Abstract
Background:
Postoperative hypocalcaemia (POH) after total thyroidectomy (TT) is a common complication. Parathyroid hormone (PTH), an accurate predictor of POH cannot assess intra-operative viability of parathyroid glands (PGs). Different dyes including indocyanine green or carbon nanoparticles have been used, but they are expensive and not widely available. Fluorescein green dye (FD) has been used as a low-cost alternative to study viability of various organs, but seldom tried in visualizing PGs. This novel study aims to assess utility of FD in determining parathyroid viability and predicting POH.
Material and Method:
Total 72 out of 88 patients undergoing TT between January and December 2019 were included. Two ml of 25% FD was given intravenously before wound closure and attempts were made to visualize PGs under blue light. A numerical score was given according to the number of PGs visualized. Intact-PTH and corrected calcium were measured on postoperative day 1 and patients observed for POH.
Results:
No PGs were visualized in 6 patients, 1 in 13, 2 in 30, 3 in 16 & 4 in 7 patients. Mean PTH was 6, 16.9, 31.6, 33.2 and 48.5 respectively. Corrected-calcium was 7.08, 7.7, 7.9, 8.5 and 8.5 respectively. All patients with score 0 received supplementary IV calcium, while 53.8% (score-1), 30% (score-2), 0% (scores-3, 4) received the same. Sensitivity, specificity and ROC of PG score of ≥2 on FD in predicting POH were 100%, 44% and 0.83 respectively.
Conclusion:
FD visualization of parathyroids post TT is feasible and can be used as low cost efficacious method to predict POH.
Keywords: Fluorescein green dye, hypocalcemia, parathyroid viability, thyroidectomy
INTRODUCTION
The most common and dreaded complication post thyroid surgery is hypocalcaemia (50% temporary and 13% permanent).[1] This may be due to inadvertent injury to parathyroid glands (PGs) or their delicate tiny blood vessels.[1] Even in specialised centres, there is up to 16% incidence of incidental parathyroidectomies while doing thyroidectomy.[2] Mere visual intra-operative identification of PGs alone does not ensure its intact vascularity.[3,4] Although post-operative day 1 (POD1) serum PTH and serum total calcium is considered to be one of the most accurate predictors of POH,[5,6] still they cannot assess the intra-operative viability of the PGs. Hence, there are more data coming forth on role of auto-fluorescence techniques to ensure that the PGs left are still viable and vascularized.[7,8] This will ensure effective way to predict whether a patient will develop hypocalcemia or not post thyroid surgery, to plan calcium supplements in a selected subset of patients, and also facilitate early safe discharge.
Indocyanine green dye and infra-red-light techniques have been used for this purpose. However, its high cost and lack of availability has resulted in its restricted use which is confined to higher centers only. In India, we use Fluorescein green (FG) dye in eye problems and it works well, easily available, and is low cost. FG dye has peak excitation at wavelength 465−490 nm (blue) and peak emission at 520−530 nm (green).[9] We planned to explore its use in identifying PG vascularity and hence planned to do a prospective cohort study.
The aim of this study is to assess the efficacy of the FG dye technique in predicting intra-operative PG vascularity and post-thyroidectomy hypocalcemia.
MATERIAL AND METHOD
This prospective cohort study was conducted during 1st January- 30th December, 2019 in the Department of Endocrine Surgery, King Georges Medical University, Lucknow.
All patients undergoing total thyroidectomies for both benign and malignant goiters were included in the study. We excluded all the patients not giving consent, previous history of thyroid or parathyroid surgery, concomitant parathyroid disease, and any known renal co-morbidities.
A total of 88 patients were admitted for total thyroidectomy, 12 patients were excluded from the study due to unavailability of POD 1 serum PTH and 4 patients were for completion thyroidectomy. Therefore, a total of 72 patients were finally enrolled in the study [Figure 1].
Figure 1.
Total number of patients enrolled after exclusion
Preoperatively patients were worked up with thyroid function test (TFT), Ultrasound neck, cytopathology of the thyroid nodule, serum calcium, S.PTH, Vitamin-D, and serum Albumin. Postoperatively patients were assessed for clinical and biochemical hypocalcemia. Morning following thyroidectomy at 8:00 am, irrespective of the time of procedure, both serum PTH and corrected calcium were obtained. Decision to start patients on calcium supplementation was based on their clinical and biochemical hypocalcemia. Biochemical POH was defined as corrected calcium level of <8 mg/dl (normal range from 8 to 10.3 mg/dl). Clinical POH was defined as those patients with signs and symptoms of hypocalcemia (Positive Trousseau-sign with tingling and numbness sensation in perioral and both upper limbs) along with a corrected calcium level of <8 mg/dl.
Protocol used for management of patients with POH is shown in Table 1. Median follow-up duration for entire cohort was 9 ± 2.6 months.
Table 1.
Departmental protocol on management of patient with post-operative hypocalcaemia
| Corrected calcium (mg/dl) | Symptoms | Sign (Trousseau sign) | Management | Calcium monitoring |
|---|---|---|---|---|
| <8 | No | No | T. calcium carbonate 1 gm BD for 1 week | Serum total calcium daily for 2 days/till normocalcemic |
| <8 | Yes | No | T. Calcium carbonate 1 gm TDS and T. Calcitriol 0.25 mcg BD for 1 week | Serum total calcium daily for 2 days/till normocalcemic |
| <8 | Yes | Yes | I.V calcium gluconate @1.6-2.2 mg/kg/hr, tapered off slowly till T-sign becomes negative+ T.Calcium carbonate 1 gm TID and Cap. Calcitriol 0.25 mcg TID and discharged once normocalcemic. T. Calcium and calcitriol to continue for 15-30 days | Serum total calcium daily for 3 days/till normocalcaemic Repeat calcium: 14 and 30 days after discharge on follow-up. |
Material
2 ml of 25% Fluorescein dye (equivalent to 500 mg, which is the recommended adult dose) [Figure 2a], LED blue light with wavelength of 465 nm [Figure 2b], and yellow-tinted night glass [Figure 2c] which was used as a filter for blocking the blue light were used for the study purpose. FG dye was diluted with 2 ml of normal saline and injected intravenous through the cubital vein. After 30 seconds of injection of FD and in complete darkness inside the operating room, PGs were looked for by using blue light and wearing yellow glasses.
Figure 2.
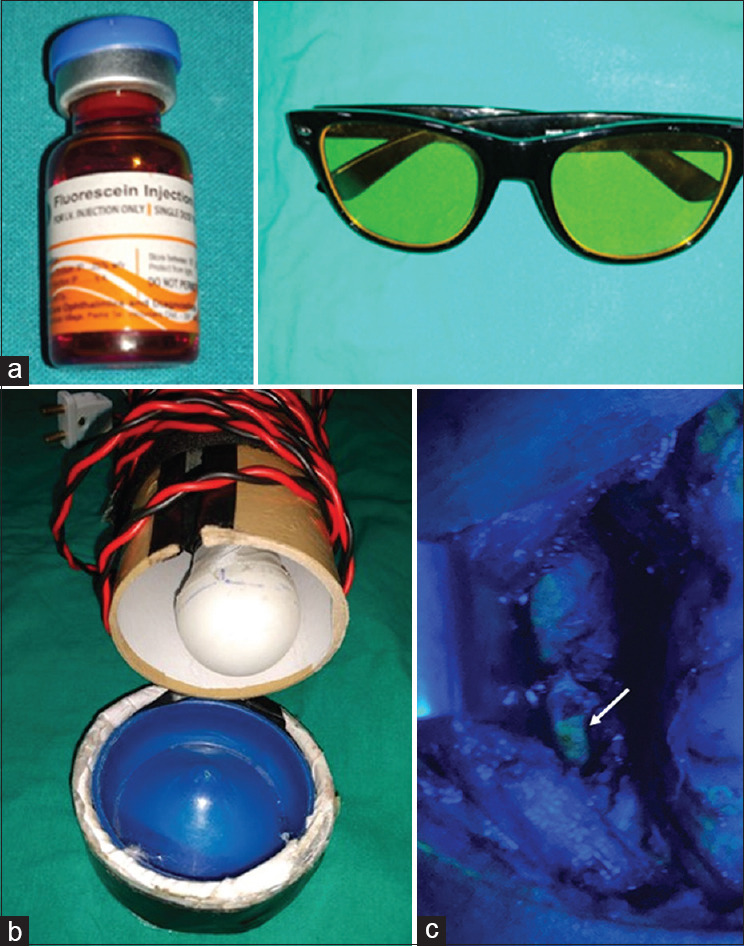
(a): 2 ml of 25% fluorescein green dye. (b): LED blue light. (c): Yellow tinted night glass used as filter. (d): Parathyroid gland visualised as yellow green (white arrow) on fluorescein green dye under blue light
After resecting the thyroid gland and before wound closure, attempts were made to visualize the parathyroid glands before and after injecting the fluorescein dye by the operating surgeon (consultant) and assistant (senior resident) surgeon, and a numerical score of 0 − 4 was given according to the number of parathyroid glands visualized. Parathyroid glands on fluorescein dye after subjecting to blue light with the top lights off were visualized as yellow-green in color (white arrow) [Figure 2d].
Statistical test
Statistical analysis was done using SPSS software (version 22.0). The descriptive statistics were performed using mean with standard deviation or frequency with percentages. ROC curve was drawn to check the sensitivity, specificity of PG score using FD in predicting POH.
RESULTS
The final study population consisted of 72 patients with female to male ratio of 5.5:1. The mean age of study population was 39.2 ± 11.9 years. The most common indication for surgery was benign euthyroid multinodular goitre in 34 (58.6%) patients. There were 58 (69%) of benign and 14 (18%) of malignant pathology in operated thyroidectomy specimens. Papillary Thyroid Carcinoma was the most common malignant pathology subtype in 9 (64.3%) patients. Total thyroidectomy was performed in 65 patients (90.5%), TT with central compartment lymph node dissection (CCLND) and U/L neck dissection in 2 patients (2.7%), TT with CCLND with B/L selective (level 2-5) neck dissection in 5 patients (6.8%).
In majority of the patients, in 30 (44.4%), 2 PGs were visualised, 0 parathyroid in 6 (6.9%) patients, 4 parathyroids in 7 (9.7%) patients on fluorescein dye. On the other hand, on a naked eye, 0 parathyroid in 1 patient, 2 PT in 29 (41.7%) patients and 4 PT in 11 (15.3%) patients were visualised [Table 2].
Table 2.
Data on mean serum PTH, corrected calcium, clinical and biochemical hypocalcaemia
| No of PT visualised | No of patients (%) | Mean PTH at POD1 (pg/ml) [dye/no dye] | Mean corrected calcium at POD 1 (mg/dl) [dye/no dye] | Clinical hypocalcaemia no (%) [dye/no dye] | Biochemical hypocalcaemia no (%) [dye/no dye] |
|---|---|---|---|---|---|
| 0 | [dye] 6 (6.9) | 6±3.5 | 7.08±0.3 | 6 (100) | 7 (53.8) |
| [no dye] 1 (1.4) | 12.3 | 7.12 | - | 1 (100) | |
| 1 | [dye] 13 (18.1) | 16.97±17.7 | 7.7±0.8 | 7 (53.8) | 3 (23.1) |
| [no dye] 6 (6.9) | 21.04±23.7 | 7.9±0.8 | 2 (40) | 3 (60) | |
| 2 | [dye] 30 (44.4) | 31.6±31.7 | 7.9±0.76 | 9 (30) | 10 (33.9) |
| [no dye] 29 (41.7) | 25.8±31.5 | 7.7±0.8 | 10 (34.5) | 7 (24.1) | |
| 3 | [dye] 16 (20.8) | 33.2±17.47 | 8.5±0.32 | 0 | 4 (25) |
| [no dye] 25 (34.2) | 29.4±21.6 | 7.8±0.6 | 7 (28) | 6 (24) | |
| 4 | [dye] 7 (9.7) | 48.5±29.9 | 8.5±0.49 | 0 | 1 (14.3) |
| [no dye] 11 (15.3) | 37.4±29.7 | 8.2±1.1 | 3 (27.3) | 2 (18.2) |
The serum PTH and corrected calcium on POD-1 increased with increase in number of parathyroid glands being visualised on dye as well as on naked eye. Clinical hypocalcaemia was observed in all patients (100%) in whom no PT was visualised on fluorescein dye (FD), whereas none of the patients in whom 3 and 4 PT was visualised on dye developed clinical hypocalcaemia. However, it was observed that even in those patients in whom 3 and 4 PT was seen on naked eye developed clinical hypocalcaemia, 7 (28%) and 3 (23.7%) patients respectively [Table 2].
In 25% and 14.3% of patients in whom 3 and 4 PGs were visualised respectively on FD developed transient biochemical hypocalcaemia (i.e., recovered with oral calcium supplements within one week).
At 6 months follow-up, only two patients developed permanent hypocalcaemia. One each in whom one and two PGs were visualised on Fluorescein dye. This false negative might be due to inter-observer variation or due to delayed vascular injury. Those patients in whom no PGs were visualised on FG did not developed permanent hypocalcaemia, the reason maybe that PGs were congested and did not take up the dye which later on became viable or might be due to some tissue obscuring the visibility.
Figure 3 shows the sensitivity, specificity and ROC of PG score of ≥2 on FD in predicting POH was 100%, 44% and 0.83 (CI 73.9%-93.0%).
Figure 3.
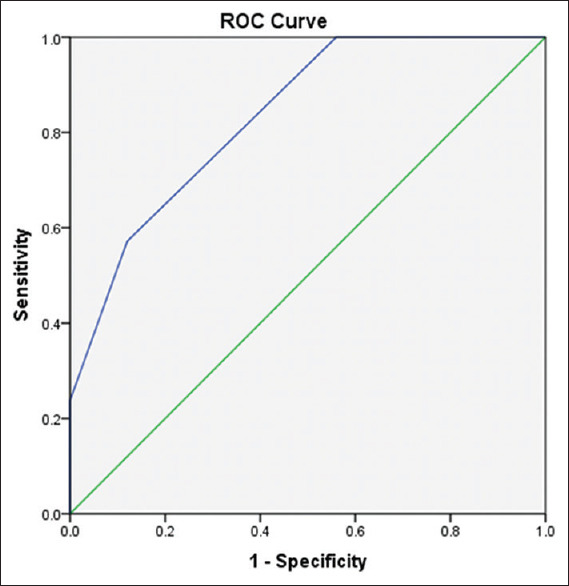
ROC curve of PG score on FD in predicting postoperative hypocalcaemia
There was no serious adverse event or anaphylactic reaction due to FG dye injection in any of the patients. Their conjunctiva becomes mild yellow in colour due to the FG dye and it normalised by itself in twenty-four hours. The cost of FG dye is Rupees 50 per vial of 2 ml dye. There was no other extra cost involved in this technique.
DISCUSSION
Post- thyroidectomy hypocalcaemia results due to varies reasons like haemodilution as a consequence of intra-operative fluid, intra-operative hypothermia which might have affected the calcium level, calcitonin release post thyroidectomy, low vitamin D level (which was not checked in all of our patients due to high cost), poor nutrition, inadvertent injury to blood supply of the parathyroid glands or removal of normal parathyroid glands.[10] In our study, we looked into quantifying and documenting intra-operative PGs viability by use of FD technique and it's co-relation with POH.
Mere identification and leaving behind PG does not ensure its intact vascularity. PGs mostly have a tiny single end artery supply from branch of inferior thyroid artery mostly and rarely from superior thyroid artery.[11] As it's an end artery, injury to this tiny vessel will result in ischemia leading to devascularized PG and postoperative hypocalcaemia. Hence, it's very important to identify intra-operatively PGs with their intact blood vessels. When PG is devascularized its colour changes and gets congested. When we give a nick on PG capsule by a sharp knife it bleeds and gets decongested. However, many a times we may not pick up early signs of devascularized PG. In our study also we had some patients in whom ≥ PGs were visualised by FD technique still patients developed POH and we may have missed these early/delayed ischemic changes in PGs in these patients.
There are many techniques used as an intra-operative adjunct like IOPTH, frozen section, giving a sharp nick on parathyroid gland to see it bleeds or not, injecting lidocaine and seeing it swells or not and use of ICG angiography to assess its viability.[12,13,14] PTH is one of the most accurate predictors of postoperative hypocalcaemia but is costly and does not tell us about the viability of parathyroid vascularity.
Indocyanine Green (ICG) dye angiography was first used to detect macular degeneration.[7] Later ICG with added infra-red camera was also used to capture perfusion of tissues example use in liver surgery, for resected margin status, sentinel lymph node biopsy, flap viability by Plastic Surgeons and even to check vascular flow after intestinal anastomosis.[15,16] As its use expanded, people also used ICG in detecting intra-operatively parathyroid vascularity while doing thyroid surgery. Recently, Laser Speckle Contrast Imaging technique has also been explored in detecting PG vascularity and to help surgeons decide when to excise the PG and auto-transplant it in muscle.[7] However, its use is confined to selected higher centres where affordability or cost is not a major issue.
Studies have shown that by naked eye intra-operatively what we think as a vascularised parathyroid gland after removal of thyroid specimen, ICG technique confirmed that it has been devascularized and helped surgeon to auto transplant that PG.[15,16] This is another benefit of using imaging technique. In our study, we did not look into the auto-transplantation of PGs factor, however we plan to incorporate this factor also in our next future RCT study.
Other benefit of using imaging technique is to confirm PG vascularity and obviate the need to routine supplement calcium or vitamin D to patient's postoperatively.[17] Problems with ICG technique are difficulty to identify PGs and camera shows in shades of grey and is a qualitative measure of PG perfusion status. Surgeons find it difficult to correctly localise the PGs and differentiate them from other anatomical structures.[17] Even in our study, we found that FD technique of identifying PGs had more positive correlation with postoperative hypocalcemia as compared to naked eye technique alone.
Some centres have used autofluorescence with near infrared light instead of dye technique for intra-operative assessment of PG vascularity.[18,19,20,21,22,23] Other intra-operative adjuncts like IOPTH and post-operative serum calcium levels are done to predict postoperative hypocalcaemia post thyroidectomy. These add to extra cost, special assay techniques for IOPTH, prolong the operative time and being invasive procedures add needle pricks to the patients. We need an intra-operative device which serve the same purpose with no postoperative needle pricks to patients and helps clinicians to decide which patient needs calcium supplements and who does not. This will guide us towards better decision making and better patient care.
In India, we need to have a safe, effective and low-cost technique which can have a wider application and can be used by all centres. We extended use of FG dye in detecting PG vascularity intra-operatively and predicting post-thyroidectomy hypocalcaemia.
Our prospective study showed that visualisation rate of PGs was more accurate with FG dye technique as compared to naked eye method. When 3/4 PGs were visualized by FG dye technique then no patient developed postoperative hypocalcaemia. However, with naked eye visualization of 3/4 PGs, 23% and 28% patients respectively developed postoperative hypocalcaemia. Hence, we conclude that mere visualization of PGs by eyes alone does not justify that left behind PGs are still viable.
In our study, the serum IOPTH levels were directly correlated to the number of identified PGs by FG dye technique. If we visualized ≥2 PGs intra-operatively by FG dye technique, then patients did not develop postoperative hypocalcaemia and we could avoid supplementing them with calcium/vitamin D medications. Patients in whom <2 PGs were visualized they had higher chance of developing postoperative hypocalcaemia and needed calcium supplements (oral or i.v.) along with Vitamin D. Hence, we need to be cautious in these subsets of patients for postoperative hypocalcaemia.
The limitations of our study are that we did not do any intervention based on our findings and it was not a randomized controlled trial (RCT). However, it is a prospective cohort study and we plan to do RCT with intervention in one arm and no intervention in the other arm in future based on our results. The strength of our study is its prospective nature and being novel as it is the first study done on the use of FG dye in assessing PG viability and predicting post-thyroidectomy hypocalcaemia.
PG visualization by FG dye technique helps surgeons in five ways: documenting how many viable PGs have been preserved in a patient post thyroidectomy, deciding how many non-viable PG are there which need to be auto transplanted, predicting postoperative hypocalcaemia, need to check postoperative serum calcium, PTH levels in selected subset of patients on high risk of developing hypocalcaemia and enable early safe discharge. This will avoid unnecessary needle pricks and added cost of biochemical investigations and help in better patient management. In this prospective cohort study, we did not do any intervention based on the findings, however we plan to check FD applicability in all these five modes of the benefits in our future prospective RCT study.
CONCLUSION
Fluorescein green dye technique for visualization of parathyroids may be used as a simple, low cost, easily available alternative method to assess intra-operative PG viability and predict postoperative hypocalcaemia. Visualisation of ≥2 parathyroid glands on fluorescein dye can rule out development of postoperative hypocalcaemia.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form the patient(s) has/have given his/her/their consent for his/her/their images and other clinical information to be reported in the journal. The patients understand that their names and initials will not be published and due efforts will be made to conceal their identity, but anonymity cannot be guaranteed.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgement
We thank the operating room staff for all the technical help needed for intra-operative documentation. There were no funding source for this work.
REFERENCES
- 1.Falco J, Dip F, Quadri P, de la Fuente M, Rosenthal R. Cutting edge in thyroid surgery: Autofluorescence of parathyroid glands. J Am Coll Surg. 2016;223:374–80. doi: 10.1016/j.jamcollsurg.2016.04.049. [DOI] [PubMed] [Google Scholar]
- 2.Awisi Gyau D, Begley CG, Daniel Nelson J. A simple and cost effective method for preparing FL and LG solutions. Ocul Surf. 2018;16:139–45. doi: 10.1016/j.jtos.2017.10.004. [DOI] [PubMed] [Google Scholar]
- 3.Park I, Rhu J, Woo JW, Choi JH, Kim JS, Kim JH. Preserving parathyroid gland vasculature to reduce post-thyroidectomy hypocalcemia. World J Surg. 2016;40:1382–9. doi: 10.1007/s00268-016-3423-3. [DOI] [PubMed] [Google Scholar]
- 4.Mehta S, Dhiwakar M, Swaminathan K. Outcomes of parathyroid glan identification and autotransplantation during total thyroidectomy. Eur Arch Otorhinolaryngol. 2020;277:2319–24. doi: 10.1007/s00405-020-05941-9. [DOI] [PubMed] [Google Scholar]
- 5.Sekar S, Belavendra A, Jacob MJ. Early discharge and selective calcium supplementation after thyroidectomy based on post-operative day 1 parathhormone and calcium level: A prospective study. Indian J Endocr Metab. 2020;24:319–24. doi: 10.4103/ijem.IJEM_172_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cherian AJ, Ramakant P, Paul TV, Abraham DT, Paul MJ. Next-day parathyroid hormone as a predictor of post-thyroidectomy hypocalcemia. World J Endoc Surg. 2016;8:203–7. [Google Scholar]
- 7.Mannoh EA, Thomas G, Solórzano CC, Mahadevan-Jansen A. Intraoperative assessment of parathyroid viability using laser speckle contrast imaging. Sci Rep. 2017;7:14798. doi: 10.1038/s41598-017-14941-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kim SW, Song SH, Lee HS, Noh WJ, Oak C, Ahn YC, Lee KD. Intraoperative real-time localization of normal parathyroid glands with autofluorescence imaging. J Clin Endocrinol Metab. 2016;101:4646–52. doi: 10.1210/jc.2016-2558. [DOI] [PubMed] [Google Scholar]
- 9.Lin P, Han P, Liang F, Cai Q, Chen R, Yu S, Zhou Z, Huang X. Characteristics of the parathyroid gland in endoscopic thyroidectomy with the application of an image enhancement system. Surg Endosc. 2018;32:3925–35. doi: 10.1007/s00464-018-6132-1. [DOI] [PubMed] [Google Scholar]
- 10.Karampinis I, Di Meo G, Gerken A, Stasiunaitis V, Lammert A, Nowak K. [Intraoperative indocyanine green fluorescence to assure vital parathyroids in thyroid resections] Zentralbl Chir. 2018;143:380–4. doi: 10.1055/a-0655-7881. [DOI] [PubMed] [Google Scholar]
- 11.Dedivitis RA, Aires FT, Cernea CR. Hypoparathyroidism after thyroidectomy: Prevention, assessment and management. Curr Opin Otolaryngol Head Neck Surg. 2017;25:142–6. doi: 10.1097/MOO.0000000000000346. [DOI] [PubMed] [Google Scholar]
- 12.Mohebati A, Shaha AR. Anatomy of thyroid and parathyroid glands and neurovascular relations. Clin Anat. 2012;25:19–31. doi: 10.1002/ca.21220. [DOI] [PubMed] [Google Scholar]
- 13.Vidal Fortuny J, Sadowski SM, Belfontali V, Guigard S, Poncet A, Ris F, et al. Randomized clinical trial of intraoperative parathyroid gland angiography with indocyanine green fluorescence predicting parathyroid function after thyroid surgery. Br J Surg. 2018;105:350–7. doi: 10.1002/bjs.10783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Abbaci M, De Leeuw F, Breuskin I, Casiraghi O, Lakhdar AB, Ghanem W, et al. Parathyroid gland management using optical technologies during thyroidectomy or parathyroidectomy: A systematic review. Oral Oncol. 2018;87:186–96. doi: 10.1016/j.oraloncology.2018.11.011. [DOI] [PubMed] [Google Scholar]
- 15.Cavicchi O, Piccin O, Caliceti U, Fernandez IJ, Bordonaro C, Saggese D, et al. Accuracy of PTH assay and corrected calcium in early prediction of hypoparathyroidism after thyroid surgery. Otolaryngol Head Neck Surg. 2018;138:594–600. doi: 10.1016/j.otohns.2008.01.016. [DOI] [PubMed] [Google Scholar]
- 16.Rudin AV, McKenzie TJ, Thompson GB, Farley DR, Lyden ML. Evaluation of parathyroid glands with indocyanine green fluorescence angiography after thyroidectomy. World J Surg. 2019;43:1538–43. doi: 10.1007/s00268-019-04909-z. [DOI] [PubMed] [Google Scholar]
- 17.Tsang SH, Sharma T. Fluorescein angiography. Adv Exp Med Biol. 2018;1085:7–10. doi: 10.1007/978-3-319-95046-4_2. [DOI] [PubMed] [Google Scholar]
- 18.Vidal Fortuny J, Belfontali V, Sadowski SM, Karenovics W, Guigard S, Triponez F. Parathyroid gland angiography with indocyanine green fluorescence to predict parathyroid function after thyroid surgery. Br J Surg. 2016;103:537–43. doi: 10.1002/bjs.10101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cavallo C, De Laurentis C, Vetrano IG, Falco J, Broggi M, Schiariti M, et al. The utilization of fluorescein in brain tumor surgery: A systematic review. J Neurosurg Sci. 2018;62:690–703. doi: 10.23736/S0390-5616.18.04480-6. [DOI] [PubMed] [Google Scholar]
- 20.Vidal Fortuny J, Karenovics W, Triponez F, Sadowski SM. Intra-operative indocyanine green angiography of the parathyroid gland. World J Surg. 2016;40:2378–81. doi: 10.1007/s00268-016-3493-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Alesina PF, Meier B, Hinrichs J, Mohmand W, Walz MK. Enhanced visualization of parathyroid glands during video-assisted neck surgery. Langenbecks Arch Surg. 2018;403:395–401. doi: 10.1007/s00423-018-1665-2. [DOI] [PubMed] [Google Scholar]
- 22.Ladurner R, Lerchenberger M, Al Arabi N, Gallwas JKS, Stepp H, Hallfeldt KKJ. Parathyroid autofluorescence-how does it affect parathyroid and thyroid surgery? A 5 year experience. Molecules. 2019;24:2560. doi: 10.3390/molecules24142560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jin H, Dong Q, He Z, Fan J, Liao K, Cui M. Application of a fluorescence imaging system with indocyanine green to protect the parathyroid gland intraoperatively and to predict postoperative parathyroidism. Adv Ther. 2018;35:2167–75. doi: 10.1007/s12325-018-0834-6. [DOI] [PubMed] [Google Scholar]


