Abstract
Kala-azar, commonly known as visceral leishmaniasis (VL), is a neglected tropical disease that has been targeted in South Asia for elimination by 2020. Presently, the Kala-azar Elimination Programme is aimed at identifying new low-endemic foci by active case detection, consolidating vector control measures, and decreasing potential reservoirs, of which Post Kala-azar Dermal Leishmaniasis (PKDL) is considered as the most important. PKDL is a skin condition that occurs after apparently successful treatment of VL and is characterized by hypopigmented patches (macular) or a mixture of papules, nodules, and/or macules (polymorphic). To achieve this goal of elimination, it is important to delineate the pathophysiology so that informed decisions can be made regarding the most appropriate and cost-effective approach. We reviewed the literature with regard to PKDL in Asia and Africa and interpreted the findings in establishing a potential correlation between the immune responses and pathophysiology. The overall histopathology indicated the presence of a dense, inflammatory cellular infiltrate, characterized by increased expression of alternatively activated CD68+ macrophages, CD8+ T cells showing features of exhaustion, CD20+ B cells, along with decreased CD1a+ dendritic cells. Accordingly, this review is an update on the overall immunopathology of PKDL, so as to provide a better understanding of host-parasite interactions and the immune responses generated which could translate into availability of markers that can be harnessed for assessment of disease progression and improvement of existing treatment modalities.
KEY WORDS: Immune response, kala-azar, post kala-azar dermal leishmaniasis, visceral leishmaniasis
Introduction
Leishmaniases are vector-borne parasitic diseases caused by at least 20 species of the genus Leishmania and are transmitted to mammalian hosts by female sandflies. The wide pleomorphism of species of Leishmania accounts for the diverse clinical presentations that range from self-curing cutaneous lesions to a life-threatening visceral variant. Visceral leishmaniasis (VL)/kala-azar has a dermal sequela, Post Kala-azar Dermal Leishmaniasis (PKDL), and usually presents in patients with a history of apparently cured VL and occurs primarily in two geographic zones, namely South Asia and East Africa (mainly Sudan).[1,2]
Although similar in their etiology as the causative species is Leishmania donovani, PKDL in these two geographic zones have striking differences in terms of their incidence, as 5%–10% of VL cases in South Asia develop PKDL 2-3 years after apparent cure, whereas in East Africa it is seen in up to 50% of cases usually during or within 1 year of treatment for VL. They also differ in their clinical features, as in Africa it appears as papular, macular, maculopapular, nodular, or plaque-like lesions, whereas in South Asia, patients present with two distinct forms, macular or polymorphic (combination of macular, papular and nodular) lesions.[3,4,5,6] In South Asia, its public health relevance lies in the fact that as the lesions harbor parasites that are exposed to the bite of sandflies, PKDL cases are considered to play a major role in disease transmission cycle.[7,8,9,10] Accordingly, PKDL is recognized as a major constraint in the kala-azar elimination programme.[7,11,12] As the outcome of VL having a PKDL sequel is determined by a complex interplay of parasite characteristics, vector biology, and host factors, with immune responses taking center stage amongst the host factors,[1] development of diagnostic and therapeutic strategies requires an understanding of the host immune responses, so that informed decisions are made.[8,13,14] Accordingly, the aim of this review is to provide an understanding of the parasite-driven immune evasion strategies that enable parasite survival in PKDL following apparent cure from VL.
The etiopathogenesis of PKDL is still unclear and there is yet to emerge a consensus regarding possible causes for the generally viscerotropic L. donovani parasite to become dermatropic.[15] An important lacuna is the absence of an animal model and therefore, information is derived solely from human studies, and understandably remains limited. It is a heterogeneous and dynamic condition, and patients differ with regard to time of onset after VL, chronicity, distribution, and appearance of the lesions, as well as immune responses (including tendency to self-heal), all of which vary over time.[6]
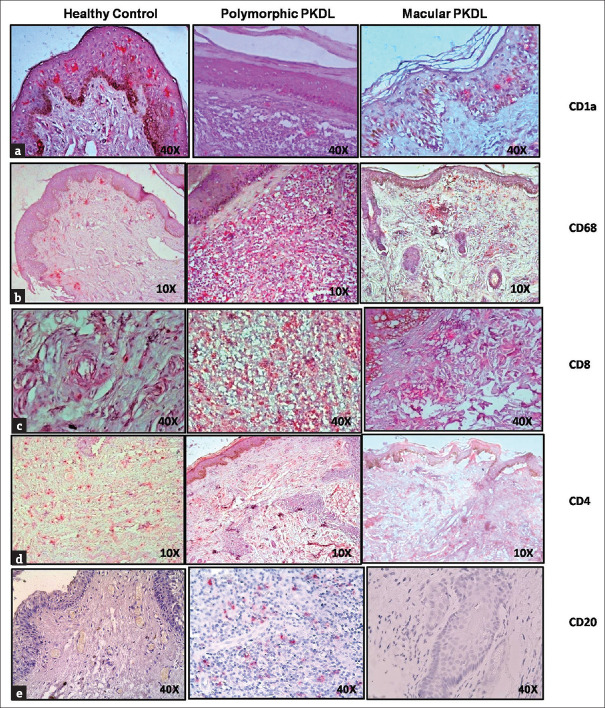
Intracellular pathogens like Leishmania have evolved innovative approaches to evade immune responses that include interference with antigen processing/presentation, altered phagocytosis, induction of immune regulatory pathways and manipulation of co-stimulatory molecules.[16] Examination of the dermal lesions has established that in PKDL cases, a consistent feature is the presence of a diffuse cell infiltrate, composed mainly of lymphocytes, macrophages, and plasma cells [Figure 1a-e].[17,18] It is expected that their detailed analysis will provide insights into its etiopathogenesis, as also suggest potential immunomodulatory approaches.
Figure 1.
Distribution of dendritic cells, macrophages, T cells, and B cells in dermal lesions of patients with polymorphic and macular PKDL. Representative immunohistochemical profiles of (a) CD1a, (b) CD68, (c) CD8, (d) CD4 and (e) CD20 from skin biopsies of a healthy control and patient with polymorphic and macular PKDL (×10 and ×40 magnification) (Source: Sengupta R, Mukherjee S, Moulik S, Mitra S, Chaudhuri SJ, Das NK, et al. In-situ immune profile of polymorphic vs. macular Indian Post Kala-azar Dermal Leishmaniasis. Int J Parasitol Drugs Drug Resist 2019;11: 166-76)
Search strategy and selection criteria
The electronic database PubMed (https://www.ncbi.nlm.nih.gov/pubmed/) was searched for articles published till date (March 2020) using the terms “PKDL,” “post-kala-azar dermal leishmaniasis.” Relevant articles from the authors' personal files were identified, and from the articles, relevant information had been included.
Dendritic cells in PKDL
Dendritic cells (DCs) are highly heterogeneous hematopoietic bone-marrow-derived leukocytes that are widely distributed, and in the skin are considered as the most professional and potent antigen-presenting cell (APC), being specialized in antigen uptake, processing, and presentation to T-cells.[19] Dermal DCs consist of at least three subpopulations, namely epidermal Langerhans cells (LCs) and two migratory dermal DC (dDCs) subsets, identifiable by CD1a staining [Figure 1a]. The unique capacity of DCs to check out sites of pathogen entry, response to microbial signals, and subsequent activation of naive T cells contributes to their critical role in initiating antimicrobial immunity.[20] Thus, a central role for DCs in modulating immune responses in leishmaniasis especially in the dermis has been proposed.[20]
In the peripheral blood of PKDL cases, the expression of plasmacytoid and myeloid DCs is comparable with healthy controls (unpublished data). However, this is not so at lesional sites, where a significant reduction of LCs and dDCs is demonstrated based on the decrease in CD1a [Figure 1a].[21,22] However, this decrease in CD1a is not restricted to selective downregulation of activated LC/dDC, as MHC-class II staining along with IL-12p40 is consistently decreased, suggesting an overall attenuation of LCs/dDCs.[22] Except for the human immunodeficiency virus and human papillomavirus,[23] a decrease in LCs has not been reported and importantly, distinguishes PKDL from lepromatous leprosy, a disease with several overlapping clinical features. This decrease in LCs and dDCs occurs in both polymorphic and macular lesions, but the decrease is more prominent in the former [Table 1].[18] Furthermore, the expression of HLA-DR is also decreased in both variants of PKDL,[18,22] [Table 1] similar to African PKDL, where Langerhans cells in the epidermis are depleted with loss of dendritic morphology, and are HLA-DR negative.[24,25] In the dermis they stain positively for B7-2 but not for B7-1. As these LCs are important initiators of the skin immune response, their pronounced decrease, along with their loss of dendritic morphology, decreased expression of HLA-DR and B7-1 collectively indicate their inability to perform their key function of antigen presentation and endorse its immunosuppressive role. It is tempting to propose that such changes could be attributed to the immunosuppressive effect of UVB rays that are known to trigger damage to epidermal LCs and inhibit contact hypersensitivity and alloantigen responses.[15,26] As UVB rays can directly damage epidermal LCs,[26] it could account for their decrease being more pronounced in polymorphic lesions that appear mainly in sun-exposed areas,[18] as opposed to the macular form, where hypopigmented patches are present even in the photo-protected regions.[6]
Table 1.
Status of immunological markers at the lesional sites in polymorphic vs. macular PKDL
| Immunological parameters | Lesional | |
|---|---|---|
| Polymorphic PKDL | Macular PKDL | |
| CD1a+ Dendritic cells (Langerhans cells and dermal dendritic cells) | Significant decrease | Significant decrease |
| HLA-DR | Marginal decrease | Significant decrease |
| CD68+ macrophages | Significant increase | Marginal increase |
| CD8+ T cells | Significant increase | Absent or comparable to healthy controls |
| CD4+ T cells | Absent | Absent |
| CD20+ B cells | Higher increase | Increase |
Role of monocyte/macrophage polarization in PKDL
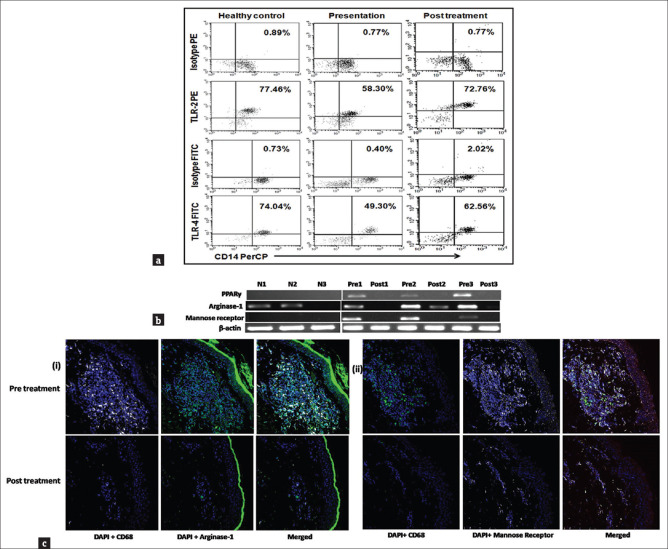
A pivotal pathogenic event in leishmaniasis is the harboring of the causative Leishmania parasite within phagolysosomes of macrophages. To achieve this, the parasite needs to deviously initiate mechanisms that can modulate the macrophage microbicidal machinery.[27] Monocytes can be classified into three subsets, namely classical (CD14++CD16-), intermediate (CD14++CD16+), and non-classical (CD14+CD16++) with discrete functions.[28] However, in PKDL, no apparent differences are seen between frequencies of the different monocyte subtypes in peripheral blood.[29] Although in PKDL, the parasites are mainly restricted to dermal sites, alterations in the systemic cellular immunity are also observed. For instance, in the peripheral blood of PKDL cases, as compared to healthy controls, the frequency of CD14+ monocytes expressing TLR2+ and TLR4+ is significantly reduced [Figure 2a].[29] As attenuation of the oxidative burst occurs secondary to reduced phosphorylation of mitogen-activated protein kinases (MAPKs) through the TLR2/4 pathway or the CD40 signalosome, it is proposed that this decreased expression of TLR2/4 account for the intramonocytic redox imbalance, and a tilt toward an anti-inflammatory pro-parasitic milieu. Indeed, this is endorsed by the reduced ex vivo levels of nitrous oxide (NO) in monocytes and attenuated generation of reactive oxygen species (ROS), along with a decrease in the production of superoxide.[29] Collectively, this altered redox balance translates into an impairment of the anti-microbial functions of monocyte-macrophages leading to enhanced parasite survival.
Figure 2.
(a) Decreased expression of TLR2 and 4 within monocytes. Representative data showing expression of TLR2 and TLR4 within CD14+ monocytes in a healthy control, a patient with PKDL (Pre t/t), and posttreatment (Post t/t). Isotype control staining is also shown. (b) Raised mRNA expression of PPAR-γ, arginase-1 and mannose receptor at the lesional sites in patients with PKDL. Representative mRNA expression profile of PPAR-γ, arginase-1, and mannose receptor in skin samples from healthy controls (N 1–3), patients with PKDL (Pre 1–3), and posttreatment (Post 1–3). The RT-PCR products were quantified by densitometric analysis after normalization with β-actin. (c) Lesional macrophages showed a raised expression of arginase-1 and mannose receptor (i) Expression of arginase-1 (green, Panels 1 and 2) in CD68+ macrophages (white, Panels 1 and 2) at the lesional site of a patient with PKDL (Pre t/t) and posttreatment (Post t/t). Nuclei are shown in blue (DAPI, Panels 1 and 2). Figures were captured in 400X magnification. (ii) Expression of mannose receptor (CD206, white, Panels 1 and 2) in CD68+ macrophages (green, Panels 1 and 2) at the lesional site of a patient with PKDL (Pre t/t) and posttreatment (Post t/t). Nuclei are shown in blue (DAPI, Panels 1 and 2). Figures were captured in 400X magnification. (Source: Mukhopadhyay D, Mukherjee S, Roy S, Dalton JE, Kundu S, Sarkar A, et al. M2 Polarization of monocytes-macrophages is a hallmark of Indian Post Kala-azar Dermal Leishmaniasis. PLoS Negl Trop Dis 2015;9: e0004145)
Conventionally, upon stimulation with Th1-associated cytokines, notably IFN-γ, monocytes/macrophages acquire a heightened effect or function against intracellular pathogens, referred to as a classically activated or M1 phenotype. Conversely, in the milieu of Th2 associated cytokines, for example, IL-4, IL-13, IL-10, IL-33, and TGF-β or by microbial triggers, M2 polarization or alternative activation occurs.[30] In general, IL-4, IL-10 and IL-13 are central mediators for skewing the macrophage function toward the M2 phenotype, and have been corroborated by an enhanced transcription of IL-4 and IL-10 driven genes, notably Mannose receptor (Mrc1), Arginase (Arg1), PPARγ (Peroxisome Proliferator Activated Receptor Gamma gene) and Fizz1 among others.[31] PPARγ is a transcription factor of the nuclear hormone receptor family which acts downstream of STAT6 signaling to regulate macrophage metabolism via the enhanced expression of CD206- mannose receptor.[32] In PKDL, there is a decreased generation of reactive oxygen and nitrogen radicals in monocytes which suggested alternative activation/M2 polarization,[29] and is endorsed by the enhanced mRNA expression of nuclear receptor PPAR γ along with ARG1 (Arginase 1 gene) [Figure 2b].[29] Furthermore, this was corroborated by an increased presence of CD68+ macrophages in dermal lesions [Figure 1b] and localization of arginase-1 and mannose receptor (CD206) within CD68+ macrophages [Figure 2c].[29] Additionally, the Vitamin D receptor signaling being linked to M2 polarization and generation of antimicrobial peptides was examined,[33] wherein raised plasma levels of 1α,25-dihydroxyvitamin D3(1,25-D3) was accompanied by an increase in VDR (responsible for nuclear signaling of 1,25-D3), CYP27B1 (encoding vitamin D-1α-hydroxylase which converted inactive prohormone to its bioactive 1,25D3 form) and LL-37 (a downstream antimicrobial effector peptide cathelecidin of the Vitamin D signaling pathway).[29] Taken together, it may be proposed that in PKDL, the alternatively activated M2 phenotype of peripheral monocytes and dermal macrophages sustain disease chronicity, and their repolarization to M1 by antileishmanial drugs, such as miltefosine,[29] represents a therapeutic opportunity.[34]
Role of T-cells in PKDL immunology
To delineate mechanisms that promote immunopathology, the contribution of T lymphocytes in PKDL has been studied in terms of the status of T-cells (CD3), T helper cells (CD3/CD4), cytotoxic T-cells (CD3/CD8), NK cells (CD56), NK T cells (CD3/CD56) and T regulatory cells (CD4/CD25).[35] In PKDL patients, before and after treatment, the percentages of T lymphocytes, T helper cells, NK cells, NK T cells, and T regulatory cells are comparable with normal individuals.[35] Similarly, the lymphoproliferative responses to phytohemagglutinin, a non-specific mitogen is no different from healthy controls as the percentages of IFN-γ, IL-2, IL-4, and IL-10 expressing lymphocytes is comparable to controls.[35] The only notable feature in peripheral blood of PKDL cases is a small but significant increase in the percentage of CD3/CD8 lymphocytes, which is retained even after treatment.[35] Additionally, in response to L. donovani antigen, PKDL cases show an 8-fold increase in the percentage of IL-10 expressing CD3/CD8 lymphocytes as compared to controls which decrease with treatment.[35] Taken together, it may be proposed that IL-10 producing CD3/CD8 lymphocytes are important protagonists in the immunopathogenesis of Indian PKDL.[35]
Dermal homing of CD8 T-cells in PKDL
In PKDL, the enhanced secretion of IL-4/IL-13 and IL-10 facilitates the emergence of alternatively activated M2 monocytes/macrophages,[29] that supportes the expression of Th2 chemoattractants CCL17 and CCL22. These chemokines following an interaction with their receptor CCR4 pave the way for migration of skin-homing Th2-cells to the pathologic site [Figure 1c], that comprise primarily of CD8+ T-cells, and this increase was more prominent in the polymorphic variant as compared to the macular group [Table 1].[18,36] These lesional CD8+ T-cells demonstrate an absence of Perforin, Granzyme and Zap-70, along with an enhanced expression of Programmed Death-1 (PD-1), IL-10 and IL-5.[36] However, in Indian PKDL, there is a notable absence of CD4+ T-cells at the lesional sites [Figure 1d],[36] which is in sharp contrast to self-healing African PKDL, where the increase in CD4+ T-cells >> CD8+ T-cells.[21] This near total absence of CD4+ T-cells is attributed to the enhanced presence of alternatively activated macrophages, that via IL-4/IL-13 mediated activation of signal transducer and activation of transcription 6 (STAT-6), can inhibit T-cell proliferation and survival which possibly facilitate disease progression.[37,38]
Role of regulatory and anergic T cells in PKDL
Regulatory T cells (Tregs) comprise 5%–10% of the circulating CD4 T-cell population and uniquely express the transcription factor, Forkhead Box P3 (FoxP3), which is crucial for their development and function.[39] It is reported that in acute infections, Tregs benefit the host by preventing immune-mediated pathology after pathogen eradication, whereas in chronic infections, their activity is unfavorable to the host, as they promote parasite survival amidst an active immune response.[40] In the peripheral blood of PKDL cases, the percentage of Tregs represented by CD4+CD25+ lymphocytes is comparable with healthy controls,[41] but at lesional sites, there is an increase in the FoxP3 positive cells along with an abundant mRNA expression of IFN-γ, IL- 10, and FoxP3 which is reduced following treatment.[41,42]
Although in PKDL, peripheral blood cells do not have increased natural T regulatory cells (CD4+CD25+FoxP3+), the absence of an antigen-specific memory or recall response, characterized by absence of T cell proliferation and IFN-γ production suggests immune anergy, attributable to the higher frequency of IL-10 producing antigen-specific circulating CD8+ T-cells.[35,41] Immune anergy is observed when the interaction between the major histocompatibility complex (on the APCs) and T cell receptor (TCR) occurs in the absence of co-stimulation, that is, an interaction between CD28 on T cells and CD80 or CD86 on APCs.[43] In PKDL, there is a conspicuous decrease in the expression of CD86 on monocytes[44] and CD28 on CD8+ T-cells[41] which supports the peripheral “immune anergy” phenotype. Furthermore, it is shown that the peripheral CD8+ T-cells express PD1, along with a decreased expression of CD127, suggesting that the peripheral CD8+ T cells acquire a regulatory T-cell phenotype.[36] Taken together, in PKDL, it may be envisaged that lack of co-stimulation between circulating monocytes and CD8+ T-cells induces an anergic state. This is supported by an increased frequency of IL-10 positivity along with enhanced plasma and lesional CCL17 that facilitates the dermal homing of CD8+CCR4+ T-cells. Furthermore, these CD8+CCR4+ T-cells show an upregulation of PD-1 and IL-10, and the overall immune exhaustion supports disease progression, emphasizing the need for designing immunotherapies capable of bolstering T-cell potency.
Cytokine profiles in PKDL
Pro-inflammatory cytokines, namely IL-6, IL-8, IL-1β, TNF-α, and IL-12 are the domain of M1 monocytes/macrophages, whereas IL-4, IL-13, IL-10, and TGF-β secreted by M2 monocytes help sustain the immunosuppressive environment.[30] In PKDL, although a dermal disease, monocytes/macrophages in circulation show an enhanced production of IL-4, IL-10, and IL-13.[29,44] Alongside, circulating monocytes in patients with PKDL generate lower amounts of IL-6, IL-1β, and IL-8,[35] and a significant population of monocytes express the pro-inflammatory IL-12p40.[29]
At the lesional sites, a raised mRNA expression of both pro- and anti-inflammatory cytokines has been reported.[35,45] Although the mRNA expression of pro-inflammatory cytokines, such as IFN-γ is augmented at disease presentation, it is associated with a significantly lowered expression of its receptor IFN-γR1, thus accounting for its inability to mediate its anticipated host protective action against leishmaniasis.[46] Furthermore, the raised mRNA expression of counter-regulatory cytokines, such as TGF-β and IL-10,[41,44,46] possibly curtails the pro-inflammatory cytokines based immune responses, and instead supports an immunosuppressive milieu accounting for parasite persistence.
Humoral immune responses in PKDL
In leishmaniasis, the pronounced Th2-anti-inflammatory response translates into a strong humoral response as evidenced by polyclonal gammaglobulinemia and raised levels of antileishmanial antibodies which have been used to develop diagnostic tools, such as the direct agglutination test (DAT) and the rK39 strip test.[47,48] Additionally, studies have been undertaken to dissect differences, if any, between the polymorphic and macular variants of PKDL. In general, the humoral response appears stronger in the polymorphic variant, evident by a greater increase in the proportion of lesional CD20+ B-cells [Table 1 and Figure 1e][18] along with a significant curtailment in levels of Ig, IgM, and IgG following their treatment. The scenario is similar with regard to IgG subclasses, as levels of IgG1 and IgG3 are higher in polymorphic PKDL at disease presentation, and with treatment, the reduction is more dramatic in the polymorphic variant.[49] These differences could be attributed simply to the higher parasite load observed in polymorphic PKDL which is also supported by higher IgG avidity[49,50] or variations in host immune responses, a question which is pertinent, but remains unexplored.
Drug responses in PKDL
Given the tilt towards a Th2 response in PKDL, the potentiation of Th1 activation through the use of immunomodulators is an important consideration.[51,52] Currently, the drug of choice for PKDL is miltefosine whose direct antileishmanial mechanism of action includes disruption of (membrane) lipid metabolism, apoptosis-like cell death, induction of mitochondrial dysfunction, along with indirect immunomodulatory effects involving augmentation of the Th1 cell response particularly via enhancement of interferon (IFN-γ) and IL-12, along with overriding of the Leishmania -driven Th2 response.[34,53] This is demonstrated in PKDL patients by Mukhopadhyay et al.[45] who report that miltefosine responses significantly increase the secretion of Th1 cytokines and reduce anti-inflammatory Th2 responses.
Amphotericin B deoxycholate (AmB) is the recommended second-line drug for the treatment of PKDL in cases when miltefosine is discontinued due to its toxic effects, or in cases that relapsed.[55] Similarly, liposomal amphotericin B (LAmB) promotes a direct leishmanicidal activity by virtue of its high intercalation affinity for ergosterol or its precursor present on the parasite, along with its ability to increase the permeability of cell membranes, resulting in leakage of ions and small solute molecules culminating in cell death.[50] However, its immunomodulatory role, if any, is yet to be documented in PKDL.
For orally administered drugs to be effective in the skin, factors contributing toward drug accumulation within lesions are influenced by the inflammatory response as (a) it can increase permeability secondary to vasodilatation and (b) the resultant capillary damage can facilitate the generation of a leaky vasculature.[55] Therefore, it can be proposed that in polymorphic PKDL, the stronger pro-inflammatory milieu vis-à-vis the macular variant[18] could account for its higher drug accumulation and consequently, its better therapeutic response.[50] Taken together, in the absence of any satisfactory canonical treatment for PKDL, the combination therapy of LAmB and miltefosine appears promising as it takes into consideration the potent leishmanicidal activity of the former coupled with the additional immunomodulatory properties of the latter.[56]
Conclusions
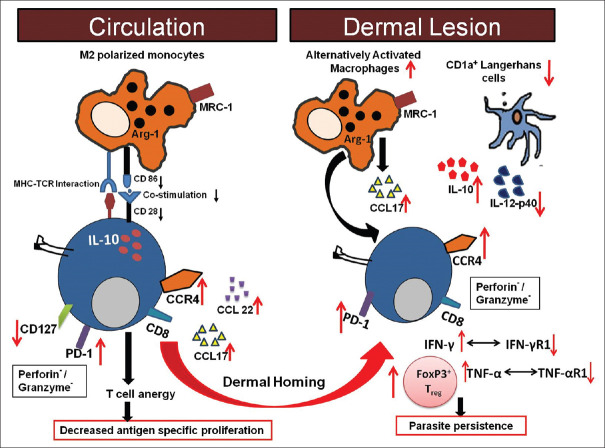
The natural history of PKDL is not completely understood and like many of the diverse forms of leishmaniasis, our view may be clouded by data derived from animal models (of CL and VL) and/or by pooling data across clinically diverse forms of disease which may not necessarily be accurate. It has been proposed that PKDL originates as an immune reconstitution inflammatory syndrome (IRIS), resulting from a loss of immune suppression following drug treatment for VL. Although data are fragmentary and no systematic picture of the immune status at presentation or after initiation of drug therapy in PKDL has yet emerged, evidence, so far as reviewed in this study, suggests that akin to VL, the pathophysiology of PKDL involves an enhanced Th1/Th2 response with a Th2 bias, as evident by increased levels of IL-4, IL-5, IL-13, IL-10, and TGF-β, along with the presence of a systemic and dermal immunosuppressive milieu and includes the presence of an increased population of antigen-specific IL-10 producing anergic T-cell population in peripheral blood, a decreased presence of dendritic cells at lesional sites, a huge infiltration of CD68+ alternatively activated macrophages and a dermal pathology dominated by IL-10 and FoxP3, that individually or more likely collectively contribute towards establishment of a pro-parasitic milieu. Furthermore, the cellular infiltrate is dominated by an increased presence of CD8+ T-cells, which exhibit features of exhaustion, along with a near total absence of CD4+ T-cells [Figure 3]. Thus, Leishmania donovani parasites have deviously evolved immune escape mechanisms emphasizing the importance of designing immunotherapeutic strategies aimed at restoring effector responses.
Figure 3.
Cellular interactions between peripheral blood and dermal lesions in patients with PKDL. In patients with PKDL, an increased presence of IL-10 and IL-5 along with immune reinforcement by alternatively activated macrophages (M2) and decreased CD1a+ dendritic cells prevented parasite elimination. Increased CCL17/22 upon interaction with CCR4 led to dermal homing of CD8+ T cells which showed exhaustion as confirmed by the increased presence of PD-1 in peripheral blood and dermal lesions and, benefitted from the milieu generated by the increased presence of IL-4/IL-5 and IL-10
Conflicts of interest
There are no conflicts of interest.
Acknowledgements
The authors have received support from Indian Council for Medical Research (ICMR) (Grant number 6/9-7(151)2017-ECD II); Department of Health Research (DHR), Government of India (Grant number DHR/HRD/Fellowship/SUG-05/2015-16); Fund for Improvement of S and T infrastructure in Universities and Higher Educational Institutions (FIST) Program, Department of Science and Technology, Government of India (DST-FIST) (Grant number SR/FST/LS1-663/2016); Department of Science and Technology, Government of West Bengal (Grant number 969 [Sanc.]/ST/P/S&T/9G-22/2016). DM and RS are recipients of Senior Research Fellowships from ICMR, Govt. of India, SMukherjee and AD are recipients of Senior Research Fellowships from DST-INSPIRE and UGC, respectively. SSG is a recipient of a Junior Research Fellowship from CSIR, Govt. of India.
References
- 1.Burza S, Croft SL, Boelaert M. Leishmaniasis. Lancet. 2018;392:951–70. doi: 10.1016/S0140-6736(18)31204-2. [DOI] [PubMed] [Google Scholar]
- 2.Zijlstra EE, Musa AM, Khalil EAG, el-Hassan IM, el-Hassan AM. Post-kala-azar dermal leishmaniasis. Lancet Infect Dis. 2003;3:87–98. doi: 10.1016/s1473-3099(03)00517-6. [DOI] [PubMed] [Google Scholar]
- 3.Zijlstra EE. The immunology of post-kala-azar dermal leishmaniasis (PKDL) Parasit Vectors. 2016;9:464. doi: 10.1186/s13071-016-1721-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ganguly S, Das NK, Barbhuiya JN, Chatterjee M. Post-kala-azar dermal leishmaniasis-an overview. Int J Dermatol. 2010;49:921–31. doi: 10.1111/j.1365-4632.2010.04558.x. [DOI] [PubMed] [Google Scholar]
- 5.Ramesh V, Kaushal H, Mishra AK, Singh R, Salotra P. Clinico-epidemiological analysis of Post kala-azar dermal leishmaniasis (PKDL) cases in India over last two decades: A hospital based retrospective study. BMC Public Health. 2015;15:1092. doi: 10.1186/s12889-015-2424-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sengupta R, Chaudhuri SJ, Moulik S, Ghosh MK, Saha B, Das NK, et al. Active surveillance identified a neglected burden of macular cases of Post Kala-azar Dermal Leishmaniasis in West Bengal. PLoS Negl Trop Dis. 2019;13:e0007249. doi: 10.1371/journal.pntd.0007249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Le Rutte EA, Zijlstra EE, de Vlas SJ. Post-Kala-Azar Dermal Leishmaniasis as a reservoir for Visceral Leishmaniasis transmission. Trends Parasitol. 2019;35:590–2. doi: 10.1016/j.pt.2019.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zijlstra EE, Alves F, Rijal S, Arana B, Alvar J. Post-kala-azar dermal leishmaniasis in the Indian subcontinent: A threat to the South-East Asia Region Kala-azar elimination programme. PLoS Negl Trop Dis. 2017;11:e0005877. doi: 10.1371/journal.pntd.0005877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Molina R, Ghosh D, Carrillo E, Monnerat S, Bern C, Mondal D, et al. Infectivity of Post-Kala-azar Dermal Leishmaniasis patients to sand flies: Revisiting a proof of concept in the context of the Kala-azar elimination program in the Indian Subcontinent. Clin Infect Dis. 2017;65:150–3. doi: 10.1093/cid/cix245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mondal D, Bern C, Ghosh D, Rashid M, Molina R, Chowdhury R, et al. Quantifying the infectiousness of post-Kala-Azar dermal leishmaniasis toward sand flies. Clin Infect Dis. 2019;69:251–8. doi: 10.1093/cid/ciy891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Matlashewski G, Arana B, Kroeger A, Battacharya S, Sundar S, Das P, et al. Visceral leishmaniasis: Elimination with existing interventions. Lancet Infect Dis. 2011;11:322–5. doi: 10.1016/S1473-3099(10)70320-0. [DOI] [PubMed] [Google Scholar]
- 12.Ganguly S, Saha P, Chatterjee M, Roy S, Ghosh TK, Guha SK, et al. PKDL-A silent parasite pool for transmission of Leishmaniasis in kala-azar endemic areas of Malda district, West Bengal, India. PLoS Negl Trop Dis. 2015;9:e0004138. doi: 10.1371/journal.pntd.0004138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sundar S, Singh OP, Chakravarty J. Visceral leishmaniasis elimination targets in India, strategies for preventing resurgence. Expert Rev Anti Infect Ther. 2018;16:805–12. doi: 10.1080/14787210.2018.1532790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Singh OP, Hasker E, Boelaert M, Sundar S. Elimination of visceral leishmaniasis on the Indian subcontinent. Lancet Infect Dis. 2016;16:e304–9. doi: 10.1016/S1473-3099(16)30140-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mukhopadhyay D, Dalton JE, Kaye PM, Chatterjee M. Post kala-azar dermal leishmaniasis: An unresolved mystery. Trends Parasitol. 2014;30:65–74. doi: 10.1016/j.pt.2013.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Walker DM, Oghumu S, Gupta G, McGwire BS, Drew ME, Satoskar AR. Mechanisms of cellular invasion by intracellular parasites. Cell Mol Life Sci. 2014;71:1245–63. doi: 10.1007/s00018-013-1491-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Singh A, Ramesh V, Ramam M. Histopathological characteristics of post kala-azar dermal leishmaniasis: A series of 88 patients. Indian J Dermatol Venereol Leprol. 2015;81:29–34. doi: 10.4103/0378-6323.148562. [DOI] [PubMed] [Google Scholar]
- 18.Sengupta R, Mukherjee S, Moulik S, Mitra S, Chaudhuri SJ, Das NK, et al. In-situ immune profile of polymorphic vs.macular Indian Post Kala-azar dermal leishmaniasis. Int J Parasitol Drugs Drug Resist. 2019;11:166–76. doi: 10.1016/j.ijpddr.2019.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Collin M, Bigley V. Human dendritic cell subsets: An update. Immunology. 2018;154:3–20. doi: 10.1111/imm.12888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.von Stebut E, Belkaid Y, Jakob T, Sacks DL, Udey MC. Uptake of Leishmania major amastigotes results in activation and interleukin 12 release from murine skin-derived dendritic cells: Implications for the initiation of anti-Leishmania immunity. J Exp Med. 1998;188:1547–52. doi: 10.1084/jem.188.8.1547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ismail A, Gadir AFA, Theander TG, Kharazmi A, El Hassan AM. Pathology of post-kala-azar dermal leishmaniasis: A light microscopical, immunohistochemical, and ultrastructural study of skin lesions and draining lymph nodes.J. Cutan Pathol. 2006;33:778–87. doi: 10.1111/j.1600-0560.2006.00531.x. [DOI] [PubMed] [Google Scholar]
- 22.Mukherjee S, Mukhopadhyay D, Braun C, Barbhuiya JN, Das NK, Chatterjee U, et al. Decreased presence of Langerhans cells is a critical determinant for Indian Post kala-azar dermal leishmaniasis. Exp Dermatol. 2015;24:232–4. doi: 10.1111/exd.12635. [DOI] [PubMed] [Google Scholar]
- 23.Levi G, Feldman J, Holman S, Salarieh A, Strickler HD, Alter S, et al. Relationship between HIV viral load and Langerhans cells of the cervical epithelium. J Obstet Gynaecol Res. 2005;31:178–84. doi: 10.1111/j.1341-8076.2005.00267.x. [DOI] [PubMed] [Google Scholar]
- 24.Ismail A, Khalil EAG, Musa AM, el Hassan IM, Ibrahim ME, Theander TG, et al. The pathogenesis of post kala-azar dermal leishmaniasis from the field to the molecule: Does ultraviolet light (UVB) radiation play a role? Med Hypotheses. 2006;66:993–9. doi: 10.1016/j.mehy.2005.03.035. [DOI] [PubMed] [Google Scholar]
- 25.Ismail A, el Hassan AM, Kemp K, Gasim S, Kadaru AE, Moller T, et al. Immunopathology of post kala-azar dermal leishmaniasis (PKDL): T-cell phenotypes and cytokine profile. J Pathol. 1999;189:615–22. doi: 10.1002/(SICI)1096-9896(199912)189:4<615::AID-PATH466>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 26.Clydesdale GJ, Dandie GW, Muller HK. Ultraviolet light induced injury: Immunological and inflammatory effects. Immunol Cell Biol. 2001;79:547–68. doi: 10.1046/j.1440-1711.2001.01047.x. [DOI] [PubMed] [Google Scholar]
- 27.Kaye P, Scott P. Leishmaniasis: Complexity at the host-pathogen interface. Nat Rev Microbiol. 2011;9:604–15. doi: 10.1038/nrmicro2608. [DOI] [PubMed] [Google Scholar]
- 28.Ziegler-Heitbrock L, Ancuta P, Crowe S, Dalod M, Grau V, Hart DN, et al. Nomenclature of monocytes and dendritic cells in blood. Blood. 2010;116:e74–80. doi: 10.1182/blood-2010-02-258558. [DOI] [PubMed] [Google Scholar]
- 29.Mukhopadhyay D, Mukherjee S, Roy S, Dalton JE, Kundu S, Sarkar A, et al. M2 Polarization of monocytes-macrophages is a hallmark of Indian Post Kala-Azar dermal leishmaniasis. PLoS Negl Trop Dis. 2015;9:e0004145. doi: 10.1371/journal.pntd.0004145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Martinez FO, Helming L, Gordon S. Alternative activation of macrophages: An immunologic functional perspective. Annu Rev Immunol. 2009;27:451–83. doi: 10.1146/annurev.immunol.021908.132532. [DOI] [PubMed] [Google Scholar]
- 31.Locati M, Curtale G, Mantovani A. Diversity, mechanisms, and significance of macrophage plasticity. Annu Rev Patho. 2020;15:123–47. doi: 10.1146/annurev-pathmechdis-012418-012718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bouhlel MA, Staels B, Chinetti-Gbaguidi G. Peroxisome proliferator-activated receptors--from active regulators of macrophage biology to pharmacological targets in the treatment of cardiovascular disease. J Intern Med. 2008;263:28–42. doi: 10.1111/j.1365-2796.2007.01892.x. [DOI] [PubMed] [Google Scholar]
- 33.Griffin MD, Xing N, Kumar R. Vitamin D and its analogs as regulators of immune activation and antigen presentation. Annu Rev Nutr. 2003;23:117–45. doi: 10.1146/annurev.nutr.23.011702.073114. [DOI] [PubMed] [Google Scholar]
- 34.Palić S, Bhairosing P, Beijnen JH, Dorlo TPC. Systematic review of host-mediated activity of Miltefosine in Leishmaniasis through immunomodulation. Antimicrob Agents Chemother. 2019;63:e02507–18. doi: 10.1128/AAC.02507-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ganguly S, Das NK, Panja M, Pal S, Modak D, Rahaman M, et al. Increased levels of interleukin-10 and IgG3 are hallmarks of Indian post-kala-azar dermal leishmaniasis. J Infect Dis. 2008;197:1762–71. doi: 10.1086/588387. [DOI] [PubMed] [Google Scholar]
- 36.Mukherjee S, Sengupta R, Mukhopadhyay D, Braun C, Mitra S, Roy S, et al. Impaired activation of lesional CD8+ T-cells is associated with enhanced expression of Programmed Death-1 in Indian Post Kala-azar Dermal Leishmaniasis. Sci Rep. 2019;9:762. doi: 10.1038/s41598-018-37144-y. Available from: https://doiorg/101038/s41598-018-37144-y . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Huber S, Hoffmann R, Muskens F, Voehringer D. Alternatively activated macrophages inhibit T-cell proliferation by Stat6-dependent expression of PD-L2. Blood. 2010;116:3311–20. doi: 10.1182/blood-2010-02-271981. [DOI] [PubMed] [Google Scholar]
- 38.Badley AD, Dockrell D, Simpson M, Schut R, Lynch DH, Leibson P, et al. Macrophage-dependent apoptosis of CD4+T lymphocytes from HIV-infected individuals is mediated by FasL and tumor necrosis factor. J Exp Med. 1997;185:55–64. doi: 10.1084/jem.185.1.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Prendergast CT, Sanin DE, Mountford AP. Alternatively activated mononuclear phagocytes from the skin site of infection and the impact of IL-4Rα signalling on CD4+T cell survival in draining lymph nodes after repeated exposure to Schistosoma mansoni cercariae. PLoS Negl Trop Dis. 2016;10:e0004911. doi: 10.1371/journal.pntd.0004911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jonuleit H, Schmitt E, Stassen M, Tuettenberg A, Knop J, Enk AH. Identification and functional characterization of human CD4(+)CD25(+) T cells with regulatory properties isolated from peripheral blood. J Exp Med. 2001;193:1285–94. doi: 10.1084/jem.193.11.1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Belkaid Y. Regulatory T cells and infection: A dangerous necessity. Nat Rev Immunol. 2007;7:875–88. doi: 10.1038/nri2189. [DOI] [PubMed] [Google Scholar]
- 42.Ganguly S, Mukhopadhyay D, Das NK, Chaduvula M, Sadhu S, Chatterjee U, et al. Enhanced lesional Foxp3 expression and peripheral anergic lymphocytes indicate a role for regulatory T cells in Indian post-kala-azar dermal leishmaniasis. J Invest Dermatol. 2010;130:1013–22. doi: 10.1038/jid.2009.393. [DOI] [PubMed] [Google Scholar]
- 43.Katara GK, Ansari NA, Verma S, Ramesh V, Salotra P. Foxp3 and IL-10 expression correlates with parasite burden in lesional tissues of post kala azar dermal leishmaniasis (PKDL) patients. PLoS Negl Trop Dis. 2011;5:e1171. doi: 10.1371/journal.pntd.0001171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Linsley PS, Ledbetter JA. The role of the CD28 receptor during T cell responses to antigen. Annu Rev Immunol. 1993;11:191–212. doi: 10.1146/annurev.iy.11.040193.001203. [DOI] [PubMed] [Google Scholar]
- 45.Mukhopadhyay D, Das NK, Roy S, Kundu S, Barbhuiya JN, Chatterjee M. Miltefosine effectively modulates the cytokine milieu in Indian post kala-azar dermal leishmaniasis. J Infect Dis. 2011;204:1427–36. doi: 10.1093/infdis/jir551. [DOI] [PubMed] [Google Scholar]
- 46.Ansari NA, Saluja S, Salotra P. Elevated levels of interferon-gamma, interleukin-10, and interleukin-6 during active disease in Indian kala azar. Clin Immunol. 2006;119:339–45. doi: 10.1016/j.clim.2006.01.017. [DOI] [PubMed] [Google Scholar]
- 47.Ansari NA, Ramesh V, Salotra P. Interferon (IFN)-gamma, tumor necrosis factor-alpha, interleukin-6, and IFN-gamma receptor 1 are the major immunological determinants associated with post-kala azar dermal leishmaniasis. J Infect Dis. 2006;194:958–65. doi: 10.1086/506624. [DOI] [PubMed] [Google Scholar]
- 48.Sundar S, Singh OP. Molecular diagnosis of Visceral Leishmaniasis. Mol Diagn Ther. 2018;22:443–57. doi: 10.1007/s40291-018-0343-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Singh R, Subba Raju BV, Jain RK, Salotra P. Potential of direct agglutination test based on promastigote and amastigote antigens for serodiagnosis of post-kala-azar dermal leishmaniasis. Clin Diagn Lab Immunol. 2005;12:1191–4. doi: 10.1128/CDLI.12.10.1191-1194.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mukhopadhyay D, Das NK, De Sarkar S, Manna A, Ganguly DN, Barbhuiya JN, et al. Evaluation of serological markers to monitor the disease status of Indian post kala-azar dermal leishmaniasis. Trans R Soc Trop Med Hyg. 2012;106:668–76. doi: 10.1016/j.trstmh.2012.07.005. [DOI] [PubMed] [Google Scholar]
- 51.Moulik S, Chaudhuri SJ, Sardar B, Ghosh M, Saha B, Das NK, et al. Monitoring of parasite kinetics in Indian Post Kala Azar dermal leishmaniasis. Clin Inf Dis. 2018;66:404–10. doi: 10.1093/cid/cix808. [DOI] [PubMed] [Google Scholar]
- 52.Singh OP, Sundar S. Immunotherapy and targeted therapies in treatment of visceral leishmaniasis: Current status and future prospects. Front Immunol. 2014;5:296. doi: 10.3389/fimmu.2014.00296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Dorlo TPC, Balasegaram M, Beijnen JH, de Vries PJ. Miltefosine: A review of its pharmacology and therapeutic efficacy in the treatment of leishmaniasis. J Antimicrob Chemother. 2012;67:2576–97. doi: 10.1093/jac/dks275. [DOI] [PubMed] [Google Scholar]
- 54.Ramesh V, Singh R, Avishek K, Verma A, Deep DK, Verma S, et al. Decline in clinical efficacy of oral Miltefosine in treatment of Post Kala-azar dermal leishmaniasis (PKDL) in India. PLoS Negl Trop Dis. 2015;9:e0004093. doi: 10.1371/journal.pntd.0004093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Asad M, Bhattacharya P, Banerjee A, Ali N. Therapeutic and immunomodulatory activities of short-course treatment of murine visceral leishmaniasis with KALSOME™10, a new liposomal amphotericin B. BMC Infect Dis. 2015;15:188. doi: 10.1186/s12879-015-0928-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ramesh V, Dixit KK, Sharma N, Singh R, Salotra P. Assessing the efficacy and safety of Liposomal Amphotericin B and Miltefosine in combination for treatment of Post Kala-Azar Dermal Leishmaniasis. J Infect Dis. 2020;221:608–17. doi: 10.1093/infdis/jiz486. [DOI] [PubMed] [Google Scholar]