Abstract
Background
Osteopontin plays critical roles in osteoarthritis (OA) by regulating the functions of osteoclasts. It is known that osteopontin can induce the expression of lncRNA HOX transcript antisense RNA (HOTAIR), indicating the involvement of HOTAIR in OA. This study was carried out to investigate the role of HOTAIR in OA.
Methods
Synovial fluid was extracted from both OA patients (n = 58) and healthy controls (n = 58). Expression of osteopontin and HOTAIR in synovial fluid was determined by RT-qPCR. Osteopontin was used to treat chondrocytes at dosages of 0, 1, 5 and 10 µg/ml, followed by measurement of HOTAIR expression by RT-qPCR. The role of osteopontin and HOTAIR overexpression, as well as HOTAIR knockdown in regulating the proliferation of chondrocytes was analyzed by cck-8 assay.
Results
HOTAIR was upregulated in OA. A positive correlation between HOTAIR and osteopontin was observed. In the primary chondrocytes, osteopontin treatment increased HOTAIR expression, while HOTAIR overexpression and knockdown failed to significantly affect osteopontin expression. In addition, osteopontin and HOTAIR overexpression increased chondrocyte proliferation, while HOTAIRE knockdown decreased chondrocyte proliferation. In addition, HOTAIR knockdown reduced the effects of osteopontin treatment on cell proliferation.
Conclusions
Osteopontin-induced HOTAIR expression is involved in osteoarthritis by regulating cell proliferation.
Keywords: Osteopontin, HOTAIR, Osteoarthritis, Chondrocyte, Proliferation
Background
Osteoarthritis (OA) is the most frequently diagnosed arthritis among middle-age to elderly people. OA occurs during the breaks of cartilage that protects or cushions the ends of bones [1, 2]. Aging is the most common cause of primary osteoarthritis. With the increased use of joints, damaged cartilages will be lead to swelling and joint pain [3]. It is estimated that more than 10% of males and 13% of females aged over 60 years are suffering from OA [4]. At present, there is no cure for OA, and medications mainly aim to relieve osteoarthritis symptoms, such as primarily pain [5]. Therefore, novel and more effective therapeutic approaches are still needed. However, molecular pathogenesis of OA remains hardly known, therefore the development of treatment strategies is limited [6, 7].
It has been well established that the initiation and progression of OA require the involvement of multiple molecular players [8, 9]. For instance, osteopontin, also known as Secreted Phosphoprotein 1 (SPP1), is a multifunctional phosphoprotein that is secreted by multiple cells types, such as chondrocytes and osteoblasts, is upregulated in OA and induces the production of matrix metalloproteinase 13 to promote the degradation of type II collagen, which is the major component of cartilage matrix [10, 11]. Therefore, osteopontin is considered as a target for the treatment of OA [10, 11]. Besides that, long non-coding RNAs (lncRNAs) also play crucial roles in OA by regulating OA-related gene expression [12]. In a recent study, osteopontin was reported to regulate lncRNA HOX transcript antisense RNA (HOTAIR) expression in cancer cells [13], suggesting the potential involvement of the interaction between osteopontin and HOTAIR in cancer. It is known that HOTAIR is overexpressed in OA, suppresses WIF-1 expression and activates Wnt pathway to promote OA progression [14]. This study was therefore carried out to explore the potential interaction between osteopontin and HOTAIR in OA.
Methods
Research subjects
From May 2018 to June 2019, 58 OA patients (23 males and 35 females, 52 to 70 years, 61.2 ± 5.7 years) and 58 healthy controls (23 males and 35 females, 52 to 70 years, 61.3 ± 5.9 years) were enrolled at Chinese Medicine Hospital of Xinjiang Uygur Autonomous Region. This study was approved by the Research Ethics Committee of Chinese Medicine Hospital of Xinjiang Uygur Autonomous Region and was in line with the Helsinki Declaration. Inclusion criteria: (1) newly diagnosed OA patients; (2) patients willing to donate synovial fluid. Exclusion criteria: (1) patients complicated with other clinical disorders; (2) recurrent OA; (3) initiated therapy within 3 months prior to admission. The diagnosis of OA was performed by both X-ray and joint fluid analysis. All healthy controls received systemic physiological exam at aforementioned hospital, and all physiological functions were normal. Written informed consent was provided by all patients and controls.
Synovial fluid and chondrocytes
Synovial fluid (1-1.5 ml) was extracted from the affected site of 58 OA patients (32 cases of knee and 26 cases of hip) before the initiation of therapy. Collection of synovial fluid samples was performed using a syringe under local anesthesia. Synovial fluid (1-1.5 ml) was also extracted from the knee of 32 healthy controls and the hip of 26 healthy controls to match OA group. All synovial fluid samples were immediately transferred to a liquid nitrogen sink for long-term storage.
Cell model used in this study were primary chondrocytes from OA patients (402OA-05A, Sigma-Aldrich, USA). Chondrocytes were cultivated with Chondrocyte Growth Medium (PromoCell) in a 5% CO2 incubator at 37 °C. Subculture was performed at a ratio of 1:8. Cells used in subsequent experiments were collected at passage 1–2. In cases of osteopontin treatment, osteopontin (Sigma-Aldrich) was used to treat chondrocytes at dosages of 0, 1, 5 and 10 µg/ml for 48 h before use.
Cell transfections
Osteopontin and HOTAIR backbone expressing vectors were constructed with pcDNA3.1 vector (Invitrogen). Negative control (NC) siRNA and HOTAIR siRNA were also purchased from Invitrogen. Vector (1 µg) or siRNA (40 nM) was transfected into 108 chondrocytes using lipofectamine 2000 (Invitrogen). Empty vector or NC miRNA transfection was performed to serve as NC, and control (C) cells were untransfected cells. Subsequent experiments were performed 48 h later.
ELISA
Levels of osteopontin in cell culture medium were measured using Osteopontin Human ELISA Kit (Catalog # BMS2066, Invitrogen). The analytical sensitivity was 0.26 ng/mL, and assay range was 0.47-30 ng/mL.
RNA preparations
Isolation of RNA from both synovial fluid and chondrocytes was performed using RNAzol reagent (Invitrogen), followed by genomic DNA removal performed by incubating all RNA samples with DNase I for 2 h at 37 °C. To determine RNA integrity, RNAs were separated using 5% Urine-PAGE gel. To determine RNA purity, OD 260 and 280 ratios were measured, and 260/280 ratio was calculated.
RT-qPCR
RNA samples with a 260/280 ratio close to 2.0 were revere transcribed into cDNA samples using ReverTra Ace™ qPCR RT Master Mix (TOYOBO). With cDNA samples as template, SYBR Premix Ex TaqTM II (Takara, Japan) was used to perform qPCRs to measure the mRNA levels of osteopontin and HOTAIR expression. GAPDH was used as internal control of all qPCRs. Three technical replicates were included in each experiment, and 2−ΔΔCt method was used to normalize mRNA expression levels of osteopontin and HOTAIR to GAPDH. Primer sequences were: forward, 5’-GGAAAGATCCAAATGGGACCA-3’ and reverse, 5’-CTAGGAATCAGCACGAAGCAAA-3’ for HOTAIR; forward, 5’-GTCTCCTCTGACTTCAACAGC-3’ and reverse, 5’-ACCACCCTGTTGCTGTAGCCA-3’ for GAPDH; forward, 5’-GCAGTCATCCTTCTCTCAGT-3’ and reverse, 5’-GTATGCAGTAGCTTGTTACTT-3’ for GAPDH.
Cell Counting Kit 8 (CCK-8) assay
Proliferation of chondrocytes in different transfection groups was analyzed by performing CCK-8 assay using a kit (ab228554, Abcam). Chondrocytes were transferred to a 96-well plate (5000 cells in 0.1 ml per well) and cells were cultivated at 37 °C. CCK-8 solution was added to reach 10% at 1.5 h before the measurement of OD values at 450 nm, which was performed every 24 h for a total of 4 days.
Statistical analysis
PCRs were performed in three technical replicates, and all other experiments were performed in three independent biological replicates. Mean ± SD values were calculated. The difference between OA and control groups was explored by unpaired t test. One-way ANOVA and Tukey’s test were used to explore differences among multiple groups. Linear regression was used to compare multiple groups. Correlations were explored by linear regression. p < 0.05 was considered statistically significant.
Results
HOTAIR and osteopontin were upregulated in OA
Synovial fluid samples isolated from both OA patients (n = 58) and healthy controls (n = 58) were subjected to RNA isolation and RT-qPCR to measure the mRNA expression levels of HOTAIR and osteopontin. Compared with Control group, the mRNA expression of HOTAIR(Fig. 1a, p < 0.01) and osteopontin (Fig. 1b, p < 0.01) was significantly upregulated in OA group. Therefore, our data suggested that upregulated expression of HOTAIR and osteopontin may involve in OA.
Fig. 1.
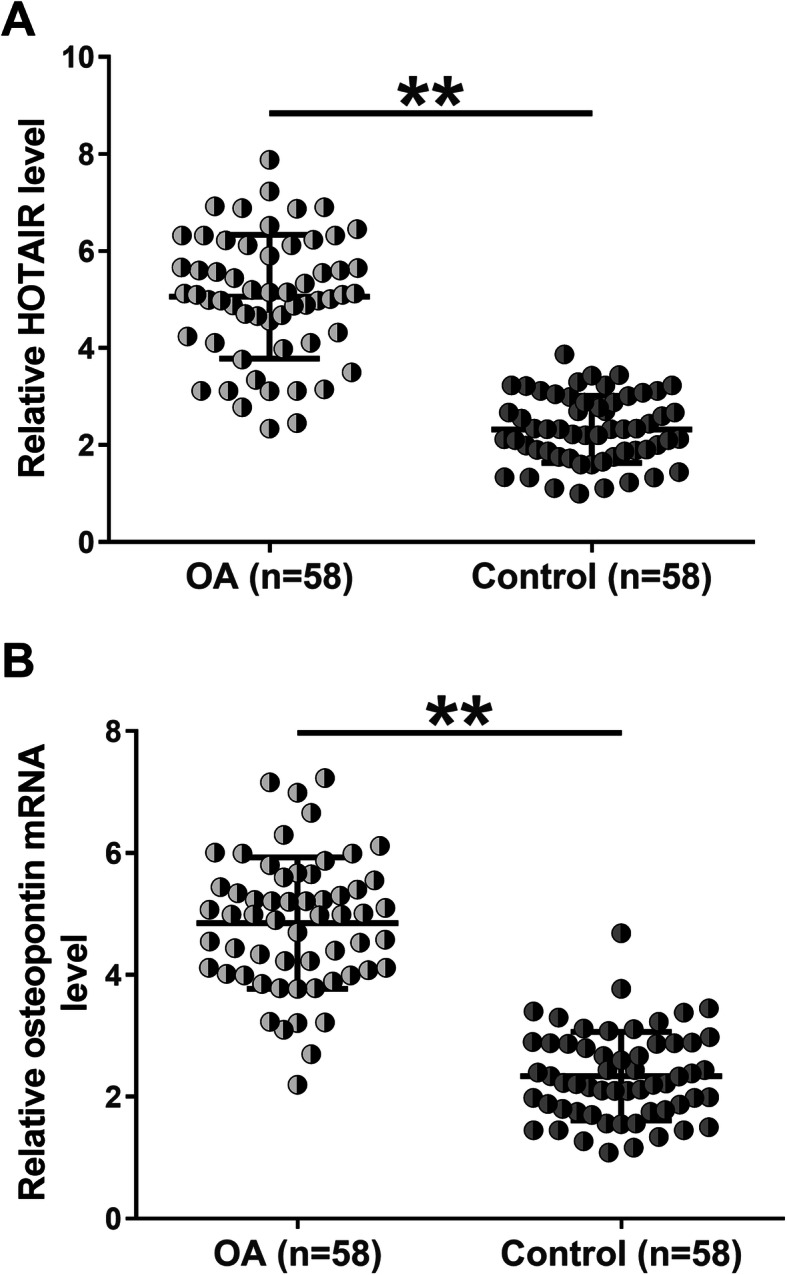
HOTAIR and osteopontin were upregulated in OA. Synovial fluid isolated from both OA patients (n = 58) and healthy controls (n = 58) was subjected to RNA isolation and RT-qPCR to measure the mRNA expression of HOTAIR and osteopontin. qPCRs were performed in triplicate and mean values were presented. **,p < 0.01
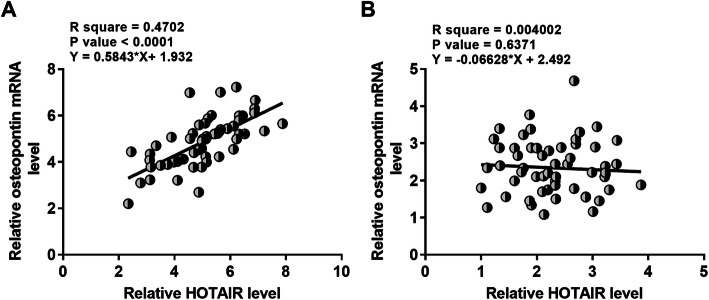
HOTAIR and osteopontin were positively correlated across OA patients
Correlation between HOTAIR and osteopontin mRNA across synovial fluid samples from both OA patients (Fig. 2a) and healthy controls (Fig. 2b) was analyzed by performing linear regression analysis. The results revealed a significant and positive correlation between HOTAIR and osteopontin mRNA across synovial fluid samples from OA patients, but not from healthy controls.
Fig. 2.
HOTAIR and osteopontin were positively correlated across OA patients. Correlations between HOTAIR and osteopontin mRNA across synovial fluid samples from both OA patients (a) and healthy controls (b) were analyzed by performing linear regression analysis
Osteopontin induced the upregulation of HOTAIR expression in chondrocytes from OA patients
The significant correlation between HOTAIR and osteopontin in OA patients suggested the potential interaction between them in OA. To explore the interaction between them, osteopontin was used to treat chondrocytes at dosages of 0, 1, 5 and 10 µg, followed by measuring expression of HOTAIR by RT-qPCR. It was observed that osteopontin treatment upregulated HOTAIR expression in a dose-dependent manner (Fig. 3a, p < 0.05). To test whether HOTAIR can also regulate osteopontin, HOTAIR overexpression and knockdown was achieved by transfecting HOTAIR expression vector or siRNA into chondrocytes, followed by confirmation of transfections by RT-qPCR (Fig. 3b, p < 0.05). Compared with C and NC groups, HOTAIR overexpression and knockdown failed to significantly affect the expression of osteopontin (Fig. 3c). ELISA data also showed that HOTAIR overexpression and knockdown failed to significantly affect the secretion of osteopontin from chondrocytes (Fig. 3d). Therefore, osteopontin is likely an upstream inducer of HOTAIR expression.
Fig. 3.
Osteopontin induced the upregulation of HOTAIR in chondrocytes from OA patients. To explore the interaction between them, osteopontin was used to treat chondrocytes at dosages of 0, 1, 5 and 10 µg/ml, followed by measuring the expression of HOTAIR by RT-qPCR (a). To test whether HOTAIR can also regulate osteopontin, HOTAIR overexpression and knockdown was achieved by transfecting HOTAIR expression vector or siRNA into chondrocytes, followed by confirmation of transfections by RT-qPCR (b). The effects of HOTAIR overexpression and knockdown on the expression of osteopontin were analyzed by RT-qPCR (c) and ELISA (d). All experiments were repeated 3 times and mean ± SD values
Osteopontin promoted the proliferation of chondrocytes through HOTAIR
The role of osteopontin and HOTAIR overexpression and knockdown in regulating the proliferation of chondrocytes was analyzed by performing CCK-8 assay. It was observed that osteopontin and HOTAIR overexpression promoted the proliferation of chondrocytes, while HOTAIRE knockdown inhibited the proliferation of chondrocytes. In addition, HOTAIR knockdown reduced the effects of osteopontin treatment on cell proliferation (Fig. 4, p < 0.05).
Fig. 4.

Osteopontin promoted the proliferation of chondrocytes through HOTAIR. The role of osteopontin and HOTAIR overexpression and HOTAIR knockdown in regulating the proliferation of chondrocytes was analyzed by performing CCK-8 assay. All experiments were repeated 3 times and mean ± SD values
Discussion
This study explored the interactions between osteopontin and HOTAIR in OA. We found that osteopontin and HOTAIR were both upregulated in OA. Moreover, osteopontin might induce the expression of HOTAIR to promote the proliferation of chondrocytes derived from OA patients.
HOTAIR is a well-established critical player with oncogenic roles in cancer biology [14]. Several recent studies also showed that HOTAIR has multiple functions in the pathology of OA. HOTAIR is overexpressed in OA and promotes IL-1β-induced MMP overexpression [15]. Overexpression of HOTIAR in OA interacts with miR-17-5p/FUT2/β-catenin axis to promote disease progression [16]. In another study, HOTAIR was reported to interact with lncRNA PACER to participate in the regulation of chondrocyte apoptosis[17]. Consistently, our results were in consistent with previous study that the expression of HOTAIR was upregulated in OA.
Although the involvement of HOTAIR in OA has been reported by several previous studies, the interaction of HOTAIR with osteopontin, a critical player in OA [10, 11], is still known. Osteopontin was reported to regulate HOTAIR in cancer cells [13]. In our study, we showed that HOTAIR was also upregulated by osteopontin in chondrocytes. However, HOTAIR overexpression and knockdown failed to regulate the expression of osteopontin. Therefore, our study characterized osteopontin as an upstream regulator of HOTAIR in OA.
Chondrocytes are the only mature cells in healthy cartilage, and reduce proliferation and increase apoptosis of chondrocytes contribute to the progression of OA [18]. Both our study and previous studies showed enhanced effects of osteopontin on the proliferation of chondrocytes [10, 11]. Therefore, osteopontin may play protective roles in OA by promoting the proliferation of chondrocytes. However, osteopontin is also a pro-inflammatory factor in OA and an inflammatory response contribute to the development of OA [10], suggesting that it may also promote the progression of OA by inducing inflammation. Therefore, the use of osteopontin as a target for the treatment of OA should be carefully evaluated. It has been well established that lncRNA expression was frequently altered in OA [19]. The altered expression of lncRNAs may participate in OA progression by regulating the proliferation of chondrocytes [20, 21]. For instance, lncRNA SNHG5 regulates the axis of miR-26a/SOX2 to increase the proliferation of chondrocytes [20]. In addition, lncRNA XIST regulates miR-211 to affect the proliferation of OA chondrocytes through CXCR4 and MAPK [21]. In this study we showed that HOTAIR could also regulate the proliferation of chondrocytes. However, the mechanism that mediates the function of HOTAIR remains to be explored.
However, this study was limited by the small sample size. Our future studies will enroll more OA patients and health controls to further confirm our conclusions. In addition, the in vivo interaction between osteopontin and HOTAIR in OA is unknown. Animal model experiments are needed to further explore their roles in OA. Besides that, levels of osteopontin in this study were only measured in cell culture, but not in synovial fluid. Although the levels of osteopontin in cell culture medium may also reflect the secretion of osteopontin under in vivo conditions, the level of osteopontin in synovial fluid should also be analyzed in future studies.
Conclusions
In conclusion, osteopontin and HOTAIR are overexpressed in OA. Moreover, osteopontin induces the expression of HOTAIR to promote the proliferation of chondrocytes.
Acknowledgements
Not applicable.
Abbreviations
- OA
Osteoarthritis
- lncRNAs
Long non-coding RNAs
- C
Control
Authors’ contributions
QZL, AA and RF: study concepts, study design, experiments studies, data analysis and writing the manuscript. YJD, JL and ZFL: experimental studies, data analysis, literature research, manuscript preparation, manuscript review and manuscript editing; All authors have read and approved the final article.
Funding
This work was supported by National Natural Science Foundation (Grant No. 81760875). The funding bodies had no role in the design of the study; collection, analysis, and interpretation of data and writing of the manuscript.
Availability of data and materials
The datasets generated and/or analyzed during the current study are not publicly available due research design but are available from the corresponding author on reasonable request.
Ethics approval and consent to participate
This study was approved by the Research Ethics Committee of Chinese Medicine Hospital of Xinjiang Uygur Autonomous Region and was in line with the Helsinki Declaration. Written informed consent was provided by all patients and controls.
Consent for publication
Not applicable.
Competing interests
All the authors have declared that in the research there was no potential conflict of interest.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Quanzhi Liang and Ailijiang Asila contributed equally to this work.
References
- 1.Glyn-Jones S, Palmer AJR, Agricola R, Price AJ, Vincent TL, Weinans H, et al. Osteoarthritis. Lancet. 2015;386(9991):376–87. doi: 10.1016/S0140-6736(14)60802-3. [DOI] [PubMed] [Google Scholar]
- 2.Zhang Y, Jordan JM. Epidemiology of osteoarthritis. Clin Geriatr Med. 2010;26(3):355–69. doi: 10.1016/j.cger.2010.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Greene MA, Loeser RF. Aging-related inflammation in osteoarthritis. Osteoarthritis Cartilage. 2015;23(11):1966–71. doi: 10.1016/j.joca.2015.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wallace I, Worthington S, Felson D, Jurmain R, Wren K, Maijanen H, et al. Knee osteoarthritis has doubled in prevalence since the mid-20th century. Proceedings of the National Academy of Sciences. 2017;114:201703856. [DOI] [PMC free article] [PubMed]
- 5.Sinusas K. Osteoarthritis: diagnosis and treatment. Am Fam Physician. 2012;85(1):49–56. [PubMed] [Google Scholar]
- 6.Kim JR, Yoo JJ, Kim HA. Therapeutics in Osteoarthritis Based on an Understanding of Its Molecular Pathogenesis. Int J Mol Sci. 2018;19(3). [DOI] [PMC free article] [PubMed]
- 7.Mobasheri A, van Spil WE, Budd E, Uzieliene I, Bernotiene E, Bay-Jensen AC, et al. Molecular taxonomy of osteoarthritis for patient stratification, disease management and drug development: biochemical markers associated with emerging clinical phenotypes and molecular endotypes. Curr Opin Rheumatol. 2019;31(1):80–9. doi: 10.1097/BOR.0000000000000567. [DOI] [PubMed] [Google Scholar]
- 8.Valdes AM, Spector TD. The clinical relevance of genetic susceptibility to osteoarthritis. Best Pract Res Clin Rheumatol. 2010;24(1):3–14. doi: 10.1016/j.berh.2009.08.005. [DOI] [PubMed] [Google Scholar]
- 9.Xia B, Di C, Zhang J, Hu S, Jin H, Tong P. Osteoarthritis pathogenesis: a review of molecular mechanisms. Calcif Tissue Int. 2014;95(6):495–505. doi: 10.1007/s00223-014-9917-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cheng C, Gao S, Lei G. Association of osteopontin with osteoarthritis. Rheumatol Int. 2014;34(12):1627–31. doi: 10.1007/s00296-014-3036-9. [DOI] [PubMed] [Google Scholar]
- 11.Liang J, Xu L, Zhou F, Liu A-M, Ge H-X, Chen Y-Y, et al. MALAT1/miR-127-5p regulates osteopontin (OPN)-mediated proliferation of human chondrocytes through PI3K/Akt pathway. Journal of cellular biochemistry. 2017;119. [DOI] [PubMed]
- 12.Fu M, Huang G, Zhang Z, Liu J, Zhang Z, Huang Z, et al. Expression profile of long noncoding RNAs in cartilage from knee osteoarthritis patients. Osteoarthritis Cartilage. 2015;23(3):423–32. doi: 10.1016/j.joca.2014.12.001. [DOI] [PubMed] [Google Scholar]
- 13.Yang G, Zhang S, Gao F, Liu Z, Lu M, Peng S, et al. Osteopontin enhances the expression of HOTAIR in cancer cells via IRF1. Biochim Biophys Acta. 2014;1839(9):837–48. doi: 10.1016/j.bbagrm.2014.06.020. [DOI] [PubMed] [Google Scholar]
- 14.Gupta RA, Shah N, Wang KC, Kim J, Horlings HM, Wong DJ, et al. Long non-coding RNA HOTAIR reprograms chromatin state to promote cancer metastasis. Nature. 2010;464(7291):1071–6. doi: 10.1038/nature08975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang C, Wang P, Jiang P, Lv Y, Dong C, Dai X, et al. Upregulation of lncRNA HOTAIR contributes to IL-1beta-induced MMP overexpression and chondrocytes apoptosis in temporomandibular joint osteoarthritis. Gene. 2016;586(2):248–53. doi: 10.1016/j.gene.2016.04.016. [DOI] [PubMed] [Google Scholar]
- 16.Hu J, Wang Z, Shan Y, Pan Y, Ma J, Jia L. Long non-coding RNA HOTAIR promotes osteoarthritis progression via miR-17-5p/FUT2/beta-catenin axis. Cell Death Dis. 2018;9(7):711. doi: 10.1038/s41419-018-0746-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jiang M, Liu J, Luo T, Chen Q, Lu M, Meng D. LncRNA PACER is down-regulated in osteoarthritis and regulates chondrocyte apoptosis and lncRNA HOTAIR expression. Biosci Rep. 2019;39(6). [DOI] [PMC free article] [PubMed]
- 18.Singh P, Marcu KB, Goldring MB, Otero M. Phenotypic instability of chondrocytes in osteoarthritis: on a path to hypertrophy. Ann N Y Acad Sci. 2019;1442(1):17–34. doi: 10.1111/nyas.13930. [DOI] [PubMed] [Google Scholar]
- 19.Abbasifard M, Kamiab Z, Bagheri-Hosseinabadi Z, Sadeghi I. The role and function of long non-coding RNAs in osteoarthritis. Exp Mol Pathol. 2020;114:104407. doi: 10.1016/j.yexmp.2020.104407. [DOI] [PubMed] [Google Scholar]
- 20.Shen H, Wang Y, Shi W, Sun G, Hong L, Zhang Y. LncRNA SNHG5/miR-26a/SOX2 signal axis enhances proliferation of chondrocyte in osteoarthritis. Acta Biochim Biophys Sin. 2018;50(2):191–8. doi: 10.1093/abbs/gmx141. [DOI] [PubMed] [Google Scholar]
- 21.Li L, Lv G, Wang B, Kuang L. The role of lncRNA XIST/miR-211 axis in modulating the proliferation and apoptosis of osteoarthritis chondrocytes through CXCR4 and MAPK signaling. Biochem Biophys Res Commun. 2018;503(4):2555–62. doi: 10.1016/j.bbrc.2018.07.015. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated and/or analyzed during the current study are not publicly available due research design but are available from the corresponding author on reasonable request.