Graphical abstract
Keywords: COVID-19, Pregnancy, Cytokine profile, Interleukin, Interferon
Abstract
Objective
To compare the levels of various cytokines between pregnant women with confirmed coronavirus disease (COVID-19) infection and pregnant women without any defined risk factor.
Materials and Methods
Pregnant women with confirmed COVID-19 infection (study group)(n = 90) were prospectively compared to a gestational age-matched control group of pregnant women without any defined risk factors (n = 90). Demographic features, clinical characteristics, laboratory parameters, interferon-gamma (IFN γ), interleukin (IL-2), IL-6, IL-10, and IL-17 levels were compared between the groups. Additionally, a correlation analysis was performed in the study group for the assessment of IFN γ, IL-2, IL-6, IL-10, and IL-17 levels with disease severity and CRP levels.
Results
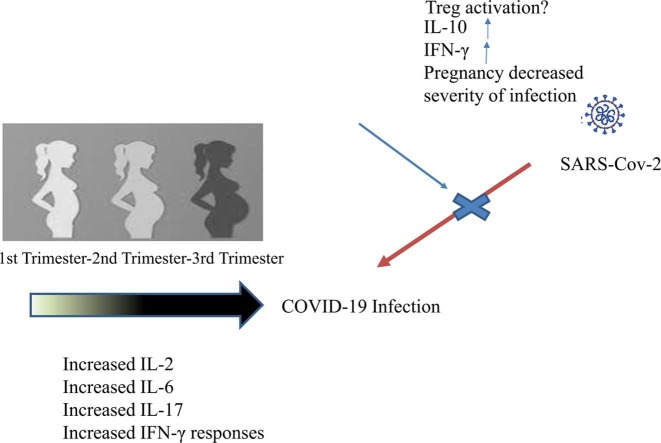
Study group had significantly higher pregnancy complication rate, erythrocyte sedimentation rate, C-reactive protein, procalcitonin, ferritin, D-dimer, lactate dehydrogenase, IFN γ, and IL-6 values (p < 0.05). On the other hand, the control group had significantly higher hemoglobin, leukocyte, platelet, lymphocyte, IL-2, IL-10, and IL-17 values (p < 0.05). Statistically significant differences were found between the groups for IFN γ, IL-2, IL-10, and IL-17 values between the trimesters (p < 0.05). Statistically significant positive correlations were found for IFN γ and IL-6 with disease severity (r = 0.41 and p < 0.001 for IFN γ and r = 0.58 and p < 0.001 for IL-6). On the other hand, a moderate negative correlation for IL-2 and a weak negative correlation for IL-10 were present (r = -0.62 and p < 0.001 for IL-2 and r = -0.19 and p = 0.01 for IL-10). A statistically significant positive moderate correlation was found between IL-6 and CRP (r = 0.40 and p < 0.001)
Conclusion
COVID-19 infection seems to have an impact on the cytokine profile of pregnant women varying according to pregnancy trimesters and cytokine levels seem to be correlated with disease severity.
1. Introduction
Coronavirus disease 2019 (COVID-2019) has been at the center of the world's attention for almost a year [1]. It has prominent social, economical, and psychological effects on the majority of people besides being a mortal disease [2]. Health-care authorities are anxious about controlling this highly contagious disease. Concerns are even greater regarding pregnant women, who are particularly vulnerable to infections. Although researchers all over the globe have been working on this novel viral infection to find an effective treatment modality or vaccine, not much progress has been achieved so far [3], [4]. For this reason, immunology-based studies enlighting the possible pathophysiological mechanisms of COVID-19 infection may be useful to establish more efficient management protocols.
Increased levels of inflammatory cytokines and excessive activation of T lymphocytes, macrophages, and endothelial cells called ''cytokine storm'' were observed in severe COVID-19 cases. Interferon-gamma (IFN γ), interleukin 6 (IL-6), tumor necrosis factor-alpha (TNF-a), IL-10, IL-1, IL-5, IL-8, IL-10, and granulocyte–macrophage colony-stimulating factor (GM-CSF) were reported to be the main mediators behind cytokine storm [5], [6]. Thus, evaluation of cytokine profiles in COVID-19 patients has attracted many researchers and many studies have been conducted on this issue [7], [8], [9]. Cytokine levels were used for the assessment of disease severity, survival, and treatment response in the previous studies [10], [11]. However, our knowledge is limited regarding the cytokine levels in pregnant women. As pregnancy is characterized by significant unique immunological changes, cytokine profile studies focusing on this particular population are very important for potential clinical implications [12], [13].
This study aims to compare the levels of various cytokines between pregnant women with confirmed COVID-19 infection and pregnant women without any defined risk factor.
2. Materials and Methods
The present prospective case-control study included pregnant women admitted to the Department of Obstetrics and Gynecology, Turkish Ministry of Health Ankara City Hospital between June 1, 2020 and August 30, 2020. Pregnant women with confirmed COVID-19 infection (study group) were compared to a gestational age-matched control group of pregnant women without any defined risk factors. All consecutive cases who gave the required informed consent for participating in the study were included. The study protocol was approved by both the Turkish Ministry of Health and the institutional ethics committee (E1-20–1008).
Turkish Ministry of Health Ankara City Hospital is the largest pandemic center in the region dealing with all types of COVID-19 patients including pregnant women [14]. All pregnant COVID-19 cases are managed within the framework of a special antenatal care program conducted by a special multidisciplinary team consisted of perinatologists, obstetricians, infectious diseases specialists, and neonatologists [14].
Confirmation of Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection was made by positive results on real-time polymerase chain reaction (RT-PCR) assays of nasopharyngeal and oropharyngeal specimens [15]. The severity of COVID-19 infection was made according to current guidelines [16], [17]. Blood samples were collected from the participants along with the initial laboratory tests upon their first admission to the hospital.
In the first part of the study, maternal age, gravidity, parity, body-mass index (BMI), gestational age at hospital admission, pregnancy complications, hemoglobin (Hb), leukocyte, platelet, lymphocyte counts, erythrocyte sedimentation rate (ESR), C-reactive protein (CRP), procalcitonin, ferritin, D-dimer, lactate dehydrogenase (LDH), neutrophil to lymphocyte ratio (NLR), IFN γ, IL-2, IL-6, IL-10, and IL-17 levels were compared between the groups. Thereafter, IFN γ, IL-2, IL-6, IL-10, and IL-17 levels were compared between the groups for each trimester. Finally, a correlation analysis was performed in the study group for the assessment of IFN γ, IL-2, IL-6, IL-10, and IL-17 levels with disease severity and CRP levels. IL-2, IL-6, IL-10, IL-17, and IFN γ levels were measured with commercially ELISA kits according to kit datasheet (eBioscience, Thermo Fisher Scientific USA).
Statistical analyses were performed by Statistical Package for the Social Sciences (SPSS.22, IBM SPSS Statistics for Windows, Version 22.0 Armonk, NY: IBM Corp.). Medians and interquartile range values were used for the non-normally distributed descriptive parameters. Kruskal-Wallis test and Mann-Whitney U tests were performed for comparing the median values between the groups. Mann-Whitney U test was also conducted for pairwise comparisons using Bonferroni correction. Categorical variables were presented with numbers and percentages. Chi-square test was used to compare categorical variables. Correlation analysis was performed by Spearman's rho test. A two-tailed P value < 0.05 was regarded as statistically significant.
3. Results
A total of 180 pregnant women were included in the present study (90 COVID-19 positives and 90 controls). There were 30 patients for each pregnancy trimester in both of the groups. Comparison of demographic features and clinical characteristics between the study and control groups was shown in Table 1 . The study group had significantly higher pregnancy complication rate, ESR, CRP, procalcitonin, ferritin, D-dimer, LDH, IFN γ, and IL-6 values (p < 0.05). On the other hand, the control group had significantly higher Hb, leukocyte, platelet, lymphocyte, IL-2, IL-10, and IL-17 values (p < 0.05).
Table 1.
Comparision of demographic features and clinical characteristics between pregnant women with COVID-19 infection and pregnant women without any defined risk factor.
| Variables | Pregnant women with COVID-19 infection (n = 90) | Pregnant women without any defined risk factor (n = 90) | P value |
|---|---|---|---|
| Maternal age (years)(median, IQR)a | 28 (6) | 27 (5) | 0.64 |
| Gravidity (median, IQR)a | 2 (1) | 2 (2) | 0.07 |
| Parity (median, IQR)a | 1 (2) | 1 (1) | 0.08 |
| BMI (median, IQR)a | 25.6 (4.5) | 25.4 (5.2) | 0.53 |
| Gestational age at hospital admission (weeks)(median, IQR)a | 24 (21) | 24 (22) | 0.69 |
| Pregnancy complication rate (n, %)b | 15 (16.6%) | 3 (3.3%) | 0.01 |
| Pregnancy complication type (n, %)b | 0.03 | ||
| Miscarriage (n, %) | 2 (2.2%) | 0 (0%) | |
| GDM (n, %) | 3 (3.3%) | 1 (1.1%) | |
| GHT (n, %) | 3 (3.3%) | 1 (1.1%) | |
| IHCP (n, %) | 2 (2.2%) | 0 (0%) | |
| Preeclampsia (n, %) | 2 (2.2%) | 0 (0%) | |
| Preterm delivery (n, %) | 3 (3.3%) | 1 (1.1%) | |
| Hb (g/dl)(median, IQR)a | 11.5 (1.7) | 12 (1.5) | 0.003 |
| Leukocyte (109/L)(median, IQR)a | 6.1 (3.4) | 8.8 (3.1) | <0.001 |
| Platelet (109/L)(median, IQR)a | 220 (84.5) | 251 (81.7) | <0.001 |
| Lymphocyte (1 0 9)(median, IQR)a | 1.2 (6.6) | 1.8 (5.8) | <0.001 |
| ESR (mm/hr)(median, IQR)a | 32 (12.5) | 24.5 (17.5) | <0.001 |
| CRP (mg/L)(median, IQR)a | 11.5 (10.5) | 4 (3.5) | <0.001 |
| Procalcitonin (ng/ml)(median, IQR)a | 0.03 (0.02) | 0.01 (0.01) | <0.001 |
| Ferritin (ng/ml)(median, IQR)a | 21 (28) | 12 (10) | <0.001 |
| D-dimer (mcg/ml)(median, IQR)a | 1.2 (1.1) | 0.6 (0.5) | <0.001 |
| LDH (IU/L)(median, IQR)a | 200 (50) | 180 (60) | 0.01 |
| NLR (median, IQR)a | 3.7 (3) | 3.4 (1.8) | 0.56 |
| IFN γ (ng/dl)(median, IQR)a | 20 (18) | 17.5 (5) | <0.001 |
| IL-2 (pg/ml)(median, IQR)a | 90 (20) | 115 (33.75) | <0.001 |
| IL-6 (pg/ml)(median, IQR)a | 6.5 (7.8) | 3.6 (1.25) | <0.001 |
| IL-10 (pg/ml)(median, IQR)a | 8.6 (4.6) | 9.6 (2.9) | 0.002 |
| IL-17 (pg/ml)(median, IQR)a | 76 (28) | 86 (31.5) | 0.03 |
BMI: Body-mass index, COVID-19: Coronavirus disease 2019, CRP: C-reactive protein, ESR: erythrocyte sedimentation rate, GDM: Gestational diabetes mellitus, GHT: Gestational hypertension, Hb: Hemoglobin, ICHP: intrahepatic cholestasis of pregnancy, IFN γ: Interferon gamma, IL: Interleukin, IQR: Interquartile-range, LDH: Lactate dehydrogenase, NLR: Neutrophil to lymphocyte ratio.
Statistical analysis was performed by Mann-Whitney U test.
Statistical analysis was performed by chi-square test.
Comparison of IFN γ, IL-2, IL-6, IL-10, and IL-17 levels between the pregnancy trimesters was shown in Table 2 . Statistically significant differences were found between the groups for IFN γ, IL-2, IL-10, and IL-17 values. The study group had significantly higher IFN γ levels in the third trimester compared to the control group (p = 0.001). On the other hand, the control group had significantly higher IL-2 levels in the first and second trimesters (p < 0.001 for both). Furthermore, significantly higher IL-10 and IL-17 levels were present in the control group for the first trimester (p values were 0.01 and 0.04, respectively).
Table 2.
Comparision of IFN γ, IL-2, IL-6, IL-10 and IL-17 levels between the pregnancy trimesters.
| Variables | COVID-19 group first trimester (n = 30) | Control group first trimester (n = 30) |
COVID-19 group second trimester (n = 30) |
Control group second trimester (n = 30) |
COVID-19 group third trimester (n = 30) | Control group third trimester (n = 30) |
P valuea |
|---|---|---|---|---|---|---|---|
| IFN γ (ng/dl)(median, IQR) | 17.5 (5) | 15 (3) | 20 (10) | 15 (2.5) | 37.5 (35) | 20 (17.5) | <0.001c |
| IL-2 (pg/ml)(median, IQR) | 80 (20) | 105 (25) | 90(23.75) | 145 (40) | 95 (15) | 110 (20) | <0.001d |
| IL-6 (pg/ml)(median, IQR) | 6.1 (4.3) | 3.3 (1.2) | 6.3 (6.2) | 3.5 (2.3) | 8.1 (15) | 3.8 (1.5) | 0.30 |
| IL-10 (pg/ml)(median, IQR) | 7 (4.5) | 10.8 (5) | 8.8 (3.5) | 9.6 (5) | 9.6 (5.6) | 9.3 (2.6) | <0.001e |
| IL-17 (pg/ml)(median, IQR) | 68 (26) | 104 (19) | 80 (26) | 76 (18) | 84 (35) | 80 (24) | 0.01f |
IFN γ: Interferon gamma, IL: Interleukin.
bPairwise comparisions was performed by Mann-Whitney U test.
Statistical analysis analysis was performed by Kruskal-Wallis test between the groups.
Statistically significant difference was found between the study and control groups in the third trimester (p = 0.001).
Statistically significant differences were found between the study and control groups in the first and second trimesters (p < 0.001 for both).
Statistically significant differences was found between the study and control groups in the first trimester (p = 0.01).
Statistically significant differences was found between the study and control groups in the first trimester (p = 0.04).
Correlation of IFN γ, IL-2, IL-6, IL-10, and IL-17 levels with disease severity in pregnant women with COVID-19 infection was shown in Table 3 . There were 63 (70%), 24 (26.6%), and 3 (3.3%) cases with mild, moderate, and severe COVID-19 infection, respectively. Statistically significant positive correlations were found for IFN γ and IL-6 with disease severity (r = 0.41 and p < 0.001 for IFN γ and r = 0.58 and p < 0.001 for IL-6). On the other hand, a moderate negative correlation for IL-2 and a weak negative correlation for IL-10 were present (r = -0.62 and p < 0.001 for IL-2 and r = -0.19 and p = 0.01 for IL-10). Correlation of IFN γ, IL-2, IL-6, IL-10, and IL-17 levels with CRP in pregnant women with COVID-19 infection was shown in Table 4 . A statistically significant positive moderate correlation was found between IL and 6 and CRP (r = 0.40 and p < 0.001).
Table 3.
Correlation of IFN γ, IL-2, IL-6, IL-10 and IL-17 levels with disease severity in pregnant women with COVID-19 infection.
| Parameter | r valuea | p valuea |
|---|---|---|
| IFN γ | 0.41 | <0.001 |
| IL-2 | −0.62 | <0.001 |
| IL-6 | 0.58 | <0.001 |
| IL-10 | −0.19 | 0.01 |
| IL-17 | −0.13 | 0.07 |
COVID-19: Coronavirus disease 2019, IFN γ: Interferon gamma, IL: Interleukin
Correlation analysis was performed by Spearman test.
Table 4.
Correlation of IFN γ, IL-2, IL-6, IL-10 and IL-17 levels with CRP in pregnant women with COVID-19 infection.
| Parameter | r valuea | p valuea |
|---|---|---|
| IFN γ | 0.04 | 0.68 |
| IL-2 | 0.18 | 0.06 |
| IL-6 | 0.40 | <0.001 |
| IL-10 | 0.16 | 0.12 |
| IL-17 | 0.008 | 0.94 |
COVID-19: Coronavirus disease 2019, CRP: C-reactive protein, IFN γ: Interferon gamma, IL: Interleukin
Correlation analysis was performed by Spearman test.
4. Discussion
The main findings of the present study indicated that pregnancy complications and inflammation markers were significantly higher in pregnant women with COVID-19 infection. Furthermore, significantly higher IFN γ and IL-6 together with significantly lower IL-2, IL-10, and IL-17 levels were observed in pregnant women with COVID-19 infection. Additionally, cytokine levels vary between pregnancy trimesters except for IL-6 and they are significantly correlated with disease severity except for IL-17. To the best of our knowledge, this is the first study in the literature evaluating the cytokine profile in pregnancies complicated by COVID-19.
Pregnancy goes together with a specific immune-adaptive process to allow the proper implantation of the semi-allograft fetus [18]. Increased production of various anti-inflammatory cytokines like IL-4 and IL-10 provides an immune-tolerant microenvironment for the conception material [19]. On the other hand, altered expression of pro-inflammatory cytokines like IL-1 and TNF-a was found to be associated with increased rates of pregnancy complications like miscarriage and preterm delivery. However, pro-inflammatory processes are also necessary for physiological events such as placental invasion and parturition [20], [21]. Thus, the balance between pro and anti-inflammatory cytokines is essential for a healthy pregnancy [12], [22].
The excessive inflammatory response to COVID-19 infection is regarded as the main pathophysiological triggering event behind mortality and morbidity in infected individuals [6]. Aberrant production of cytokines like TNF-a, IL-1, IFN γ, IL-4, and IL-10 have been reported in severe cases with increased rates of adverse outcomes [23]. For this reason, researchers have been working on novel treatment options focusing on the alteration of immune responses in COVID-19 patients [24], [25]. However, as pregnancy is a unique process characterized by various immunologic changes and each immunomodulatory event is associated with a specific condition, it is challenging to predict the impact of immune-mediated therapies on pregnant women [12], [22]. Thus, more data is necessary to establish more appropriate management protocols for pregnant women with COVID-19. Furthermore, additional data about the immunomodulatory changes in COVID-19 patients during pregnancy may reveal pathophysiological events behind this deadly disease.
Trophoblasts, specialized natural killer cells, and decidual leukocytes secrete IFN γ during pregnancy [26]. It takes part in the differentiation of decidual natural killer cells, the formation of the placenta, and the maintenance of the decidua [27]. However, IFN γ is also associated with pregnancy loss, especially in congenital infections [28]. IFN γ levels vary in patients with COVID-19 infection according to disease severity. While increased levels are reported in mild and moderate cases, slightly lower levels are observed in patients with severe COVID-19 infection [29]. Significantly higher levels of IFN γ were found for pregnant women with COVID-19 infection in the present study and this difference was most prominent in the third trimester. These findings seem to be associated with the higher frequency of mild COVID-19 cases and the shift of anti-inflammatory balance to a more pro-inflammatory state in the third trimester of pregnancy.
Altered levels of IL-2 is associated with various obstetric complications like pregnancy loss and preeclampsia [30]. Increased levels of IL-2 are observed in COVID-19 infection and this increase is in parallel with disease severity [6]. However, significantly lower levels of IL-2 were found in the pregnant women with COVID-19 infection in the present study and this difference was more obvious for the first and second trimesters. These findings were not consistent with the literature. However, as our knowledge is limited with regard to the impact of COVID-19 infection on the cytokine profile of pregnant women, the mentioned findings may indicate an altered immune response or an adaptive immunologic change in this specific population.
Excessive production of IL-6 is associated with adverse pregnancy outcomes like preterm delivery, preterm premature rupture of the membranes, and chorioamnionitis [31]. Additionally, increased IL-6 levels are observed in severe COVID-19 cases and it is regarded as one of the key actors of the famous ''cytokine storm'' [5], [23], [24]. Significantly higher IL-6 levels were found for the study group in the present study and no significant difference was observed between the pregnancy trimesters. These findings were consistent with the literature.
Placental villous trophoblasts, uterine natural killer cells, and decidual monocytes are the main producers of IL-10 during pregnancy. As it is an anti-inflammatory cytokine, it mainly takes part in the immune-tolerant stages of pregnancy [32]. Early production of IL-10 in COVID-19 infected individuals was found to be associated with poor prognosis [33]. However, in the present study, the control group had significantly higher IL-10 levels which were more prominent in the first trimester. In our opinion, decreased IL-10 levels in COVID-19 pregnancies may be one of the factors behind the impaired immune tolerance in this population and it may be associated with pregnancy losses [34].
Increased levels of IL-17 during pregnancy was reported to be associated with favorable obstetric outcomes [35]. Excessive IL-17 may cause alveolar injury and fibrosis in COVID-19 cases and it is considered as a potential therapeutic target [36]. As both pregnancy and COVID-19 infection may increase IL-17 levels in infected individuals, it is expected that COVID-19 positive cases should have higher levels of IL-17. However, contrary to the literature, higher levels were found in the control group of the present study, especially in the first trimester. This may be associated with a higher rate of pregnancy complications and a higher frequency of mild cases in the study group.
The most important part of the present study was the correlation of cytokine levels with disease severity. It may help physicians in two aspects. Firstly, cytokine levels may be used for the assessment of disease prognosis in COVID-19 positive pregnancies. Secondly, novel medications may be developed targeting the pathways of cytokine responses in these patients. However, future studies with larger populations and more number of cytokine types are necessary to obtain more precisive results.
The main strength of the present study was its novelty, prospective design, and relatively high number of study parameters. However, a relatively low number of cytokine types and a higher number of mild cases were the main limitations.
In conclusion, COVID-19 infection seems to have an impact on the cytokine profile of pregnant women varying according to pregnancy trimesters and cytokine levels seem to be correlated with disease severity.
CRediT authorship contribution statement
Atakan Tanacan: Conceptualization, Writing - original draft, Formal analysis. Nuray Yazihan: Investigation, Methodology, Writing - review & editing. Seyit Ahmet Erol: Data curation, Writing - review & editing. Ali Taner Anuk: Data curation, Writing - review & editing. Fatma Didem Yucel Yetiskin: Formal analysis, Writing - review & editing. Derya Biriken: Methodology, Writing - review & editing. A.Seval Ozgu-Erdinc: Formal analysis, Writing - review & editing. Huseyin Levent Keskin: Visualization, Writing - review & editing. Ozlem Moraloglu Tekin: Supervision, Writing - review & editing. Dilek Sahin: Conceptualization, Writing - original draft, Supervision.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
Acknowledgments
None.
Funding
No funding was used for this study.
References
- 1.Xiong J., Lipsitz O., Nasri F., Lui L.M., Gill H., Phan L., Chen-Li D., Iacobucci M., Ho R., Majeed A. Impact of COVID-19 pandemic on mental health in the general population: A systematic review. J. Affect. Disord. 2020 doi: 10.1016/j.jad.2020.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nicola M., Alsafi Z., Sohrabi C., Kerwan A., Al-Jabir A., Iosifidis C., Agha M., Agha R. The socio-economic implications of the coronavirus and COVID-19 pandemic: a review. Int. J. Surg. 2020 doi: 10.1016/j.ijsu.2020.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Song Y., Zhang M., Yin L., Wang K., Zhou Y., Zhou M., Lu Y. COVID-19 treatment: close to a cure?–a rapid review of pharmacotherapies for the novel coronavirus. Int. J. Antimicrob. Agents. 2020;106080 doi: 10.1016/j.ijantimicag.2020.106080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gravett C.A., Gravett M.G., Martin E.T., Bernson J.D., Khan S., Boyle D.S., Lannon S.M., Patterson J., Rubens C.E., Steele M.S. Serious and life-threatening pregnancy-related infections: opportunities to reduce the global burden. PLoS Med. 2012;9(10) doi: 10.1371/journal.pmed.1001324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pedersen S.F., Ho Y.C. SARS-CoV-2: a storm is raging. J. Clin. Investig. 2020;130(5):2202–2205. doi: 10.1172/JCI137647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mehta P., McAuley D.F., Brown M., Sanchez E., Tattersall R.S., Manson J.J. COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet (London, England) 2020;395(10229):1033–1034. doi: 10.1016/S0140-6736(20)30628-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Akbari H., Tabrizi R., Lankarani K.B., Aria H., Vakili S., Asadian F., Noroozi S., Keshavarz P., Faramarz S. The role of cytokine profile and lymphocyte subsets in the severity of coronavirus disease, (COVID-19): A systematic review and meta-analysis. Life Sci. 2019;2020 doi: 10.1016/j.lfs.2020.118167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ye Q., Wang B., Mao J. Cytokine storm in COVID-19 and treatment. J. Infect. 2020 doi: 10.1016/j.jinf.2020.03.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mangalmurti N., Hunter C.A. Cytokine storms: understanding COVID-19. Immunity. 2020 doi: 10.1016/j.immuni.2020.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Del Valle D.M., Kim-Schulze S., Huang H.-H., Beckmann N.D., Nirenberg S., Wang B., Lavin Y., Swartz T.H., Madduri D., Stock A. An inflammatory cytokine signature predicts COVID-19 severity and survival. Nat. Med. 2020;26(10):1636–1643. doi: 10.1038/s41591-020-1051-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ghazavi A., Ganji A., Keshavarzian N., Rabiemajd S., Mosayebi G. Cytokine profile and disease severity in patients with COVID-19. Cytokine. 2020;137 doi: 10.1016/j.cyto.2020.155323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Erlebacher A. Immunology of the maternal-fetal interface. Annu. Rev. Immunol. 2013;31:387–411. doi: 10.1146/annurev-immunol-032712-100003. [DOI] [PubMed] [Google Scholar]
- 13.La Rocca C., Carbone F., Longobardi S., Matarese G. The immunology of pregnancy: regulatory T cells control maternal immune tolerance toward the fetus. Immunol. Lett. 2014;162(1):41–48. doi: 10.1016/j.imlet.2014.06.013. [DOI] [PubMed] [Google Scholar]
- 14.Sahin D., Tanacan A., Erol S.A., Anuk A.T., Eyi E.G., Ozgu-Erdinc A.S., Yucel A., Keskin H.L., Tayman C., Unlu S. A pandemic center's experience of managing pregnant women with COVID-19 infection in Turkey: A prospective cohort study. Int. J. Gynecol. Obstet. 2020 doi: 10.1002/ijgo.13318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang X., Tan L., Wang X., Liu W., Lu Y., Cheng L., Sun Z. Comparison of nasopharyngeal and oropharyngeal swabs for SARS-CoV-2 detection in 353 patients received tests with both specimens simultaneously. Int J Infect Dis. 2020;94:107–109. doi: 10.1016/j.ijid.2020.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Turkish Ministry of Health, General Directorate of Public Health, COVİD-19 (SARS-CoV-2 infection) Guideline, Scientific Committee Report. https://covid19bilgi.saglik.gov.tr/depo/rehberler/COVID-19_Rehberi.pdf?type=file. (Accessed 21.08.2020.
- 17.Rasmussen S.A., Smulian J.C., Lednicky J.A., Wen T.S., Jamieson D.J. Coronavirus Disease 2019 (COVID-19) and Pregnancy: What obstetricians need to know. American J. Obstet. Gynecol. 2020 doi: 10.1016/j.ajog.2020.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Robertson S.A. Immune regulation of conception and embryo implantation-all about quality control? J. Reprod. Immunol. 2010;85(1):51–57. doi: 10.1016/j.jri.2010.01.008. [DOI] [PubMed] [Google Scholar]
- 19.Chow S.S., Craig M.E., Jones C.A., Hall B., Catteau J., Lloyd A.R., Rawlinson W.D. Differences in amniotic fluid and maternal serum cytokine levels in early midtrimester women without evidence of infection. Cytokine. 2008;44(1):78–84. doi: 10.1016/j.cyto.2008.06.009. [DOI] [PubMed] [Google Scholar]
- 20.Raghupathy R., Kalinka J. Cytokine imbalance in pregnancy complications and its modulation. Front. Biosci. 2008;13(1):985–994. doi: 10.2741/2737. [DOI] [PubMed] [Google Scholar]
- 21.Azizieh F., Dingle K., Raghupathy R., Johnson K., VanderPlas J., Ansari A. Multivariate analysis of cytokine profiles in pregnancy complications. Am. J. Reprod. Immunol. 2018;79(3) doi: 10.1111/aji.12818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.PrabhuDas M., Bonney E., Caron K., Dey S., Erlebacher A., Fazleabas A., Fisher S., Golos T., Matzuk M., McCune J.M. Immune mechanisms at the maternal-fetal interface: perspectives and challenges. Nat. Immunol. 2015;16(4):328. doi: 10.1038/ni.3131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Castelli V., Cimini A., Ferri C. Cytokine Storm in COVID-19:“When You Come Out of the Storm, You Won't Be the Same Person Who Walked in”. Front. Immunol. 2020;11 doi: 10.3389/fimmu.2020.02132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Y. Jamilloux, T. Henry, A. Belot, S. Viel, M. Fauter, T. El Jammal, T. Walzer, B. François, P. Sève, Should we stimulate or suppress immune responses in COVID-19? Cytokine and anti-cytokine interventions, Autoimmunity Reviews (2020) 102567. [DOI] [PMC free article] [PubMed]
- 25.Monteleone G., Sarzi-Puttini P.C., Ardizzone S. Preventing COVID-19-induced pneumonia with anticytokine therapy. Lancet Rheumatol. 2020;2(5):e255–e256. doi: 10.1016/S2665-9913(20)30092-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Casazza R.L., Lazear H.M., Miner J.J. Protective and pathogenic effects of interferon signaling during pregnancy. Viral Immunol. 2020;33(1):3–11. doi: 10.1089/vim.2019.0076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhou J.Z., Way S.S., Chen K. Immunology of the uterine and vaginal mucosae. Trends Immunol. 2018;39(4):302–314. doi: 10.1016/j.it.2018.01.007. [DOI] [PubMed] [Google Scholar]
- 28.Senegas A., Villard O., Neuville A., Marcellin L., Pfaff A., Steinmetz T., Mousli M., Klein J., Candolfi E. Toxoplasma gondii-induced foetal resorption in mice involves interferon-γ-induced apoptosis and spiral artery dilation at the maternofoetal interface. Int. J. Parasitol. 2009;39(4):481–487. doi: 10.1016/j.ijpara.2008.08.009. [DOI] [PubMed] [Google Scholar]
- 29.Lagunas-Rangel F.A., Chávez-Valencia V. High IL-6/IFN-γ ratio could be associated with severe disease in COVID-19 patients. J. Med. Virol. 2020 doi: 10.1002/jmv.25900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fasouliotis S.J., Spandorfer S.D., Witkin S.S., Schattman G., Liu H.C., Roberts J.E., Rosenwaks Z. Maternal serum levels of interferon-γ and interleukin-2 soluble receptor-α predict the outcome of early IVF pregnancies. Hum. Reprod. 2004;19(6):1357–1363. doi: 10.1093/humrep/deh169. [DOI] [PubMed] [Google Scholar]
- 31.Qiu X., Zhang L., Tong Y., Qu Y., Wang H., Mu D. Interleukin-6 for early diagnosis of neonatal sepsis with premature rupture of the membranes: A meta-analysis. Medicine. 2018;97(47) doi: 10.1097/MD.0000000000013146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chatterjee P., Chiasson V.L., Bounds K.R., Mitchell B.M. Regulation of the anti-inflammatory cytokines interleukin-4 and interleukin-10 during pregnancy. Front. Immunol. 2014;5:253. doi: 10.3389/fimmu.2014.00253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhao Y., Qin L., Zhang P., Li K., Liang L., Sun J., Xu B., Dai Y., Li X., Zhang C. Longitudinal COVID-19 profiling associates IL-1RA and IL-10 with disease severity and RANTES with mild disease. JCI insight. 2020;5(13) doi: 10.1172/jci.insight.139834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Juan J., Gil M.M., Rong Z., Zhang Y., Yang H., Poon L.C. Effects of coronavirus disease 2019 (COVID-19) on maternal, perinatal and neonatal outcomes: a systematic review. Ultrasound Obstet Gynecol. 2020 doi: 10.1002/uog.22088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kaminski V.L., Ellwanger J.H., Matte M.C.C., Savaris R.F., Vianna P., Chies J.A.B. IL-17 blood levels increase in healthy pregnancy but not in spontaneous abortion. Mol. Biol. Rep. 2018;45(5):1565–1568. doi: 10.1007/s11033-018-4268-7. [DOI] [PubMed] [Google Scholar]
- 36.Mendoza V.M.M. Interleukin-17: a potential therapeutic target in COVID-19. J. Infect. 2020 doi: 10.1016/j.jinf.2020.05.072. [DOI] [PMC free article] [PubMed] [Google Scholar]