Abstract
Chemical leucoderma, an under-diagnosed common condition often mimicking idiopathic vitiligo, represents an acquired depigmentation induced by repeated exposure to specific chemical compounds in subjects with genetic susceptibility to vitiligo. This has been increasing rapidly in incidence in recent decades in developing countries like India. The term 'chemical vitiligo' was first coined by us to indicate the possible relationship between chemical leucoderma and vitiligo, which has been supported recently by other authors to designate the term 'chemical-induced vitiligo'. The largest case series showed that household chemical exposure was the major etiological factor. Causative chemicals are mostly phenolic and catecholic derivatives. Vitiligo pathogenesis is induced by genetic and environmental factors like many other autoimmune diseases. Innate immunity acts as a bridge between cellular stress and adaptive immunity. Multiple patches are commonly seen; children below 12 years are also affected in good numbers. The most common presence of confetti macules indicates these as characteristic, although not pathognomonic, of chemical leucoderma. Chemical leucoderma has been broadened into 'chemical leucoderma syndrome' with proper staging. The clinical criteria for diagnosis of chemical leucoderma have been specifically outlined. Same pathomechanism of chemical leucoderma might elucidate trigger factors and reasons for progression and chronicity in idiopathic vitiligo. Depigmentation in chemical vitiligo spreads to distant sites, in the same way as generalized idiopathic vitiligo. The study showed that chemical triggering factors played a very significant role in the induction and progression of vitiligo. Thus it should be rational to consider chemical vitiligo not as a separate entity but as a major subset of vitiligo spectrum.
KEY WORDS: Chemical leucoderma, chemical vitiligo, contact leucoderma, vitiligo
Introduction
Chemical leucoderma denotes an acquired vitiligo-like depigmentation triggered by repeated exposure to specific chemical compounds, independent of their sensitizing potential. These chemicals are toxic for melanocytes only in subjects having specific genetic susceptibility to vitiligo. This entity is distinctly separate from postinflammatory depigmentation and koebnerization in vitiligo.[1,2,3]
Chemical leucoderma remains an under-diagnosed common condition often mimicking idiopathic vitiligo in dermatological practice. This ailment has been increasing rapidly in incidence in recent decades in developing countries like India.[3]
The nomenclature regarding the disease has created a lot of confusion. The term 'contact leucoderma' or 'occupational leucoderma' does not signify the exact ailment. Instead of more applicable term, 'chemical leucoderma' has been used for practical purposes. However, recent clinical and laboratory investigations have thrown much light into the grey zone, which suggest more scientific term 'chemical vitiligo' focusing its resemblance to idiopathic vitiligo.[3,4]
The Journey from 'contact Leucoderma' to 'chemical Vitiligo'
The term 'contact leucoderma' is misleading as the adjective 'contact' may signify that the lesion is confined only to the site of contact, which is not true.[2,3] The term 'contact' may also falsely signify that its pathogenesis is similar to contact dermatitis. The term 'occupational leucoderma' remains misnomer as most of our cases[3] were induced by non-occupational household exposure. Thus, 'chemical leucoderma' might be the more rational and justified nomenclature. The term 'contact/occupational vitiligo', has been used by some authors[5] to represent cases where initial cutaneous depigmentation extends from the site of chemical depigmentation to develop into progressive generalized vitiligo. This term is confusing as chemical leucoderma, as shown in our and others reports,[2,3,4,6] can spread to remote areas of the body. We first coined the term 'chemical vitiligo' for some patients who, despite restricting the offending toxic chemicals for more than 1 year, continue to develop vitiliginous patches in different parts of body.[3] This observation suggested clinically for the first time about a possible relationship between chemical leucoderma and vitiligo.
Harris,[4] in the tune of our thinking,[3] proposed that as chemically induced vitiligo and non-chemically induced vitiligo appear on the same spectrum clinically, histologically, and pathogenically, chemical-induced depigmentation should be called “chemical-induced vitiligo,” rather than by other terms that have been used. This conception and terminology seem to be quite rational and scientific. However, the term “chemical vitiligo” as we designated[3,7] would be more appropriate, practical and easy to use.
Contributory Chemicals
Oliver et al. in 1939[8] first diagnosed chemical leucoderma in workers who used “acid cured” rubber gloves in a leather manufacturing company. Monobenzyl ether of hydroquinone (MBEH), an antioxidant used in the rubber industry, was the offending agent. Subsequently, multiple cases of occupational leucoderma induced by phenolic compounds were reported from various countries.
Taylor et al. first reported chemical leucoderma from semi-permanent and permanent hair colors and rinses in 1993[9]; contributory chemicals were paraphenylenediamine (PPD) and benzyl alcohol. From India, chemical leucoderma was first reported by Pandhi and Kumar[10] caused by adhesive “bindi” (decorative color used on the forehead by Asian females) and footwear. Bajaj et al. were first to document chemical leucoderma from free paratertiary butylphenol (PTBP) in “bindi” adhesive,[11] from MBEH depigmentation of the breast by the habit of keeping synthetic wallets inside blouses,[12] from MBEH causing footwear depigmentation,[13] from PPD in hair dye,[14] from azo dye in “alta” (a decorative color used by Asian females on their feet)[15] and from other domestic objects, such as watch straps, spectacles, and hearing aids.[16]
From a new skin-lightening cream marketed by a Japanese cosmetic company about 16,000 users developed vitiligo (about 2% of all users), the active ingredient being rhododendrol. Remote depigmentation was noted in 5% of the patients. Lesions induced by rhododendrol were mostly indistinguishable from vitiligo clinically and histologically.[4,6,17]
The largest case series in the world till date reported by us[3] comprising of 864 patients during 5 years showed that household chemical exposure was the major etiological factor in 70.7% cases [Table 1], the rest were of industrial origin.
Table 1.
Causative common household products among 864 chemical leucoderma patients[3]
| Consumer | Percentage |
|---|---|
| Hair dye | 27.4 |
| Deodorant/perfume | 21.6 |
| Detergent/cleanser | 15.4 |
| Adhesive ‘bindi’ (decorative color on forehead) | 12.0 |
| Rubber sandal | 9.4 |
| Black socks/shoes | 9.1 |
| Eyeliner | 8.2 |
| Lip liner | 4.8 |
| Rubber condom | 3.5 |
| Lipstick | 3.3 |
| Fur toys | 3.1 |
| Toothpaste | 1.9 |
| Insecticide | 1.7 |
| Alta (decorative color on feet) | 1.2 |
| Amulet (holy material) string color | 0.9 |
| Multiple chemicals | 67.8 |
Household chemicals versus industrial chemicals
Our study[3] first showed that household chemical exposure was much more common than occupational chemical exposure to produce chemical leucoderma in developing countries like India [Figures 1-5]. Western textbook on occupational dermatology[18] also confirmed this. However, in later years, the number of common household products from different parts of the world has been shown to trigger and/or deteriorate vitiligo in many patients although the contributory chemical ingredients have not been identified in all cases.[4,6,17]
Figure 1.
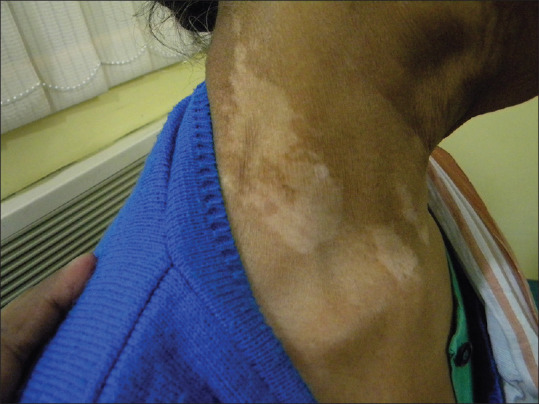
Chemical vitiligo at the neck from a blue sweater (azo dye)
Figure 5.
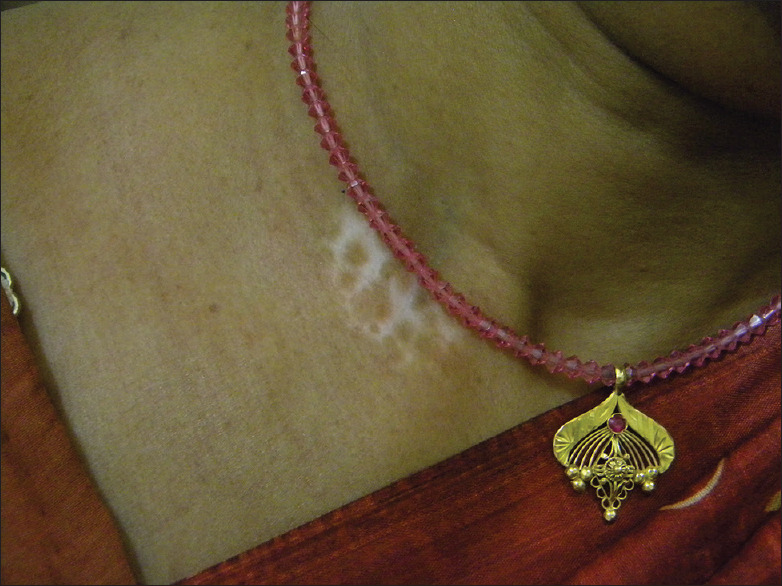
Chemical vitiligo on the neck from reddish necklace beads
Figure 2.
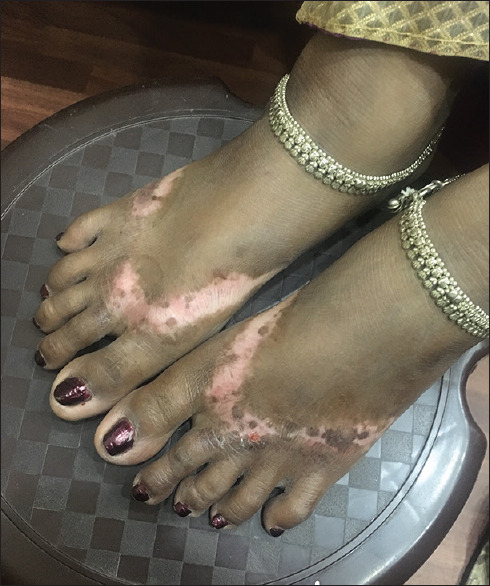
Chemical vitiligo on feet from rubber chappals
Figure 3.

Chemical vitiligo on the glans penis from rubber condom
Figure 4.
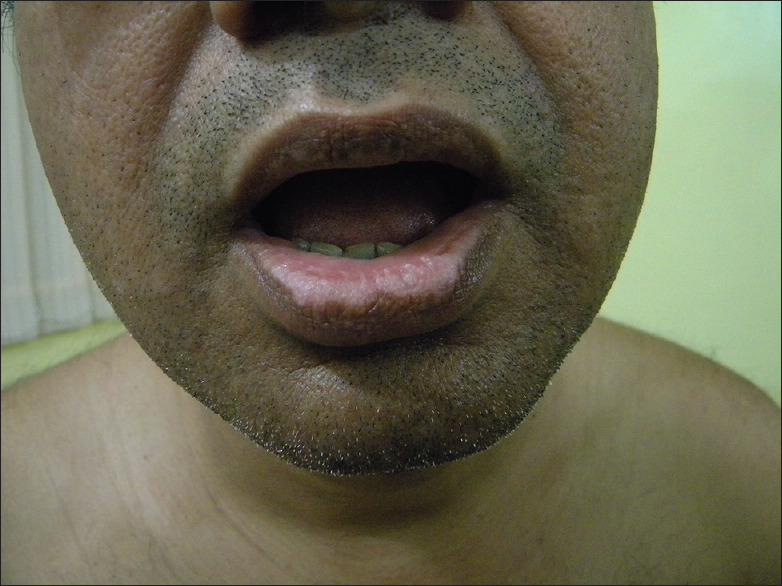
Chemical vitiligo on lower lip from toothpaste
Pathomechanism
Vitiligo develops when the functioning melanocytes are lost from the epidermis. A single dominant pathway is not playing the role in all cases of melanocyte loss in vitiligo. It results from complex interactions of genetic, biochemical, environmental and immunological events, in a susceptible with genetic background.[19]
Vitiligo pathogenesis is induced by genetic and environmental factors like many other autoimmune diseases. The environment strongly influences the likelihood of development of autoimmunity.[4]
Genetic background
Vitiligo is inherited as a polygenic pattern; more than 30 genes have been identified as predisposing factor.[20] As both arms of immune system are jointly inducing the vitiligo pathomechanism, genes that control innate immunity (NLRP1, IFIH1, CASP7, TICAM1, and others), as well as genes modulating adaptive immunity (CTLA4, CD80, HLA, GZMB, FOXP3, and others) play synergistically in this process.[20,21] The chance of development of the disease in siblings of vitiligo sufferers is 6.1%, while the same in identical twin is 23%.[20,22] Appearance of vitiligo as clusters in families also indicates role of genetic propensity.[20]
Environmental factors
Non-genetic factors play a sizable role in the induction of disease process of vitiligo as mentioned earlier that chances of developing disease in identical twins is low.[20] A family history of vitiligo was found in 28.3% cases of chemical leucoderma with preexisting vitiligo, whereas it was seen only in 4.8% cases in chemical leucoderma without preexisting vitiligo. Thus chemical leucoderma probably represents an environmental disease rather than a genetic one.[3]
Causative chemicals are mostly phenolic and catecholic derivatives,[23] which resemble tyrosine and can occupy the catalytic center of tyrosinase as surrogate substrate in the melanin synthesis pathway.[1] These are oxidized by tyrosinase or tyrosinase-related protein to more reactive o -quinones, with the generation of reactive oxygen species (ROS), which contribute to oxidative stress. In the presence of excess H2O2, this process is accelerated.[19]
Kroll et al.[24] first documented that phenolic compounds could lead to melanocyte death indirectly by stimulating an inflammatory cascade in dendritic cells when simultaneously cultured with exposed melanocytes. By adding tertiary butyl phenol (4-TBP) to melanocytes they observed cellular stress response, which caused the secretion of heat shock protein (HSP70) and, in turn, elevated tumor necrosis factor-related apoptosis-inducing ligands (TRAIL) death receptor expression. HSP70, the proinflammatory protein-activated dendritic cells, which when cultured along with melanocytes, killed the later. Dendritic cell (DC) effector functions also take pivotal role in spread of leucoderma by systemic autoimmunity [Figure 6].[7]
Figure 6.
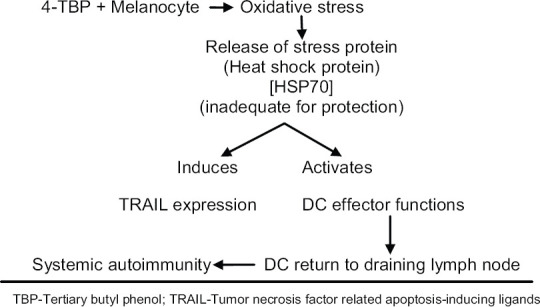
The study by Manga et al. showed that 4-TBP triggered oxidative stress in melanocytes. The melanocytes of vitiligo patients were shown to be more sensitive to 4-TBP compared to normal melanocytes. This sensitivity was reduced in considerable amount by addition of antioxidant in the culture medium.[25]
Tyrosinase-related protein-1 (Tyrp1), by catalytic conversion of chemicals, produces radical oxygen species. This oxidative stress triggers activation of cellular free radical scavenging pathway to prevent cell death. Genetic inability of melanocytes to tolerate and/or respond to oxidative stress may underlie etiology of chemical leucoderma.[1,7]
Vitiligo: Autoimmune theory
Elevated levels of melanocyte-specific antibodies with ability to kill melanocytes were detected both in vivo and in vitro in vitiligo patients, which led to autoimmune theory of vitiligo.[21] Serum level of these antibodies, however, did not correlate with the disease activity. Despite systemic presence of antibodies in the serum, skin patches were very distinct. These facts led to believe that autoantibodies were not the real answer to the mystery. New journey began to search for the new theories and to detect the exact cause of vitiligo.[21]
Melanocytic-intrinsic pathogenesis: Cellular stress
Melanocytes are intrinsically abnormal in vitiligo patients, damaged internally from stress. Thus although melanocytes in all persons are subjected to stress, vitiligo patients either have a lower sensitivity threshold to normal stress level or face an increased level of stress than normal individuals.[20,21]
Role of innate immunity
Several types of innate immune cells, including Natural Killer (NK) cells and inflammatory cells, accumulate in the lesional skin of the vitiligo patients, particularly during the active stage. Innate immune cells, particularly antigen-presenting cells, travel out of the skin to reach the draining lymph nodes to present melanocyte-specific antigens to T-cells and activate them. In turn, these autoreactive T-cells secrete cytokines that invite more autoreactive T-cells, which directly kill melanocytes, thus creating important crosstalk between adaptive and innate immunity in vitiligo.[20]
Innate immunity acts as a bridge between cellular stress and adaptive immunity.[20,26] The activation of several innate immune cells, the release of cytokines and chemokines, that recruit autoreactive cytotoxic T-cells, ultimately lead to T-cell-mediated autoimmune destruction of melanocytes.[20]
Role of adaptive immunity: autoimmune pathogenesis (T-cell cytotoxicity)
Cytotoxic, autoreactive CD8+ T-cells have definitive pathogenic role in vitiligo. Apart from that, CD4+ T regulatory cells (Tregs) appear to play a vital role in pathomechanism of the disease. Tregs protect humans from development of vitiligo. A recent study has documented that vitiligo patients lack Treg-mediated control of autoreactive CD8+ T-cells that is normally seen in healthy individuals.[20]
Chemical vitiligo: The convergence of two theories
In chemical vitiligo, loss of melanocytes starts at the site of exposure but in approximately 26% of the patients spreads to distant areas [Figure 7].[3,6,27] So, offending chemicals are not directly cytotoxic to melanocytes. Thus importance of environmental influences on autoimmunity provides a fresh insight into a novel conception of linking cell stress to the immune response. Furthermore, this can serve as a template for other autoimmune diseases.[21]
Figure 7.
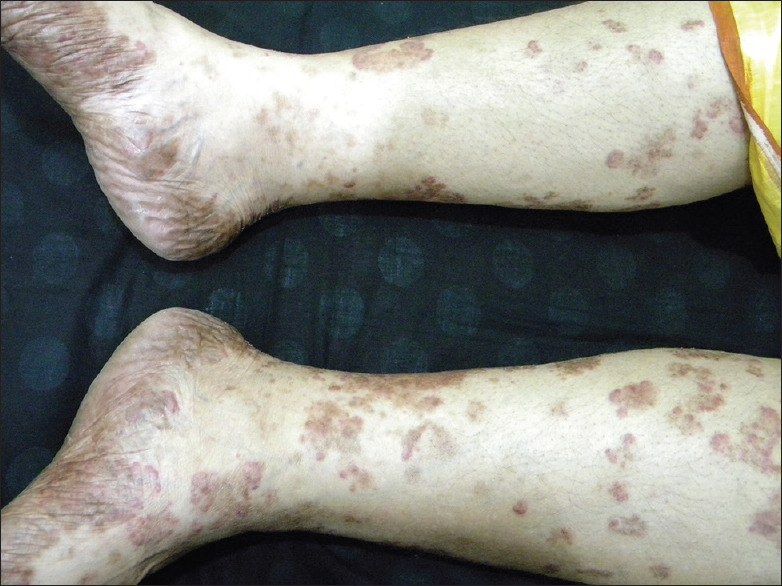
Chemical vitiligo from black socks involving at first feet and then spreading to upper parts of the legs
Clinical Presentation
Multiple patches (69.1%) are commonly seen than a solitary patch in chemical vitiligo. The age of involvement was 74 days to 84 years (median 36 years). A considerable number of children below the age of 12 years (14.1%) were affected thus indicating exposure to non-industrial chemicals. The face was the commonest area of involvement and the scalp was the least.[3] Some investigators[4,27] found that women who started using hair dye before the age of 30 years and those who used hair dye for more than 5 years (irrespective of starting age) had a 50% higher risk of developing chemical vitiligo.
Confetti macules [Figure 8] were present in 88.4% of the patients.[3] The presence of numerous acquired confetti or pea-sized macules is characteristic, although not diagnostic, of chemical vitiligo.[2,3] However, the presence of confetti-like depigmentation has been recently reported to be a clinical sign of highly active vitiligo.[4,28] As chemical vitiligo is a rapidly progressive process,[4] confetti macules may be far more commonly seen in these cases compared to non-chemical vitiligo. However, its diagnostic significance has been laid down in Table 2, which should only be considered along with other diagnostic criteria of chemical leucoderma syndrome.[7]
Figure 8.
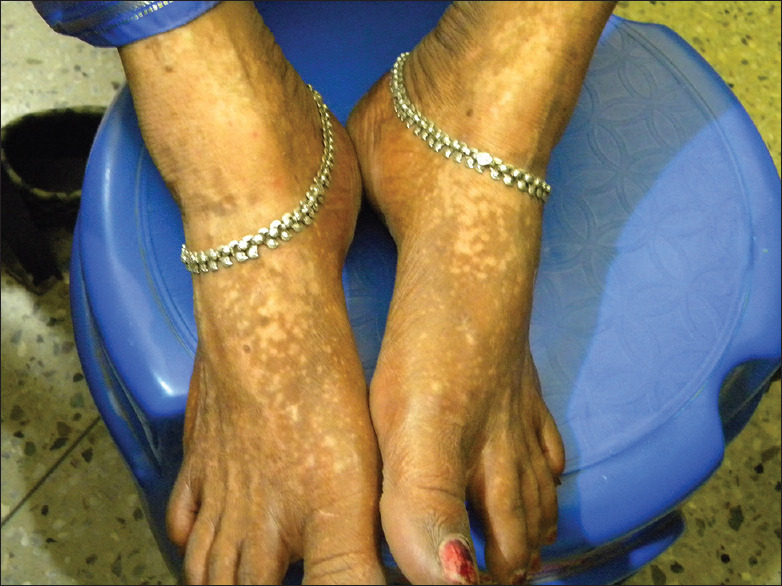
Chemical vitiligo on both feet from black shoes with numerous acquired confetti macules along with larger depigmented patches
Table 2.
Clinical diagnostic criteria of ‘chemical leucoderma syndrome (CLS)’3
| 1. Acquired vitiligo-like depigmented lesion(s) |
| 2. History of repeated exposure to specific chemical compounds |
| 3. Patterned vitiligo-like macules conforming to site of exposure [Figure 9] |
| 4. Confetti macules |
| Any three of the above four criteria should be present to diagnose a case of chemical leucoderma |
Figure 9.
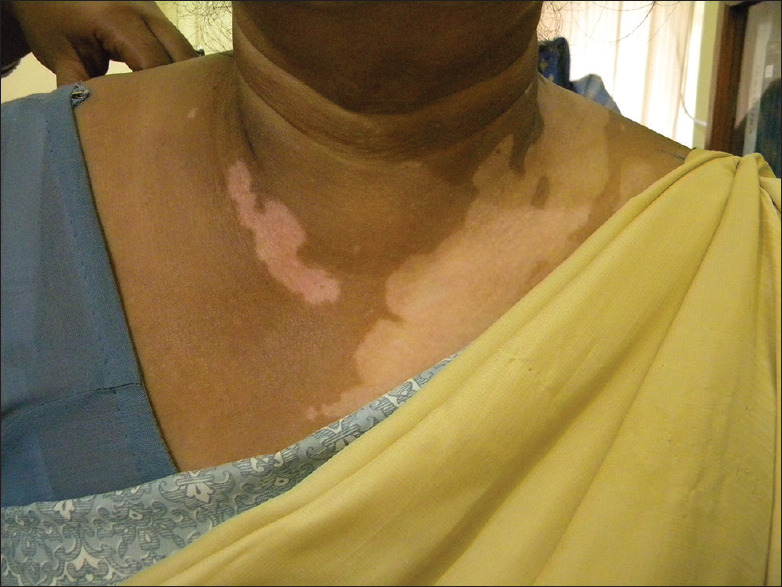
Chemical vitiligo from artificial necklace showing patterned vitiligo-like macules conforming to the site of exposure
Pruritus was present in 21.8% of patients but associated contact dermatitis was seen in only 5.4% cases. However, eczematous eruption and leucoderma were not congruent in such cases. Liver pathology and hypothyroidism were seen in 7.9% and 3.9% of patients, respectively.[3]
A favorable therapeutic response was more commonly seen in 'pure' chemical leucoderma cases (73.4%) rather than in chemical leucoderma associated with pre-existing vitiligo (20.9%).[3]
Syndromic staging of chemical leucoderma syndrome
From our studies and experience on this topic, we could formulate a syndromic staging in chemical leucoderma similar to allergic contact dermatitis syndrome[29] and designated the whole syndrome as 'chemical leucoderma syndrome (CLS)'. The detail of this proposal is outlined in Table 3. Dr Boissy[30] a legendary author in this field has welcome this staging as 'this staging system seems very appropriate for occupational/contact leukoderma'.
Table 3.
Chemical leucoderma syndrome (CLS)[3]
| Stage I. Chemical leucoderma only at the site of contact |
| Stage II. Local spread of chemical leucoderma through the lymphatics beyond the site of contact |
| Stage IIIA. Distant spread of chemical leucoderma through hematogenous spread beyond the site of contact |
| Stage IIIB. Distant spread of chemical leucoderma through hematogenous spread beyond the site of contact along with systemic organ involvement |
| Stage IIICa. Systemic introduction (injection, inhalation or ingestion) other than skin contact causing chemical leucoderma with or without systemic organ involvement |
| Stage IV. Distant spread of vitiligo-like patches even after 1 year of strictly withholding exposure to offending chemicals (‘chemical vitiligo’) |
aThis stage is a hypothesis or probability; not yet proved.
One aspect that remains to be documented is whether any systemic exposure of chemicals through inhalation, ingestion or injection can cause chemical leucoderma in a way similar to systemic contact dermatitis.[29] Some cases of idiopathic vitiligo, however, may be due to unsuspected inhalation or ingestion of chemicals that produce contact leucoderma.[31] Chemical factors, such as food contaminants/additives/preservatives could aggravate vitiligo because they produce oxidative stress in the skin.[32] Prevalence of vitiligo was found to be higher in villagers living near dyeing, printing and carpet industries. Like us, many authors reported various systemic abnormalities, including thyroid, liver, and splenic changes.[33] Based on these observations, they suggested that the chemicals might be capable of inducing inflammation in organs beyond the skin.[4]
Clinical criteria of diagnosis of chemical leucoderma syndrome
Chemical leucoderma has to be excluded with certainty from other causes of depigmentary diseases. The clinical criteria of diagnosis of chemical leucoderma have not yet been specifically outlined. We proposed the clinical diagnostic criteria of chemical leucoderma as outlined in Table 2. This has been accepted and quoted by standard and reputed textbook of dermatology.[34]
Chemical Vitiligo and Vitiligo: Interlinking
Chemical-induced vitiligo is indistinguishable from vitiligo.[4,35] Cummings and Norland[5] concluded that some form of vitiligo vulgaris are chemical leucoderma caused by unidentified melanocytotoxic chemicals in the environment. Glassman[19] emphasized the role of exogenous oxidants in vitiligo and highlighted pathogenesis of chemical leucoderma, as same pathomechanism might elucidate trigger factors and reasons for progression and chronicity in idiopathic vitiligo. Depigmentation in chemical vitiligo spreads to distant sites, in the same way as generalized idiopathic vitiligo.[3,4,19,27]
In another study,[36] we have been able to show that chemical triggering factors play a very significant role in the induction and propagation of vitiligo. History of household chemical exposure and industrial (occupational) chemical exposure were found in 94.6% and 0.4% of the patients, respectively. The duration of disease and duration of chemical exposure had no correlation. Regarding avoidance of chemical exposure on the progression of vitiligo, the difference between fully compliant versus noncompliant was statistically significant but between partial compliant versus noncompliant was not significant. Correlation of site of chemical exposure with the site of onset of disease was found in 64.9% of the patients. Thus the distinction between chemical vitiligo and idiopathic vitiligo is gradually becoming blurred day by day by continuous clinical and laboratory observations.
Conclusion
Chemical leucoderma was previously conceptualized as a distinctly separate disease from vitiligo, which was conventionally accepted as an idiopathic one. Recent scientific exploration has proved beyond doubt that both the ailments are almost similar in etiology, clinical feature and histopathology. Indeed diagnosis of chemical vitiligo by proper history taking could exclude in many cases the idiopathic nature of vitiligo. However, a small number of non–chemical-induced vitiligo may require further elaboration and careful history taking and clinical assessment to prove other etiology before stamping it as idiopathic. So at the present scenario, it should be rational to consider chemical vitiligo not as a separate entity but a major subset of vitiligo spectrum.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Miyamoto L, Taylor JS. Chemical leukoderma. In: Hann SK, Nordlund JJ, editors. A Comprehensive Monograph on Basic and Clinical Science. London: Blackwell Science; 2000. pp. 269–80. [Google Scholar]
- 2.Mosher DB, Fitizpatrick TB. Chemical leukoderma. In: Sober AJ, Fitzpatrick TB, editors. Yearbook of Dermatology. Mosby: Boston; 1994. pp. 3–13. [Google Scholar]
- 3.Ghosh S, Mukhopadhyay S. Chemical leucoderma: A clinico- aetiological study of 864 cases in the perspective of a developing country. Br J Dermatol. 2009;160:40–7. doi: 10.1111/j.1365-2133.2008.08815.x. [DOI] [PubMed] [Google Scholar]
- 4.Harris JE. Chemical-induced vitiligo. Dermatol Clin. 2017;35:151–61. doi: 10.1016/j.det.2016.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cummings MP, Norlund JJ. Chemical leukoderma: Fact or fancy. Am J Contact Dermatitis. 1995;6:122–6. [Google Scholar]
- 6.Tokura Y, Fujiyama T, Ikeya S, Tatsuno K, Aoshima M, Kasuya A, et al. Biochemical, cytological, and immunological mechanisms of rhododendrol-induced leukoderma. J Dermatol Sci. 2015;77:146–9. doi: 10.1016/j.jdermsci.2015.02.001. [DOI] [PubMed] [Google Scholar]
- 7.Ghosh S. Chemical Leukoderma: What's new on etiopathological and clinical aspects? Indian J Dermatol. 2010;55:255–8. doi: 10.4103/0019-5154.70680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Oliver EA, Schwartz L, Warren LH. Occupation leukoderma. J Am Med Assoc. 1939;113:927–8. [Google Scholar]
- 9.Taylor JS, Maibach HI, Fisher AA, Bergfeld WF. Contact leukoderma associated with the use of hair colors. Cutis. 1993;52:273–80. [PubMed] [Google Scholar]
- 10.Pandhi RK, Kumar AS. Contact leukoderma due to 'bindi' and footwear. Dermatologica. 1985;170:260–2. doi: 10.1159/000249545. [DOI] [PubMed] [Google Scholar]
- 11.Bajaj AK, Gupta SC, Chatterjee AK. Contact depigmentation from free para-tertiary-butylphenol in bindi adhesive. Contact Dermatitis. 1990;22:99–102. doi: 10.1111/j.1600-0536.1990.tb01525.x. [DOI] [PubMed] [Google Scholar]
- 12.Bajaj AK, Gupta SC, Chatterjee AK. Contact depigmentation of the breast. Contact Dermatitis. 1991;24:58. doi: 10.1111/j.1600-0536.1991.tb01635.x. [DOI] [PubMed] [Google Scholar]
- 13.Bajaj AK, Gupta SC, Chatterjee AK. Footwear depigmentation. Contact Dermatitis. 1996;35:117–8. doi: 10.1111/j.1600-0536.1996.tb02313.x. [DOI] [PubMed] [Google Scholar]
- 14.Bajaj AK, Gupta SC, Chatterjee AK, Singh KG, Basu S, Kant A. Hair dye depigmentation. Contact Dermatitis. 1996;35:56–7. doi: 10.1111/j.1600-0536.1996.tb02278.x. [DOI] [PubMed] [Google Scholar]
- 15.Bajaj AK, Pandey RK, Misra K, Chatterjee AK, Tiwari A, Basu S. Contact depigmentation caused by an azo dye in alta. Contact Dermatitis. 1998;38:189–93. doi: 10.1111/j.1600-0536.1998.tb05705.x. [DOI] [PubMed] [Google Scholar]
- 16.Bajaj AK, Misra A, Misra K, Rastogi S. The azo dye solvent yellow 3 produces depigmentation. Contact Dermatitis. 2000;42:237–8. [PubMed] [Google Scholar]
- 17.Nishigori C, Aoyama Y, Ito A, Suzuki K, Suzuki T, Tanemura A, et al. Guide for medical professionals (i.e, dermatologists) for the management of rhododenol-induced leukoderma. J Dermatol. 2015;42:113–28. doi: 10.1111/1346-8138.12744. [DOI] [PubMed] [Google Scholar]
- 18.Wietze van der Veen JP, Yazdani M. Pigment Disorders. In: Rustemeyer T, Elsner P, John SM, Maibach HI, editors. Kanerva's Occupational Dermatology. 2nd ed. Vol. 1. Berlin: Springer; 2012. pp. 285–93. [Google Scholar]
- 19.Glassman SJ. Vitiligo, reactive oxygen species and T-cells. Clin Sci. 2011;120:99–120. doi: 10.1042/CS20090603. [DOI] [PubMed] [Google Scholar]
- 20.Maggi R, Harris J. Emerging treatments for vitiligo: Gaining insight from pathogenesis. J Egypt Women's Dermatol Soc. 2017;14:1–8. [Google Scholar]
- 21.Harris JE. Cellular stress and innate inflammation in organ-specific autoimmunity: Lessons learned from vitiligo. Immunol Rev. 2016;269:11–25. doi: 10.1111/imr.12369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Alkhateeb A, Fain PR, Thody A, Bennett DC, Spritz RA. Epidemiology of vitiligo and associated autoimmune diseases in Caucasian probands and their families. Pigment Cell Res. 2003;16:208–14. doi: 10.1034/j.1600-0749.2003.00032.x. [DOI] [PubMed] [Google Scholar]
- 23.Boissy RE, Manga P. On the etiology of contact /occupational vitiligo. Pigment Cell Res. 2004;17:208–14. doi: 10.1111/j.1600-0749.2004.00130.x. [DOI] [PubMed] [Google Scholar]
- 24.Kroll TM, Bommiasamy H, Boissy RE, Hernandez C, Nickoloff BJ, Mestril R, et al. 4-Tertiary butyl phenol exposure sensitizes human melanocytes to dendritic cell-mediated killing: Relevance to vitiligo. J Invest Dermatol. 2005;124:798–806. doi: 10.1111/j.0022-202X.2005.23653.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Manga P, Sheyn D, Yang F, Sarangarajan R, Boissy RE. A role for Tyrosinase-related protein 1 in 4-tert -Butylphenol-induced toxicity in melanocytes: Implications for vitiligo. Am J Pathol. 2006;169:1652–62. doi: 10.2353/ajpath.2006.050769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Richmond JM, Frisoli ML, Harris JE. Innate immune mechanisms in vitiligo: Danger from within. Curr Opin Immunol. 2013;25:676–82. doi: 10.1016/j.coi.2013.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wu S, Li WQ, Cho E, Harris JE, Speizer F, Qureshi AA. Use of permanent hair dye and risk of vitiligo in women. Pig Cell Melanoma Res. 2015;28:744–6. doi: 10.1111/pcmr.12402. [DOI] [PubMed] [Google Scholar]
- 28.Sosa JJ, Currimbhoy SD, Ukoha U, Sirignano S, O'Leary R, Vandergriff T, et al. Confetti-like depigmentation: A potential sign of rapidly progressing vitiligo. J Am Acad Dermatol. 2015;73:272–5. doi: 10.1016/j.jaad.2015.05.014. [DOI] [PubMed] [Google Scholar]
- 29.Lachapelle JM. The spectrum of diseases for which patch testing is recommended. In: Lachapelle JM, Maibach HI, editors. Patch Testing, Prick Testing: A Practical Guide. Berlin: Springer; 2003. pp. 7–26. [Google Scholar]
- 30.Boissy RE. Occupational vitiligo. In: Picardo M, Taieb A, editors. Vitiligo Berlin: Springer; 2009. pp. 175–80. [Google Scholar]
- 31.Fisher AA. Vitiligo due to contactants. Cutis. 1976;17:431–8. [PubMed] [Google Scholar]
- 32.Bickers DR, Athar M. Oxidative stress in the pathogenesis of skin disease. J Invest Dermatol. 2006;126:2565–75. doi: 10.1038/sj.jid.5700340. [DOI] [PubMed] [Google Scholar]
- 33.Behl PN, Bhatia RK. 400 cases of vitiligo– A clinico-therapeutic analysis. Indian J Dermatol. 1972;17:51–6. [PubMed] [Google Scholar]
- 34.James WD, Elston DM, Treat JR, Rosenbach MA, Neuhaus IM. Andrews' Diseases of the Skin. 13th ed. New Delhi: Elsevier; 2020. pp. 862–80. [Google Scholar]
- 35.Alikhan A, Felsten LM, Daly M, Petronic-Rosic V. Vitiligo: a comprehensive overview Part I.Introduction, epidemiology, quality of life, diagnosis, differential diagnosis, associations, histopathology, etiology, and work-up. J Am Acad Dermatol. 2011;65:473–91. doi: 10.1016/j.jaad.2010.11.061. [DOI] [PubMed] [Google Scholar]
- 36.Alam M, Ghosh S. Effect of chemical exposure in induction and evolution of vitiligo: Correlation between duration of exposure and disease, site of exposure and onset, and impact upon avoidance. Clin Epidemiol Glob Health. 2015;3(Suppl 1):S91–5. [Google Scholar]


