Abstract
Background:
Podoplanin, an important protein, has been implicated in various cellular processes, including lymphangiogenesis. Podoplanin is a mucin-type transmembrane glycoprotein that is accepted as a novel marker of lymphatic endothelial cells.
Objectives:
To study the immunohistochemical expression of podoplanin in the different stages of mycosis fungoides (MF) in comparison to control and to correlate their expression with disease severity and progression.
Materials and Methods:
The study included 50 patients of MF, clinically diagnosed and assessed by World Health Organization/European Organization for Research And Treatment Of Cancer Consensus and 20 normal persons as control. Skin biopsy specimens were taken from all and examined for expression of podoplanin immunohistochemically.
Results:
Significant upregulation of podoplanin expression was detected in all studied patients of MF in comparison to control group. Podoplanin expression in malignant lymphocytes and also lymph vessel density showed significant upregulation in the aggressive clinical presentations as well as the highest stages regarding TNMB staging of MF.
Conclusions:
Evaluation of podoplanin expression may be taken into consideration in the future as a useful tool to identify high-risk MF patients. Furthermore, it may open new therapeutic options for the clinical management of those patients.
KEY WORDS: Lymphangiogenesis, mycosis fungoides, podoplanin
Introduction
Lymphangiogenesis is a multistep process of formation of new lymphatic vessels from the existing vascular network through extension and branching in response to local stimulation.[1] It plays a fundamental role in metastasis of malignancies.[2]
Podoplanin is a mucin-type transmembrane glycoprotein[3,4,5] that was accepted as a novel marker of lymphatic endothelial cells.[6] In normal human tissue, it is expressed in kidney, skeletal muscle, placenta, lung, heart, myofibroblasts of the breast, salivary glands, osteoblasts, and mesothelial cells.[7,8] Occasionally, focal expression of podoplanin can be found in circumscribed areas of the basal layer of the human epidermis.[9]
As podoplanin is expressed on lymphatic but not on blood vessel endothelium, it is widely used as a specific marker for lymphatic endothelial cells and for lymphangiogenesis in many species.[10] It is involved in the process of adhesion and migration of endothelial cells, and its expression is observed on lymphatic endothelium and in oncologic lesions of breast, esophageal and testicular cancers, as well as in germ cell tumors.[11]
Mycosis fungoides (MF) is classified as cutaneous T-cell lymphoma (CTCL).[12] It arises in peripheral T lymphocytes and develops in the skin, evolving through consecutive stages, from patches, plaques to tumors.[13] Studies suggest involvement of lymphangiogenesis in the development and progression of the CTCL.[14,15]
The aim of this work was to study the immunohistochemical expression of podoplanin in different stages of MF in comparison to normal skin and to correlate their expression with disease severity and progression.
Materials and Methods
The study included 50 patients of MF, clinically diagnosed and assessed by World Health Organization/European Organization for Research and Treatment of Cancer Consensus and confirmed by histopathological and immunohistochemical examinations in addition to 20 normal skin specimens from age- and sex-matched healthy individuals. The patients were collected from Dermatology and Venereology Department and Oncology Institute, Tanta University Hospitals. Ethical approval was obtained from Ethics Committee before commencement of the study with approval number (31264) (2/2018).
All subjects included in the study were either newly diagnosed patients or who stopped systemic treatment for at least 6 weeks and topical treatment for 4 weeks before biopsy. Patients with any other dermatological or systemic diseases and pregnant and lactating females were excluded from the study.
All participants were subjected to complete careful history taking, thorough general and dermatological examinations. Detailed dermatological examination of all MF lesions was performed to assess the clinical type of MF and to classify the different stages of MF patients according to TNMB classification.[16,17]
After taking written informed consent, routine laboratory investigations were done for all participants. Four mm punch skin biopsies were taken from each MF patient and normal control skin specimens from matched sites were taken, fixed in 10% formalin and routinely processed paraffin-embedded tissue sections (3-4 μ) were prepared on charged glass slides for:
- Hematoxylin and eosin (H and E) staining was performed to confirm the diagnosis of MF and to demonstrate the histopathological stage of lesion
- Immunohistochemical staining: The method used for immunostaining was the standard avidin biotin peroxidase complex using primary monoclonal mouse anti-human podoplanin antibody (clone D2-40, RTU, IR072; Dako).[18] The slides were submitted to subsequent steps of deparaffinization and rehydration. Antigen retrieval was done by boiling in citrate buffer saline at pH 6.0 followed by cooling at room temperature. The primary antibody was incubated overnight at room temperature, and then the secondary antibody was applied with diaminobenzidine as a chromogen substrate and Meyer's hematoxylin as a counter stain. All slides were examined by image analysis by Leica Qwin at the same magnification. For each specimen, four separate fields of view were evaluated, for:
- Density scoring of podoplanin expression:[19] Positive staining lymphocytes (light-brown to deep-brown color) were classified according to the staining intensity, which was rated on a scale of 0 to 3 as: 0-negative, 1-mild (less than 25% positive cells), 2-moderate (25%-75% positive cells), and 3-severe (more than 75% positive cells)
- Quantification of lymphatic vessel density (LVD):[20] Evaluation of positive reactions was performed by counting podoplanin positive lymphatic vessels, sitting around a visible lumen under 100 magnification, to find three hot spots with the greatest number of lymphatic vessels. Then, total amount of lymphatic vessels in each area was counted and average score for each section was determined.
Statistical analysis
Data were analyzed using IBM SPSS software package version 20.0 (Armonk, NY: IBM Corp). Qualitative data were described using number and percent. Quantitative data were described using range, mean, standard deviation, and median. Comparison between different groups regarding categorical variables was tested using Chi-square test. Correction for Chi-square was conducted using Fisher's Exact test or Monte-Carlo correction. For normally distributed data, comparison between two independent populations was done using independent t -test. Significance of the obtained results was considered with P < 0.05.
Results
Clinical results
There were 30 males and 20 females among the patients and 12 males and 8 females among the controls (P = 1.000). The average age among the patients was 34.08 ± 17.62 (range 6–65) years and that among the control was 28.50 ± 11.12 (range 12–50) years (P = 0.272).
The duration of MF lesions ranged from 0.25 to 20 years with a mean ± SD of 9.96 ± 5.48.
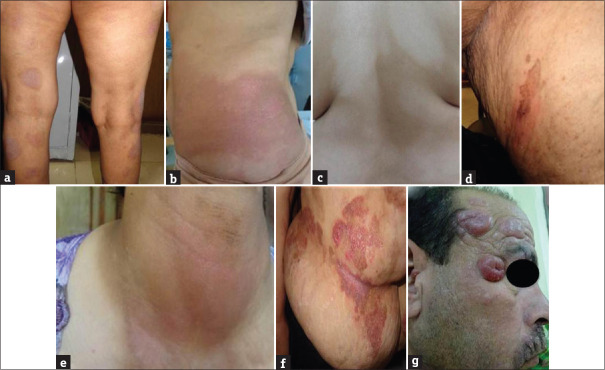
The MF patients were classified into the following clinical types: 38 patients had patch stage (18 classic, 10 hypopigmented, 2 hyperpigmented, 4 poikilodermatous, 2 folliculotropic and 2 erythrodermic); 10 patients had plaque stage (classic type), and 2 patients had tumor stage (classic type) [Table 1 and Figure 1a-g].
Table 1.
The clinical presentation and the clinical types of mycosis fungoides among the patients
| Clinical type | Clinical presentation | |||||
|---|---|---|---|---|---|---|
| Patch stage (n=38) | Plaque stage (n=10) | Tumor stage (n=2) | ||||
| No. | % | No. | % | No. | % | |
| Classic type | 18 | 47.4 | 10 | 100.0 | 2 | 100.0 |
| Hypopigmented MF | 10 | 26.3 | 0 | 0.0 | 0 | 0.0 |
| Hyperpigmented MF | 2 | 5.3 | 0 | 0.0 | 0 | 0.0 |
| Poikilodermatous MF | 4 | 10.4 | 0 | 0.0 | 0 | 0.0 |
| Folliculotropic MF | 2 | 5.3 | 0 | 0.0 | 0 | 0.0 |
| Erythrodermic MF | 2 | 5.3 | 0 | 0.0 | 0 | 0.0 |
MF: Mycosis fungoides
Figure 1.
(a) Patch stage, classic mycosis fungoides. (b) Patch stage, classic mycosis fungoides. (c) Patch stage, hypopigmented mycosis fungoides. (d) Patch stage, hyperpigmented mycosis fungoides. (e) Patch stage, folliculotropic mycosis fungoides. (f) Plaque stage, classic mycosis fungoides. (g) Tumor stage, classic mycosis fungoides
Regarding the grading of MF according to TNMB staging, the patients were classified into: 24 (48%) patients at stage IA, 16 (32%) patients at stage IB, 4 (8%) patients at stage IIA, 2 (4%) patients at stage IIB, and 4 (8%) patients at stage IIIA.
Histopathological and immunohistochemical results
The tissue sections of the patients with MF showed that 18 (31.8%) had epidermal atrophy, 24 (54.5%) had Pautrier's microabscess, and all (100%) had intact hair follicles, epidermotropism, and atypical dermal infiltrate with variable density.
In normal control skin, there was mild podoplanin expression (+1), in basal cell layer of epidermis only in 12 (60%) stained sections, while the dermis was negatively stained for podoplanin and the mean lymph vessel density (LVD) was 7.0.
In MF patient group, the basal cell layer of epidermis showed moderate expression (+2) in 24 (48%) patients and severe expression (+3) in 26 (52%) patients. The malignant lymphocytes in dermis showed mild expression (+1) in 12 (24%) patients, moderate expression (+2) in 14 (28%) patients, and severe expression (+3) in 24 (48%) patients [Figures 2-4]. The mean LVD was 22.4 (range 15–43).
Figure 2.
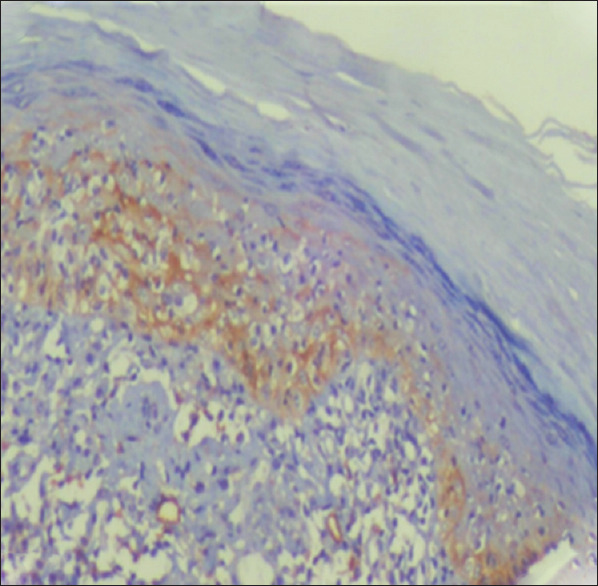
Patch stage MF showed moderate podoplanin expression in the epidermis (basal cell layer), epidermotropic malignant lymphocytes and in lymph vessels density (IHC, ×200)
Figure 4.
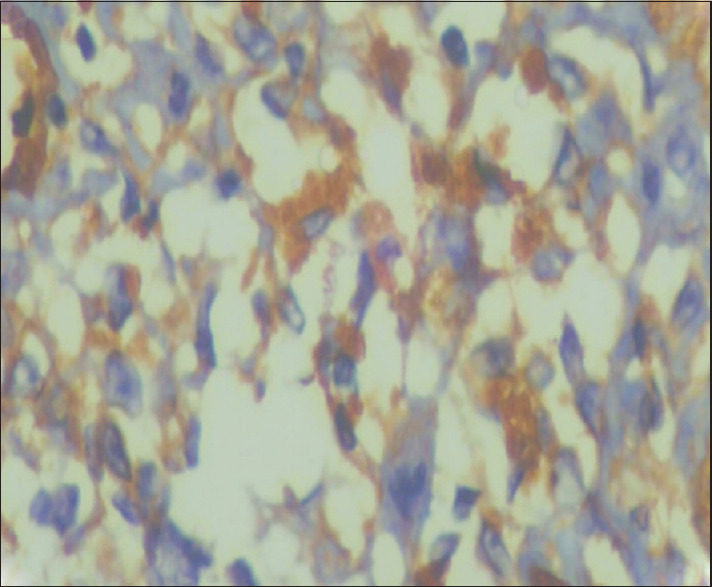
Tumor stage MF showed severe podoplanin expression in the dermal malignant lymphocytes (IHC, ×400)
Figure 3.

Plaque stage MF showed severe podoplanin expression in the epidermis (basal cell layer), moderately expressed in dermal malignant lymphocytes and severe expression of lymph vessels density (IHC, ×200)
There was a statistically highly significant increase of podoplanin expression and also the mean of LVD in MF patients in comparison to normal control skin (P < 0.001, <0.001 respectively) [Table 2].
Table 2.
Comparison between the patient and control groups according to intensity of podoplanin expression
| Podoplanin intensity score | Patients (n=50) | Control (n=20) | P | ||
|---|---|---|---|---|---|
| No. | % | No. | % | ||
| Epidermis (basal cell layer) | |||||
| Negative | 0 | 0 | 8 | 40.0 | <0.001 |
| Mild | 0 | 0 | 12 | 60.0 | |
| Moderate | 24 | 48.0 | 0 | 0 | |
| Severe | 26 | 52.0 | 0 | 0 | |
| Dermis (Malignant lymphocyte) | |||||
| Negative | 0 | 0 | 20 | 100.0 | <0.001 |
| Mild | 12 | 24.0 | 0 | 0 | |
| Moderate | 14 | 28.0 | 0 | 0 | |
| Severe | 24 | 48.0 | 0 | 0 | |
| Dermis (LVD) | |||||
| Min-Max | 15.0-43.0 | 7.0-7.0 | <0.001 | ||
| Mean±SD | 22.40±7.71 | 7.0±0.0 | |||
| Median | 20.0 | 7.0 | |||
LVD: Lymph vessel density
The relationship between the intensity score of podoplanin expression and clinical type of MF and MF staging according to TNMB classification were illustrated in Tables 3 and 4. There was a statistically highly significant increase of podoplanin expression in the malignant lymphocytes and also the mean LVD with progression of skin lesion from patch to plaque and tumor stage with (P < 0.05, <0.001 respectively).
Table 3.
Comparison between intensity of podoplanin expression and clinical types of mycosis fungoides among the patients
| Intensity of podoplanin expression | Clinical presentation | P | |||||
|---|---|---|---|---|---|---|---|
| Patch stage (No.=38) | Plaque stage (No.=10) | Tumor stage (No.=2) | |||||
| No. | % | No. | % | No. | % | ||
| Epidermis (basal cell layer) | |||||||
| Negative | 0 | 0 | 0 | 0 | 0 | 0 | 0.239 |
| Mild | 0 | 0 | 0 | 0 | 0 | 0 | |
| Moderate | 22 | 57.9 | 2 | 20.0 | 0 | 0 | |
| Severe | 16 | 42.1 | 8 | 80.0 | 2 | 100.0 | |
| Dermis (malignant lymphocyte) | |||||||
| Negative | 0 | 0 | 0 | 0 | 0 | 0 | 0.05* |
| Mild | 12 | 31.6 | 0 | 0 | 0 | 0 | |
| Moderate | 14 | 36.8 | 0 | 0.0 | 0 | 0 | |
| Severe | 12 | 31.6 | 10 | 100.0 | 2 | 100.0 | |
| LVD | |||||||
| Min. - Max. | 15.0-27.0 | 24.0-40.0 | 43.0# | <0.001* | |||
| Mean±SD. | 18.95±3.57 | 31.40±5.98 | |||||
| Median | 18.0 | 30.0 | |||||
*Statistically significant, #Excluded from the relation due to small number of case (n=1), LVD: Lymphatic vessel density
Table 4.
Comparison between intensity of podoplanin expression and TNMB staging of mycosis fungoides
| Podoplanin expression | TNMB Staging | P | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| IA (No. = 24) | IB (No. = 16) | IIA (No. = 4) | IIB (No. = 2) | IIIA (No.=4) | |||||||
| No. | % | No. | % | No. | % | No. | % | No. | % | ||
| Epidermis (basal cell layer) | |||||||||||
| Negative | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0.006* |
| Mild | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | |
| Moderate | 20 | 83.3 | 4 | 25.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | |
| Severe | 4 | 16.7 | 12 | 75.0 | 4 | 100.0 | 2 | 100.0 | 4 | 100.0 | |
| Dermis (malignant lymphocyte) | |||||||||||
| Negative | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0.068 |
| Mild | 12 | 50.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | |
| Moderate | 6 | 25.0 | 8 | 50.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | |
| Severe | 6 | 25.0 | 8 | 50.0 | 4 | 100.0 | 2 | 100.0 | 4 | 100.0 | |
| LVD | |||||||||||
| Min - Max | 15.0-18.0 | 20.0-27.0 | 29.0-30.0 | 34.0# | 40.0-43.0 | <0.001* | |||||
| Mean±SD. | 16.67±1.30 | 23.0±2.45 | 29.50±0.71 | 41.50±2.12 | |||||||
| Median | 17.0 | 23.0 | 29.50 | 41.50 | |||||||
*Statistically significant, #Excluded from the relation due to small number of case (No.=1), LVD: Lymph vessel density
There was a statistically significant positive correlation between intensity score of podoplanin expression in basal cell layer of epidermis, malignant lymphocyte, and the mean of LVD in the dermis with clinical types of MF and also with TNMB staging of MF.
Discussion
MF is the most common form of primary CTCL; accounting for approximately 50% of cases. It arises in peripheral T lymphocytes and develops solely in the skin, evolving through consecutive stages, from patches through plaques to tumors.[13] MF is similar to solid tumors and other hematological malignancies as the lymhangiogenesis is involved in the development and progression of the disease. The importance of this process in the dissemination of malignant neoplasm has been suggested due to the discovery of specific markers of lymphatic vessels as podoplanin.[9,21]
The current study revealed a statistically highly significant upregulation of podoplanin expression in basal layer of epidermis and malignant lymphocytes and the mean of LVD in MF patients in comparison to normal control skin. These findings were in agreement with Jankowska-Konsuret et al.[18] who found that the immunohistochemical expression of podoplanin was higher in MF patient group than in the control group. MF lesions are characterized by mixed infiltration consisting of malignant lymphocytes and increased expression of podoplanin-enhanced LVD. Also, Karpova et al.[22] found that podoplanin antibodies were expressed in the presence of lymphatic vasculature in all tested skin biopsies of erythrodermic MF and there was a higher density of lymphatic vasculature, detected by podoplanin antibodies.
The current study revealed a statistically significant positive correlation between the intensity score of podoplanin expression in the basal cell layer of epidermis, malignant lymphocyte, and LVD of the MF studied patients and progression of MF regarding the clinical types (patch, plaque, and tumor) and TNMB staging of MF.
These findings were in agreement with Jankowska-Konsur et al.[18] who reported that increased podoplanin expression was found in advanced MF. Podoplanin expression increased with more infiltrated cutaneous lesions (tumors, stage T3) and with more extensive skin involvement (erythroderma, stage T4), compared with less infiltrated skin changes with limited involvement of the body surface (patches and plaques, stages T1, T2). The role of lymphangiogenesis had been illustrated in the progression of disease, skin and nodal involvement of MF as they found increased podoplanin expression in higher stages of MF clinical advancement.
Karpova et al.[22] reported a positive correlation between increased number of podoplanin positive vessels and disease progression in Sézary syndrome. LVD was also associated with aggressive histology in non-Hodgkin's lymphoma.[23] Intratumoral lymphatics and lymphangiogenesis were also detected in head and neck cancer, thyroid carcinoma, and melanoma, and might contribute to lymph node metastasis.[24,25,26] Moreover, peritumoral lymphatics located immediately adjacent to tumors or in the peritumoral stroma, which can be dilated or enlarged, are known to be associated with human tumors.[27,28,29]
So far, only few studies have analyzed the process of lymphangiogenesis in CTCL. The current study demonstrated that the higher podoplanin expression was significantly correlated with disease progression regarding the cutaneous lesions and nodal involvement in TNMB staging.
The limitation in our study was the small sample size of the patients with different clinical presentations of MF. Also, the lack of prior research on the same topic of the current study reduced the scope of data comparison.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Wigle J, Harvey N, Dermar M, Lagutina I, Grosveld G, Gunn MD, et al. An essential role for Prox1 in the induction of lymphatic endothelial cell phenotype. EMBO J. 2002;21:1505–13. doi: 10.1093/emboj/21.7.1505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rutkowski J, Ihm J, Lee S, Kilarski WW, Greenwood VI, Pasquier MC, et al. VEGFR-3 neutralization inhibits ovarian lymphangiogenesis, follicle maturation, and murine pregnancy. Am J Pathol. 2013;183:1596–607. doi: 10.1016/j.ajpath.2013.07.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Marsee D, Pinkus G, Hornick JL. Podoplanin (D2-40) is a highly effective marker of follicular dendritic cells. Appl Immunohistchem Mol Morphol. 2009;2:102–7. doi: 10.1097/PAI.0b013e318183a8e2. [DOI] [PubMed] [Google Scholar]
- 4.Wicki A, Lehembre F, Wick N, Hantusch B, Kerjaschki D, Christofori G, et al. Tumor invasion in the absence of epithelial-mesenchymal transition: Podoplanin-mediated remodeling of the actin cytoskeleton. Cancer Cell. 2006;9:261–72. doi: 10.1016/j.ccr.2006.03.010. [DOI] [PubMed] [Google Scholar]
- 5.Astarita J, Acton S, Turley S. Podoplanin emerging functions in development, the immune system and cancer. Front Immunol. 2012;3:1–11. doi: 10.3389/fimmu.2012.00283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Raica M, Cimpean A, Ribatti D. The role of podoplanin in tumor progression and metastasis. Anticancer Res. 2008;28:2997–3006. [PubMed] [Google Scholar]
- 7.Martín-Villar E, Megías D, Castel S, Yurrita MM, Vilaró S, Quintanilla M. Podoplanin binds ERM proteins to activate RhoA and promote epithelial-mesenchymal transition. J Cell Sci. 2006;119:4541–53. doi: 10.1242/jcs.03218. [DOI] [PubMed] [Google Scholar]
- 8.Ordóñez NG. Podoplanin: A novel diagnostic immunohistochemical marker. Adv Anat Pathol. 2006:83–8. doi: 10.1097/01.pap.0000213007.48479.94. [DOI] [PubMed] [Google Scholar]
- 9.Schacht V, Dadras S, Johnson L, Jackson D, Hong Y, Detmar M. Up-regulation of the lymphatic marker podoplanin, a mucin-type transmembrane glycoprotein, in human squamous cell carcinomas and germ cell tumors. Am J Pathol. 2005;166:913–21. doi: 10.1016/S0002-9440(10)62311-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Breiteneder-Geleff S, Soleiman A, Kowalski H, Horvat R, Amann G, Kriehuber E, et al. Angiosarcomas express mixed endothelial phenotypes of blood and lymphatic capillaries: Podoplanin as a specific marker for lymphatic endothelium. Am J Pathol. 1999;154:385–94. doi: 10.1016/S0002-9440(10)65285-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Niemiec JA, Adamczyk A, Ambicka A, Mucha-Malecka A, Wysocki W, Ryś J. Triple-negative basal marker-expressing and high-grade breast carcinomas are characterized by high lymphatic vessel density and the expression of podoplanin in stromal fibroblast. Appl Immunohistochem Mol Morphol. 2014;22:10–6. doi: 10.1097/PAI.0b013e318286030d. [DOI] [PubMed] [Google Scholar]
- 12.Wilcox RA. Cutaneous T-cell lymphoma: 2017 update on diagnosis, risk-stratification, and management. Am J Hematol. 2017;92:1085–102. doi: 10.1002/ajh.24876. [DOI] [PubMed] [Google Scholar]
- 13.Ralfkiaer U, Hagedorn P, Bangsgaard N, Lovendorf MB, Ahler CB, Svensson L, et al. Diagnostic microRNA profiling in cutaneous T-cell lymphoma (CTCL) Blood. 2011;118:5891–900. doi: 10.1182/blood-2011-06-358382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ambrosio M, Rocca B, Barone A, Ginori A, Crivelli F, Pirtoli L, et al. Lymphatic vascularization in prostate adenocarcinoma: Correlation with tumor grade, androgen withdrawal and prognosis. Anticancer Res. 2015;35:5595–600. [PubMed] [Google Scholar]
- 15.Ernst B, Mikstas C, Stover T, Stauber R, Strieth S. Association of elf4e and SPARC expression with lymphangiogenesis and lymph node metastasis in hypopharyngeal cancer. Anticancer Res. 2018;38:699–706. doi: 10.21873/anticanres.12275. [DOI] [PubMed] [Google Scholar]
- 16.Olsen E, Vonderheid E, Pimpinelli N, Willemze R, Kim Y, Knobler R, et al. Revisions to the staging and classification of mycosis fungoides and Sézary syndrome: a proposal of the International Society of Cutaneous Lymphomas (ISCL) and the cutaneous lymphoma task force of the European Organization of Research and Treatment of Cancer (EORTC) Blood. 2007;110:1713–22. doi: 10.1182/blood-2007-03-055749. [DOI] [PubMed] [Google Scholar]
- 17.Jawed SI, Myskowski PL, Horwitz S, Moskowitz A, Querfeld C. Primary cutaneous T-cell lymphoma (mycosis fungoides and Sezary syndrome), part I.Diagnosis: Clinical and histopathologic features and new molecular and biologic markers. J Am Acad Dermatol. 2014;70:1–16. doi: 10.1016/j.jaad.2013.07.049. [DOI] [PubMed] [Google Scholar]
- 18.Jankowska-Konsur A, Kobierzycki C, Grzegrzółka J, Piotrowska A, Gomulkiewicz A, Glatzel-Plucinska N, et al. Podoplanin expression correlates with disease progression in mycosis fungoides. Acta Derm Venereol. 2017;97:235–41. doi: 10.2340/00015555-2517. [DOI] [PubMed] [Google Scholar]
- 19.Kaur H, Gupta S. An analysis of the expression of Bcl-2, podoplanin and lymph angiogenesis in benign and malignant salivary gland tumours. J Clin Exp Pathol. 2013;3:145–51. [Google Scholar]
- 20.Wojciechowsha-Zdrojowy M, Szepietowski J, Matusiak L, Dziegial P, Pula B. Expression of podoplanin in non-melanoma skin cancers and actinic keratosis. Anticancer Res. 2016;36:159–7. [PubMed] [Google Scholar]
- 21.Niemiec J, Sas-Kprczynska B, Harazin-Lechowska A, Martynow D, Adamczyk A. Lymphatic and blood vessels in male breast cancer. Anticancer Res. 2015;35:1041–8. [PubMed] [Google Scholar]
- 22.Karpova M, Fujii K, Jenni D, Dummer R, Urosevic-Maiwald M. Evaluation of lymphangiogenic markers in Sézary syndrome. Leuk Lymphoma. 2011;52:491–501. doi: 10.3109/10428194.2010.517877. [DOI] [PubMed] [Google Scholar]
- 23.Ma S, Lin M, Liu H, Yu J. Lymphangiogenesis in non-Hodgkin's lymphoma and its correlation with cyclooxygenase-2 and vascular endothelial growth factor-C. Oncol Lett. 2012;4:695–700. doi: 10.3892/ol.2012.817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Beasley N, Prevo R, Banerji S, Leek RD, Moore J, van Trappen P, et al. Intratumoral lymphangiogenesis and lymph node metastasis in head and neck cancer. Cancer Res. 2002;62:1315–20. [PubMed] [Google Scholar]
- 25.Hall F, Freeman J, Asa S, Jackson D, Beasley N. Intratumoral lymphatics and lymph node metastases in papillary thyroid carcinoma. Arch Otolaryngol Head Neck Surg. 2003;129:716–9. doi: 10.1001/archotol.129.7.716. [DOI] [PubMed] [Google Scholar]
- 26.Straume O, Jackson D, Akslen L. Independent prognostic impact of lymphatic vessel density and presence of low-grade lymphangiogenesis in cutaneous melanoma. Clin Cancer Res. 2003;9:250–6. [PubMed] [Google Scholar]
- 27.Pepper MS. Lymphangiogenesis and tumor metastasis: Myth or reality? Clin Cancer Res. 2001;7:462–8. [PubMed] [Google Scholar]
- 28.Dadras S, Bertoncini P, Brown L, Muzikansky A, Jackson DG, Ellwanger U, et al. Tumor lymphangiogenesis: A novel prognostic indicator for cutaneous melanoma metastasis and survival. Am J Pathol. 2004;162:1951–60. doi: 10.1016/S0002-9440(10)64328-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bono P, Wasenius V, Heikkilä P, Lundin J, Jackson D, Joensuu H. High LYVE-1-positive lymphatic vessel numbers are associated with poor outcome in breast cancer. Clin Cancer Res. 2004;10:7144–9. doi: 10.1158/1078-0432.CCR-03-0826. [DOI] [PubMed] [Google Scholar]