SARS-CoV-2 antibodies persist
As the number of daily COVID-19 cases continues to mount worldwide, the nature of the humoral immune response to severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) remains uncertain. Wajnberg et al. used a cohort of more than 30,000 infected individuals with mild to moderate COVID-19 symptoms to determine the robustness and longevity of the anti–SARS-CoV-2 antibody response. They found that neutralizing antibody titers against the SARS-CoV-2 spike protein persisted for at least 5 months after infection. Although continued monitoring of this cohort will be needed to confirm the longevity and potency of this response, these preliminary results suggest that the chance of reinfection may be lower than is currently feared.
Science, this issue p. 1227
Antibodies generated after mild to moderate SARS-CoV-2 infection are neutralizing and relatively stable for at least 5 months.
Abstract
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has caused a global pandemic with millions infected and more than 1 million fatalities. Questions regarding the robustness, functionality, and longevity of the antibody response to the virus remain unanswered. Here, on the basis of a dataset of 30,082 individuals screened at Mount Sinai Health System in New York City, we report that the vast majority of infected individuals with mild-to-moderate COVID-19 experience robust immunoglobulin G antibody responses against the viral spike protein. We also show that titers are relatively stable for at least a period of about 5 months and that anti-spike binding titers significantly correlate with neutralization of authentic SARS-CoV-2. Our data suggest that more than 90% of seroconverters make detectable neutralizing antibody responses. These titers remain relatively stable for several months after infection.
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has infected millions of individuals globally and, as of October 2020, has led to the death of >1 million individuals. Although the antibody responses in severe COVID-19 cases have been relatively well characterized (1, 2), assessing the response in mild and asymptomatic cases is of great importance because they constitute most infections. It will be critical to understand the robustness of the antibody response in these mild cases, including its longevity and functionality, so as to inform serosurveys and to determine levels and duration of antibody titers that may be protective against reinfection (3).
Antibodies to SARS-CoV-2 can target many of its encoded proteins, including structural and nonstructural antigens. Thus far, two structural proteins have been used as target antigens for serological assays. One is the abundant nucleoprotein (NP), which is found inside the virus or inside infected cells. However, because of the biological function of NP and because it is shielded from antibodies by viral or cellular membranes, it is unlikely that NP antibodies can directly neutralize SARS-CoV-2. The second structural protein often used as a target for characterizing the immune response to SARS-CoV-2 is the spike protein. The spike is a large trimeric glycoprotein that contains the receptor binding domain, which the virus uses to dock to its cellular receptor, angiotensin-converting enzyme 2, and for fusion of viral and cellular membranes (4, 5). It is known from other coronaviruses—and it holds true for SARS-CoV-2—that the spike is the main, and potentially the only, target for neutralizing antibodies (6). Therefore, the assay used in this study to characterize the antibody response to SARS-CoV-2 is based on the trimerized, stabilized ectodomain of the spike protein (7). An enzyme-linked immunosorbent assay (ELISA) initially developed in early 2020 has been extensively used in research (7–10). This so-called Mount Sinai ELISA has high sensitivity (92.5%) and specificity (100%), as determined with an initial validation panel of samples (table S1). Furthermore, it has a positive predictive value (PPV) of 100%, with a negative predictive value (NPV) of 99.6%.
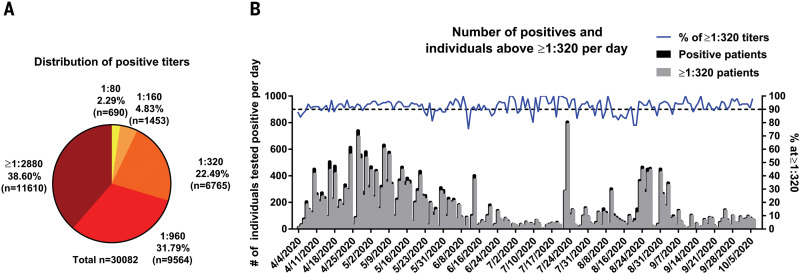
In March 2020, the Mount Sinai Health System began screening individuals for antibodies to SARS-CoV-2 to recruit volunteers as donors for convalescent plasma therapy (11). Screened patients had either polymerase chain reaction (PCR)–confirmed SARS-CoV-2 infections or suspected disease, which is defined as being told by a physician that symptoms may be related to SARS-CoV-2 or exposure to someone with confirmed SARS-CoV-2 infection. Most of the symptomatic patients who were screened experienced mild-to-moderate disease, with <5% requiring emergency department evaluation or hospitalization. Mount Sinai also offered the antibody test to all employees in its health system on a voluntary basis. By 6 October 2020, Mount Sinai had screened 72,401 individuals, with a total of 30,082 individuals testing positive (defined as detectable antibodies to the spike protein at a titer of 1:80 or higher) and 42,319 testing negative. The clinical laboratory ELISA set up results in discrete titers of either 1:80, 1:160, 1:320, 1:960, or ≥1:2880. Titers of 1:80 and 1:160 were categorized as low titers, 1:320 as moderate, and 1:960 and ≥1:2880 as high titers. For plasma therapy, titers of 1:320 or higher were initially deemed eligible. Of the 30,082 positive samples, 690 (2.29%) had a titer of 1:80; 1453 (4.83%), 1:160; 6765 (22.49%), 1:320; 9564 (31.79%), 1:960; and 11,610 (38.60%), ≥1:2880 (Fig. 1). Thus, we conclude that the vast majority of positive individuals have moderate-to-high titers of anti-spike antibodies. Of course, the argument could be made that we could be missing a number of individuals who had been infected with SARS-CoV-2 and did not produce antibodies, given that many individuals included in our dataset had never been tested by a nucleic acid amplification test for the virus. An earlier analysis performed with a smaller subset of 568 PCR-confirmed individuals using the same ELISA showed that >99% developed an anti-spike antibody response (8). In a later dataset of 2347 patients who self-reported positive PCR, 95% had positive antibody titers, which indicates that we did not miss large numbers of patients and confirms our prior sensitivity findings. Thus, the rate of individuals who do not seroconvert after SARS-CoV-2 infection is low, although such individuals may exist, and the majority of responders mount titers of 1:320 or higher.
Fig. 1. SARS-CoV-2 spike antibody titers in 30,082 individuals.
(A) The percentage of individuals with antibody titers of 1:80 (low), 1:160 (low), 1:320 (moderate), 1:960 (high), and ≥1:2880 (high). (B) Absolute numbers of individuals testing positive and percent of individuals with titers of 1:320 over time. Testing of each sample was performed once in a Clinical Laboratory Improvement Amendments (CLIA)–certified laboratory using an assay that received emergency use authorization (EUA) from the U.S. Food and Drug Administration (FDA).
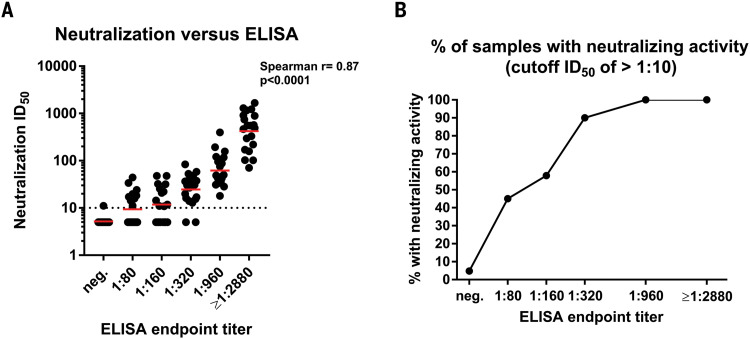
Determining the neutralizing effects of SARS-CoV-2 spike antibodies is critical to understanding possible protective effects of the immune response. Therefore, we performed a well-established quantitative microneutralization assay (12) based on authentic SARS-CoV-2 with 120 samples of known ELISA titers ranging from negative to ≥1:2880. Neutralization titers significantly correlated (Spearman ρ = 0.87, P < 0.0001) with spike-binding titers (Fig. 2A). Although there was some variability, sera with 1:320, 1:960, and ≥1:2880 ELISA titers had geometric mean 50% inhibitory dilutions (ID50) of about 1:30, 1:75, and 1:550, respectively. If any and all neutralizing activity above background is considered, then ~50% of sera in the 1:80 to 1:160 titer range, 90% in the 1:320 range, and all sera in the 1:960 to ≥1:2880 range had neutralizing activity (Fig. 2B). Only one of the negative samples showed activity slightly above background, which was potentially an ELISA false negative.
Fig. 2. Neutralizing activity of serum samples in relation to ELISA titers.
(A) A correlation analysis between ELISA titers on the x axis and neutralization titers in a microneutralization assay on the y axis. The Spearman ρ was determined. Red bars indicate the geometric mean. (B) The proportion of sera that exert any neutralizing activity in each of the ELISA titer categories. Testing was performed once, using an FDA EUA ELISA in a CLIA laboratory, or twice, following a standardized neutralization protocol.
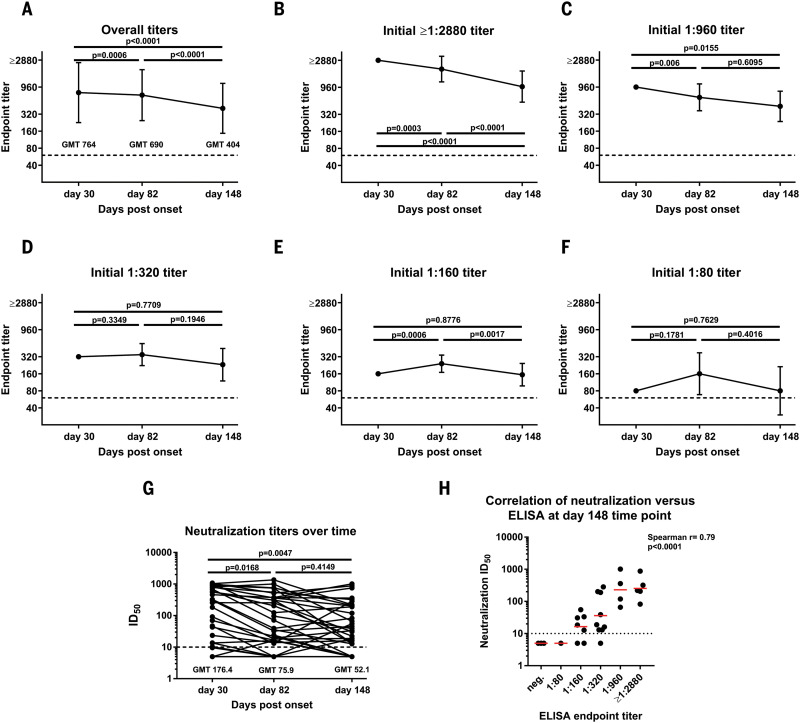
Another important question is longevity of the antibody response to the spike. To assess the medium-range stability of serum antibody titers against the spike protein, we recalled 121 plasma donors at a variety of titer levels who had initially been screened at around day 30 after symptom onset for two additional time points. The mean interval between the initial titer measurement and the second was 52 days (range: 33 to 67 days). This set the second time point at a mean of 82 days after symptom onset (range: 52 to 104 days) and the third time point at 148 days after symptom onset (range: 113 to 186 days). In comparing overall titers, we observed a slight drop from a geometric mean titer (GMT) of 764 to a GMT of 690 from the first to the second time point and another drop to a GMT of 404 for the last time point (Fig. 3A). In the higher titer range of ≥1:2880 and 1:960, we also observed a slow decline in titer over time (Fig. 3, B and C). Unexpectedly, but in agreement with earlier observations that seroconversion in mild COVID-19 cases might take a longer time to mount (8), we saw an increase in individuals who had an initial titer of 1:320, 1:160, or 1:80 (Fig. 3, D to F) from day 30 to day 82. Titers in these groups declined to about day 30 levels on day 148. Notably, one individual in the initial 1:80 group dropped from a 1:80 titer to being negative at the day 82 time point, and two others lost reactivity at the day 148 time point, indicating that very low initial titers might drop to undetectable levels over time. Neutralizing antibody titers followed titers measured by ELISA (Fig. 3G), and a good correlation between neutralization and ELISA titers was still observed on day 148 (Fig. 3H). The initial serum antibody titer was likely produced by plasmablasts, and plasmablast-derived antibodies peak 2 to 3 weeks after symptom onset. Given an immunoglobulin G half-life of ~21 days, the sustained antibody titers observed here over time are likely produced by long-lived plasma cells in the bone marrow. Note that our observations contrast with a recent report that found waning titers at 8 weeks after virus infection (13). Especially in asymptomatic cases, antibody responses disappeared after 8 weeks in 40% of individuals in that study. However, the antibodies measured in that paper targeted the NP plus a single linear spike epitope. The same paper also reported relatively stable (slightly declining) neutralizing antibody titers, which shows much higher concordance with our present findings. Thus, the stability of the antibody response over time may also depend on the target antigen.
Fig. 3. Antibody titer stability over time.
(A) Titers of 121 volunteers whose blood was initially drawn ~30 days after COVID-19 symptom onset and who were then recalled for additional blood draws at ~82 days and 148 days after symptom onset. (B to F) The same data as in (A), but stratified by the initial (day 30) titer. Titers are graphed as geometric mean titers (GMT) with geometric standard error. (G) Neutralization titers of 36 individuals over time. A paired one-way analysis of variance corrected for multiple comparison was used to determine statistical significance. (H) A correlation analysis between ELISA titers on the x axis and neutralization titers in a microneutralization assay on the y axis at day 148. Red bars indicate the geometric mean. The Spearman ρ was determined. Testing was performed once, using an FDA EUA ELISA in a CLIA-certified laboratory, or twice, following a standardized neutralization protocol.
Correlates of protection have been established for many different viral infections. These correlates are usually based on a specific level of antibody acquired through vaccination or natural infection that substantially reduces the risk of (re)infection. One example is the hemagglutination inhibition titer for the influenza virus, where a 1:40 titer reduces the risk of getting infected by 50% (14). Similar titers have been established for the measles virus (an ID50 titer of 1:120), hepatitis A virus, hepatitis B virus, and many others (15). These titers have facilitated vaccine development considerably. For some viruses and vaccines, the kinetics of the antibody response is also known, allowing for an accurate prediction of how long protection will last (16).
It is still unclear whether infection with SARS-CoV-2 in humans protects from reinfection and, if it does, for how long. We know from work with common human coronaviruses that neutralizing antibodies are induced and that these antibodies can last for years and provide protection from reinfection or, in the event of reinfection, attenuate disease (17). Furthermore, we now know from nonhuman primate models that infection with SARS-CoV-2 does protect from reinfection for at least some time (18, 19). We also know that transferring serum of convalescent animals or neutralizing monoclonal antibodies to naïve animals can be protective and reduces virus replication significantly (20, 21). Finally, vaccine-induced neutralizing antibody titers have been established as a correlate of protection in nonhuman primates (22). Notably, these vaccine-induced titers were relatively low and in the lower range of the titers observed in this study. Our data reveal that individuals who have recovered from mild COVID-19 experience relatively robust antibody responses to the spike protein, which correlate significantly with neutralization of authentic SARS-CoV-2 virus. Furthermore, the vast majority of individuals with antibody titers of 1:320 or higher show neutralizing activity in their serum. We also find stable antibody titers over a period of at least 3 months and only modest declines at the 5-month time point, which is consistent with data for the human coronaviruses SARS-CoV-1 and Middle East respiratory syndrome–related coronavirus (MERS-CoV) (17). We plan to follow this cohort over longer intervals of time. Although we cannot provide conclusive evidence that these antibody responses protect from reinfection, we believe it is very likely that they will decrease the odds ratio of reinfection and may attenuate disease in the case of breakthrough infection. We believe that it is imperative to swiftly perform studies to investigate and establish a correlate of protection from SARS-CoV-2 infection. A correlate of protection, combined with a better understanding of antibody kinetics to the spike protein, would inform policy regarding the COVID-19 pandemic and would be beneficial to vaccine development efforts.
Acknowledgments
We are grateful for the continuous expert guidance provided by the ISMMS Program for the Protection of Human Subjects (PPHS). We also thank R. A. Albrecht for oversight of the conventional Biosafety Level 3 biocontainment facility, the medical students involved in the plasma convalescence program, and D. Adhimoolam for invaluable help with data curation. Furthermore, we thank E. Lium and team at Mount Sinai Innovation Partners for continuous support and V. Sarić and team at Mount Sinai’s Development Office for fundraising and for taking many little things off our shoulders during this difficult time. Finally, we thank P. Palese and the Department of Microbiology and D. Charney and the Icahn School of Medicine at Mount Sinai’s Dean’s Office for strong institutional support of our work. Funding: This work was partially supported by NIAID Centers of Excellence for Influenza Research and Surveillance (CEIRS) contract HHSN272201400008C (F.K.) and by Collaborative Influenza Vaccine Innovation Centers (CIVIC) contract 75N93019C00051 (F.K.). Support was also provided by the JPB Foundation, the Open Philanthropy Project (#2020-215611), and other philanthropic donations. This effort was deliberated with Leidos Biomedical Research Inc./NCI over the last several months and is now going to be supported by the Serological Sciences Network (SeroNet). This project will be funded in whole or in part with federal funds from the National Cancer Institute, National Institutes of Health, under contract 75N91019D00024, task order 75N91020F00003, as well as U54 CA260560. The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the U.S. government. Author contributions: A.W., D.R.A., M.J.B., M.Ma., K.M., K.S., and J.A. performed clinical activities. A.W., J.A., D.L.R., F.K., and C.C.-C. designed the study. A.W. and F.K. analyzed the data. A.F., M.Mc., P.M., D.R.M., D.S., and S.S. performed experiments. A.W., C.C.-C., and F.K. wrote the manuscript. All authors edited and approved the manuscript. Competing interests: Mount Sinai has licensed serological assays to commercial entities and has filed for patent protection for serological assays. J.A. reports grants for multicenter clinical trials: Atea (COVID), Frontier Technology (HIV), Gilead Sciences (COVID and HIV), Janssen (COVID and HIV), Merck (HIV), Pfizer (COVID), Regeneron (COVID), and Viiv (HIV). J.A. has received personal fees for serving on the HIV scientific advisory boards of Gilead, Janssen, Merck, Theratechnology, and Viiv. Data and materials availability: All data are available in the manuscript or the supplementary materials. This work is licensed under a Creative Commons Attribution 4.0 International (CC BY 4.0) license, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. To view a copy of this license, visit https://creativecommons.org/licenses/by/4.0/. This license does not apply to figures/photos/artwork or other content included in the article that is credited to a third party; obtain authorization from the rights holder before using such material.
Supplementary Materials
science.sciencemag.org/content/370/6521/1227/suppl/DC1
Materials and Methods
Table S1
References
MDAR Reproducibility Checklist
References and Notes
- 1.Liu L., To K. K.-W., Chan K.-H., Wong Y.-C., Zhou R., Kwan K.-Y., Fong C. H.-Y., Chen L.-L., Choi C. Y.-K., Lu L., Tsang O. T.-Y., Leung W.-S., To W.-K., Hung I. F.-N., Yuen K.-Y., Chen Z., High neutralizing antibody titer in intensive care unit patients with COVID-19. Emerg. Microbes Infect. 9, 1664–1670 (2020). 10.1080/22221751.2020.1791738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Long Q. X., Liu B.-Z., Deng H.-J., Wu G.-C., Deng K., Chen Y.-K., Liao P., Qiu J.-F., Lin Y., Cai X.-F., Wang D.-Q., Hu Y., Ren J.-H., Tang N., Xu Y.-Y., Yu L.-H., Mo Z., Gong F., Zhang X.-L., Tian W.-G., Hu L., Zhang X.-X., Xiang J.-L., Du H.-X., Liu H.-W., Lang C.-H., Luo X.-H., Wu S.-B., Cui X.-P., Zhou Z., Zhu M.-M., Wang J., Xue C.-J., Li X.-F., Wang L., Li Z.-J., Wang K., Niu C.-C., Yang Q.-J., Tang X.-J., Zhang Y., Liu X.-M., Li J.-J., Zhang D.-C., Zhang F., Liu P., Yuan J., Li Q., Hu J.-L., Chen J., Huang A.-L., Antibody responses to SARS-CoV-2 in patients with COVID-19. Nat. Med. 26, 845–848 (2020). 10.1038/s41591-020-0897-1 [DOI] [PubMed] [Google Scholar]
- 3.Krammer F., Simon V., Serology assays to manage COVID-19. Science 368, 1060–1061 (2020). 10.1126/science.abc1227 [DOI] [PubMed] [Google Scholar]
- 4.Wrapp D., Wang N., Corbett K. S., Goldsmith J. A., Hsieh C.-L., Abiona O., Graham B. S., McLellan J. S., Cryo-EM structure of the 2019-nCoV spike in the prefusion conformation. Science 367, 1260–1263 (2020). 10.1126/science.abb2507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Letko M., Marzi A., Munster V., Functional assessment of cell entry and receptor usage for SARS-CoV-2 and other lineage B betacoronaviruses. Nat. Microbiol. 5, 562–569 (2020). 10.1038/s41564-020-0688-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Amanat F., Krammer F., SARS-CoV-2 vaccines: Status report. Immunity 52, 583–589 (2020). 10.1016/j.immuni.2020.03.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Amanat F., Stadlbauer D., Strohmeier S., Nguyen T. H. O., Chromikova V., McMahon M., Jiang K., Arunkumar G. A., Jurczyszak D., Polanco J., Bermudez-Gonzalez M., Kleiner G., Aydillo T., Miorin L., Fierer D. S., Lugo L. A., Kojic E. M., Stoever J., Liu S. T. H., Cunningham-Rundles C., Felgner P. L., Moran T., García-Sastre A., Caplivski D., Cheng A. C., Kedzierska K., Vapalahti O., Hepojoki J. M., Simon V., Krammer F., A serological assay to detect SARS-CoV-2 seroconversion in humans. Nat. Med. 26, 1033–1036 (2020). 10.1038/s41591-020-0913-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wajnberg A., Mansour M., Leven E., Bouvier N. M., Patel G., Firpo-Betancourt A., Mendu R., Jhang J., Arinsburg S., Gitman M., Houldsworth J., Sordillo E., Paniz-Mondolfi A., Baine I., Simon V., Aberg J., Krammer F., Reich D., Cordon-Cardo C., Humoral response and PCR positivity in patients with COVID-19 in the New York City region, USA: An observational study. Lancet 1, e283–e289 (2020). 10.1016/S2666-5247(20)30120-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.D. Stadlbauer, J. Tan, K. Jiang, M. Hernandez, S. Fabre, F. Amanat, C. Teo, G. A. Arunkumar, M. McMahon, J. Jhang, M. Nowak, V. Simon, E. Sordillo, H. van Bakel, F. Krammer, Seroconversion of a city: Longitudinal monitoring of SARS-CoV-2 seroprevalence in New York City. medRxiv 2020.06.2028.20142190 [Preprint]. 29 June 2020. 10.1101/2020.06.28.20142190. 10.1101/2020.06.28.20142190 [DOI]
- 10.Stadlbauer D., Amanat F., Chromikova V., Jiang K., Strohmeier S., Arunkumar G. A., Tan J., Bhavsar D., Capuano C., Kirkpatrick E., Meade P., Brito R. N., Teo C., McMahon M., Simon V., Krammer F., SARS-CoV-2 seroconversion in humans: A detailed protocol for a serological assay, antigen production, and test setup. Curr. Protoc. Microbiol. 57, e100 (2020). 10.1002/cpmc.100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liu S. T. H., Lin H.-M., Baine I., Wajnberg A., Gumprecht J. P., Rahman F., Rodriguez D., Tandon P., Bassily-Marcus A., Bander J., Sanky C., Dupper A., Zheng A., Nguyen F. T., Amanat F., Stadlbauer D., Altman D. R., Chen B. K., Krammer F., Mendu D. R., Firpo-Betancourt A., Levin M. A., Bagiella E., Casadevall A., Cordon-Cardo C., Jhang J. S., Arinsburg S. A., Reich D. L., Aberg J. A., Bouvier N. M., Convalescent plasma treatment of severe COVID-19: A propensity score-matched control study. Nat. Med. 10.1038/s41591-020-1088-9 (2020). 10.1038/s41591-020-1088-9 [DOI] [PubMed] [Google Scholar]
- 12.Amanat F., White K. M., Miorin L., Strohmeier S., McMahon M., Meade P., Liu W.-C., Albrecht R. A., Simon V., Martinez-Sobrido L., Moran T., García-Sastre A., Krammer F., An in vitro microneutralization assay for SARS-CoV-2 serology and drug screening. Curr. Protoc. Microbiol. 58, e108 (2020). 10.1002/cpmc.108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Long Q. X., Tang X.-J., Shi Q.-L., Li Q., Deng H.-J., Yuan J., Hu J.-L., Xu W., Zhang Y., Lv F.-J., Su K., Zhang F., Gong J., Wu B., Liu X.-M., Li J.-J., Qiu J.-F., Chen J., Huang A.-L., Clinical and immunological assessment of asymptomatic SARS-CoV-2 infections. Nat. Med. 26, 1200–1204 (2020). 10.1038/s41591-020-0965-6 [DOI] [PubMed] [Google Scholar]
- 14.Krammer F., Weir J. P., Engelhardt O., Katz J. M., Cox R. J., Meeting report and review: Immunological assays and correlates of protection for next-generation influenza vaccines. Influenza Other Respir. Viruses 14, 237–243 (2020). 10.1111/irv.12706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Plotkin S. A., Correlates of protection induced by vaccination. Clin. Vaccine Immunol. 17, 1055–1065 (2010). 10.1128/CVI.00131-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Van Herck K., Van Damme P., Inactivated hepatitis A vaccine-induced antibodies: Follow-up and estimates of long-term persistence. J. Med. Virol. 63, 1–7 (2001). [DOI] [PubMed] [Google Scholar]
- 17.Huang A. T., Garcia-Carreras B., Hitchings M. D. T., Yang B., Katzelnick L. C., Rattigan S. M., Borgert B. A., Moreno C. A., Solomon B. D., Trimmer-Smith L., Etienne V., Rodriguez-Barraquer I., Lessler J., Salje H., Burke D. S., Wesolowski A., Cummings D. A. T., A systematic review of antibody mediated immunity to coronaviruses: Kinetics, correlates of protection, and association with severity. Nat. Commun. 11, 4704 (2020). 10.1038/s41467-020-18450-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Deng W., Bao L., Liu J., Xiao C., Liu J., Xue J., Lv Q., Qi F., Gao H., Yu P., Xu Y., Qu Y., Li F., Xiang Z., Yu H., Gong S., Liu M., Wang G., Wang S., Song Z., Liu Y., Zhao W., Han Y., Zhao L., Liu X., Wei Q., Qin C., Primary exposure to SARS-CoV-2 protects against reinfection in rhesus macaques. Science 369, 818–823 (2020). 10.1126/science.abc5343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chandrashekar A., Liu J., Martinot A. J., McMahan K., Mercado N. B., Peter L., Tostanoski L. H., Yu J., Maliga Z., Nekorchuk M., Busman-Sahay K., Terry M., Wrijil L. M., Ducat S., Martinez D. R., Atyeo C., Fischinger S., Burke J. S., Slein M. D., Pessaint L., Van Ry A., Greenhouse J., Taylor T., Blade K., Cook A., Finneyfrock B., Brown R., Teow E., Velasco J., Zahn R., Wegmann F., Abbink P., Bondzie E. A., Dagotto G., Gebre M. S., He X., Jacob-Dolan C., Kordana N., Li Z., Lifton M. A., Mahrokhian S. H., Maxfield L. F., Nityanandam R., Nkolola J. P., Schmidt A. G., Miller A. D., Baric R. S., Alter G., Sorger P. K., Estes J. D., Andersen H., Lewis M. G., Barouch D. H., SARS-CoV-2 infection protects against rechallenge in rhesus macaques. Science 369, 812–817 (2020). 10.1126/science.abc4776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shi R., Shan C., Duan X., Chen Z., Liu P., Song J., Song T., Bi X., Han C., Wu L., Gao G., Hu X., Zhang Y., Tong Z., Huang W., Liu W. J., Wu G., Zhang B., Wang L., Qi J., Feng H., Wang F.-S., Wang Q., Gao G. F., Yuan Z., Yan J., A human neutralizing antibody targets the receptor-binding site of SARS-CoV-2. Nature 584, 120–124 (2020). 10.1038/s41586-020-2381-y [DOI] [PubMed] [Google Scholar]
- 21.Imai M., Iwatsuki-Horimoto K., Hatta M., Loeber S., Halfmann P. J., Nakajima N., Watanabe T., Ujie M., Takahashi K., Ito M., Yamada S., Fan S., Chiba S., Kuroda M., Guan L., Takada K., Armbrust T., Balogh A., Furusawa Y., Okuda M., Ueki H., Yasuhara A., Sakai-Tagawa Y., Lopes T. J. S., Kiso M., Yamayoshi S., Kinoshita N., Ohmagari N., Hattori S. I., Takeda M., Mitsuya H., Krammer F., Suzuki T., Kawaoka Y., Syrian hamsters as a small animal model for SARS-CoV-2 infection and countermeasure development. Proc. Natl. Acad. Sci. U.S.A. 117, 16587–16595 (2020). 10.1073/pnas.2009799117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yu J., Tostanoski L. H., Peter L., Mercado N. B., McMahan K., Mahrokhian S. H., Nkolola J. P., Liu J., Li Z., Chandrashekar A., Martinez D. R., Loos C., Atyeo C., Fischinger S., Burke J. S., Slein M. D., Chen Y., Zuiani A., Lelis F. J. N., Travers M., Habibi S., Pessaint L., Van Ry A., Blade K., Brown R., Cook A., Finneyfrock B., Dodson A., Teow E., Velasco J., Zahn R., Wegmann F., Bondzie E. A., Dagotto G., Gebre M. S., He X., Jacob-Dolan C., Kirilova M., Kordana N., Lin Z., Maxfield L. F., Nampanya F., Nityanandam R., Ventura J. D., Wan H., Cai Y., Chen B., Schmidt A. G., Wesemann D. R., Baric R. S., Alter G., Andersen H., Lewis M. G., Barouch D. H., DNA vaccine protection against SARS-CoV-2 in rhesus macaques. Science 369, 806–811 (2020). 10.1126/science.abc6284 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
science.sciencemag.org/content/370/6521/1227/suppl/DC1
Materials and Methods
Table S1
References
MDAR Reproducibility Checklist