Abstract
Isoegomaketone (IK) is a known component of Perilla frutescens that reportedly exhibits anti-inflammatory, anti-cancer and anti-allergic properties. A novel compound known as 9-HIK has been isolated from the extract of a radiation mutant P. frutescens var. crispa using supercritical carbon dioxide. In the present study, 9-HIK induced heme oxygenase-1 (HO-1) mRNA expression in RAW264.7 cells, with maximal levels observed 4 h after 9-HIK treatment. In addition, 9-HIK inhibited the mRNA and protein expression of pro-inflammatory mediators, such as IL-6 and interferon-β, as well as the production of nitric oxide (NO) in lipopolysaccharide-stimulated RAW264.7 cells. Furthermore, N-acetyl-L-cysteine, a reactive oxygen species scavenger, inhibited NO production and HO-1 mRNA expression levels through the nuclear factor erythroid 2-related factor 2 pathway. Overall, 9-HIK displayed anti-inflammatory properties in LPS-induced RAW264.7 cells via direct suppression of inflammatory mediators and HO-1 induction.
Keywords: 9-hydroxy-isoegomaketone, Perilla frutescens, inflammation, heme oxygenase-1, nuclear factor erythroid 2-related factor 2
Introduction
Inflammation is an organism's defense response to extrinsic stimuli or diseases. It is mediated by multiple processes involving various cells and cytokines. External stimuli, such as lipopolysaccharide (LPS), or internal stimuli, such as arachidonic acid metabolites, induce the infiltration of inflammatory cells, such as macrophages and granulocytes, into target sites (1).
Macrophages are inflammatory cells that protect organisms from external pathogens by releasing inflammatory mediators and cytokines, such as nitric oxide (NO), inducible NO synthase (iNOS), TNF-α and IL-6 (2). Activated macrophages are involved in the early phase of infection by producing NO or reactive oxygen species (ROS), as well as cytokines, leading to an inflammatory cascade (1). Under inflammatory conditions, the excessive release of pro-inflammatory mediators and cytokines induces injury of cells and tissues, and may lead to chronic inflammatory diseases (2). Therefore, the control of inflammatory responses is necessary for the prevention of chronic inflammatory diseases (2). The currently employed non-steroidal anti-inflammatory drugs are known to cause side effects, such as heartburn and indigestion. Therefore, previous study has aimed to develop anti-inflammatory drugs from natural products (2).
Heme oxygenase (HO) is an enzyme induced by oxidative stress that promotes heme degradation. HO exists in three isoforms: HO-1, −2 and −3 (1). HO-1 protects cells from harmful free radicals and NO, and controls inflammatory reactions. HO-1 is regulated at the transcriptional level and is related to nuclear factor erythroid 2-related factor 2 (Nrf2), a basic leucine zipper protein that regulates the expression of antioxidant proteins to prevent oxidative damage induced by wounds and inflammation (3). Under physiological conditions, Nrf2 is bound to Kelch-like ECH-associated protein 1 (Keap1); however, it is released from Keap1 for nuclear translocation following HO-1 stimulation through antioxidant response elements (3).
Perilla frutescens is a member of the Labiatae family (4). It is native to the highlands of Southeast Asia and India and traditionally grown as crops in Korea, Japan, India and China. As a medicinal plant, P. frutescens is used as a treatment for cough, vomiting, cold, constipation and abdominal pain (5). P. frutescens emits a unique odor owing to the presence of aromatic ingredients, such as perillaketones and perillaldehydes, in addition to other compounds, including luteolins, catechins, ferulins, rosmarinic acids and apigenin (6). Furthermore, it reportedly exhibits various pharmacological properties, including antioxidant, antiallergic, and anti-inflammatory effects (6).
The plant material used in the present study was the radiation mutant P. frutescens var. crispa. This mutant obtained by exposing the seeds to γ-ray irradiation carries a higher content of isoegomaketone (IK) compared with the original cultivar (7). In previous studies, IK was identified as an essential component of P. frutescens, exhibiting various anti-inflammatory (8), anti-cancer (9), antioxidant (8,10), anti-arthritic (11) and anti-obesity (12) effects. 9-HIK is a novel compound isolated from the radiation mutant P. frutescens var. crispa extract, in which it is present in 8.8-fold higher concentrations than those of the wild-type (13). 9-HIK was shown to inhibit the production of NO in LPS-treated RAW264.7 cells, although with less potency than IK (13). The 9-HIK structure has a hydroxyl group attached to the carbon 9 of IK (13).
The leaves and seeds of P. frutescens are important for the development of natural drugs, owing to the presence of substances with various biological activities, including antioxidant, anti-inflammatory and antiallergic effects (6). Therefore, the aim of the present study was to determine whether 9-HIK could attenuate inflammation in RAW264.7 cells.
Materials and methods
Materials
In the present study, 9-HIK was isolated as described previously (13). Dulbecco's modified Eagle's medium (DMEM), FBS and penicillin-streptomycin were purchased from Hyclone (Cytiva). Reagents, such as LPS, DMSO, Griess reagent, N-acetyl-L-cysteine (NAC, an ROS scavenger), NP40 cell lysis buffer and protease inhibitor cocktail were purchased from Sigma-Aldrich (Merck KGaA). An EZ-Cytox cell viability assay kit was purchased from Daeil Lab Service, Co., Ltd. An RNeasy kit was purchased from Qiagen GmbH. A PrimeScript™ II 1st strand cDNA synthesis kit and TB Green® premix Ex Taq™ (Tli RNaseH Plus) were obtained from Takara Bio, Inc. A Chromo 4 RT-PCR detection system was purchased from Bio-Rad Laboratories, Inc. Rabbit polyclonal antibodies against β-tubulin (cat. no. SC-9104) and HO-1 (cat. no. SC-10789) were obtained from Santa Cruz Biotechnology, Inc. Rabbit polyclonal antibodies against iNOS (cat. no. 2977S), Nrf2 (cat. no. 12721S) and lamin B (cat. no. 13435S) were purchased from Cell Signaling Technology, Inc. Goat anti-rabbit IgG HRP-conjugated secondary antibody (cat. no. A16110) was supplied by Invitrogen (Thermo Fisher Scientific, Inc.). ELISA kits for IL-6 detection (cat. no. SM6000B) were purchased from R&D Systems, Inc. The ELISA kit for interferon (IFN)-β (cat. no. 42400-2) was obtained from Pestka Biomedical Laboratories, Inc.
Reagent preparation
9-HIK was dissolved in DMSO at a stock concentration of 50 mM, then further diluted in DMSO for further use. Griess reagent was dissolved in distilled water. LPS was dissolved in distilled water to make 1 mg/ml stock solution and then further diluted in DMSO for subsequent use. NAC was dissolved in DMSO to make 50 mM stock solution and then further diluted in DMEM for subsequent use. DMSO alone was used as a control and the final DMSO concentration in the cell culture was ≤0.1% (v/v).
Cell culture
The RAW264.7 macrophage cell line was purchased from the American Type Culture Collection. RAW264.7 cells were cultured in DMEM supplemented with 10% FBS, 100 U/ml penicillin and 100 µg/m streptomycin at 37°C with 5% CO2.
Cytotoxicity assay
Cell viability was measured using the EZ-Cytox assay kit. RAW264.7 macrophages were seeded at a density of 2.0×105 cells/ml in 96-well plates. The cells were then treated with 5, 10, 15, 20 or 40 µM 9-HIK. After 24 h of incubation, 10 µl EZ-Cytox assay reagent was added to each well and incubated at 37°C and 5% CO2 for 4 h. The cell viability index was determined by using a Benchmark Plus spectrophotometer (Bio-Rad Laboratories, Inc.) at 480 nm with a reference wavelength of 650 nm. The results are presented as the mean ± SD of six replicates from a single experiment.
NO production assay
RAW264.7 cells were seeded at a density of 2.0×105 cells/ml in 96-well plates. The cells were pre-treated with 5, 10 or 20 µM 9-HIK for 2 h, then stimulated with 1 µg/ml LPS for an additional 18 h at 37°C with 5% CO2 (14). The culture supernatant (100 µl) was mixed with an equal volume of Griess reagent (100 µl) in a 96-well plate and incubated for 15 min at room temperature. Absorbance was measured at 540 nm using a spectrophotometer. The results are presented as the mean ± SD of three replicates from one experiment.
Western blot analysis
RAW264.7 cells were cultured in a 100-mm culture dish at a density of 2.0×105 cells/ml for 24 h. The cells were pre-treated with 5, 10 or 20 µM 9-HIK for 2 h, then stimulated with 1 µg/ml LPS for 18 h to induce iNOS protein expression. In a separate experiment, the cells were treated with 5, 10 or 20 µM 9-HIK for 12 h to determine HO-1 protein expression. Cells were treated with 20 µM 9-HIK for 0, 1, 2 or 4 h and then Nrf2 protein expression levels were measured.
The collected cells were washed once with cold PBS, then lysed using NP40 cell lysis buffer (with 1 mM phenylmethylsulfonyl fluoride and 1X protease inhibitor cocktail) for 30 min on ice. The protein was obtained via centrifugation for 15 min at 16,853 × g at 4°C. The concentration of the cell lysate was measured using the Bio-Rad Protein Assay kit (Bio-Rad Laboratories, Inc.). Equal amounts of protein (30 µg) were separated by SDS-PAGE on 10% gels, then transferred onto a nitrocellulose membrane (Hybond ECL Nitrocellulose; GE Healthcare). After blocking the membrane using blocking buffer (PBS with 5% skimmed milk and 0.05% Tween 20) at room temperature for 1 h, the membranes were incubated with primary antibodies specific for HO-1 (cat. no. sc-10789), iNOS (cat. no. 2977s), Nrf2 (cat. no. 12721s), Lamin B (cat. no. 13435s) and β-tubulin (cat. no. sc-9104) at 4°C overnight. Rabbit polyclonal primary antibodies against iNOS, Nrf2, and Lamin B were diluted 1:1,000 in 0.1% TBS solution with Tween® 20 (TBS-T) buffer. Rabbit polyclonal primary antibodies against HO-1 and β-tubulin were diluted to 1:200 in 0.1% TBS-T buffer. The membranes were washed three times with TBS-T buffer for 15 min, then incubated with HRP-conjugated secondary antibodies for 2 h on a shaker at room temperature. The goat anti-rabbit IgG HRP-conjugated secondary antibody (cat. no. A16110) was diluted 1:5,000 in 5% skim milk. The membranes were washed again, and the protein bands were detected using Amersham ECL™ Prime Western Blotting Detection reagent (Cytiva). The results are presented as the mean ± standard deviation (SD) of three replicates for one representative experiment. Protein expression levels were semi-quantified using ImageJ software (version 1.52; National Institutes of Health).
Fractionation of cytosol and nuclear extracts
RAW264.7 cells were cultured in a 60-mm culture dish at a density of 4.0×105 cells/ml for 24 h. Cells were pre-treated with 20 µM 9-HIK for 0, 1, 2 or 4 h. The cells were washed once with cold PBS and harvested by pipetting. The collected cells were mixed in buffer A (10 mM HEPES pH 7.9; 10 mM KCl; 1.5 mM MgCl2, 0.5 mM DTT; 0.2 mM PMSF) for 10 min on ice, then collected by centrifugation at 1,873 × g for 5 min at 4°C. To prepare the cytosolic fraction, the collected cells were mixed with buffer B (10 mM HEPES pH 7.9; 10 mM KCl; 1.5 mM MgCl2; 0.1% NP40; 0.5 mM DTT; 0.2 mM PMSF). After vortexing for 10 sec, the cytosolic fractions were centrifuged at 1,873 × g for 5 min at 4°C. To collect the nuclear fraction, the pellets were re-suspended in buffer C (20 mM HEPES pH 7.9; 420 mM NaCl; 1.5 mM MgCl2; 25% glycerol; 0.2 mM EDTA; 0.5 mM DTT; 0.2 mM PMSF) and maintained on ice for 30 min. The nuclear fractions were centrifuged at 1,873 × g for 15 min at 4°C. The concentration of the cytosolic and nuclear fraction was measured using the Bio-Rad Protein Assay kit (Bio-Rad Laboratories, Inc.).
Reverse-transcription quantitative (RT-q)PCR
RAW264.7 cells were cultured in 6-well plates at a density of 2.0×105 cells/ml for 24 h. Cells were pre-treated with 5, 10 or 20 µM 9-HIK for 2 h, then stimulated with 1 µg/ml LPS for 4 h (for IL-6 and IFN-β) or 18 h (for iNOS). For the measurement of HO-1 expression, cells were treated with 5, 10 or 20 µM 9-HIK for 4 h. To measure HO-1 mRNA expression levels, RAW264.7 cells treated with 20 µM 9-HIK for 8 h. Also, to measure the level of HO-1 mRNA expression in ROS scavenger treated cells, RAW264.7 cells were co-treated with 20 µM 9-HIK and 5 mM NAC for 4 h. Total RNA was isolated using the RNeasy kit. The PrimeScript™ II 1st strand cDNA synthesis kit was used for reverse transcription at 30°C for 10 min, 42°C for 60 min and 95°C for 5 min. The Chromo 4 RT-PCR detection system and TB Green premix were used qPCR amplification of iNOS, HO-1, IL-6, IFN-β and β-actin using 50 cycles at 94°C for 20 sec, 60°C for 20 sec and 72°C for 30 sec. Primer sequences are listed in Table I. The specificity of the amplified PCR products was assessed via melting curve analysis. Real-time PCR data were calculated as relative values using GeneXpression Macro chromo4 software program (version 1.1; Bio-Rad Laboratories, Inc.). The results are presented as the mean ± SD of three replicates from one experiment.
Table I.
Primer sequences used for reverse transcription-quantitative PCR.
| Gene | Forward primer sequence (5′→3′) | Reverse primer sequence (5′→3′) |
|---|---|---|
| IL-6 | AGTTGCCTTCTTGGGACTGA | TCCACGATTTCCCAGAGAAC |
| IFN-β | GGAAAGATTGACGTGGGAGA | AGGCATCAACTGACAGG |
| iNOS | GGAAAGATTGACGTGGGAGA | CTCCAATCTCTGCCTATCCGTCTC |
| HO-1 | TCCTACACCACACCAAACTGTGTG | CTCCAATCTCTGCCTATCCGTCTC |
| β-actin | GGCTGTATTCCCCTCCATCG | CCAGTTGGTAACAATGCCATGT |
IFN-β, interferon-β; iNOS, inducible nitric oxide synthase; HO-1, heme oxygenase-1.
Measurement of IL-6 and IFN-β levels
RAW264.7 cells were cultured in 6-well plates at a density of 2.0×105 cells/ml for 24 h. The cells were pre-treated with 5, 10 or 20 µM 9-HIK for 2 h, then stimulated with 1 µg/ml LPS for 4 h. IL-6 and IFN-β levels in the culture supernatant were measured using ELISA kits, according to the manufacturer's protocol. The results are presented as the mean ± SD of three replicates from a single experiment.
Statistical methods
Data are presented as the mean ± SD of three or six replicates from a single experiment. Differences between groups were assessed using one-way ANOVA followed by Tukey's post hoc test. P<0.05 was considered to indicate a statistically significant difference.
Results
Cytotoxicity of 9-HIK
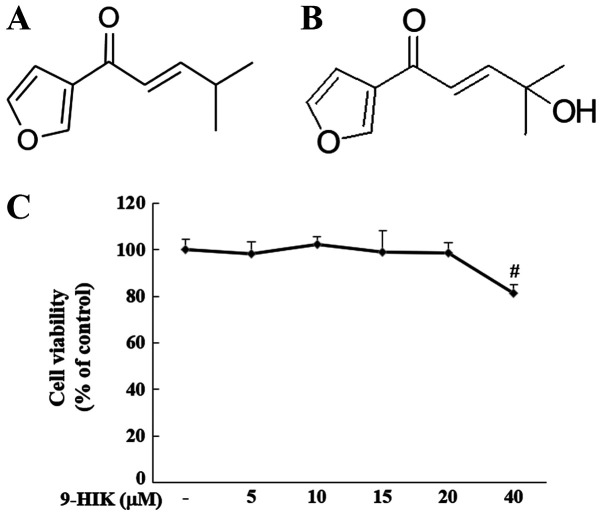
The effect of 9-HIK on cell viability was evaluated by treating RAW264.7 cells with 5, 10, 15, 20 and 40 µM 9-HIK for 24 h. Treatment with 9-HIK did not result in cytotoxicity at concentrations ≤20 µM but reduced cell viability to 81.27% at 40 µΜ (Fig. 1C). Based on these results, 9-HIK was used at concentrations ≤20 µM in subsequent experiments. Chemical structure of IK and chemical structure of 9-HIK (Fig. 1A and B).
Figure 1.
Chemical structure of IK and 9-HIK and cytotoxic effect of 9-HIK. (A) Chemical structure of IK. (B) Chemical structure of 9-HIK. (C) Cell viability of RAW264.7 cells treated with various concentrations of 9-HIK for 24 h. Data are presented as the mean ± SD; n=6. #P<0.05 vs. control. IK, isoegomaketone; 9-HIK, 9-hydroxy-isoegomaketone.
Effect of 9-HIK on NO production and iNOS expression in LPS-stimulated RAW264.7 cells
NO production by 9-HIK was analyzed in RAW264.7 cells treated with 5, 10 or 20 µM 9-HIK for 2 h, followed by stimulation with LPS for 18 h. LPS-treated RAW264.7 cells displayed a 6.4-fold increase in NO production compared with the negative control. However, NO production was inhibited with an IC50 value of 14.4 µM by 9-HIK treatment compared with cells treated with LPS alone (Fig. 2A). To determine whether the inhibition of NO synthesis by 9-HIK could be attributed to the suppression of iNOS expression, the protein and mRNA expression levels of this enzyme were measured in LPS-stimulated RAW264.7 cells. 9-HIK treatment inhibited mRNA and protein expression of iNOS compared with LPS stimulation alone (Fig. 2B and C). These results suggested that 9-HIK inhibited NO production in LPS-stimulated RAW264.7 cells via suppression of iNOS expression.
Figure 2.
Effects of 9-HIK on NO production and iNOS expression in LPS-treated RAW264.7 cells. RAW264.7 cells were pre-treated with various concentrations of 9-HIK for 2 h, then stimulated with LPS for 18 h. (A) NO production. (B) iNOS mRNA expression levels. (C) iNOS protein expression levels. Data are presented as the mean ± SD; n=3. #P<0.05 vs. control; *P<0.05 vs. LPS-treated cells. 9-HIK, 9-hydroxy-isoegomaketone; LPS, lipopolysaccharide; iNOS, inducible nitric oxide synthase; NO, nitric oxide.
Effect of 9-HIK on LPS-induced pro-inflammatory cytokine production in RAW264.7 cells
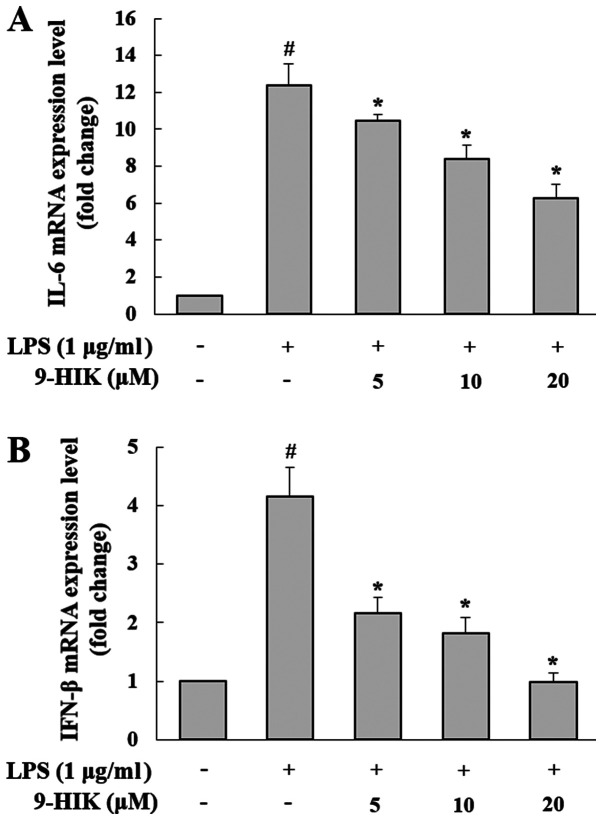
To determine the effects of 9-HIK on the synthesis of pro-inflammatory mediators, such as IL-6 and IFN-β, RAW264.7 cells were pre-treated with 9-HIK for 2 h at concentrations of 5, 10 and 20 µM, then stimulated with LPS for 4 h. As shown in Fig. 3, the mRNA expression levels of both cytokines were increased in LPS-induced RAW264.7 cells, compared with the control group. However, 9-HIK treatment reduced pro-inflammatory cytokine transcriptional levels compared with LPS challenge alone.
Figure 3.
Effect of 9-HIK on IL-6 and IFN-β mRNA expression in LPS-stimulated RAW264.7 cells. RAW264.7 cells were pre-treated with various concentrations of 9-HIK for 2 h, then stimulated with LPS for 4 h. (A) IL-6 and (B) IFN-β mRNA expression levels. Data are presented as the mean ± SD; n=3. #P<0.05 vs. control; *P<0.05 vs. LPS-treated cells. 9-HIK, 9-hydroxy-isoegomaketone; IFN-β, interferon-β; LPS, lipopolysaccharide.
Effects of 9-HIK on IL-6 and IFN-β protein levels in LPS-stimulated RAW264.7 cells
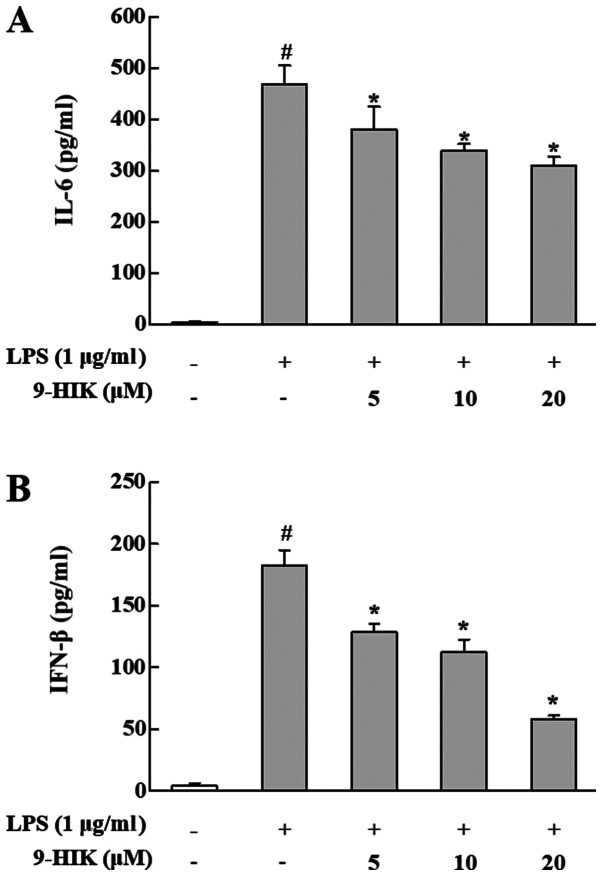
To further examine the inhibitory effect of 9-HIK on inflammation in LPS-stimulated RAW264.7 cells, the protein levels of IFN-β and IL-6 were measured in culture supernatants. RAW264.7 cells were pre-treated with 9-HIK for 2 h at concentrations of 5, 10 and 20 µM, then stimulated with LPS for 4 h. As shown in Fig. 4, the protein levels of both cytokines were increased in LPS-induced RAW264.7 cells, compared with control cells. However, 9-HIK treatment attenuated protein expression of both cytokines, compared with LPS stimulation alone. Thus, 9-HIK inhibited the expression of pro-inflammatory cytokines at the protein level in LPS-stimulated RAW264.7 cells.
Figure 4.
Effect of 9-HIK on IL-6 and IFN-β protein expression in LPS-stimulated RAW264.7 cells. RAW264.7 cells were pre-treated with various concentrations of 9-HIK for 2 h, then stimulated with LPS for 4 h. (A) IL-6 and (B) IFN-β protein expression levels. Data are presented as the mean ± SD; n=3. #P<0.05 vs. control; *P<0.05 vs. LPS-treated cells. 9-HIK, 9-hydroxy-isoegomaketone; IFN-β, interferon-β; LPS, lipopolysaccharide.
Effects of 9-HIK on HO-1 expression in RAW264.7 cells
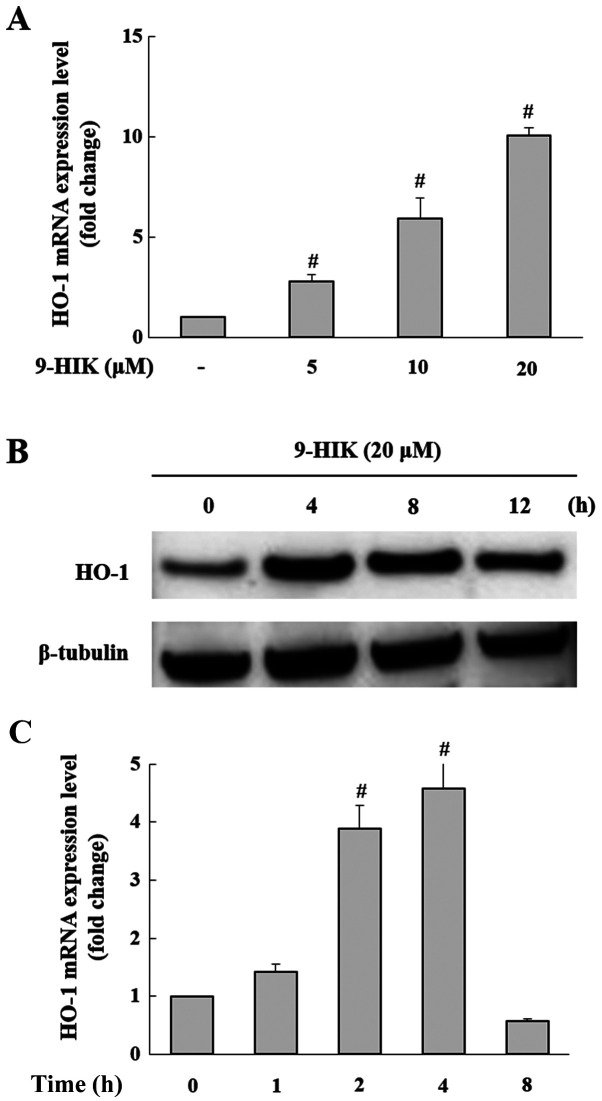
A previous study demonstrated that IK exerted anti-inflammatory effects through HO-1 expression (10). Therefore, it was hypothesized that the inhibitory effect of 9-HIK on LPS-stimulated inflammation may also be mediated by HO-1. Treatment with 9-HIK significantly increased the HO-1 mRNA (Fig. 5A) and protein expression levels (Fig. 5B) compared with control cells. HO-1 mRNA and protein expression reached a maximal increase following 9-HIK treatment for 4 h (Fig. 5C).
Figure 5.
Effect of 9-HIK on HO-1 expression in RAW264.7 cells. (A) HO-1 mRNA expression levels in RAW264.7 cells incubated with various concentrations of 9-HIK for 4 h. (B) RAW264.7 cells were treated with 20 µM 9-HIK for 0, 4, 8 and 12 h and then HO-1 protein expression levels were measured. (C) RAW264.7 cells were treated with 20 µM 9-HIK for 0, 1, 2, 4 and 8 h and then HO-1 mRNA expression levels were measured. Data are presented as the mean ± SD; n=3. #P<0.05 vs. control. 9-HIK, 9-hydroxy-isoegomaketone; HO-1, heme oxygenase-1.
Effects of 9-HIK on Nrf2 expression levels in RAW264.7 cells
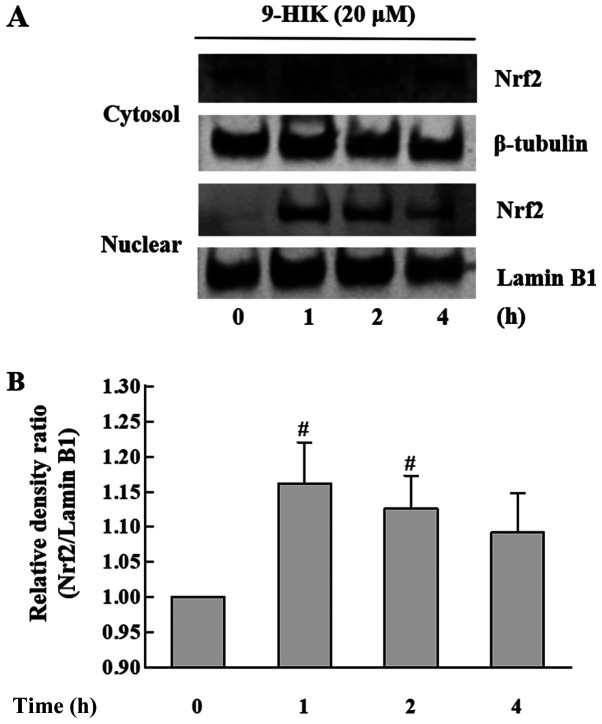
In subsequent experiments, whether 9-HIK could affect the expression of the Nrf2 transcription factor in RAW264.7 cells was investigated. Nrf2 plays an important role in protecting cells from the oxidative stress (3). RAW264.7 cells were treated with 20 µM 9-HIK for 0, 1, 2 or 4 h. The subcellular localization of Nrf2 in 9-HIK-treated RAW264.7 cells was measured via western blotting. As shown in Fig. 6, 9-HIK increased Nrf2 protein levels in the nuclear fraction, showing a maximal increase after 1 h of treatment. However, Nrf2 protein levels remained unchanged in cytosolic extracts. These results indicated that 9-HIK might induce the translocation of Nrf2 from the cytosol to the nucleus in RAW264.7 cells. However, 9-HIK may not affect overall Nrf2 expression levels.
Figure 6.
Effects of 9-HIK on Nrf2 translocation. Cytosolic and nuclear protein extracts were isolated from RAW264.7 cells following treatment with 20 µM 9-HIK for 0, 1, 2 and 4 h. (A) Nrf2 protein expression levels in cytosolic and nuclear extracts. (B) Nrf2/Lamin B1 density ratio was calculated. Data are presented as the mean ± SD; n=3. #P<0.05 vs. control. 9-HIK, 9-hydroxy-isoegomaketone; Nrf2, nuclear factor erythroid 2-related factor 2.
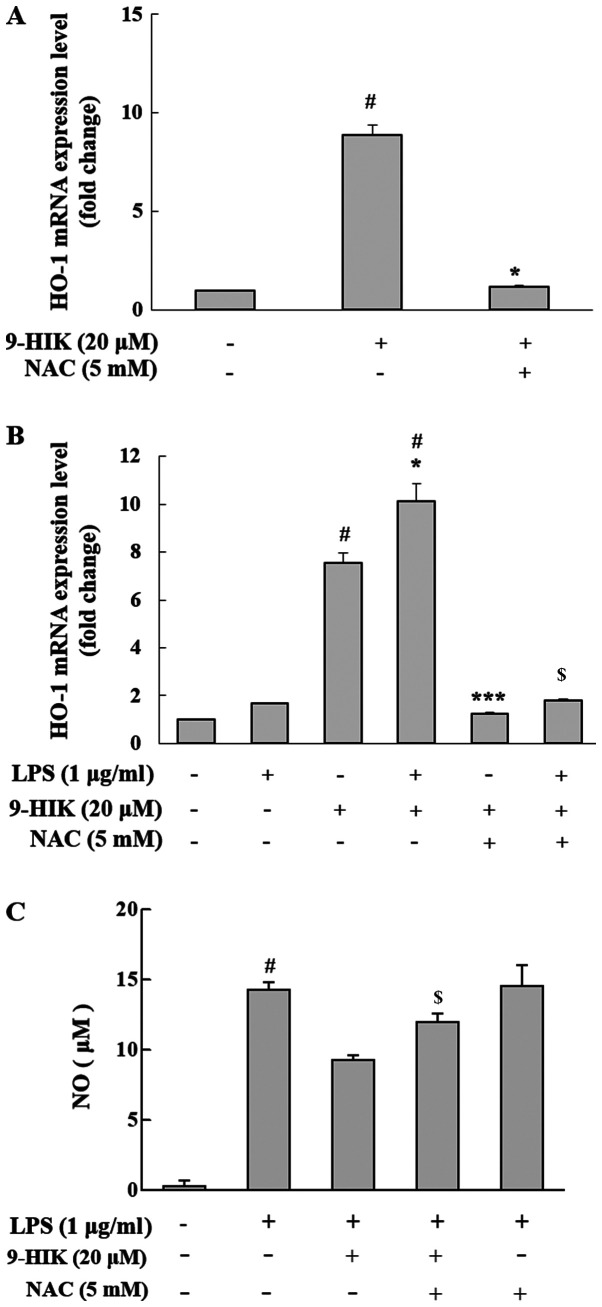
Effect of ROS scavenger on HO-1 induction in 9-HIK-treated RAW264.7 cells
Nrf2 activation is reportedly induced by ROS (15). To measure the level of HO-1 mRNA expression in ROS scavenger treated cells, RAW264.7 cells were co-treated with 20 µM 9-HIK and 5 mM NAC for 4 h. NAC is a potent ROS scavenger that inhibits intracellular ROS synthesis (2). 9-HIK-induced HO-1 mRNA expression levels decreased following NAC co-treatment in RAW264.7 cells (Fig. 7A). Also, RAW264.7 cells were pretreated with 20 µM 9-HIK and 5 mM NAC for 2 h, then stimulated LPS for 4 h. Treatment with LPS alone did not affect the mRNA expression levels of HO-1; however, treatment with LPS and 9-HIK resulted in a greater increase in HO-1 mRNA compared with either LPS or 9-HIK alone. Additionally, treatment with NAC reduced HO-1 mRNA levels compared with LPS and 9-HIK-treated cells (Fig. 7B).
Figure 7.
Effect of ROS scavenger on HO-1 expression and NO production in 9-HIK-treated RAW264.7 cells. (A) HO-1 mRNA expression levels in RAW264.7 cells treated with 20 µM 9-HIK and 5 mM NAC for 4 h. (B) HO-1 mRNA expression levels in RAW264.7 cells pre-treated with 20 µM 9-HIK and 5 mM NAC, then stimulated with 1 µg/ml LPS for 2 h. (C) NO production in RAW264.7 cells pretreated with 20 µM 9-HIK and 5 mM NAC for 2 h, then stimulated with 1 µg/ml LPS for 18 h. Data are presented as the mean ± SD; n=3. #P<0.05 vs. control; *P<0.05 vs. LPS-treated cells; $P<0.05 vs. LPS + 9-HIK-treated cells; ***P<0.05 vs. 9-HIK-treated cells. ROS, reactive oxygen species; HO-1, heme oxygenase-1; NO, nitric oxide; 9-HIK, 9-hydroxy-isoegomaketone; NAC, n-acetyl-L-cysteine; LPS, lipopolysaccharide.
Furthermore, to determine whether 9-HIK could inhibit LPS-induced NO production, RAW264.7 cells were treated with LPS and 9-HIK in the presence or absence of NAC, and NO levels were measured. Compared with LPS and 9-HIK co-treatment, NAC addition increased NO levels. However, LPS and NAC treatment did not affect NO levels compared with LPS alone (Fig. 7C). Thus, 9-HIK inhibited LPS-induced NO synthesis.
Discussion
Plant components used in traditional medicine are proposed to have nutritional value, as well as various physiological effects on the immune, endocrine and nervous systems (16). Therefore, they are frequently used in the context of inflammation, allergy and autoimmune disease. The leaves and seeds of P. frutescens contain small amounts of flavonoids and phenolic acids, such as catechins, apigenin, caffeic acid and luteolin (5). The radiation mutant P. frutescens var. crispa (cv. Antisperill) used in the present study was generated via 200-Gy γ-ray irradiation of the wild-type cultivar (7). Previous studies have reported that IK, a compound found in P. frutescens essential oil, exhibits pharmacological properties, including anti-inflammatory, antioxidant (8), anti-cancer (9), anti-arthritic (11) and anti-obesity effects (12). The concentrations of IK, Prilla Ketone (PK), and 9-HIK in the mutant cultivar obtained through radiation breeding are increased relative to the wild-type, and their predicted biosynthetic mechanisms have been suggested in a previous study (13). All three compounds are also present in the wild-type, albeit in extremely small amounts (particularly IK and 9-HIK); thus, few studies have been carried out using the wild-type cultivar. In a study using natural resources, the results demonstrated that the smallest amount of active ingredients offered the greatest disadvantage and radiation breeding might be an alternative solution to these challenges. Based on the structure of IK and PK, it is hypothesized that the α, β-unsaturated ketone, a carbon-carbon double bond conjugated to a ketone (Cβ=Cα-C=O) present in IK and 9-HIK, plays an important role in mediating the anti-inflammatory activity of these compounds (10). Moreover, the addition of a hydroxyl group to the 9th carbon of IK does not increase the anti-inflammatory effect, but rather marginally decreases it; however, cytotoxicity is reduced compared with IK (13). In a previous study, 9-HIK reportedly suppressed NO production in LPS-induced RAW264.7 cells (13), but no study on its mechanism of action has been reported. Although 9-HIK has a structure similar to IK, whether it inhibits NO production in a manner similar to IK in RAW264.7 cells remains unclear. 9-HIK was separated in large quantities using centrifugal partition chromatography (17). It has been demonstrated that 9-HIK exerted anti-inflammatory effects indirectly through HO-1 induction, or via a direct pathway by inhibiting IFN-β production, both pathways are similar to IK (10). Our previous study aimed to determine the intracellular targets of IK that could mediate its anti-inflammatory effects through a direct pathway; however, this currently remains unknown (10). Identification of the targets of IK may be extrapolated to 9-HIK, as the targets of both compounds are expected to be identical.
Inflammation is a complex pathological reaction caused by harmful stimuli, infection, or tissue damage. At the molecular level, the inflammatory response is initiated by cytokines, chemokines and ROS, which are water-soluble pro-inflammatory mediators released by inflammatory cells (12). Acute inflammation is an immediate response to external stimuli, whereby macrophages recognize the infection and secrete pro-inflammatory cytokines to mobilize other immune cells and trigger inflammation (2). The findings of the present study suggested that 9-HIK was a potent agent involved in acute inflammatory responses through inhibition of IL-6 and IFN-β production in LPS-stimulated RAW264.7 cells. 9-HIK inhibited NO production in LPS-treated RAW264.7 cells with an IC50 value of 14.4 µM, which was marginally less effective than IK (IC50=8.8 µM), but demonstrated advantages over IK in terms of cytotoxicity (13).
NO is enzymatically generated from l-arginine by NOS (18). NO is generated by intracellular NOS. NO produced by endothelial NOS and neuronal NOS is beneficial for physical health, as it regulates cell signaling and survival and has antibacterial activity (19). In contrast, NO synthesized by iNOS is associated with inflammatory responses, as it interacts with other free radicals to produce cytotoxic molecules (20). Therefore, agents that inhibit NO production could be used to attenuate inflammatory disease (21,22). In the present study, iNOS and NO production increased in LPS-stimulated RAW264.7 cells. However, 9-HIK inhibited NO synthesis and iNOS expression. Therefore, 9-HIK may represent a potential therapeutic candidate for inflammatory diseases. Macrophages and neutrophils both play an important role in the inflammatory response. Because neutrophils are short-lived, the present study only examined macrophages. However, it is necessary to study the anti-inflammatory effects of 9-HIK in various cell lines, including neutrophils, in the future.
LPS can directly activate RAW264.7 macrophages via Toll-like receptor 4, and induce the expression of pro-inflammatory cytokines and mediators (23). NO, prostaglandin E2 and cytokines are essential mediators of immune responses and host responses to inflammation (24). Overproduction of pro-inflammatory cytokines such as IL-6 and IFN-β causes fever, inflammation and tissue destruction (25). Therefore, studies investigating the inhibition of inflammatory cytokine expression are crucial for the development of anti-inflammatory agents (25). In the current study, 9-HIK inhibited the mRNA and protein expression levels of IL-6 and IFN-β in LPS-stimulated RAW264.7 cells. The effect of 9-HIK on NF-κB activation was not investigated; however, based on the present findings, it is hypothesized that the effect of 9-HIK on NF-κB activation would be suppressed at a lower level than the IFN-β pathway, similar to IK (10). Thus, the present data may provide evidence of a link between Nrf2/HO-1 signaling and anti-inflammatory activity of 9-HIK. Nevertheless, as NF-κB is an important anti-inflammatory transcription factor, the effect of 9-HIK on this pathway will require further study.
Nrf2-mediated signaling pathways protect cells from various external stimuli, including oxidative stress or LPS (26). Furthermore, activated Nrf2 is considered a potential therapeutic agent for the treatment of various inflammatory diseases, such as autoimmune diseases, rheumatoid arthritis, gastritis and atherosclerosis (2). Nrf2 binds to Keap1 in the cytoplasm and is readily degraded via ubiquitin (24). However, oxidative stress induces the expression of HO-1, a gene encoding an antioxidant enzyme, by increasing the nuclear accumulation of Nrf2 (24). In addition, HO-1 and its metabolites exhibit important anti-inflammatory effects mediated by Nrf2 (27). Activation of NF-κB by oxidative stress releases pro-inflammatory cytokines. Nrf2 activation plays an important role in inhibiting the transcription of pro-inflammatory mediators through NF-κB (27). The present study demonstrated that HO-1 expression increased with 9-HIK treatment in a concentration-dependent manner, with maximal induction occurring at 4 h. Moreover, HO-1 mRNA expression and protein levels increased following 9-HIK treatment, possibly via nuclear translocation of Nrf2. However, as the observed differences were small, it may be hypothesized that other pathways additionally participate in this response. Furthermore, the inhibitory effect of 9-HIK on NO production in LPS-stimulated RAW264.7 cells was attenuated following treatment with NAC, a ROS scavenger; thus, the HO-1 induction by 9-HIK was linked to ROS generation. HO-1 expression was inhibited by treatment with a ROS scavenger. Collectively, these results suggested that 9-HIK may increase HO-1 mRNA and protein levels through the translocation of Nrf2 into the nucleus. Therefore, 9-HIK may represent a potential therapeutic candidate for inflammatory diseases. Preclinical studies using animal models are required to validate the anti-inflammatory properties of 9-HIK in vivo before development as a natural anti-inflammatory drug.
Acknowledgements
Not applicable.
Funding
This work was supported by The National Research Foundation of Korea grant funded by the Korean government (Ministry of Science, ICT and Future Planning); grant no. 2017M2A2A6A05018541).
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Authors' contributions
HMK designed the study, performed experiments and wrote the manuscript. BN, SBP, ARH and JWN drafted the manuscript and helped with understanding the structural difference between 9-HIK and IK. HGJ assisted in drafting and revising the manuscript. CHJ designed and supervised the study. HMK and CHJ confirmed the authenticity of all the raw data. All authors read and approved the final manuscript.
Ethics approval and consent to participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Elbirt KK, Bonkovsky HL. Heme oxygenase: Recent advances in understanding its regulation and role. Proc Assoc Am Physicians. 1999;111:438–447. doi: 10.1111/paa.1999.111.5.438. [DOI] [PubMed] [Google Scholar]
- 2.An MY, Eo HJ, Son HJ, Geum NG, Park GH, Jeong JB. Anti-inflammatory effects of leaf and branch extracts of honeyberry (Lonicera caerulea) on lipopolysaccharide-stimulated RAW264.7 cells through ATF3 and Nrf2/HO-1 activation. Mol Med Rep. 2020;22:5219–5230. doi: 10.3892/mmr.2020.11638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Johnson JA, Johnson DA, Kraft AD, Calkins MJ, Jakel RJ, Vargas MR, Chen PC. The Nrf2-ARE pathway: An indicator and modulator of oxidative stress in neurodegeneration. Ann NY Acad Sci. 2008;1147:61–69. doi: 10.1196/annals.1427.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Seo WH, Baek HH. Characteristic aroma-active compounds of Korean perilla (Perilla frutescens Britton) leaf. J Agric Food Chem. 2009;57:11537–11542. doi: 10.1021/jf902669d. [DOI] [PubMed] [Google Scholar]
- 5.Osakabe N, Yasuda A, Natsume M, Sanbongi C, Kato Y, Osawa T, Yoshikawa T. Rosmarinic acid, a major polyphenolic component of Perilla frutescens, reduces lipopolysaccharide (LPS)-induced liver injury in D-galactosamine (D-GalN)-sensitized mice. Free Radic Biol Med. 2002;33:798–806. doi: 10.1016/S0891-5849(02)00970-X. [DOI] [PubMed] [Google Scholar]
- 6.Toshiaki M, Furuta Y, Wakushima H, Fujii H, Saito KI, Kano Y. Anti-allergic effect of Perilla frutescens and its active constituents. Phytother Res. 2003;17:240–243. doi: 10.1002/ptr.1115. [DOI] [PubMed] [Google Scholar]
- 7.Park YD, Lee YM, Kang MA, Lee HJ, Jin CH, Choi DS, Kim DS, Kang SY, Kim WG, Jeong IY. Phytochemical profiles and in vitro anti-inflammatory properties of Perilla frutescens cv. Chookyoupjaso mutants induced by mutagenesis with γ-ray. Food Sci Biotechnol. 2010;19:305–311. doi: 10.1007/s10068-010-0044-8. [DOI] [Google Scholar]
- 8.Chung BH, Lee HY, Lee JS, Young CYF. Perillyl alcohol inhibits the expression and function of the androgen receptor in human prostate cancer cells. Cancer Lett. 2006;236:222–228. doi: 10.1016/j.canlet.2005.05.023. [DOI] [PubMed] [Google Scholar]
- 9.Cho BO, Jin CH, Park YD, Ryu HW, Byun MW, Seo KI, Jeong IY. Isoegomaketone induces apoptosis through caspase-dependent and caspase-independent pathways in human DLD1 cells. Biosci Biotechnol Biochem. 2011;75:1306–1311. doi: 10.1271/bbb.110088. [DOI] [PubMed] [Google Scholar]
- 10.Jin CH, Lee HJ, Park YD, Choi DS, Kim DS, Kang SY, Seo KI, Jeong IY. Isoegomaketone inhibits lipopolysaccharide-induced nitric oxide production in RAW 264.7 macrophages through the heme oxygenase-1 induction and inhibition of the interferon-beta-STAT-1 pathway. J Agric Food Chem. 2010;58:860–867. doi: 10.1021/jf9033333. [DOI] [PubMed] [Google Scholar]
- 11.Jin CH, So Y, Nam B, Han SN, Kim JB. Isoegomaketone alleviates the development of collagen antibody-induced arthritis in male Balb/c Mice. Molecules. 2017;22:1209. doi: 10.3390/molecules22071209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.So YK, Jo YH, Nam BM, Lee SY, Kim JB, Kang SY, Jeong HG, Jin CH. Anti-obesity effect of isoegomaketone isolated from Perilla frutescens (L.) Britt. cv. Leaves. Korean J Pharmacogn. 2015;46:1–6. [Google Scholar]
- 13.Nam B, So Y, Kim HY, Kim JB, Jin CH, Han AR. A New Monoterpene from the leaves of a radiation mutant cultivar of perilla frutescens var. crispa with Inhibitory activity on LPS-Induced NO production. Molecules. 2017;22:1471. doi: 10.3390/molecules22091471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Baek SH, Park T, Kang MG, Park D. Anti-inflammatory activity and ROS regulation effect of sinapaldehyed in LPS-stimulated RAW264.7 macrophages. Molecules. 2020;25:4089. doi: 10.3390/molecules25184089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Alam J, Cook JL. Transcriptional regulation of the heme oxygenase-1 gene via the stress response element pathway. Pharm Des. 2003;9:2499–2511. doi: 10.2174/1381612033453730. [DOI] [PubMed] [Google Scholar]
- 16.Alam J, Stewart D, Touchard C, Boinapally S, Choi AM, Cook JL. Nrf2, a Cap‘n’Collar transcription factor, regulates induction of the heme oxygenase-1 gene. J Biol Chem. 1999;274:26071–26078. doi: 10.1074/jbc.274.37.26071. [DOI] [PubMed] [Google Scholar]
- 17.Nam B, Paudel SB, Kim JB, Jin CH, Lee D, Nam JW, Han AR. Preparative Separation of Three Monoterpenes from Perilla frutescens var. crispa using centrifugal partition chromatography. Int J Anal Chem. 2019;2019:8751345. doi: 10.1155/2019/8751345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lo Faro ML, Fox B, Whatmore JL, Winyard PG, Whiteman M. Hydrogen sulfide and nitric oxide interactions in inflammation. Nitric Oxide. 2014;41:38–47. doi: 10.1016/j.niox.2014.05.014. [DOI] [PubMed] [Google Scholar]
- 19.Wei ZY, Chi KQ, Wang KS, Wu J, Liu LP, Piao HR. Design, synthesis, evaluation, and molecular docking of ursolic acid derivatives containing a nitrogen heterocycle as anti-inflammatory agents. Bioorg Med Chem Lett. 2018;28:1797–1803. doi: 10.1016/j.bmcl.2018.04.021. [DOI] [PubMed] [Google Scholar]
- 20.Zhang Y, Li C, Zhou C, Hong P, Zhang Y, Sun S, Qian ZJ. 2′-Hydroxy-5′-methoxyacetophenone attenuates the inflammatory response in LPS-induced BV-2 and RAW264.7 cells via NF-κB signaling pathway. J Neuroimmunol. 2019;330:143–151. doi: 10.1016/j.jneuroim.2019.03.009. [DOI] [PubMed] [Google Scholar]
- 21.Tripathi P, Tripathi P, Kashyap L, Singh V. The role of nitric oxide in inflammatory reactions. FEMS Immunol Med Microbiol. 2007;51:443–452. doi: 10.1111/j.1574-695X.2007.00329.x. [DOI] [PubMed] [Google Scholar]
- 22.Hofseth LJ. Nitric oxide as a target of complementary and alternative medicines to prevent and treat inflammation and cancer. Cancer Lett. 2008;268:10–30. doi: 10.1016/j.canlet.2008.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jin B, Jin H. Oxymatrine attenuates lipopolysaccharide-induced acute lung injury by activating the epithelial sodium channel and suppressing the JNK signaling pathway. Exp Anim. 2018;67:337–347. doi: 10.1538/expanim.17-0121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kwon DH, Choi EO, Hwang H, Kim KJ, Hong SH, Lee DH, Choi YH. Socheongja and Socheong 2 extracts suppress lipopolysaccharide-induced inflammation and oxidative stress in RAW 264.7 macrophages through activating Nrf2/HO-1 signaling and suppressing MAPKs pathway. J Life Sci. 2018;28:207–215. [Google Scholar]
- 25.Dinarello CA. Proinflammatory cytokines. Chest. 2000;118:503–508. doi: 10.1378/chest.118.2.503. [DOI] [PubMed] [Google Scholar]
- 26.Huang Y, Li W, Su ZY, Kong AN. The complexity of the Nrf2 pathway: Beyond the antioxidant response. J Nutr Biochem. 2015;26:1401–1413. doi: 10.1016/j.jnutbio.2015.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vomund S, Schafer A, Parnham MJ, Brune B, von Knethen A. Nrf2, the master regulator of anti-oxidative responses. Int J Mol Sci. 2017;18:2772. doi: 10.3390/ijms18122772. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.