Abstract
Acute intermittent porphyria (AIP) is a rare autosomal dominant metabolic disease with a broad spectrum of clinical manifestations, and can be easily confused with other diseases. Many patients with porphyria may have symptoms of peripheral nerve damage during an AIP attack, but most such patients are usually only mildly affected. Herein, we describe the case of an undiagnosed woman who developed overall weakness and respiratory failure within 48 hours, leading to her referral to the intensive care unit. Her neuropathy rapidly deteriorated, leading to quadriplegia and bulbar palsy within 14 days. Finally, the reddish color of her urine and further genetic analysis led to a diagnosis of AIP. The patient was treated with intravenous glucose infusion and her condition gradually improved; however, severe neurological sequelae remained. To the best of our knowledge, the AIP reported in this case, involving rapid and severe neuropathy, is extremely rare worldwide. A diagnosis of AIP should therefore be considered when patients present with severe progressive neuropathy. Moreover, early diagnosis may considerably improve patient prognosis.
Keywords: Acute intermittent porphyria, severe neuropathy, intensive care unit, paralysis, case report, genetic analysis, diagnostic features
Introduction
Acute intermittent porphyria (AIP) is a rare autosomal dominant metabolic disease that is caused by a partial deficiency of hydroxymethylbilane (HMBS), the third enzyme in the heme biosynthesis pathway. This deficiency results in the accumulation of upstream metabolic products, such as delta-aminolevulinic acid (ALA) and porphobilinogen (PBG). ALA is believed to be neurotoxic and affects the autonomic, peripheral, and central nervous systems, thereby causing related symptoms, including severe abdominal pain, hypertension, respiratory failure, and quadriplegia. Because AIP has a broad spectrum of clinical manifestations, it can be easily confused with other diseases.
The reported prevalence of HMBS gene mutations is high (1/1,675);1 however, the clinical penetrance is comparatively low: in Europe, AIP is estimated to affect approximately 5.4 people per million (1/185,000).2 Moreover, 10% to 40% of patients may have symptoms of peripheral nerve damage during an AIP attack, but they are usually only mildly affected.3 However, in the present case, the patient had neuropathy that presented intensely, with a relatively unusual clinical picture and severe, long-lasting sequelae.
Case report
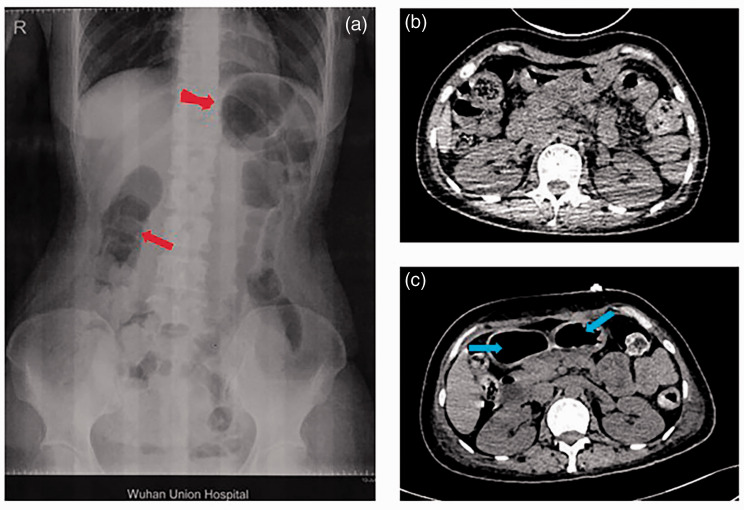
A 37-year-old woman presented to the surgical unit of a general teaching hospital with abdominal pain that she experienced while walking to the hospital (May 2019). Her symptoms had started 4 months previously (January 2019). The initial symptoms included recurring abdominal pain and bloating (almost once per month). During this period, the patient visited her local clinic several times, without obtaining a clear diagnosis. The patient received Chinese medicine over a long period of time and also received symptomatic treatments (e.g., enema, tramadol as analgesia), but her condition did not improve considerably. She had a history of a cesarean section in 2013, and no other notable medical history or family history of disease. On her admission assessment, an abdominal flat plate X-ray showed incomplete intestinal obstruction (Figure 1a), but no obvious abnormalities were observed in any other inspections.
Figure 1.
Imaging results of the patient with acute intermittent porphyria. (a) Abdominal X-ray showing incomplete intestinal obstruction when the patient initially presented to the surgical unit. Obvious gastrointestinal gas is indicated by red arrows. (b) Abdominal computed tomography (CT) showing no obvious lesions when the patient was transferred to the intensive care unit. (c) Abdominal CT 14 days after the patient was transferred to the intensive care unit, showing unspecific intestinal obstruction. Extensive colonic expansion is indicated by blue arrows.
Forty-eight hours after admission, the patient suddenly developed dyspnea, muscle weakness, and a disorder of consciousness, with uncorrectable hypoxemia (SPO2 86%), tachycardia (130 bpm), and hypotension (blood pressure 80/59 mmHg). She was transferred to the intensive care unit (ICU) for further management. Urgent arterial blood gas analysis on ICU admission indicated severe hypoxemia and respiratory acidosis (pH: 7.153, PO2: 66 mmHg, PCO2: 50.2 mmHg, and PO2/FiO2: 110 mmHg); tracheal intubation was then performed.
On day 2 of ICU admission, the patient’s awareness gradually recovered and her abdominal pain spontaneously disappeared. Further examination revealed stubborn respiratory failure, meaning that the patient was unable to be removed from the ventilator. Muscle power of the limbs was 0/5 proximally and 2/5 distally. Furthermore, sensation and cranial nerve examinations were normal. Cranial computed tomography (CT) and abdominal CT did not indicate any abnormalities (Figure 1b), although the chest CT showed patchy exudation of the lower lungs, which did not explain the patient’s respiratory failure. Bedside ultrasound revealed that diaphragm displacement during spontaneous breathing mode was 5.4 mm, whereas normal displacement is considered to be greater than 10 mm. Blood tests showed that serum creatinine was elevated at 350.5 µmol/L (normal range: 44–133 µmol/L), and urea nitrogen was elevated at 22.23 mmol/L (normal range: 2.9–7.5 mmol/L), although these levels returned to normal soon after hemodialysis.
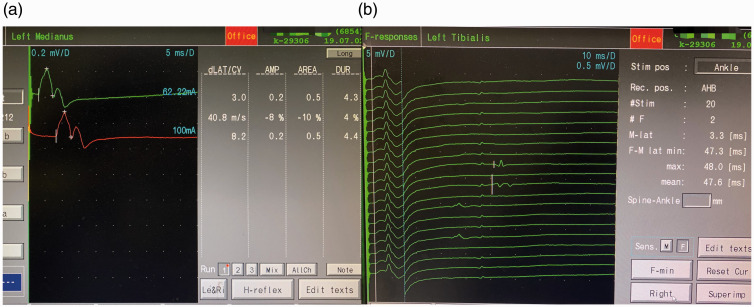
The initial cerebrospinal fluid (CSF) examination was within the normal range, and anti-ganglioside antibodies, such as GM1 and GD1a, were negative. Furthermore, electromyography findings showed unspecific peripheral nerve damage (Figure 2). The patient was treated with a course of intravenous immunoglobulin for a probable diagnosis of Guillain–Barré syndrome (GBS), but no relief was achieved.
Figure 2.
Electromyography results of the patient with acute intermittent porphyria. (a) Traces showing that the motor conduction velocity of the left median nerve slowed down and the amplitude was decreased. (b) Traces showing that the F wave of the left tibial nerve was less than 10%.
The patient’s condition continued to worsen, and on day 14 after ICU admission, her abdominal pain and constipation returned, along with changes in her neurological examination. Her overall weakness worsened, with muscle power of 0/5 (absolutely no movement) in all four limbs. Furthermore, a cranial nerve examination showed new nasal and labial asymmetry, and her eyelids were unable to close. Abdominal CT at this time showed extensive colonic expansion (Figure 1c). Another CSF examination was performed, but the results remained normal, with no cytoalbuminologic dissociation. It was also noted that the color of the patient’s urine turned visibly reddish on exposure to sunlight (Figure 3). A diagnosis of AIP was finally confirmed by genetic analysis, which identified a missense mutation: c.541C>T, encoding p. Gln181 in the HMBS gene (Figure 4). Targeted mutation analysis was then performed in her two children, but neither of them carried the disease-causing gene.
Figure 3.
Urine analysis of the patient with acute intermittent porphyria. (a) Urine sample of the patient. (b) The same urine sample after exposure to sunlight for 3 hours.
Figure 4.
Genetic analysis of the patient with acute intermittent porphyria and her two sons. A missense mutation, c.541C > T encoding p. Gln181 in the HMBS gene, is indicated by a red arrow in the proband. Red arrows in the results of the genetic analyses of the patient’s two sons indicate that they do not carry the mutant gene.
Because heme arginate is not available in mainland China, the patient was treated with intravenous glucose infusion (50%). Twenty-four hours after glucose medication, her abdominal pain was alleviated and her urine returned to a normal color; however, her peripheral neuropathy did not markedly improve. After 2 weeks of treatment, the tracheal tube was removed. The patient was then transferred back to her local hospital for rehabilitation training because of the poor muscle strength in her limbs. At the 1-year follow-up, her overall muscle power had gradually improved to grade 3/5, and she was able to walk with the aid of a rehabilitation walking assistance vehicle. To date, the patient has not experienced any other AIP attacks, but she still has severe neurological sequelae.
Discussion
AIP is a rare metabolic disease caused by HMBS mutations. Although AIP occurs in all races, it is thought to be most common in northern Europe, with a calculated prevalence of 5.4 per million.4,5 Between 10% and 40% of patients with porphyria may exhibit symptoms of peripheral nerve damage during an AIP attack, and such patients are usually mildly affected.3 It is rare for a patient to experience severe peripheral nerve damage, such as that which caused the quadriplegia described in the present case report. Cranial nerve involvement accompanies 75% of all cases of porphyric neuropathy, and usually develops after motor symptoms appear,6 which is consistent with our case.
There were several indications that could have led to the diagnosis of AIP in the present case because the patient had recurrent abdominal pain and peripheral nerve damage, but an accurate diagnosis was still delayed. In practice, 11% of patients diagnosed with AIP have previously undiagnosed acute porphyria; according to Pischik et al.,7 this is because physicians commonly misdiagnose this cause of acute polyneuropathy or encephalopathy. Neuropathic symptoms in most patients reach their maximal intensity at 2 weeks after onset.8 In the present case, severe neuropathy, including quadriplegia and respiratory failure, developed in 48 hours. Moreover, when the patient entered the ICU, the symptoms of abdominal pain had disappeared, urine color was normal, and the period of the AIP attack was therefore probably missed. Furthermore, both the analysis of precursors and porphyrins and the genetic analysis were not available in the local hospital. The relatively long duration between suspecting AIP and obtaining the results of the genetic analysis, which was outsourced to Wuhan Kindstar Diagnostics Co., Ltd. (Wuhan, China), delayed the patient’s diagnosis by 10 days.
The mechanisms of porphyric neuropathy remain unclear. The two major proposed mechanisms are related to a lack of heme, leading to dysfunction of the energy-dependent Na+/K+ ATPase,9 as well as the direct neurotoxicity of accumulated porphyrin precursors, including ALA and PBG.10,11 The early administration of heme arginate may prevent the onset of neuropathy by reducing the expression of the rate-limiting liver enzyme ALA synthase 1 (ALAS1), thus impeding further progression.12 Although hemin is the standard of treatment, especially when neurological symptoms occur, our patient was treated with intravenous glucose infusion during the AIP attack because heme arginate is not available in mainland China (Table 1). Fortunately, the high-carbon water therapy effectively relieved the patient’s symptoms and curbed the progression of the disease, but it did not reverse her severe nerve damage. The relationship between the lack of hemin availability (as the standard of treatment) and the control of the crisis and evolution of the patient remains unknown. Previously, 15 uncontrolled studies of 420 patients with acute hepatic porphyrias have demonstrated that a significant drop in urine ALA and PBG is usually achieved by heme therapy, but that not all patients respond well to this treatment.12 Moreover, McColl et al.13 reported that only half of all patients benefit from heme infusion. Delayed onset of therapy and irreversible neurological damage may contribute to the poor effects of heme treatment.14,15 The present patient’s neuropathy deteriorated to the extent of quadriplegia, mechanical ventilation, and bulbar palsy, which may be correlated with a poor outcome.16 Similarly, O’Malley et al.17 reported that, in a woman with complete flaccid paralysis, most muscle groups had returned to full strength after 3 years. Accordingly, the recovery period of porphyric neuropathy is calculated to be at least several months, and each patient’s prognosis needs continuous attention.18
Table 1.
Comparison between the standard treatment for acute intermittent porphyria and the treatment the patient received in this case.
| Standard treatment | Description of standard treatment | Treatment received |
|---|---|---|
| Remove incentives | Discontinuation of porphyrinogenic drugs | Same as standard treatment |
| High carbohydrate support | High carbohydrate diet or intravenous infusion of glucose (300–500 g/day) | Intravenous infusion of glucose (about 400 g/day) |
| Heme treatment | For severe cases, heme treatment should be given immediately, for up to 4 days (3–4 mg/kg/day) | Not available |
| Symptom measures | ||
| For pain | Acetylsalicylic acid, morphine derivatives, gabapentin | Morphine |
| For tachycardia and hypertension | Propranolol, metoprolol, valsartan | Metoprolol |
| For symptoms of ileus | Neostigmine or enema | Enema |
| For respiratory relief | Assisted or controlled ventilation (possibly tracheotomy) | Controlled ventilation including endotracheal intubation and tracheotomy |
| Early physiotherapeutic measures | Active and passive training of limbs |
We reported rare clinical symptoms of AIP: rapidly progressive neuropathy attacks with severe manifestations that are life-threatening and often difficult to diagnose. Through careful neurophysiological examinations, complete laboratory examinations (cerebrospinal fluid analysis, electromyography, urine exposure test, genetic testing), imaging examinations (CT of the head, chest, and abdomen, and ultrasound of the diaphragm), we finally confirmed the diagnosis of our patient and effectively contained her disease progression. This report may provide some reference for similar cases in the future. The main limitation of this case is that the patient was unable to receive heme treatment, which may have affected her prognosis.
Conclusion
The clinical presentations of AIP are nonspecific and may mimic many common surgical and medical conditions, such as appendicitis and GBS, which can result in the underdiagnosis and misdiagnosis of this disease. When a patient presents with severe progressive neuropathy, AIP should be taken into consideration, despite the appearance of symptoms of common diseases in the ICU, such as hypokalemic periodic paralysis, myasthenia gravis, and severe GBS. Early diagnosis can avoid serious peripheral nerve damage, prevent recurrent episodes, and even save lives.
Ethics statement
All treatments and procedures were performed in accordance with the Declaration of Helsinki of 1975, as revised in 2008, and the patient’s consent for treatment was obtained. The patient provided written informed consent for publication of this case report. Written informed consent was also obtained from the patient’s husband for the publication of any potentially identifiable images or data contained in this article.
Footnotes
Author contributions: SH, RL, and YY contributed substantially to the article concept. SH and RL retrieved the related literature and drafted the manuscript. SH, RL, and YY provided care for critically ill patients and collected clinical data. YY revised and approved the final version before submission. All of the listed authors participated actively in the study, and have read and approved the final manuscript.
Acknowledgements: The authors thank the doctors of the Department of Neurology, Union Hospital of Huazhong University of Science and Technology, for their assistance with diagnosis and treatment.
Declaration of conflicting interest: The authors declare that there is no conflict of interest.
Funding: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
ORCID iD: Yin Yuan https://orcid.org/0000-0003-3392-4026
References
- 1.Nordmann Y, Puy H, Da Silva V, et al. Acute intermittent porphyria: prevalence of mutations in the porphobilinogen deaminase gene in blood donors in France. J Intern Med 1997; 242: 213–217. [DOI] [PubMed] [Google Scholar]
- 2.Kauppinen R, Mustajoki P. Prognosis of acute porphyria: occurrence of acute attacks, precipitating factors, and associated diseases. Medicine (Baltimore) 1992; 71: 1–13. [PubMed] [Google Scholar]
- 3.Albers JW, Robertson WC, Jr, Daube JR. Electrodiagnostic findings in acute porphyric neuropathy. Muscle Nerve 1978; 1: 292–296. [DOI] [PubMed] [Google Scholar]
- 4.Elder G, Harper P, Badminton M, et al. The incidence of inherited porphyrias in Europe. J Inherit Metab Dis 2013; 36: 849–857. [DOI] [PubMed] [Google Scholar]
- 5.Ramanujam VM, Anderson KE. Porphyria diagnostics-part 1: a brief overview of the porphyrias. Curr Hum Genet 2015; 86: 1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lin CS, Lee MJ, Park SB, et al. Purple pigments: the pathophysiology of acute porphyric neuropathy. Clin Neurophysiol 2011; 122: 2336–2344. [DOI] [PubMed] [Google Scholar]
- 7.Pischik E, Kazakov V, Kauppinen R. Is screening for urinary porphobilinogen useful among patients with acute polyneuropathy or encephalopathy? J Neurol 2008; 255: 974–979. [DOI] [PubMed] [Google Scholar]
- 8.Simon NG, Herkes GK. The neurologic manifestations of the acute porphyrias. J Clin Neurosci 2011; 18: 1147–1153. [DOI] [PubMed] [Google Scholar]
- 9.Albers JW, Fink JK. Porphyric neuropathy. Muscle Nerve 2004; 30: 410–422. [DOI] [PubMed] [Google Scholar]
- 10.Bonkovsky HL, Dixon N, Rudnick S. Pathogenesis and clinical features of the acute hepatic porphyrias (AHPs). Mol Genet Metab 2019; 128: 213–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.D'Avola D, López-Franco E, Sangro B, et al. Phase I open label liver-directed gene therapy clinical trial for acute intermittent porphyria. J Hepatol 2016; 65: 776–783. [DOI] [PubMed] [Google Scholar]
- 12.Stolzel U, Doss MO, Schuppan D. Clinical guide and update on porphyrias. Gastroenterology 2019; 157: 365–381. [DOI] [PubMed] [Google Scholar]
- 13.McColl KE, Moore MR, Thompson GG, et al. Treatment with haematin in acute hepatic porphyria. Q J Med 1981; 50: 161–174. [PubMed] [Google Scholar]
- 14.Mustajoki P, Nordmann Y. Early administration of heme arginate for acute porphyric attacks. Arch Intern Med 1993; 153: 2004–2008. [PubMed] [Google Scholar]
- 15.Anderson KE, Collins S. Open-label study of hemin for acute porphyria: clinical practice implications. Am J Med 2006; 119: 801.e19–801.e24. [DOI] [PubMed] [Google Scholar]
- 16.Pischik E, Bulyanitsa A, Kazakov V, et al. Clinical features predictive of a poor prognosis in acute porphyria. J Neurol 2004; 251: 1538–1541. [DOI] [PubMed] [Google Scholar]
- 17.O'Malley R, Rao G, Stein P, et al. Porphyria: often discussed but too often missed. Pract Neurol 2018; 18: 352–358. [DOI] [PubMed] [Google Scholar]
- 18.Schutte CM, Van Der Meyden CH, Van Niekerk L, et al. Severe porphyric neuropathy–importance of screening for porphyria in Guillain-Barre syndrome. S Afr Med J 2015; 106: 44–47. [DOI] [PubMed] [Google Scholar]