularly diagnosed cases, WES is a valuable tool for molecular investigation in paediatric-onset MDs with unrevealing, comprehensive neurometabolic workup especially aiming for potentially treatable inborn metabolic diseases. This study demonstrated that identification of genetic etiologies of MDs allows a more accurate prediction of clinical course and guides the use of potential therapies for better clinical outcomes. As such, there is an increasing potential to develop precision medicine for treatment of MDs.
Methods
Patient cohort
The study was conducted in Queen Mary Hospital and Duchess of Kent Children’s Hospital, two affiliated hospitals of The University of Hong Kong (HKU). Over 4 years (2016–2019), 140 patients who were followed up longitudinally in a specialized and tertiary neurometabolic / movement disorder clinic were examined. The inclusion criterion was the diagnosis of a paediatric-onset (≤ 18 years of age) MD or combination of MDs including chorea, athetosis, dystonia, tremor, myoclonus, parkinsonism, cerebellar ataxia and spasticity as the main clinical sign(s) with unrevealing etiologies after extensive investigations. Such investigations included neuroimaging studies (Magnetic Resonance Imaging of the brain) and neurometabolic workup such as blood for lactate, gas, ammonia, amino acid, acylcarnitine profile, cholestanol, creatine, guanidinoacetate, lipid profile, vacuolated lymphocytes, lysosomal enzymes, biotinidase, copper, caeruloplasmin, very long chain fatty acids, pristanic and phytanic acids, vitamin E, total homocysteine, manganese, urate, iron profile; and urine for amino acid, organic acid, creatine, guanidinoacetate, purine and pyrimidines, oligosaccharides and glycosaminoglycans. Cerebrospinal fluid (CSF) for routine microscopy, glucose, protein, amino acids, lactate and neurotransmitter profiling were performed in 23 patients (74%). The reasons for not performing lumbar puncture include decline by the families or the results of WES were already available. Targeted gene sequencing was performed for patients with a clearly abnormal biochemical and / or radiological phenotype suggestive of a neurometabolic disorder. The exclusion criterion was disorders with acquired or other secondary causes such as cerebral palsy (CP) with a clear history of brain insult, malformation of cortical development, or brain tumors. A cohort of 31 patients from 30 families (patient 6 and 7 are siblings) was finally recruited into our present study.
Genetic analyses
Genomic DNA were extracted from peripheral blood using Flexigene DNA Kit (Qiagen GmbH, Germany). Quality of genomic DNA was evaluated by agarose gel analysis and quantity was measured by Qubit® dsDNA assay (Thermo Fisher Scientific, Waltham, MA).
WES was performed in Genome Diagnostics Nijmegen and our local setting (HKU). WES and the data analysis in Genome Diagnostic Nijmegen were performed as described previously [13]. In our local setting, WES was performed as described in our previous study [34]. Exome libraries preparation and quality control were performed according to the manufacturer instructions. The libraries were sequenced by Illumina HiSeq 1500 or NextSeq 500 sequencing platform with a targeted sequencing coverage of 100x. Data processing has been done by our in-house developed bioinformatics pipeline. Briefly, filtered raw reads were mapped to the reference human genome (GRCh37/hg19) by Burrow-Wheeler Aligner (BWA) 0.7.10. Genome Analysis Toolkit (GATK) best practices v3.4-46 was used for variant calling by HaplotypeCaller and the variants were annotated by Annotate Variation (ANNOVAR). First-tier variant analysis was based on a virtual gene panel consist of 272 movement disorder-related genes (Additional file 1: Table 1), 244 mitochondrial disease-related genes (Additional file 1: Table 2) and genes in MitoCarta 2.0 encoding protein with strong support of mitochondrial localization (http://www.broadinstitute.org/pubs/MitoCarta). Even though patients with clinical suspicion of a mitochondrial disorders were excluded, the analysis also included mitochondrial-related genes as the phenotypic presentation for both movement and mitochondrial disorders are highly overlapping. If pathogenic variant(s) could not be identified in these genes, open-exome analysis will be performed. Some of the data was jointly analysis by collaborators at Yale University. Raw WES data from HKU was transferred. Reads were aligned to hg19 reference genome using bwa-mem and processed according to the GATK best practice guidelines. Copy-number variants (CNV) were called using gcnv (part of GATK4). The variants were annotated using Variant Effect Predictor through Hail and then uploaded to seqr (https://seqr.broadinstitute.org/) for analysis. Rare variants were assessed for pathogenicity based on the American College of Medical Genetics (ACMG) guideline [35]. Potential disease-causing variants and segregation analysis were performed by Sanger sequencing.
Supplementary information
Additional file 1: Supplementary table 1: 272 movement disorder-related genes; Supplementary table 2: 244 mitochondrial disease-related genes; Supplementary table 3: Clinical features of patients with no genetic variant found.
Acknowledgements
We would like to thank the Society for the Relief of Disabled Children, the Joshua Hellmann Foundation for Orphan Disease and the Edward and Yolanda Wong Fund for the support.
Abbreviations
- MDs
Movement disorders
- NGS
Next-generation sequencing
- WES
Whole exome sequencing
- SPG
Spastic paraplegia
- MRI
Magnetic resonance imaging
- VUS
Variants of uncertain significance
- GPi-DBS
Globus pallidus interna deep brain stimulation
- ATP
Adenosine-5′-triphosphate
- AHC
Alternating hemiplegia of childhood
- KD
Ketogenic diet
Authors’ contributions
AKYK and MHYT contributed equally to the manuscript. AKYK and MHYT involved in data acquisition, data analysis, drafting the manuscript. JLFF analyzed the data and revised the manuscript. CCYM, KLSC and JS revised the manuscript. RJTR, ML, SH, SP analyzed the data. MMY, CT, SF, KTL, CKM, EKCY, SMT, ELWF, NSPW and LYT involved in patient recruitment and management. SW involved in patient recruitment, management and revised the manuscript. BHYC and CWF designed and conceptualized study, involved in patient recruitment and management, critically reviewed the manuscript with suggestions for improvement. All of the authors reviewed and approved the final manuscript.
Funding
We would like to acknowledge the Society for the Relief of Disabled Children, the Joshua Hellmann Foundation for Orphan Disease and the Edward and Yolanda Wong Fund for donations to financially support this study.
Availability of data and materials
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Ethics approval and consent to participate
This study has been approved by the Institutional Review Board of the Hong Kong West Cluster and the University of Hong Kong (IRB Ref. Nos.: UW 11-190 and UW 12-221). Written informed consent was obtained from the patients and/or parents.
Consent for publication
Consent was obtained from all patients for publication.
Competing interests
Anna Ka-Yee KWONG, Mandy Ho-Yin TSANG, Jasmine Lee-Fung FUNG, Christopher Chun-Yu Mak, Kate Lok-San Chan, Richard J.T. RODENBURG, Monkol LEK, Shushu HUANG, Sander PAJUSALU, Man-Mut YAU, Cheung TSOI, Sharon FUNG, Kam-Tim LIU, Che-Kwan MA, Sheila Wong, Eric Kin-Cheong YAU, Shuk-Mui TAI, Eva Lai-Wah FUNG, Nick Shun-Ping WU, Li-Yan TSUNG, Brian Hon-Yin CHUNG, Cheuk-Wing FUNG report no conflict of interest. Jan SMEITINK is the CEO of Khondrion, a pharmaceutical company developing compounds to potentially treat mitochondrial disease.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Brian Hon-Yin Chung, Email: bhychung@hku.hk.
Cheuk-Wing Fung, Email: fcw1209m@hku.hk.
Supplementary information
The online version contains supplementary material available at 10.1186/s13023-021-01688-6.
References
- 1.Sanger TD, Chen D, Fehlings DL, Hallett M, Lang AE, Mink JW, et al. Definition and classification of hyperkinetic movements in childhood. Mov Disord. 2010;25(11):1538–1549. doi: 10.1002/mds.23088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Garcia-Cazorla A, Duarte ST. Parkinsonism and inborn errors of metabolism. J Inherit Metab Dis. 2014;37(4):627–642. doi: 10.1007/s10545-014-9723-6. [DOI] [PubMed] [Google Scholar]
- 3.Elert-Dobkowska E, Stepniak I, Krysa W, Ziora-Jakutowicz K, Rakowicz M, Sobanska A, et al. Next-generation sequencing study reveals the broader variant spectrum of hereditary spastic paraplegia and related phenotypes. Neurogenetics. 2019;20(1):27–38. doi: 10.1007/s10048-019-00565-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nibbeling EA, Delnooz CC, de Koning TJ, Sinke RJ, Jinnah HA, Tijssen MA, et al. Using the shared genetics of dystonia and ataxia to unravel their pathogenesis. Neurosci Biobehav Rev. 2017;75:22–39. doi: 10.1016/j.neubiorev.2017.01.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Synofzik M, Schule R. Overcoming the divide between ataxias and spastic paraplegias: shared phenotypes, genes, and pathways. Mov Disord. 2017;32(3):332–345. doi: 10.1002/mds.26944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mink JW, Sanger TD, et al. 93. Movement disorders: an overview. In: Swaiman KF, Ashwal S, Ferriero DM, Schor NF, Finkel RS, Gropman AL, et al., editors. Swaiman's pediatric neurology. 6. New York: Elsevier; 2017. pp. 706–717. [Google Scholar]
- 7.Bushart DD, Chopra R, Singh V, Murphy GG, Wulff H, Shakkottai VG. Targeting potassium channels to treat cerebellar ataxia. Ann Clin Transl Neurol. 2018;5(3):297–314. doi: 10.1002/acn3.527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vanderver A, Tonduti D, Auerbach S, Schmidt JL, Parikh S, Gowans GC, et al. Neurotransmitter abnormalities and response to supplementation in SPG11. Mol Genet Metab. 2012;107(1–2):229–233. doi: 10.1016/j.ymgme.2012.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Danti FR, Galosi S, Romani M, Montomoli M, Carss KJ, Raymond FL, et al. GNAO1 encephalopathy. Broadening the phenotype and evaluating treatment and outcome. Neurol Genet. 2017;3(2):e143. doi: 10.1212/NXG.0000000000000143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Meyer E, Carss KJ, Rankin J, Nichols JM, Grozeva D, Joseph AP, et al. Mutations in the histone methyltransferase gene KMT2B cause complex early-onset dystonia. Nat Genet. 2017;49(2):223–237. doi: 10.1038/ng.3740. [DOI] [PubMed] [Google Scholar]
- 11.Mihalek I, Park M, Kelly M, Waugh JL, Poduri A, Bodamer O. Molecular map of GNAO1-related disease phenotypes and reactions to treatment. bioRxiv. 2017:232058.
- 12.Pipo-Deveza J, Fehlings D, Chitayat D, Yoon G, Sroka H, Tein I. Rationale for dopa-responsive CTNNB1/ss-catenin deficient dystonia. Mov Disord. 2018;33(4):656–657. doi: 10.1002/mds.27320. [DOI] [PubMed] [Google Scholar]
- 13.Neveling K, Feenstra I, Gilissen C, Hoefsloot LH, Kamsteeg EJ, Mensenkamp AR, et al. A post-hoc comparison of the utility of sanger sequencing and exome sequencing for the diagnosis of heterogeneous diseases. Hum Mutat. 2013;34(12):1721–1726. doi: 10.1002/humu.22450. [DOI] [PubMed] [Google Scholar]
- 14.van Egmond ME, Lugtenberg CHA, Brouwer OF, Contarino MF, Fung VSC, Heiner-Fokkema MR, et al. A post hoc study on gene panel analysis for the diagnosis of dystonia. Mov Disord. 2017;32(4):569–575. doi: 10.1002/mds.26937. [DOI] [PubMed] [Google Scholar]
- 15.Graziola F, Garone G, Stregapede F, Bosco L, Vigevano F, Curatolo P, et al. Diagnostic yield of a targeted next-generation sequencing gene panel for pediatric-onset movement disorders: a 3-year cohort study. Front Genet. 2019;10:1026. doi: 10.3389/fgene.2019.01026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Montaut S, Tranchant C, Drouot N, Rudolf G, Guissart C, Tarabeux J, et al. Assessment of a targeted gene panel for identification of genes associated with movement disorders. JAMA Neurol. 2018;75(10):1234–1245. doi: 10.1001/jamaneurol.2018.1478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Reale C, Panteghini C, Carecchio M, Garavaglia B. The relevance of gene panels in movement disorders diagnosis: a lab perspective. Eur J Paediatr Neurol. 2018;22(2):285–291. doi: 10.1016/j.ejpn.2018.01.013. [DOI] [PubMed] [Google Scholar]
- 18.Seleman M, Hoyos-Bachiloglu R, Geha RS, Chou J. Uses of next-generation sequencing technologies for the diagnosis of primary immunodeficiencies. Front Immunol. 2017;8:847. doi: 10.3389/fimmu.2017.00847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cordeiro D, Bullivant G, Siriwardena K, Evans A, Kobayashi J, Cohn RD, et al. Genetic landscape of pediatric movement disorders and management implications. Neurol Genet. 2018;4(5):e265. doi: 10.1212/NXG.0000000000000265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tucci V, Kleefstra T, Hardy A, Heise I, Maggi S, Willemsen MH, et al. Dominant beta-catenin mutations cause intellectual disability with recognizable syndromic features. J Clin Investig. 2014;124(4):1468–1482. doi: 10.1172/JCI70372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Solowska JM, Baas PW. Hereditary spastic paraplegia SPG4: what is known and not known about the disease. Brain J Neurol. 2015;138(Pt 9):2471–2484. doi: 10.1093/brain/awv178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ananth AL, Robichaux-Viehoever A, Kim YM, Hanson-Kahn A, Cox R, Enns GM, et al. Clinical course of six children with GNAO1 mutations causing a severe and distinctive movement disorder. Pediatr Neurol. 2016;59:81–84. doi: 10.1016/j.pediatrneurol.2016.02.018. [DOI] [PubMed] [Google Scholar]
- 23.Jiang M, Bajpayee NS. Molecular mechanisms of go signaling. Neurosignals. 2009;17(1):23–41. doi: 10.1159/000186688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kuechler A, Willemsen MH, Albrecht B, Bacino CA, Bartholomew DW, van Bokhoven H, et al. De novo mutations in beta-catenin (CTNNB1) appear to be a frequent cause of intellectual disability: expanding the mutational and clinical spectrum. Hum Genet. 2015;134(1):97–109. doi: 10.1007/s00439-014-1498-1. [DOI] [PubMed] [Google Scholar]
- 25.Tang M, Miyamoto Y, Huang EJ. Multiple roles of beta-catenin in controlling the neurogenic niche for midbrain dopamine neurons. Development (Cambridge, England) 2009;136(12):2027–2038. doi: 10.1242/dev.034330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ju J, Hirose S, Shi XY, Ishii A, Hu LY, Zou LP. Treatment with oral ATP decreases alternating hemiplegia of childhood with de novo ATP1A3 Mutation. Orphanet J Rare Dis. 2016;11(1):55. doi: 10.1186/s13023-016-0438-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wei Z, Wang L, Deng Y. Treatment of myoclonic-atonic epilepsy caused by SLC2A1 de novo mutation with ketogenic diet: a case report. Medicine. 2019;98(18):e15428. doi: 10.1097/MD.0000000000015428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Koch H, Weber YG. The glucose transporter type 1 (Glut1) syndromes. Epilepsy & behavior : E&B. 2019;91:90–93. doi: 10.1016/j.yebeh.2018.06.010. [DOI] [PubMed] [Google Scholar]
- 29.Klepper J, Diefenbach S, Kohlschutter A, Voit T. Effects of the ketogenic diet in the glucose transporter 1 deficiency syndrome. Prostaglandins Leukot Essent Fatty Acids. 2004;70(3):321–327. doi: 10.1016/j.plefa.2003.07.004. [DOI] [PubMed] [Google Scholar]
- 30.Zech M, Boesch S, Maier EM, Borggraefe I, Vill K, Laccone F, et al. Haploinsufficiency of KMT2B, encoding the lysine-specific histone methyltransferase 2B, results in early-onset generalized dystonia. Am J Hum Genet. 2016;99(6):1377–1387. doi: 10.1016/j.ajhg.2016.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Eggink H, van Egmond ME, Verschuuren-Bemelmans CC, Schonherr MC, de Koning TJ, Oterdoom DL, et al. Dystonia-deafness syndrome caused by a beta-actin gene mutation and response to deep brain stimulation. Mov Disord. 2017;32(1):162–165. doi: 10.1002/mds.26842. [DOI] [PubMed] [Google Scholar]
- 32.Skogseid IM, Rosby O, Konglund A, Connelly JP, Nedregaard B, Jablonski GE, et al. Dystonia-deafness syndrome caused by ACTB p.Arg183Trp heterozygosity shows striatal dopaminergic dysfunction and response to pallidal stimulation. J Neurodev Disord. 2018;10(1):17. doi: 10.1186/s11689-018-9235-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zech M, Jech R, Wagner M, Mantel T, Boesch S, Nocker M, et al. Molecular diversity of combined and complex dystonia: insights from diagnostic exome sequencing. Neurogenetics. 2017;18(4):195–205. doi: 10.1007/s10048-017-0521-9. [DOI] [PubMed] [Google Scholar]
- 34.Tsang MH, Leung GK, Ho AC, Yeung KS, Mak CC, Pei SL, et al. Exome sequencing identifies molecular diagnosis in children with drug-resistant epilepsy. Epilepsia Open. 2019;4(1):63–72. doi: 10.1002/epi4.12282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Richards S, Aziz N, Bale S, Bick D, Das S, Gastier-Foster J, et al. Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet Med. 2015;17(5):405–424. doi: 10.1038/gim.2015.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Supplementary table 1: 272 movement disorder-related genes; Supplementary table 2: 244 mitochondrial disease-related genes; Supplementary table 3: Clinical features of patients with no genetic variant found.
Data Availability Statement
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.