Abstract
Background:
Asbestos-related lung diseases are a group of heterogeneous disorders with different pathogenesis and prognosis. Very few studies investigated the BALF cell profile of asbestos exposed workers. The existence of a relationship between bronchoalveolar lavage fluid (BALF) cellular pattern and specific diagnosis and/or asbestos exposure biomarkers would allow the identification of effect biomarkers useful in the follow up of asbestos-exposed workers and in the diagnosis of asbestos-related diseases.
Objectives:
To assess BALF cell profile in formerly asbestos-exposed workers and its relationship with asbestos fibre (amphibole and chrysotile) and asbestos body (AB) concentrations.
Methods:
113 male workers formerly exposed to asbestos underwent bronchoscopy with bronchoalveolar lavage and were retrospectively enrolled. 35 of them were affected by pleural plaques and 10 were affected by asbestosis. Pulmonary functional tests (PFT), BALF cellular pattern, BALF mineralogical analysis with asbestos fibres and AB counting were performed in each patient. A statistical analysis with a multivariate linear regression model was adopted.
Results:
From the statistical analysis of data a direct correlation between pack-years and BALF macrophages was found. Inversely correlation between pack-years and BALF lymphocytes was detected. There was not relationship among BALF cellular pattern, PFT values, specific diagnosis, BALF AB count or BALF asbestos fibre concentration.
Discussion:
BALF cellular pattern does not seem to be related to asbestos exposure biomarkers like AB and asbestos fibre concentration in BALF. Instead, smoke habit can induce an increase in BALF macrophages and a decrease of BALF lymphocytes count.
Key words: Bronchoalveolar lavage, asbestos, asbestosis, neutrophils, lymphocytes, macrophages
Abstract
«Analisi citologica del liquido di lavaggio broncoalveolare nei lavoratori esposti ad asbesto».
Introduzione:
Le patologie asbesto-correlate costituiscono un gruppo eterogeneo con differente patogenesi e prognosi. Sono rari gli studi che hanno indagato il profilo cellulare del BALF dei lavoratori esposti ad asbesto. L’esistenza di una relazione tra pattern cellulare del liquido di lavaggio broncoalveolare (BALF) e diagnosi specifica e/o indicatori di esposizione ad asbesto potrebbe consentire l’individuazione di indicatori di effetto utili nel follow-up degli ex-esposti ad asbesto e nella diagnosi delle patologie asbesto-correlate.
Obiettivi:
Valutare il profilo cellulare del BALF negli ex-esposti ad asbesto e il suo rapporto con le concentrazioni di fibre di asbesto (anfiboli e crisotilo) e corpuscoli dell’asbesto (AB) nel BALF.
Metodi:
Sono stati arruolati retrospettivamente 113 lavoratori maschi ex-esposti ad asbesto sottoposti a broncoscopia con lavaggio broncoalveolare. 35 di loro erano affetti da placche pleuriche da asbesto e 10 da asbestosi. Per ogni paziente venivano effettuate prove di funzionalità respiratoria, esame citologico del BALF, esame mineralogico del BALF con determinazione della concentrazione di fibre di asbesto e AB. L’analisi statistica è stata effettuata con un modello di regressione lineare multivariata.
Risultati:
Dall’analisi statistica dei dati è risultata una correlazione tra pacchetti/anno e macrofagi nel BALF, nonché una correlazione inversa tra pacchetti/anno e linfociti. Non vi erano relazioni tra profilo cellulare del BALF, indici di funzionalità respiratoria, diagnosi specifica e concentrazioni di AB e fibre di asbesto nel BALF.
Discussione:
Il profilo cellulare del BALF non sembra essere in relazione con gli indicatori di esposizione ad asbesto quali le concentrazioni di AB e fibre nel BALF. Al contrario l’abitudine al fumo può indurre un aumento dei macrofagi e una riduzione di linfociti nel BALF.
Abbreviations:
- asbestos bodies (AB)
- pulmonary functional tests (PFT)
- bronchoalveolar lavage fluid (BALF)
- high resolution computed tomography (HRCT)
- forced vital capacity (FVC)
- probability value (P).
Introduction
Bronchoalveolar lavage is a minimally invasive procedure performed during flexible bronchoscopy that allows cells and non-cellular elements to be recovered from the epithelial surface of the alveoli and terminal bronchioles (18). Bronchoalveolar lavage fluid (BALF) is obtained through instillation and subsequent recovery of a saline solution from one or more lung segments. Since BALF comes into close contact with lung tissue, it provides useful information about lung status and alveolar environment (7).
Mineralogical analysis of BALF is used for in vivo assessment of asbestos lung burden (11, 24) and for identification and quantification of asbestos fibre burden in patients with asbestosis (9). Asbestos bodies (AB) in BALF are correlated with AB in the lung and with occupational exposure (3), although AB mainly reflect exposure to amphiboles. Fibre concentrations in BALF can be considered a reliable biomarker of asbestos exposure, even many years after exposure has ceased, while AB concentrations in BALF have good specificity but low sensitivity, being poorly correlated with chrysotile exposure (27, 28, 29). A relationship between higher concentrations of amphibole in BALF and non-malignant asbestos-related diseases was also demonstrated (24).
Asbestos-exposed non-smokers and patients with asbestosis can have a BALF cell profile characterized by dramatically elevated cellularity. It was also reported that patients with asbestosis tend to have high BALF macrophage percentages with a mild increase in neutrophils and eosinophils (21). BALF neutrophilia is related to detection of crackles during physical examination and is more pronounced in patients with advanced disease (3). BALF lymphocyte percentage is also increased in patients with asbestosis. BALF lymphocyte CD4/CD8 ratio, when reported, ranges from 1.0 to 3.9 in asbestos-exposed workers and from 0.8 to 6.9 in patients with asbestosis (21).
Very few studies investigated the cell profile of healthy workers with long exposure to asbestos. The existence of a relationship between BALF cellular pattern and specific diagnosis and/or asbestos exposure biomarkers would allow the identification of effect biomarkers useful in the follow up of asbestos-exposed workers and in the diagnosis of asbestos-related diseases. Kokkinis et al. investigated the cell profile in BALF of 39 apparently healthy workers employed by a company that used chrysotile asbestos for an extended period (20). The finding of AB in BALF multiplied the possibility of lymphocytic alveolitis by three, otherwise alveolitis was polymorphonuclear. Smoking seemed to suppress lymphocytic alveolitis.
A study on 25 formerly asbestos-exposed workers showed a homogeneous distribution of smokers and ILO radiological pattern in the various exposure classes, suggesting that smoking history was not a confounding factor (6). Immunocytological evaluation of BALF showed a significantly higher percentage of lymphocytes in the group with lower exposure and a radiological picture characterized predominantly by pleural plaques, than in the other exposure groups and in subjects with pulmonary fibrosis. In early stages of asbestosis an inflammatory phase characterized by BALF lymphocytic alveolitis was demonstrated. In more advanced stages there was alveolar neutrophilia (6).
In view of such controversial findings regarding the effects of asbestos exposure on the cell composition of BALF, the aim of the study was to assess BALF cell profile and its relationship with asbestos fibres (amphibole and chrysotile) and with AB concentrations in 113 formerly asbestos-exposed workers.
Methods
Subjects
One hundred and thirteen occupationally exposed male workers (n=113, mean age 55 years ± 6.03, range 42–72 years) suspected to have asbestos-related diseases, were hospitalized in the period 2010-2018 at the Occupational Medicine Unit of the University Hospital of Siena. All workers had previous occupational exposure to asbestos documented by an accurate occupational history.
According to the case selection criteria, patients with a diagnosis of idiopathic interstitial pneumonia, non asbestos-related occupational interstitial lung diseases, hypersensitivity pneumonitis, sarcoidosis, collagen vascular diseases and allergic asthma were excluded from the study.
All workers underwent pulmonary functional tests (PFT) and high resolution computed tomography (HRCT) of the chest to assess the possibility of asbestos-related diseases.
The study was approved by the Local Ethics Committee for Clinical Experimentation of Siena University Hospital (SACCM12, 19/07/2013) and all participants gave written informed consent.
Pulmonary Functional Tests
Lung function measurements were recorded according to ATS/ERS standards (22) using a water-sealed spirometer equipped with a helium analyzer (Biomedin, Padua, Italy) with corrections for temperature and barometric pressure. The following parameters were considered: forced vital capacity (FVC), forced expiratory volume in the first second (FEV1), FEV1/FVC, total lung capacity (TLC), residual volume (RV), carbon monoxide lung transfer factor (DLCO) and carbon monoxide lung transfer factor/alveolar volume (DLCO /VA).
Chest CT
HRCT examination consisted of a series (57 images) of 0.625 mm sections separated by 10 mm gaps, obtained in axial scanning mode (0.625/4 images 4 row, 1:10 mm stop and shoot) using a 64 multidetector CT (VCT, General Electric Healthcare, Milwaukee, USA). The following parameters were used: subject position: prone; breathing instructions: suspended full inspiration; scanning mode: axial; display FOV: to encompass lungs at the largest anatomical location; interval: 10 mm; collimation 0.625 mm; reconstruction algorithm: high spatial frequency (bone plus); kV: 140; mAS: 330; time per tube rotation: 0.8 sec; intravenous contrast medium: none. Scanning was performed from the base to apex of the lung in order to avoid diaphragmatic movements. A standard reconstruction algorithm was also used to evaluate pleural alteration. All the 113 CT examinations were retrospectively examined by a Radiologist with more than 12 years of experience in thoracic imaging. The CT examinations were evaluated for evidence of pleural plaques and parenchymal abnormalities according to the International Classification of HRCT for Occupational and Environmental Respiratory Diseases (ICOERD) (32).
A sum grade of ≥2–3 bilateral irregular opacities in lower zones according to the reference film or bilateral honeycombing (sum grade ≥2) would be sufficient to represent fibrosis according to the ICOERD system (33). Subpleural curvilinear lines or dots, both findings of bronchiolar fibrosis at HRCT, were also considered as signs of asbestosis (2).
Mineralogical analysis of BALF
AB in BALF were counted with a phase contrast microscope at a magnification of ×250, as described by De Vuyst (12). Ten ml of BALF were used for asbestos fiber counting with Scanning Electron Microscope (SEM) at a magnification of ×10,000. The samples were filtered onto a 0.45 μm Millipore filter. All reagents were pre-filtered onto a 0.22 μm Millipore filter to avoid asbestos contamination. The sample was treated according to the Dodson method (13). Only particles with an aspect ratio of 3:1 or more were counted not using the lower limits of length and diameter. The detection limit was <160 fibres/ml BALF.
Bronchoalveolar lavage processing and flow cytometric analysis
Bronchoscopy with bronchoalveolar lavage was performed for diagnostic purposes as recommended by the European Respiratory Society Task Force Group on BAL (21). Briefly, BALF was filtered through sterile gauze and cells were counted by cytocentrifuge smear (600 rpm for 5 min) with a Diff quik stain kit (DiaPath, Italy); a total of 500 cells were counted. Cell viability was determined by Trypan blue exclusion in a Burker Chamber. The samples were processed by flow cytometry using a panel of monoclonal antibodies (BD Multitest™ 6-color TBNK, San Jose, CA, USA), including FITC-labeled CD3, PerCP-Cy5.5-labeled CD45, PE-Cy7-labeled CD4, APC-labeled CD19 and APC-Cy7-labeled CD8 according to the manufacturer’s instructions. At least 30,000 events were collected for each sample. The data was analysed using DIVA software (BD-biosciences San Jose, CA, USA). Lymphocytes were distinguished on the basis of forward (FSC) versus side (SSC) scatters and additional gating was applied using SSC versus CD45 to distinguish lymphocytes from cell debris. Specific panels were subsequently assessed to identify T lymphocytes and B lymphocytes. T lymphocyte subpopulations were gated in order to distinguish CD3+CD4+ (T-helper) and CD3+CD8+ (T-cytotoxic).
Statistical analysis
For each of the 113 workers, 11 explanatory variables were considered (age, asbestos-related diagnosis, years between first asbestos exposure and bronchoscopy, smoking in pack/years, % predicted FEV1, % predicted FEV1/FVC, % predicted TLC, % predicted DLCO, BALF concentration of chrysotile fibres, BALF concentration of amphibole fibres, BALF concentration of AB) and five response variables (BALF-macrophages/ml, BALF-lymphocytes/ml, BALF-neutrophil granulocytes/ml, BALF-eosinophil granulocytes/ml, CD4/CD8 ratio). Years between first asbestos exposure and bronchoscopy were included in the considered variables because in previous studies the time from the end of exposure did not significantly influence the fibre concentration in BALF (27, 28). Data of the response variables (i.e. macrophages, lymphocytes, neutrophils and eosinophils) were considered as counts, not as compositional data (i.e. in terms of percentages). Indeed, when compositional data is used for response variables in a regression model, technical difficulties occur and highly non-standard techniques have to be adopted (15, 25). Since the aim of the statistical analysis was to assess dependence between the two groups of variables, it would be reasonable to use a multivariate regression model with five dependent variables and 11 regressors. However, two dependent variables (i.e. neutrophil and eosinophil granulocytes/ml) showed zero inflated distributions (i.e. there was a non-negligible number of zeroes in the data for these variables). A global multivariate linear regression model was therefore not suitable for fitting the data, so 3 separate regression models were used with the same regressors. More precisely, we used a multivariate linear regression model (1, 31) with 3 dependent variables (macrophages/ml, lymphocytes/ml and CD4/CD8 ratio) and 2 zero-inflated Poisson regression models (8) for the dependent variables neutrophils and eosinophils. Parameter estimation and hypothesis testing of the 3 models were performed with R software (26), using the car library to implement the multivariate linear regression and the pscl library to implement the zero-inflated Poisson regression.
Results
Demographic, functional and radiological results
Table 1 reports age, smoking history, history of asbestos exposure, lung function test and BALF features of the study population. With regard to the smoking habit, 19 workers were smokers, 36 were former smokers and 58 non smokers. They all had a certain occupational history of exposure to asbestos. According to the occupational history 8 main industrial activities were singled out: insulation removal and rolling stock production in the railway industry (13 subjects), building industry (12 subjects), shipbuilding yards (4 subjects), metal foundry (25 subjects), engineering industry (13 subjects), transport (5 subjects), electric energy production (24 subjects), other work activities such as chemical industry, typography, sugar refinery, fire fighters (17 subjects). BALF cell analysis did not show any alteration. A direct correlation was found between CD4/CD8 ratio and number of pack/years (p=0.01). Radiological and clinical results indicated that 45 patients (40%) had non-malignant asbestos-related disease: 35 with pleural plaques and 10 with asbestosis with or without pleural plaques (Table 1). Out of the 35 patients with pleural plaques, 28 had a bilateral form, 13 with calcifications, 3 apical, 6 basal, 16 costal and 21 diaphragmatical (in the same subject plaques could have different characteristics simultaneosly). In the whole population of exposed workers BALF samples showed geometric mean (GM) concentrations of 1.11 AB/ml, 332.44 chrysotile fibres/ml and 458.07 amphibole fibres/ml (Table 1). Total asbestos fibre concentration (chrysotile and amphiboles) was above the detection limit in all patients with asbestosis, while in 27 out of 35 patients with only pleural plaques the fibre counting was positive and 50 subjects out of 68 workers without asbestos-related diseases were positive for fibres. Table 2 shows BALF concentration of AB, amphibole and chrysotile fibres in the asbestos exposed workers grouped for diagnosis.
Table 1.
Variables measured in the studied population of male workers formerly exposed to asbestos. Chrysotile and amphiboles fibre concentrations in BALF were below the detection limit in 73 and 49 of the 113 examined workers respectively, while AB were undetectable in 69 subjects out of 113 (these subjects were not considered)
| Mean ± SD | Median | Range | |
| Age (years) | 55.48 ± 6.03 | 55 | 42-72 |
| Years from the first asbestos exposure to bronchoscopy | 34.56 ± 7.52 | 34 | 19-57 |
| Smoke habit (pack/years) in smokers or former smokers | 21.25 ± 13.91 | 19.25 | 2-60 |
| % predicted FEV1 | 101.08 ± 17.36 | 99 | 43-150 |
| % predicted FEV1/FVC | 103.33 ± 8.14 | 104 | 73-120 |
| % predicted TLC | 97.96 ± 11.15 | 97 | 70-126 |
| % predicted DLCO | 94.57 ± 13.91 | 94 | 67-128 |
| Geometric Mean | Range | ||
| BALF concentration of chrysotile (ff/ml BALF) | 332.44 | - | 124-640 |
| BALF concentration of amphiboles (ff/ml BALF) | 458.07 | - | 200-9614 |
| BALF concentration of asbestos bodies (AB/ml BALF) | 1.11 | - | 0.1-231 |
Table 2.
GM (range) of AB, amphiboles and chrysotile fibre concentration/ml BALF in the professionally asbestos exposed workers grouped for diagnosis
| Diagnosis | Chrysotile | Amphyboles | AB |
| Non asbestos-related diseases | 336.10 (200-640) | 398.17 (200-1365) | 0.51 (53.8-0.1) |
| Pleural Plaques | 294.36 (124-496) | 447.78 (160-2361) | 2.84 (125.2-0.1) |
| Pleural Plaques and Asbestosis | 412.14 (248-640) | 777.78 (248-9614) | 2.78 (231-0.2) |
Cytological results
Table 3 describes the BALF cytological parameters in the studied population. As to the output of the multivariate linear regression on the basis of the Pillai test, the pack/years coefficient was solely significant (p= 0.02). As to the output of the two zero-inflated Poisson regression, no coefficient was significant. Hence, results of the analysis lead to conclude that the two groups of variables were not dependent. As to the pack/years, the corresponding estimated regression coefficient was positive for the response variable macrophages, while in contrast it was negative for the response variable lymphocytes (Figure 1).
Table 3.
Description of BALF cytological parameters in the studied population
| Mean ± SD | Reference Values % | |
| Cells/ml | 68287.70 ± 37652.27 | |
| Macrophages % | 78.54 ± 16.91 | >85 |
| Lymphocytes % | 17.72 ± 15.78 | 10-15 |
| Neutrophils % | 3.36 ± 6.54 | ≤3 |
| Eosinophils % | 0.38 ± 0.79 | ≤1 |
| CD4/CD8 % * | 2.49 ± 1.75 | <1 |
* CD4/CD8 ratio was calculated in 100 samples
Figure 1.
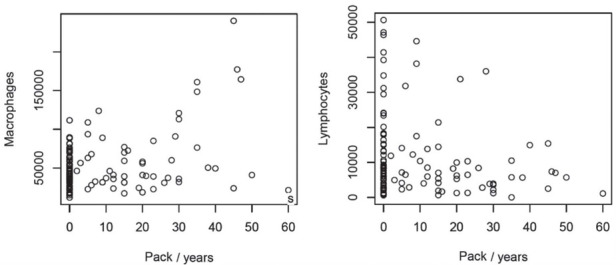
Cell count in BALF vs pack/years (estimated regression slope 940.9 and 151.4 for macrophages and lymphocytes respectively)
Despite the BALF cell profile of the studied population was normal according to our lab parameters, a not significant trend in lymphocytes quote increase and macrophages quote decrease can be observed. Since further statistical analysis failed to find any correlation between this element and specific features, this can be explained considering the natural dispersion of results. Anyway it cannot be excluded that this trend may be in agreement with previous studies (20, 30).
Discussion
The results of the present study showed that the degree of occupational exposure to asbestos in the study population did not affect BALF cellularity. Concentrations of AB, chrysotile and amphibole fibres were not related to the number of BALF cell populations or types of BALF cells, nor to years since the beginning of exposure. These results may be influenced by the relatively low exposure of the studied population. The mean concentration value of amphiboles in the examined workers consisted of 409,23 fibers/ml of BALF. This level was slightly lower than the Confidence Intervals 95% lower limit of amphibole concentration observed in the same laboratory in BALF of patients with asbestosis (24). The GM of 1.11 AB/ml BALF would confirm the hypothesis of a medium/low asbestos exposure, since levels < 1 AB ml BALF are indicative of low exposure.
The radiological evidence of pleural plaques and asbestosis does not seem to affect BALF cell composition in the studied population. This may be due to the small size of the asbestosis subgroup (only 10 patients). Furthermore most asbestosis were represented by early HRCT findings (i.e. dot-like opacities and subpleural curvilinear lines) in asymptomatic patients.
Long biopersistence of asbestos fibres in the lung was demonstrated for amphibole and chrysotile fibres (14), and for most of our workers exposure dated back many years. It is unclear whether cell responses to fibres can change over time. Moreover, the effects of asbestos exposure on BALF cell populations are not only quantitative, but also qualitative and functional. Nishimura et al. demonstrated that asbestos fibres affect alveolar macrophages of exposed rats, triggering an increase in TGF-β production not only by fibroblast stimulation, but also in a non-inflammatory way through direct production (23).
Keskitalo et al. described an increase in BAL neutrophils in patients with asbestosis (19). They also found that a high neutrophil count was a predictor of mortality in these patients. Only patients with asbestosis were investigated, while our population of patients with asbestosis was too limited to compare with the Finnish findings. Unfortunately Keskitalo et al, considering data for the BALF cell populations as percentage and not as counts, failed to adopt ad hoc techniques, as required for a correct compositional data analysis. Thus their conclusions were not supported by a sound statistical analysis (4).
There were no statistically significant correlations between respiratory function parameters and cytological findings in BALF. This is easy to explain considering that the study focused on exposed workers and not solely on patients with asbestos-related diseases, thus more than a half of the studied population was apparently healthy and most part of subjects were asymptomatic.
The only statistically significant variable examined was smoking. Pack/years were directly correlated with BALF macrophages/ml, and inversely correlated with BALF lymphocytes/ml and CD4/CD8 ratio. This suggests that smoking modifies BALF cell profiles and in line with our results others have demonstrated that smoking usually increases the total cell recovery, BALF macrophage numbers and BALF cell proportions, whereas BALF lymphocyte numbers and percentages tend to decrease in smokers (16).
Schwartz et al. compared BALF cells in 73 asbestos-exposed workers, divided into patients with asbestosis (n=25), patients with asbestos-induced pleural fibrosis (n=28) and healthy asbestos-exposed subjects (n=20) (30). Patients with asbestosis had elevated concentrations of BALF macrophages, neutrophils and eosinophils, and patients with asbestos-induced pleural disease showed an increase in BALF lymphocytes. Most of the BALF cytology features related to asbestosis were also linked to smoking history. Authors concluded that cigarette smoking strongly influenced BALF cellularity (macrophages and eosinophils) in patients with asbestosis but did not appear to affect the type or concentration of BALF cells in patients with asbestos-induced pleural fibrosis. In the studied population BALF macrophages were not related to any specific diagnosis, except smoking history, specifically a significant correlation between macrophage count and pack/years. This result may be due to the relatively few patients with asbestosis in the worker population, making the effect of smoking on BALF cytology significantly more evident. Scwartz et al. also recognized a greater effect of smoking than of asbestosis on BALF macrophage count.
The present study confirmed the previously described role of smoking on BALF cytology (5, 10, 17). Smoking induces macrophage alveolitis with a great quantity of cells. Asbestos exposure was not associated with any specific hallmark in terms of BALF cell profile. Thus mineralogical analysis of BALF should be considered essential because BALF cytology alone does not provide sufficient information regarding asbestos exposure and specific pathological diagnoses of non malignant asbestos-related diseases at least in not heavily exposed workers.
No potential conflict of interest relevant to this article was reported by the authors
References
- 1.Agresti A. New York: Wiley; 2015. Foundations of Linear and Generalized Linear Models. [Google Scholar]
- 2.Akira M, Yokoyama K, Yamamoto S, et al. Early asbestosis: evaluation with high-resolution CT. Radiology. 1991;178:409–441. doi: 10.1148/radiology.178.2.1987601. [DOI] [PubMed] [Google Scholar]
- 3.American Thoracic Society: Diagnosis and initial management of nonmalignant diseases related to asbestos. Am J Respir Crit Care Med. 2004;170:691–715. doi: 10.1164/rccm.200310-1436ST. [DOI] [PubMed] [Google Scholar]
- 4.Barabesi L, Sartorelli P. Compositional data analysis. Occup Environ Med. 2020:pii. doi: 10.1136/oemed-2019-106370. oemed-2019-106370; doi: 10.1136/oemed-2019-106370 [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 5.Bronchoalveolar lavage constituents in healthy individuals, idiopathic pulmonary fibrosis, and selected comparison groups. The BAL Cooperative Group Steering Committee. Am Rev Respir Dis. 1990;141(2 Pt 2):169–202. doi: 10.1164/ajrccm/141.5_Pt_2.S169. [DOI] [PubMed] [Google Scholar]
- 6.Brunetti G, Delmastro M, Fonte R, et al. Immunocytological and mineralogical study of bronchoalveolar lavage in a group of subjects exposed to asbestos. G Ital Med Lav Ergon. 2003;25:152–160. [PubMed] [Google Scholar]
- 7.Callejon-Leblic B, Garcia-Barrera T, Pereira-Vega A, Gomez-Ariza JL. Metabolomic study of serum, urine and bronchoalveolar lavage fluid based on gas chromatography mass spectrometry to delve into the pathology of lung cancer. J Pharm Biomed Anal. 2019;163:122–129. doi: 10.1016/j.jpba.2018.09.055. [DOI] [PubMed] [Google Scholar]
- 8.Cameron AC, Trivedi PK. Cambridge: University Press; 2005. Microeconometrics: Methods and Applications. [Google Scholar]
- 9.Cordeiro CR, Jones JC, Alfaro T, Ferreira AJ. Bronchoalveolar lavage in occupational lung diseases. Semin Respir Crit Care Med. 2007;28:504–513. doi: 10.1055/s-2007-991523. [DOI] [PubMed] [Google Scholar]
- 10.Costabel U, Guzman J. Effect of smoking on bronchoalveolar lavage constituents. Eur Respir J. 1992;5:776–779. [PubMed] [Google Scholar]
- 11.Cruz MJ, Curull V, Pijuan L, et al. Utility of Bronchoalveolar Lavage for the Diagnosis of Asbestos-Related Diseases. Arch Bronconeumol. 2017;53:318–323. doi: 10.1016/j.arbres.2016.08.016. [DOI] [PubMed] [Google Scholar]
- 12.De Vuyst P, Dumortier P, Moulin E, et al. Diagnostic value of asbestos bodies in bronchoalveolar lavage fluid. Am Rev Respir Dis. 1987;136:1219–1224. doi: 10.1164/ajrccm/136.5.1219. [DOI] [PubMed] [Google Scholar]
- 13.Dodson RF, Garcia JG, O’Sullivan M, et al. The usefulness of bronchoalveolar lavage in identifying past occupational exposure to asbestos: a light and electron microscopy study. Am J Ind Med. 1991;19:619–628. doi: 10.1002/ajim.4700190506. [DOI] [PubMed] [Google Scholar]
- 14.Feder IS, Tischoff I, Theile A, et al. The asbestos fibre burden in human lungs: new insights into the chrysotile debate. Eur Respir J. 2017;49:pii: 1602534. doi: 10.1183/13993003.02204-2017. doi: 10.1183/13993003.02534-2016. [DOI] [PubMed] [Google Scholar]
- 15.Filzmoser P, Hron K, Templ M. Applied Compositional Data Analysis with Worked Examples in R. New York: Springer. 2018 [Google Scholar]
- 16.Heron M, Grutters JC, ten Dam-Molenkamp KM, et al. Bronchoalveolar lavage cell pattern from healthy human lung. Clin Exp Immunol. 2012;167:523–531. doi: 10.1111/j.1365-2249.2011.04529.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Karimi R, Tornling G, Grunewald J, et al. Cell recovery in bronchoalveolar lavage fluid in smokers is dependent on cumulative smoking history. PLoS One. 2012;7:e34232. doi: 10.1371/journal.pone.0034232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kebbe J, Abdo T. Interstitial lung disease: the diagnostic role of bronchoscopy. J Thorac Dis. 2017;9:S996–S1010. doi: 10.21037/jtd.2017.06.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Keskitalo E, Varis L, Bloigu R, Kaarteenaho R. Bronchoalveolar cell differential count and the number of asbestos bodies correlate with survival in patients with asbestosis. Occup Environ Med. 2019;76:765–771. doi: 10.1136/oemed-2018-105606. [DOI] [PubMed] [Google Scholar]
- 20.Kokkinis FP, Bouros D, Hadjistavrou K, et al. Bronchoalveolar lavage fluid cellular profile in workers exposed to chrysotile asbestos. Toxicol Ind Health. 2011;27:849–856. doi: 10.1177/0748233711399315. [DOI] [PubMed] [Google Scholar]
- 21.Meyer KC, Raghu G, Baughman RP, et al. An official American Thoracic Society clinical practice guideline: the clinical utility of bronchoalveolar lavage cellular analysis in interstitial lung disease. Am J Respir Crit Care Med. 2012;185:1004–1014. doi: 10.1164/rccm.201202-0320ST. [DOI] [PubMed] [Google Scholar]
- 22.Miller MR, Crapo R, Hankinson J, et al. General considerations for lung function testing. Eur Respir J. 2005;26:153–161. doi: 10.1183/09031936.05.00034505. [DOI] [PubMed] [Google Scholar]
- 23.Nishimura Y, Maeda M, Kumagai-Takei N, et al. Altered functions of alveolar macrophages and NK cells involved in asbestos-related diseases. Environ Health Prev Med. 2013;18:198–204. doi: 10.1007/s12199-013-0333-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Paolucci V, Romeo R, Sisinni AG, et al. Asbestos exposure biomarkers in the follow-up of asbestos-exposed workers. Ind Health. 2018;56:249–254. doi: 10.2486/indhealth.2017-0125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pawlowsky-Glahn V, Egozcue J.J. Tolosana-Delgado R: Modeling and Analysis of Compositional Data. Chichester, UK: Wiley. 2015 [Google Scholar]
- 26.R Development Core Team: A Language and Enviroment for Statistical Computing. Vienna, Austria: Foundation for Statistical Computing. 2019 [Google Scholar]
- 27.Romeo R, Scancarello G, Cassano P, et al. Assessment of asbestos exposure via mineralogical analysis of bronchoalveolar lavage fluid. Med Lav. 2004;95:17–31. [article in Italian] [PubMed] [Google Scholar]
- 28.Sartorelli P, Scancarello G, Romeo R, et al. Asbestos exposure assessment by mineralogical analysis of bronchoalveolar lavage fluid. J Occup Environ Med. 2001;43:871–881. doi: 10.1097/00043764-200110000-00006. [DOI] [PubMed] [Google Scholar]
- 29.Sartorelli P, Romeo R, Scancarello G, et al. Measurement of asbestos fibre concentrations in fluid of repeated bronchoalveolar lavages of exposed workers. Ann Occup Hyg. 2007;51:495–500. doi: 10.1093/annhyg/mem014. [DOI] [PubMed] [Google Scholar]
- 30.Schwartz DA, Galvin JR, Merchant RK, et al. Influence of cigarette smoking on bronchoalveolar lavage cellularity in asbestos-induced lung disease. Am Rev Respir Dis. 1992;145:400–405. doi: 10.1164/ajrccm/145.2_Pt_1.400. [DOI] [PubMed] [Google Scholar]
- 31.Seber GAF. New York: Springer; 2015. The linear Model and Hypothesis. [Google Scholar]
- 32.Suganuma N, Kusaka Y, Hering KG, et al. Reliability of the proposed international classification of high-resolution computed tomography for occupational and environmental respiratory diseases. J Occup Health. 2009;51:210–222. doi: 10.1539/joh.l8030. [DOI] [PubMed] [Google Scholar]
- 33.Wolff H, Vehmas T, Oksa P, et al. Asbestos, asbestosis, and cancer, the Helsinki criteria for diagnosis and attribution 2014: recommendations. Scand J Work Environ Health. 2015;41:5–15. doi: 10.5271/sjweh.3462. [DOI] [PubMed] [Google Scholar]


