Abstract
Background
Patients infected by SARS-CoV-2 can develop interstitial pneumonia, requiring hospitalisation or mechanical ventilation. Increased levels of inflammatory biomarkers are associated with development of acute respiratory distress syndrome (ARDS). The aim of the present study was to determine which cytokines are associated with respiratory insufficiency in patients hospitalised for COVID-19.
Patients and methods
Data on 67 consecutive patients were collected between March 8 and March 30, 2020. PaO2/FiO2 ratio (P/F) was calculated at hospital admission. The following cytokines were analysed: interleukin (IL)-6, IL-1α, IL-18, tumour necrosis factor (TNF)-β, macrophage colony-stimulating factor (M-CSF), macrophage migration inhibitory factor (MIF), soluble IL-2 receptor alpha (sIL-2Rα; CD25), IL-12β, IL-3, interferon (IFN) α2a, monokine induced by gamma interferon (MIG), monocyte-chemotactic protein 3 (MCP3) and hepatocyte growth factor (HGF).
Results
P/F lower than 300 was recorded in 22 out of 67 patients (32.8%). P/F strongly correlated with IL-6 (r = −0.62, P < 0.0001), M-CSF (r = −0.63, P < 0.0001), sIL-2Rα (r = −0.54, P < 0.0001), and HGF (r = −0.53, P < 0.0001). ROC curve analyses for IL-6 (AUC 0.83, 95% CI 0.73–0.93, P < 0.0001), M-CSF (AUC 0.87, 95% CI 0.79–0.96, P < 0.0001), HGF (AUC 0.81, 95% CI 0.70–0.93, P < 0.0001), and sIL-2Rα (AUC 0.80, 95% CI, 0.69–0.90, P < 0.0001) showed that these four soluble factors were highly significant. All four soluble factors correlated with LDH, white blood cell count, neutrophil count, lymphocyte count, and CRP.
Conclusion
IL-6, M-CSF, sIL-2Rα, and HGF are possibly involved in the main biological processes of severe COVID-19, mirroring the level of systemic hyperinflammatory state, the level of lung inflammation, and the severity of organ damage.
Keywords: Coronavirus, COVID-19, Pneumonia, Interleukin 6, Hepatocyte growth factor, M-CSF, Interleukin 2
1. Introduction
Novel coronavirus (SARS-CoV-2) infection causes a severe respiratory syndrome called COVID-19. COVID-19 symptoms are similar to those observed in previous coronavirus outbreaks [1] and have led to alarmingly large numbers of patients admitted to the intensive care unit (ICU), where lethality rates are high [2]. Histopathological investigations have revealed that the virus spreads into many organs due to effective engagement of the virus spike protein with the ubiquitous human ACE-2 receptor [3], [4]. COVID-19 shows three stages of severity [5], [6]. The early stage is characterised by infection with SARS-CoV-2 when flu-like symptoms can occur. The second phase is characterised by viral pneumonia, which can cause patients to require hospitalisation and mechanical ventilation. During this phase, pulmonary inflammation and coagulopathy can develop. The worst outcome in this phase is often associated with increased levels of inflammatory biomarkers such as C-reactive protein (CRP), ferritin, IL-6, IL-1, and D-dimer [5], [6], [7]. Finally, the third stage is characterised by fibrosis. Recent studies on SARS-CoV-2 pathogenesis have highlighted the role of proinflammatory monocytes and lymphocytes as key mediators of the hyperinflammatory response produced by viral shedding in the first phases; these proinflammatory monocytes and lymphocytes are also involved in the hyperinflammatory state observed in severe cases [8]. The aim of the present study was to determine the cytokine profile associated with respiratory failure in COVID-19 patients at time of hospitalisation by evaluating a set of cytokines associated with hyperinflammation, monocyte and lymphocyte activation, and organ damage.
2. Patients and methods
This study retrospectively analysed blood samples from 67 consecutive COVID-19 patients. All patients were diagnosed for SARS-CoV-2 infection by positive RT-PCR test on nasopharyngeal swab samples. Inclusion criteria were age ≥ 18 years and admission to our hospital S. Maria della Misericordia of Udine, Italy at the Infectious Disease Clinic or ICU. All patients were admitted between March 8 and March 30, 2020. The mean age of patients was 58.6 ± 13.4 years, and 49 of the 67 patients were male. Hospitalisation occurred after a mean time of 6.3 ± 4.1 days from disease onset. In 9 patients (13.4%), Charlson’s comorbidity index was above 2. Hypertension was recorded in 26 patients (38.8%). The mean weight of this cohort was 92.3 ± 17.3 kg. Eighteen patients (26.9%) were treated with intravenous tocilizumab due to increased IL-6 levels [9]. Thirteen patients (19.4%) were admitted or transferred to the ICU. Thirty-four patients (50.7%) required oxygen therapy, 18 patients (26.9%) required non-invasive ventilation (NIV), and 12 patients (17.9%) were intubated. As of June 30, 2020, none of the 67 patients had died and all were discharged. PaO2/FiO2 ratio (P/F) was calculated in all of patients upon hospital admission. Routine laboratory test results were collected. On the basis of previous findings [8] on the main soluble drivers of systemic inflammation, organ damage, and immune response to the virus, the following cytokines, chemokines, and growth factors were analysed in all patients: IL-6, IL-1α, IL-18, tumour necrosis factor (TNF)-β, macrophage colony-stimulating factor (M-CSF), macrophage migration inhibitory factor (MIF), soluble IL-2 receptor alpha or CD25 (sIL-2Rα), IL-12β, IL-3, interferon (IFN) α2a, monokine induced by gamma interferon (MIG), monocyte-chemotactic protein 3 (MCP3), and hepatocyte growth factor (HGF).
Serum IL-6 (pg/mL) was measured by CE-IVD electrochemiluminescence immunoassay (Elecsys IL6, Cobas, Roche; physiological range < 7 pg/mL). All other soluble factors were quantified in the same sera of patients with a magnetic bead-based multiplex assay (Bio-Plex Pro™ Custom Human Cytokines and Chemokine Panel, procarta-Plex, Bio-Rad Laboratories) according to the manufacturer’s instructions. All dosages were done in sera stored in aliquots at −80 °C.
Continuous variables are reported as mean and standard deviation or median and interquartile range (IQR); categorical variables are reported as frequency. For continuous variables, comparisons between groups were made by parametric tests (t-test for independent samples) or non-parametric tests (Mann-Whitney test); chi-square test or Fisher’s exact test was used for comparison of proportions. Bivariate correlations were made by two-tailed Pearson or Spearman tests. All statistical analyses were performed in SPSS version 15.0 software (SPSS Inc.). A 2-sided α < 0.05 was considered statistically significant. Correction for multiple comparisons was not performed due to the explorative nature of the study. Only cytokines that demonstrated a moderate to high level of correlation (r > 0.5) were selected for ROC curve analyses. P/F < 300 was chosen as the cut-off value to distinguish patients with the most severe respiratory symptoms and highest risk of mortality [10]. Ethical approval for the present retrospective observational study was given by Comitato Etico Unico Regionale (CEUR) (registration number: CEUR-2020-Os-102).
3. Results
The median P/F for patients was 319.5 (IQR = 277–374.5). P/F<300 was recorded in 22 out of 67 patients (32.8%).P/F was significantly lower in patients undergoing tocilizumab treatment (median = 254, IQR = 150.25–291) compared with other patients (median = 341, IQR = 309.75–380; P < 0.0001); 14 out of 18 patients
(77.8%) undergoing tocilizumab treatment had a P/F value<300. Characteristics of patient cohorts with P/F < 300 and ≥ 300 are reported in Table 1 . Notably, the two groups of patients did not show any difference with regard to age, sex, comorbidity index, and time from disease onset to hospitalisation. On the other hand, routine laboratory parameters were significantly different between the two groups, with the exception of D-dimer values. Significant differences were also observed in all other soluble factors, with the exception of IL-1α and MIF (Table 1).
Table 1.
Characteristics of COVID-19 patients.
| Features | Patients with P/F < 300 (N = 22) |
Patients with P/F ≥ 300 (N = 45) |
P value |
|---|---|---|---|
| Age at admission, years | 57.9 ± 11.3 | 58.9 ± 14.4 | 0.77 |
| Proportion of male patients | 17/22 (77.3%) | 32/45 (71.1%) | 0.59 |
| Charlson’s index ≥ 2 | 1/21 (4.5%) | 8/45 (17.8%) | 0.25 |
| Patients requiring ventilators | 11/22 (50%) | 1/45 (2.2%) | <0.0001 |
| Patients requiring NIV | 14/22 (63.6%) | 4/41 (8.9%) | <0.0001 |
| Patients requiring oxygen therapy (non-NIV) | 20/22 (90.9%) | 14/45 (31.1%) | <0.0001 |
| Time from onset to hospitalisation, days | 5.7 ± 3.9 | 6.6 ± 4.2 | 0.41 |
| WBC, cell/mm3 | 6440.4 ± 1759.1 | 4935.3 ± 1723.7 | 0.001 |
| Neutrophil count, cell/mm3 | 5143.2 ± 1734.9 | 3453.9 ± 1724.9 | 0.0003 |
| Lymphocyte count, cell/mm3 | 792.3 ± 311.2 | 964.1 ± 306.8 | 0.04 |
| CRP, mg/L | 112.5 (53.9–201.05) | 24.1 (9.8–67.34) | 0.001 |
| D-dimer*, ng/mL | 939 (479–1923) | 754 (308.5–932) | 0.39 |
| LDH**, IU/L | 573 (439.25–847.75) | 442 (375.75–581.5) | 0.005 |
| CK***, IU/L | 134 (78.5–246.5) | 93 (56.75–150) | 0.008 |
| IL-6, pg/mL | 51.5 (32.75–106.5) | 19 (8.5–34.5) | <0.0001 |
| IL-1α, pg/mL | 0.01 (0.01–0.01) | 0.01 (0.01–0.01) | 0.89 |
| IL-18, pg/mL | 192.89 (97.04–292.35) | 108.58 (77.8–181.47) | 0.02 |
| TNFβ, pg/mL | 1.6 (0.8–7.6) | 0.29 (0.01–1.88) | 0.001 |
| M-CSF, pg/mL | 55.5 (42.32–75.92) | 24.52 (16.16–33.78) | <0.0001 |
| MIF, pg/mL | 220.81 (144.89–321.46) | 158.88 (121.49–203.82) | 0.06 |
| sIL-2Rα, pg/mL | 191.82 (141.13–269.78) | 119.33 (84.84–165.52) | <0.0001 |
| IL-12b p40, pg/mL | 0.01 (0.01–231.27) | 0.01 (0.01–0.01) | 0.003 |
| IL-3, pg/mL | 104.41 (41.93–146) | 34 (1.74–86.19) | 0.001 |
| IFNα2a, pg/mL | 76.7 (60.1–95.93) | 63.38 (46.24–86.48) | 0.03 |
| MIG | 1496.97 (863.11–2649.01) | 1036 (573.45–1378.56) | 0.002 |
| MCP-3 | 0.01 (0.01–104.46) | 0.01 (0.01–0.01) | 0.04 |
| HGF, pg/mL | 1438.42 (957.94–2707.33) | 715.22 (532.09–1123.53) | 0.001 |
Results are expressed in mean ± standard deviation or median (25–75 interquartile range).
Legend: NIV, non-invasive ventilation; WBC, white blood cell; CRP, C-Reactive Protein; LDH, lactate dehydrogenase; CK, creatine kinase; IL, interleukin; HGF, hepatocyte growth factor; IFN, interferon; M-CSF, monocyte colony stimulator factor; MIF, migration inhibitory factor; MIG, monokine induced by gamma interferon; MCP-3, monocyte-chemotactic protein; TNF, tumour necrosis factor.
*Measured in 29 patients (11 and 18 in P/F < 300 and P/F ≥ 300, respectively); **measured in 66 patients (22 and 42 in P/F < 300 and P/F ≥ 300, respectively); ***measured in 63 patients (21 and 42 in P/F < 300 and P/F ≥ 300, respectively).
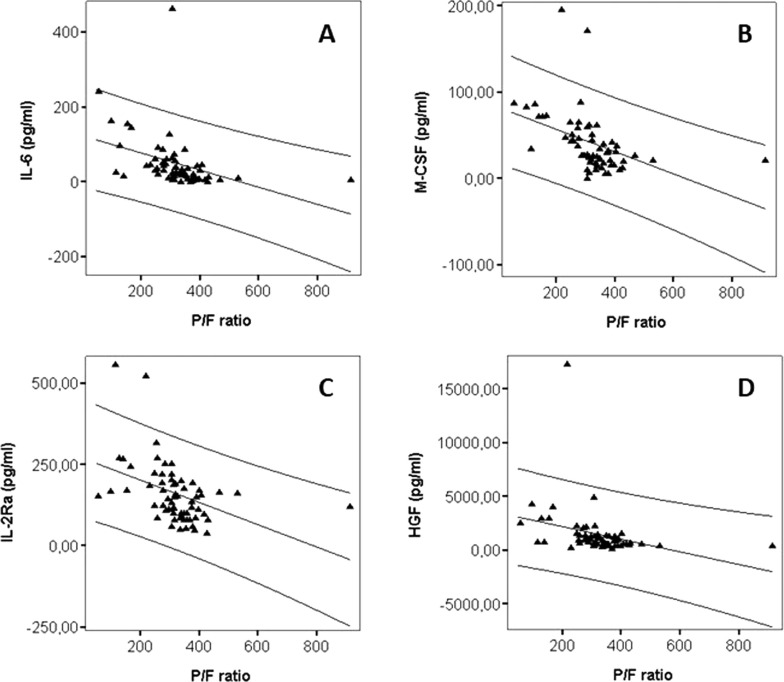
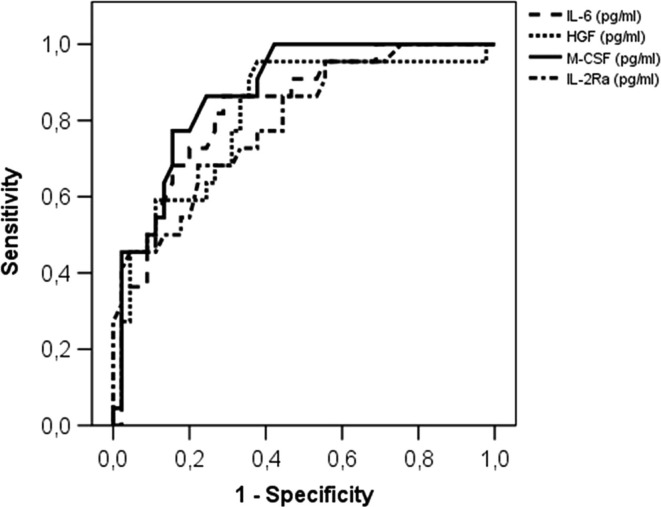
P/F significantly correlated with IL-6 (r = −0.62, P < 0.0001) (Fig. 1 A), IL-18 (r = −0.37, P = 0.003), TNFβ (r = −0.40, P = 0.001), M-CSF (r = −0.63, P < 0.0001) (Fig. 1 B), MIF (r = −0.37, P = 0.002), sIL-2Rα (r = −0.54, P < 0.0001) (Fig. 1 C), IL-3 (r = −0.29, P = 0.02), MIG (r = −0.40, P = 0.001), and HGF (r = −0.53, P < 0.0001) (Fig. 1 D); there was no significant correlation of P/F with IL-12β (r = −0.24, P = 0.06), IFNα2a (r = −0.22, P = 0.08), MCP3 (r = −0.14, P = 0.26), and IL-1α (r = −0.11, P = 0.37). Of note, IL-6 highly correlated with both M-CSF (r = 0.67, P < 0.0001) and HGF (r = 0.67, P < 0.0001), and M-CSF was highly correlated with HGF (r = −0.80, P < 0.0001). In addition, sIL-2Rα correlated with IL-6 (r = 0.44, P = 0.0002), HGF (r = 0.54, P < 0.0001) and M-CSF (r = 0.67, P < 0.0001). ROC curve analyses for IL-6 (AUC 0.83, 95% CI 0.73–0.93, P < 0.0001), M-CSF (AUC 0.87, 95% CI 0.79–0.96, P < 0.0001), HGF (AUC 0.81, 95% CI 0.70–0.93, P < 0.0001), and sIL-2Rα (AUC 0.80, 95% CI, 0.69–0.90, P < 0.0001) showed that these four soluble factors were highly significant (Fig. 2 ). The best cut-off values to distinguish patients with P/F lower than 300 from patients with P/F higher than 300 were: 37.5 pg/mL (sensitivity 73%, specificity 80%) for IL-6, 1002.5 pg/mL (sensitivity 73%, specificity 69%) for HGF, 43.1 pg/mL (sensitivity 77%, specificity 84%) for M-CSF, and 152.0 pg/mL for sIL-2Rα (sensitivity 73%, specificity 67%).
Fig. 1.
Correlation between P/F values and IL-6 (A), M-CSF (B), sIL-2α (C), and HGF (D).
Fig. 2.
ROC curve analysis of IL-6, M-CSF, sIL-2Rα and HGF for predicting P/F below 300.
All four soluble factors correlated with LDH, white blood cell count, neutrophil count, lymphocyte count, and CRP. All but sIL-2Rα correlated with D-dimer, whereas only HGF slightly correlated with creatine kinase (CK) (Table 2 ).
Table 2.
Spearman's Rho correlation between soluble factors and laboratory parameters in COVID-19 patients.
| IL-6 |
M-CSF |
sIL-2Rα |
HGF |
|||||
|---|---|---|---|---|---|---|---|---|
| Rho | P value | Rho | P value | Rho | P value | Rho | P value | |
| CRP | 0.547 | <0.0001 | 0.731 | <0.0001 | 0.474 | <0.0001 | 0.722 | <0.0001 |
| LDH | 0.525 | <0.0001 | 0.416 | 0.0005 | 0.292 | 0.02 | 0.549 | <0.0001 |
| CK | 0.246 | 0.05 | 0.245 | 0.05 | 0.166 | 0.19 | 0.315 | 0.01 |
| D-dimer | 0.451 | 0.01 | 0.389 | 0.04 | −0.017 | 0.93 | 0.414 | 0.025 |
| WBC | 0.368 | 0.002 | 0.585 | <0.0001 | 0.377 | 0.002 | 0.601 | <0.0001 |
| Neutrophil | 0.461 | <0.0001 | 0.659 | <0.0001 | 0.452 | 0.0001 | 0.648 | <0.0001 |
| Lymphocyte | −0.343 | 0.005 | −0.319 | 0.009 | −0.293 | 0.02 | −0.246 | 0.046 |
Legend: CRP, C-Reactive Protein; LDH, lactate dehydrogenase; CK, creatine kinase; WBC, white blood cell; IL, interleukin; HGF, hepatocyte growth factor; M-CSF, monocyte colony-stimulating factor.
4. Discussion
During SARS-CoV-2 infection, 25% of patients develop pneumonia and 10% require mechanical ventilation and ICU admission [1]. Virus lethality has been linked to the occurrence of a hyperinflammatory state or “cytokine storm” [2] characterised by abnormal production of proinflammatory cytokines which leads to respiratory failure and widespread tissue damage, resulting in multiorgan failure and death [3]. Therapies that target specific cytokines could improve survival rates from COVID-19, although results are variable and still preliminary [11], [12].
Early analyses of plasma cytokine levels in Chinese cases showed that COVID-19 patients had increased levels of many cytokines, including IL-1β, IL-8, G-CSF, GM-CSF, IFNγ, IP-10, MCP-1, MIP, PDGF, TNFα, and VEGF, compared with healthy controls [1]. Increased cytokines recruit macrophages, neutrophils, and T-lymphocytes to the infection site, which can cause vascular barrier damage, capillary and endothelial damage, diffuse alveolar damage, multiorgan failure, and death [3]. The lungs are the main target organ of the hyperinflammatory state, and this process may progress into acute lung injury [12]. Although the mechanisms underlying severe pneumonia in COVID-19 patients are not yet fully understood, excessive proinflammatory cytokine production is considered to be one of the most important contributing factors [13], [14], [15].
In the present work, in patients admitted to the hospital with COVID-19 pneumonia, the soluble factors IL-6, M-CSF, sIL2Rα, and HGF showed the strongest association with P/F levels below 300, the recognised cut-off value for the definition of respiratory failure [10]. Moreover, ROC curve analyses determined the best cut-off values for these four soluble factors, including IL-6 (37.5 pg/mL), which is the target of several biologic agents (i.e., tocilizumab, sarilumab, sirukumab, and siltuximab) used to treat COVID-19 patients. IL-6 together with IL-8 and TNFα have been correlated with survival prediction in COVID-19 patients [16]. Importantly, in this study, all four soluble factors correlated with biomarkers that are clearly emerging as crucial factors for the definition of severe forms of COVID-19 disease [17].
Therapies that target GM-CSF could be a suitable approach to reduce the hyperinflammatory state upstream of IL-1 and IL-6 and target both neutrophils and macrophages. In fact, GM-CSF can be included in the group of proinflammatory cytokines. GM-CSF is synthesised by many immune cells, including macrophages, T-cells, fibroblasts, endothelial cells, and epithelial cells [18], [19], and it is released in large amounts at sites of inflammation [20]. GM-CSF is also implicated in lung physiology. Increased circulating levels of GM-CSF have been described in patients with COVID-19 compared with healthy controls [1]. A recent study reported rapid activation of lung CD4+ T lymphocytes to pathogenic T helper (Th) 1 cells in the lungs of COVID 19 patients, especially in those admitted to the ICU, resulting in production of GM-CSF and IL-6. This potent proinflammatory environment strongly induced CD14+CD16+ monocytes, which in turn also contributed to GM-CSF and IL-6 release, further exacerbating the cytokine storm. These aberrant and numerous GM-CSF+-IL-6+ cells may access the pulmonary environment, which could explain the detrimental effects of hyperinflammation in severe and fatal COVID-19 cases [21]. While we did not directly study GM-CSF, GM-CSF and M-CSF share some biological functions, although it is currently unclear whether they perform redundant functions in the body [22]. Both GM-CSF and M-CSF play important roles in alveolar macrophage differentiation and proliferation [23], [24], [25]. In mice, lung levels of GM-CSF and M-CSF are low but increase during inflammation, and these cytokines may have a role in macrophage polarisation [22]. M-CSF, but not GM-CSF, showed increased serum levels in symptomatic COVID-19 patients compared with asymptomatic or convalescent patients [8]. Thus, elevated M-CSF levels in serum may mirror lung inflammation status in COVID-19 infections. Targeting GM-CSF in COVID-19 patients has shown encouraging results, which indirectly supports this hypothesis [26].
Another cytokine, sIL-2Rα, also known as CD25, appears to play a role in the biology of COVID-19. High levels of sIL-2Rα are associated with low levels of circulating lymphocytes characteristic of COVID-19 patients, particularly severely ill patients [27]. Exhausted lymphocytes have been associated with elevated inflammatory cytokines in COVID-19 [28]. However, the mechanism of lymphopenia in COVID-19 is unclear. IL-2 is critical for proliferation, differentiation, and function of many subsets of T cells, including Tregs, CD4+, and CD8+ cells [29]. Recently, a negative correlation between serum sIL-2Rα levels and T-cell number in COVID-19 patients has been described [27]. It has been hypothesised that IL-2 signalling induces lymphopenia in COVID-19. Severe COVID-19 patients show increased levels of sIL-2Rα/CD25 [30], which could be released from cell surfaces due to inflammation-induced enhanced proteolytic cleavage, leading to lymphopenia often reported in severe COVID-19 pneumonia. Therefore, agents that enhance activity of T cells such as Tregs might represent a possible strategy for management of COVID-19 pneumonia [31].
Finally, the present study reports for the first time elevated HGF levels in COVID-19 pneumonia patients and correlates HGF with P/F. Only a non-peer-reviewed preprint study has addressed this issue [32]. HGF is produced by stromal cells and stimulates epithelial cell proliferation, motility, morphogenesis, and angiogenesis in several organs [33]. Endogenous HGF is required for self-repair after organ damage in the liver, lungs, and kidneys [33]. During organ damage, plasma HGF levels significantly increased, while anti-HGF antibody administration strongly increased tissue destruction in animal models. Thus, HGF is required for limiting disease extent, and insufficient HGF production leads to organ failure [33]. Therefore, HGF supplementation ameliorates pathological conditions [33], inhibiting inflammation by interfering with proinflammatory NF-κB signalling [33]. In COVID-19, this may represent a consequence of inflammation leading to organ damage, particularly in the lung. IL-6 and TNFα seem to upregulate HGF in a negative feedback loop of damage and repair [34]. Interestingly, in our study, HGF was the sole soluble factor that correlated with CK, a possible index of cardiac damage. This feature could contribute to novel approaches to address treatment of severe COVID-19 cases by favouring biological repairing processes.
This study has some limitations. It included only patients with COVID-19 pneumonia, and thus analysis of other subpopulations of infected patients have not been performed (e.g., asymptomatic or convalescent patients). The cytokine profile panel was chosen on the basis of current knowledge of COVID-19 pathophysiology and biology; however, many other soluble factors such as IL-8 are probably implicated in the disease [35]. In addition, the patient cohort was small, and thus the results need further validation in larger, independent studies.
To conclude, we identified four soluble biomarkers involved in the main biological processes leading to severe COVID-19 manifestations. Some of these biomarkers are currently being tested in ongoing clinical trials as potential targets for future therapies. IL-6 could mirror the levels of systemic hyperinflammatory state, and sIL-2Rα can be directly implicated in lymphopenia; M-CSF may reflect levels of lung inflammation. Moreover, HGF appears to be a consequence of inflammation and an indirect biomarker of widespread organ damage and healing process attempt.
Contributions
LQ, MF, and CT designed the study and data collection tools, monitored data collection for the study, wrote the statistical analysis plan, cleaned and analysed the data, and drafted and revised the paper. They are the guarantors. AS collected and analysed the data and revised the paper. MP collected and analysed the data and revised the paper. FC monitored data collection for the study, analysed the data, and revised the paper.
Credit author statement
LQ, MF, and CT designed the study and data collection tools, monitored data collection for the study, wrote the statistical analysis plan, cleaned and analysed the data, and drafted and revised the paper. They are the guarantors. AS collected and analysed the data and revised the paper. MP collected and analysed the data and revised the paper. FC monitored data collection for the study, analysed the data, and revised the paper.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
Acknowledgement
We thank the following colleagues for their valued contribution to this work: Daniela Cesselli, prof, MD, Roberta Giacomello, BS, Federica D’Aurizio, MD.
Funding info
This research received no external funding.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.cyto.2021.155438.
Appendix A. Supplementary material
The following are the Supplementary data to this article:
References
- 1.Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y.i., Zhang L.i., Fan G., Xu J., Gu X., Cheng Z., Yu T., Xia J., Wei Y., Wu W., Xie X., Yin W., Li H., Liu M., Xiao Y., Gao H., Guo L.i., Xie J., Wang G., Jiang R., Gao Z., Jin Q.i., Wang J., Cao B. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wiersinga W.J., Rhodes A., Cheng A.C., Peacock S.J., Prescott H.C. Pathophysiology, transmission, diagnosis, and treatment of Coronavirus Disease 2019 (COVID-19): a review. JAMA. 2020;324(8):782–793. doi: 10.1001/jama.2020.12839. [DOI] [PubMed] [Google Scholar]
- 3.Polak S.B., Van Gool I.C., Cohen D., von der Thüsen J.H., van Paassen J. A systematic review of pathological findings in COVID-19: a pathophysiological timeline and possible mechanisms of disease progression. Mod. Pathol. 2020;33(11):2128–2138. doi: 10.1038/s41379-020-0603-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Falasca L., Nardacci R., Colombo D., Lalle E., Di Caro A., Nicastri E., Antinori A., Petrosillo N., Marchioni L., Biava G., D’Offizi G., Palmieri F., Goletti D., Zumla A., Ippolito G., Piacentini M., Del Nonno F. Postmortem findings in Italian patients with COVID-19: a descriptive full autopsy study of cases with and without comorbidities. J. Infect. Dis. 2020;222(11):1807–1815. doi: 10.1093/infdis/jiaa578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Solomon I.H., Normandin E., Bhattacharyya S., Mukerji S.S., Keller K., Ali A.S., Adams G., Hornick J.L., Padera R.F., Sabeti P. Neuropathological features of Covid-19. N. Engl. J. Med. 2020;383(10):989–992. doi: 10.1056/NEJMc2019373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wu C., Chen X., Cai Y., Xia J., Zhou X., Xu S., Huang H., Zhang L.i., Zhou X., Du C., Zhang Y., Song J., Wang S., Chao Y., Yang Z., Xu J., Zhou X., Chen D., Xiong W., Xu L., Zhou F., Jiang J., Bai C., Zheng J., Song Y. Risk factors associated with acute respiratory distress syndrome and death in patients with Coronavirus Disease 2019 pneumonia in Wuhan, China. JAMA Intern. Med. 2020;180(7):934. doi: 10.1001/jamainternmed.2020.0994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhou F., Yu T., Du R., Fan G., Liu Y., Liu Z., Xiang J., Wang Y., Song B., Gu X., Guan L., Wei Y., Li H., Wu X., Xu J., Tu S., Zhang Y.i., Chen H., Cao B. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395(10229):1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chi Y., Ge Y., Wu B., Zhang W., Wu T., Wen T., Liu J., Guo X., Huang C., Jiao Y., Zhu F., Zhu B., Cui L. Serum cytokine and chemokine profile in relation to the severity of Coronavirus Disease 2019 in China. J. Infect. Dis. 2020;222(5):746–754. doi: 10.1093/infdis/jiaa363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Quartuccio L., Sonaglia A., McGonagle D., Fabris M., Peghin M., Pecori D., De Monte A., Bove T., Curcio F., Bassi F., De Vita S., Tascini C. Profiling COVID-19 pneumonia progressing into the cytokine storm syndrome: results from a single Italian centre study on tocilizumab versus standard of care. J. Clin. Virol. 2020;129:104444. doi: 10.1016/j.jcv.2020.104444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Definition Task Force A.R.D.S., Ranieri V.M., Rubenfeld G.D., Thompson B.T., Ferguson N.D., Caldwell E., et al. Acute respiratory distress syndrome: the Berlin definition. JAMA. 2012;307(23):2526–2533. doi: 10.1001/jama.2012.5669. [DOI] [PubMed] [Google Scholar]
- 11.Rizk J.G., Kalantar-Zadeh K., Mehra M.R., Lavie C.J., Rizk Y., Forthal D.N. Pharmaco-immunomodulatory therapy in COVID-19. Drugs. 2020;80(13):1267–1292. doi: 10.1007/s40265-020-01367-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cantini F., Goletti D., Petrone L., Najafi Fard S., Niccoli L., Foti R. Immune therapy, or antiviral therapy, or both for COVID-19: a systematic review. Drugs. 2020;80(18):1929–1946. doi: 10.1007/s40265-020-01421-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Asrani P., Hassan M.I. SARS-CoV-2 mediated lung inflammatory responses in host: targeting the cytokine storm for therapeutic interventions. Mol. Cell. Biochem. 2020 doi: 10.1007/s11010-020-03935-z. Oct16:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen N., Zhou M., Dong X., Qu J., Gong F., Han Y., Qiu Y., Wang J., Liu Y., Wei Y., Xia J., Yu T., Zhang X., Zhang L.i. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395(10223):507–513. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lai C.-C., Shih T.-P., Ko W.-C., Tang H.-J., Hsueh P.-R. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and coronavirus disease-2019 (COVID-19): the epidemic and the challenges. Int. J. Antimicrob. Agents. 2020;55(3):105924. doi: 10.1016/j.ijantimicag.2020.105924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Del Valle D.M., Kim-Schulze S., Huang H.-H., Beckmann N.D., Nirenberg S., Wang B.o., Lavin Y., Swartz T.H., Madduri D., Stock A., Marron T.U., Xie H., Patel M., Tuballes K., Van Oekelen O., Rahman A., Kovatch P., Aberg J.A., Schadt E., Jagannath S., Mazumdar M., Charney A.W., Firpo-Betancourt A., Mendu D.R., Jhang J., Reich D., Sigel K., Cordon-Cardo C., Feldmann M., Parekh S., Merad M., Gnjatic S. An inflammatory cytokine signature predicts COVID-19 severity and survival. Nat. Med. 2020;26(10):1636–1643. doi: 10.1038/s41591-020-1051-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cheng M.P., Papenburg J., Desjardins M., Kanjilal S., Quach C., Libman M., Dittrich S., Yansouni C.P. Diagnostic testing for severe acute respiratory syndrome-related coronavirus 2: a narrative review. Ann. Intern. Med. 2020;172(11):726–734. doi: 10.7326/M20-1301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hamilton J.A. Colony-stimulating factors in inflammation and autoimmunity. Nat. Rev. Immunol. 2008;8(7):533–544. doi: 10.1038/nri2356. [DOI] [PubMed] [Google Scholar]
- 19.Griffin J.D., Cannistra S.A., Demetri G.D., Ernst T.J., Kanakura Y., Sullivan R. The biology of GM-CSF: regulation of production and interaction with its receptor. Int J Cell Cloning. 1990;8(S1):35–45. doi: 10.1002/stem.5530080705. [DOI] [PubMed] [Google Scholar]
- 20.Shiomi A., Usui T. Pivotal roles of GM-CSF in autoimmunity and inflammation. Mediators Inflamm. 2015;2015:1–13. doi: 10.1155/2015/568543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhou Y., Fu B., Zheng X., Wang D., Zhao C., Qi Y., Sun R., Tian Z., Xu X., Wei H. Pathogenic T-cells and inflammatory monocytes incite inflammatory storms in severe COVID-19 patients. Natl. Sci. Rev. 2020;7(6):998–1002. doi: 10.1093/nsr/nwaa041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Draijer C., Penke L.R.K., Peters-Golden M. Distinctive effects of GM-CSF and M-CSF on proliferation and polarization of two major pulmonary macrophage populations. J. Immunol. 2019;202(9):2700–2709. doi: 10.4049/jimmunol.1801387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Guilliams M., De Kleer I., Henri S., Post S., Vanhoutte L., De Prijck S., Deswarte K., Malissen B., Hammad H., Lambrecht B.N. Alveolar macrophages develop from fetal monocytes that differentiate into long-lived cells in the first week of life via GM-CSF. J. Exp. Med. 2013;210(10):1977–1992. doi: 10.1084/jem.20131199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chen B.D., Mueller M., Chou T.H. Role of granulocyte/macrophage colony-stimulating factor in the regulation of murine alveolar macrophage proliferation and differentiation. J. Immunol. 1988;141(1):139–144. [PubMed] [Google Scholar]
- 25.Akagawa K.S., Kamoshita K., Tokunaga T. Effects of granulocyte-macrophage colony-stimulating factor and colony-stimulating factor-1 on the proliferation and differentiation of murine alveolar macrophages. J. Immunol. 1988;141(10):3383–3390. [PubMed] [Google Scholar]
- 26.Bonaventura A., Vecchié A., Wang T.S., Lee E., Cremer P.C., Carey B., Rajendram P., Hudock K.M., Korbee L., Van Tassell B.W., Dagna L., Abbate A. Targeting GM-CSF in COVID-19 pneumonia: rationale and strategies. Front. Immunol. 2020;11 doi: 10.3389/fimmu.2020.0162510.3389/fimmu.2020.01625.s001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang Y., Wang X., Li X., Xi D., Mao R., Wu X., Cheng S., Sun X., Yi C., Ling Z., Ma L., Ning Q., Fang Y., Sun B., Wu D.i. Potential contribution of increased soluble IL-2R to lymphopenia in COVID-19 patients. Cell. Mol. Immunol. 2020;17(8):878–880. doi: 10.1038/s41423-020-0484-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zheng H.-Y., Zhang M.i., Yang C.-X., Zhang N., Wang X.-C., Yang X.-P., Dong X.-Q., Zheng Y.-T. Elevated exhaustion levels and reduced functional diversity of T cells in peripheral blood may predict severe progression in COVID-19 patients. Cell. Mol. Immunol. 2020;17(5):541–543. doi: 10.1038/s41423-020-0401-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ross S.H., Cantrell D.A. Signaling and function of interleukin-2 in T lymphocytes. Annu. Rev. Immunol. 2018;36(1):411–433. doi: 10.1146/annurev-immunol-042617-053352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chen G., Wu D.i., Guo W., Cao Y., Huang D.a., Wang H., Wang T., Zhang X., Chen H., Yu H., Zhang X., Zhang M., Wu S., Song J., Chen T., Han M., Li S., Luo X., Zhao J., Ning Q. Clinical and immunological features of severe and moderate coronavirus disease 2019. J. Clin. Invest. 2020;130(5):2620–2629. doi: 10.1172/JCI137244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stephen-Victor E., Das M., Karnam A., Pitard B., Gautier J.-F., Bayry J. Potential of regulatory T-cell-based therapies in the management of severe COVID-19. Eur. Respir. J. 2020;56(3):2002182. doi: 10.1183/13993003.02182-202010.1183/13993003.02182-2020.Shareable1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.H.-J. Deng, Q.-X. Long, B.-Z. Liu, J.-H. Ren, P. Liao, J.-F. Qiu, et al., Cytokine biomarkers of COVID-19, medRxiv. 2020. doi: https://www.medrxiv.org/content/10.1101/2020.05.31.20118315v1.
- 33.Nakamura T., Mizuno S. The discovery of hepatocyte growth factor (HGF) and its significance for cell biology, life sciences and clinical medicine. Proc. Jpn. Acad. Ser. B Phys. Biol. Sci. 2010;86(6):588–610. doi: 10.2183/pjab.86.588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Khan K.N., Masuzaki H., Fujishita A., Kitajima M., Hiraki K., Sekine I., et al. Interleukin-6- and tumour necrosis factor alpha-mediated expression of hepatocyte growth factor by stromal cells and its involvement in the growth of endometriosis. Hum. Reprod. 2005;20(10):2715–2723. doi: 10.1093/humrep/dei156. [DOI] [PubMed] [Google Scholar]
- 35.L. Quartuccio, M. Benucci, S. De Vita, Answer to Vieira et al. “Cytokine profile as a prognostic tool in coronavirus disease 2019”, Joint Bone Spine. 2020;Sep:105076. doi:10.1016/j.jbspin.2020.09.006. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.