Abstract
This work aimed to optimize carbon and nitrogen sources for the growth of Enterobacter cloacae B14 and its biosurfactant (BS) production via One-Variable-At-a-Time (OVAT) method. The BS stability under a range of pH and temperatures was assessed. Antimicrobial activity against Gram-positive and Gram-negative pathogens was determined by the agar well diffusion method. The results showed that the optimum carbon and nitrogen sources for BS production were maltose and yeast extract, respectively, with a maximum BS yield of (39.8 ± 5.2) mg BS/g biomass. The highest emulsification activity (E24) was 79%, which is significantly higher than in the previous studies. We found that B14 BS can withstand a wide range of pH values from 2 to10. It could also function under a range of temperatures from 30–37°C. Thin Layer Chromatography (TLC) and Fourier Transform Infrared Spectrometry (FTIR) analysis confirmed that B14 BS is a glycolipid-like compound, which is rarely found in Enterobacter spp. Cell-free broth showed inhibition against various pathogens, preferable to Gram-positive ones. It had better antimicrobial activity against Bacillus subtilis than a commonly-used antibiotic, tetracycline. Furthermore, B14 broth could inhibit the growth of a tetracycline-resistant Serratia marcescens. Our results showed promising B14 BS applications not only for bioremediation but also for the production of antimicrobial products.
Key words: biosurfactant, cultivation media, Enterobacter cloacae, antimicrobial activity, stability
Introduction
Biosurfactants (BS) are amphiphilic molecules containing hydrophobic and hydrophilic moieties produced by microorganisms. These compounds have a broad diversity of chemical compounds such as glycolipids, lipopeptides, lipoproteins, lipopolysaccharides, and phospholipids (Walter et al. 2010; Gudiňa et al. 2013). BS exhibit various functional properties, including emulsification and surface tension reduction (Jahan et al. 2020).
BS have many advantages over their chemical counterparts (Fracchia et al. 2015; Nurfarahin et al. 2018). They have lower toxicity, higher biodegradability, and require milder production conditions, which makes them more environmentally friendly. They are more stable at high salinity, pH, and temperature (Sobrinho et al. 2014). Owing to these properties, BS is beneficial for several applications such as bioremediation of hydrocarbons in the environment, therapeutic agents in the pharmaceutical industry, and as a solubilizer in dairy products (Gharaei-Fathabad 2011; Bhadoriya et al. 2013; Sekhon Randhawa and Rahman 2014). Due to its variety of applications, the demand for BS in many industries becomes increasing.
Several bacterial species, including Bacillus, Pseudomonas and Enterobacter, are capable of producing BS (Ranasalva et al. 2014; Ekprasert et al. 2019). However, there are only a few BS whose production have been successfully maximized to an industrial scale due to their cost of production and recovery (Shete et al. 2006). To overcome this limitation and obtain higher yield and BS efficiency, the sources of carbon and nitrogen for microbial growth should be optimized to decrease the overall costs associated with a large-scale production (Wu et al. 2008; Muller et al. 2012).
In our previous work (Ekprasert et al. 2019), we found that strain B14 can grow on spent engine oil and perform relatively higher emulsification activity of BS compared to most of the BS produced by other E. cloacae strains. The strain B14, therefore, has the potential for bioremediation of petroleum contaminants in the environment. Here, we investigated further how the B14 BS activity can be improved by selecting the optimum carbon and nitrogen sources for microbial growth. One of the frequently used approaches to optimizing the composition of cultivation medium for BS production is through the OVAT (one-variable-at-a-time) method. This serial optimization approach is conducted by varying one factor at a time, while all other factors are kept at their chosen level. The optimum level of a parameter will be used for the next round of OVAT studies conducted with the other factor, and so on (Rane et al. 2017). Higher BS activity obtained will be beneficial for applications in industrial scales. We aimed to optimize carbon and nitrogen sources using the OVAT method. A comparison of BS activity and yield derived from cells grown on different growth media was assessed. Additionally, the stability of BS under varied pH and temperatures were investigated. The type of BS was analyzed using Thin Layer Chromatography (TLC) and Fourier Transform Infrared Spectrometry (FTIR). Additionally, the antimicrobial activity was examined to investigate another potential use of BS as a therapeutic agent.
Experimental
Materials and Methods
Effect of carbon sources on bacterial growth and the production of biosurfactant. These experiments were conducted to investigate the effects of different carbon sources on the growth and BS production of E. cloacae B14, whose stock culture has been preserved in the Culture Collection Center at the Department of Microbiology, Faculty of Science, Khon Kaen University. The inoculum was grown in 100 ml of Nutrient broth (NB) and incubated at 30°C by shaking at 150 rpm for 24 hours. The biomass was harvested and washed twice with equal volumes of Mineral Salt medium (MS) (Whittenbury et al. 1970). Cell suspension was inoculated into MS medium to an OD600 of 0.1. Various carbon sources, including glucose, sucrose, lactose, maltose, and glycerol, were individually added at a concentration of 1% (w/v). Note that biosurfactant can be produced during both exponential phase and stationary phase of growth depending upon bacterial strains and medium compositions (Nurfarahin et al. 2018), so the incubation was conducted on a 7-day period, the duration of which covered those two phases where biosurfactant could be produced. The experiment was carried out in triplicate. MS medium without inoculum was set up as a negative control. Samples were taken every 12–24 hr for growth measurement (OD600) by spectrophotometry analysis and determination of emulsification activity (E24). The E24 test was performed according to (Cooper and Goldenburg 1987) and calculated by using the equation below:
Effect of nitrogen sources on bacterial growth and the production of biosurfactant. The One-variable-at-a-time (OVAT) method was carried out to determine the nitrogen source appropriate for BS production by E. cloacae B14. The carbon source supporting the best growth and BS production was used in this experiment. The nitrogen sources used were yeast extract, urea, NH4NO3, NH4Cl, and (NH4)2SO4. Each nitrogen compound was added at a concentration of 1% (w/v) in 100 ml MS medium. Cultures were incubated at 30°C with shaking at 150 rpm for seven days. Each culture was carried out in triplicate. MS medium without inoculum was set up as a negative control. Samples were taken every 12–24 hr for measurement of growth at OD600 and determination of emulsification activity.
Extraction, purification, and quantification of biosurfactant. Crude BS was extracted from cell-free supernatant of 500 ml cultures grown for four days. The cell pellet was removed by centrifugation at 8,000 g for 20 min. The pH of the supernatant was adjusted to 2.0 with 6N HCl solution. The mixture was kept at 4°C overnight. Crude biosurfactant was collected by centrifugation to remove supernatant and then dissolved in 10 ml sterile water. Purification was carried out twice by vigorously mixing 10 ml of chloroform with crude extract for 3 min, and then centrifuged to collect the solvent phase. Then, chloroform was evaporated at 40°C using a rotary evaporator (LaboGene, China). Purified BS was weighed and kept at 4°C until use.
TLC analysis. Characterization of the produced biosurfactants was performed by using TLC analysis. Samples were prepared by dissolving 0.1 g of purified BS in 20 µl of methanol. BS were separated on a silica gel plate using chloroform: methanol: acetic acid = 65 : 15 : 2 (v/v/v) as mobile phase. The spots which developed on the TLC plate were visualized by spraying 0.5% (w/v) of ninhydrin solution and phenol-H2SO4 (95 ml ethanol mixed with 5 ml of H2SO4 and 3 g of phenol) for amino acids and carbohydrates, respectively. The TLC plates were exposed to iodine in an iodine-saturated chamber in order to visualize lipid fraction. After spraying, the TLC plates were heated in a 110°C oven for 15 min, or until colors developed.
FTIR analysis. The BS characteristics were confirmed by FTIR analysis (Tensor-27, Bruker, USA). The analysis was performed with a resolution of 4 cm–1. The FTIR spectra were collected from 600–4000 wave-numbers (cm–1).
Stability studies. The stability of BS under a range of pH and temperatures was investigated. To determine its thermal stability, purified BS was tested for its E24 under different temperatures ranging from 30–100°C. BS was exposed to the temperatures tested for 15 min, then cooled down to room temperature before conducting the E24 experiment. To determine the stability of BS under various pH conditions, the pH of the BS solution was adjusted to 2–10 using 1N HCl or 1N NaOH. At that point, the E24 was determined. All experiments were carried out in triplicate.
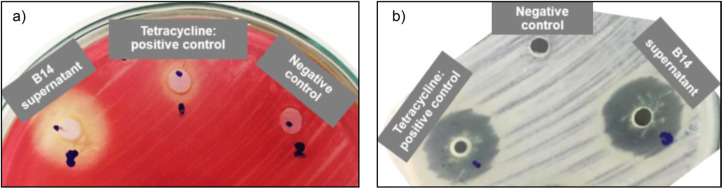
Determination of antimicrobial activities by the agar well diffusion method. Antimicrobial activities of cell-free supernatant from strain B14 were analyzed against Gram-positive and Gram-negative pathogenic bacterial strains, including Escherichia coli, Serratia marcescens, Pseudomonas aeruginosa, Staphylococcus aureus, Bacillus subtilis, and Bacillus cereus. These pathogenic strains were obtained from the Medical Culture Collection Center at the Department of Microbiology, Faculty of Medical Science, Khon Kaen University. The NB cultures of pathogenic bacteria grown overnight were diluted to OD600 = 0.1 (equivalent to 0.5 on the McFarland scale) and then swabbed onto Mueller-Hinton agar. Holes with 4 mm diameter were created using sterile cork borer (4 mm) and filled with 20 µl of B14 cell-free supernatant of the optimized cultures. Plates were incubated at 37°C for 24 hr. The zone of inhibition was determined by measuring a clear zone around the holes. Tetracycline (30 µg/ml) was used as a positive control.
Results
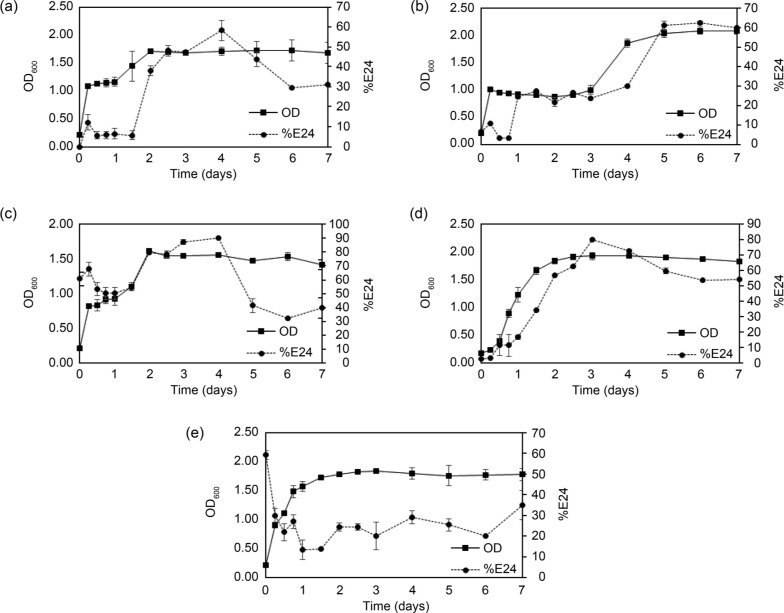
Effect of carbon sources on growth and biosurfactant production by strain B14. In order to determine a suitable carbon source for the growth of strain B14, we used MS medium where various types of carbon compounds were added as sole carbon substrates. These carbon compounds are glucose, sucrose, maltose, lactose, and glycerol. Fig. 1 shows that strain B14 could grow well on all tested carbon compounds. The biomass obtained after seven days was quite large and approximately the same in all cultures (OD600 ~ 1.5 – 2.0). It suggested that strain B14 can utilize various carbon compounds for its growth.
Fig. 1.
Growth of E. cloacae B14 using different carbon sources (solid lines with filled square markers) – (a) glucose; (b) sucrose; (c) maltose; (d) lactose and (e) glycerol. Emulsification activity (%E24) of cell-free supernatant obtained from those cultures are shown as dashed lines with filled circle markers. Error bars indicate standard deviations of triplicate data.
This work aimed to investigate the emulsification properties of BS, in particular, so the E24 test was mainly used to determine the presence of BS in the samples. The results also showed that strain B14 could produce BS with E24 higher than 50% in all cases. In particular, E24 reached the highest at 89% when strain B14 was grown on maltose. The emulsification activity in a control experiment where bacterial inoculum was not added was 50–60%. It is because maltose and glycerol themselves can assist the formation of emulsion such as in creamy food products and in soap, respectively (Li et al. 2013; Zhang et al. 2018). An increase of E24 in B14 culture grown with maltose was about 90%, which was significantly higher than those in control, and it could be due to microbial growth. Contrarily, in the case of glycerol, the E24 of the inoculated cultures decreased as cell biomass increased, suggesting that strain B14 consumed glycerol, and the E24 detected throughout the incubation period was not directly related to the growth of strain B14. Maltose- and lactose-derived BS showed the highest E24 within four days of incubation, while BS from other C-sources showed maximum E24 after five days of incubation. Also, the maximum E24 obtained when using other C-sources (approximately 60%) was much lower than the E24 from maltose (89%) and lactose (80%). Therefore, maltose was the best carbon source for B14 BS production and was used in the next experiment where nitrogen sources were varied.
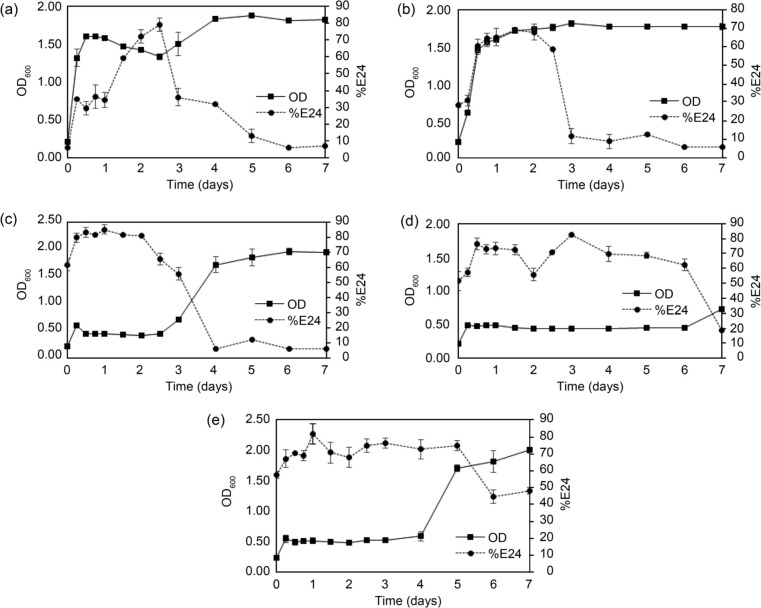
Effect of organic and inorganic nitrogen sources on growth and biosurfactant production. The effects of nitrogen sources on the growth of strain B14 when using maltose as a carbon source were investigated. The nitrogen sources used here are yeast extract, urea, NH4NO3, NH4Cl, and (NH4)2SO4.
Fig. 2 shows that strain B14 could grow well in almost all nitrogen sources (OD600 ~ 1.5 – 2.0), except NH4Cl (OD600 ~ 0.6). Also, the E24 of BS is almost the same in all cases (~ 70–90%). Both yeast extract and urea provided relatively high OD600 and the E24 within the shortest incubation time. Therefore, we further quantified the amount of BS in mg BS/g cell dry weight to determine the optimum nitrogen sources for BS production by E. cloacae B14 in the next experiment.
Fig. 2.
Growth of E. cloacae B14 on maltose using different nitrogen sources (solid lines with filled square markers) – (a) yeast extract; (b) urea; (c) NH4NO3; (d) NH4Cl and (e) (NH4)2SO4. Emulsification activity (%E24) of cell-free supernatant obtained from those cultures are shown as dashed lines with filled circle markers. Error bars indicate standard deviations of triplicate data.
Quantification of BS produced using maltose as a carbon source. In order to compare the BS yield, the weights of extracted BS, after being purified, were measured per gram of the B14 cell dry weight. The results shown in Fig. 3 indicated that B14 grown using yeast extract could produce the largest BS (39.8 ± 5.2 mg BS/g cell dry weight). It is most likely because yeast extract can serve as both carbon and nitrogen sources for cell growth and thus effectively produce BS.
Fig. 3.
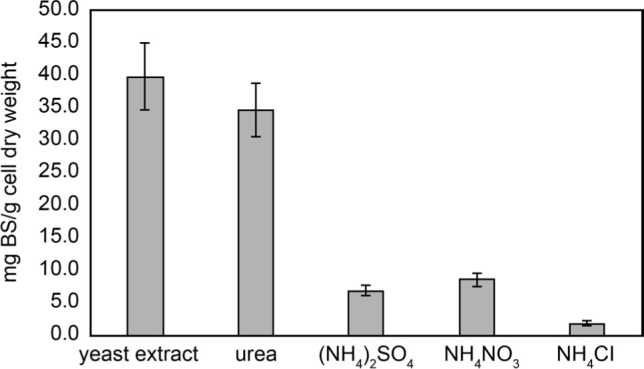
Biosurfactant yielded from E. cloacae B14 grown on maltose media with different nitrogen sources. Error bars indicate standard deviations of triplicate data.
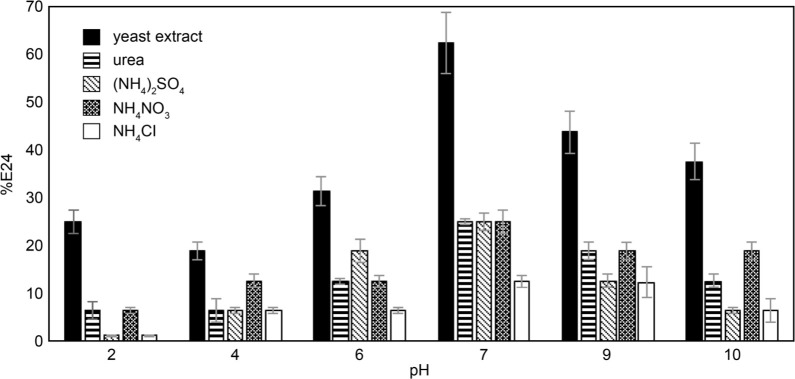
Stability of BS under a range of pH and temperatures. Cell-free supernatant of B14 grown in different media was tested for its stability to emulsify oil-water mixture under various pH and temperature conditions. All cell-free broth showed good emulsification activity under a wide range of pH (Fig. 4).
Fig. 4.
Stability of biosurfactant under a range of pH. Error bars indicate standard deviations of triplicate data.
The best activity was obtained when the pH of samples was maintained at the value of 7. It is because pH 7 facilitated the best growth of B14 and, therefore, is the most suitable working condition for its BS activity. Interestingly, the emulsification activity was still relatively high (up to 43%) even at alkaline pH (pH 9–10). It suggested the potential application of B14 BS in an alkaline environment. The highest E24 was still found when using yeast extract as a nitrogen source. Although the concentration of BS from urea- and yeast extract-containing media were relatively similar, their E24 during a range of pH exposure was different. It is probably because urea-derived BS did not behave as an emulsifier, while yeast extract-derived BS showed distinct characteristics. We remark that different nitrogen sources resulted in the production of BS with different emulsification activity is probably due to nutrient transport efficiency (Onwosi and Odibo 2012), but not exactly the types of biosurfactant produced. Zhang et al. (2016) reported that Bacillus atrophaeus 5–2a produced BS with different levels of emulsification activity when grown on different nitrogen sources. Khopade et al. 2012 suggested that some nitrogen sources affected the pH of the culture medium and resulted in a change in the efficiency of BS.
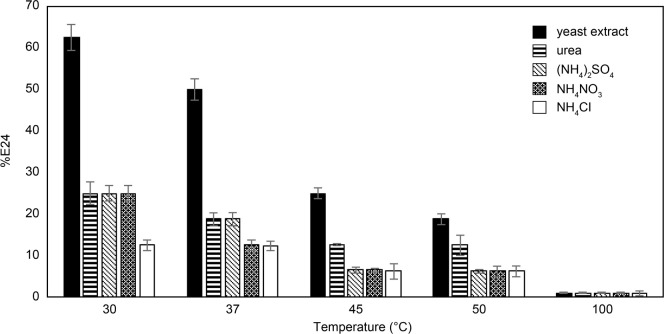
The results of BS stability, depending on temperature, are shown in Fig. 5. The E24 decreased with increasing temperature in all media. BS was still active when the temperature was increased to 37°C. However, the E24 was quite low at 100°C due to structural alteration in the BS molecules according to extreme temperature (Aparna et al. 2012). Cell-free supernatant from maltose-yeast extract cultures exhibited a significantly higher E24 than that from cells grown on other culture media. The highest %E24 (62.5 ± 3.2%) was obtained when tested at 30°C. According to these results (Fig. 1–5), we selected to use maltose and yeast extract as the optimum carbon and nitrogen sources for BS production by E. cloacae B14. The BS derived from this optimized medium was then used in the next experiments.
Fig. 5.
Stability of biosurfactant under a range of temperatures. Error bars indicate standard deviations of triplicate data.
Antimicrobial activities. The antimicrobial activity test was also investigated (Table I). In this work, we selected tetracycline as a positive control because it is a widely-used antibiotic with a broad spectrum against pathogenic bacteria. The activity of cell-free supernatant containing B14 BS was better against Gram-positive, showing clear zone diameters of 20.7–26.7 mm. In contrast, the activity against Gram-negative pathogenic bacteria was in a range of 9.7–17.0 mm. The cell-free broth showed more significant inhibition against B. subtilis (a clear zone diameter of 22.0 ± 1.8 mm) than the commonly-used antibiotic tetracycline (a clear zone diameter of 20.0 ± 1.1 mm) (Fig. 8b). Furthermore, the cell-free broth could inhibit the growth of a tetracycline-resistant strain of S. marcescens (Fig. 8a). It suggested the potential use of B14 biosurfactant for the production of antimicrobial products in the future.
Table I.
Antimicrobial activity of cell-free supernatant of E. cloacae B14 against Gram-positive and Gram-negative pathogens using 30 µg/ml tetracycline as a control. Standard deviations were calculated from the data obtained from triplicate experiments.
| Pathogenic bacteria | Inhibition zone diameter (mm) ± standard deviation | |
|---|---|---|
| Cell-free supernatant of B14 cultures | Tetracycline 30 μg/ml (control) | |
| Gram-negative bacteria | ||
| Escherichia coli | 12.3 ± 1.1 | 21.1 ± 0.5 |
| Pseudomonas aeruginosa | 17.0 ± 1.4 | 20.0 ± 0.7 |
| Serratia marcescens | 9.7 ± 1.5 | 0.0 ± 0.0 |
| Gram-positive bacteria | ||
| Bacillus cereus | 20.7 ± 2.0 | 30.0 ± 2.0 |
| Bacillus subtilis | 22.0 ± 1.8 | 20.0 ± 1.1 |
| Staphylococcus aureus | 26.7 ± 2.1 | 30.0 ± 1.8 |
Fig. 8.
Antimicrobial activity of B14 supernatant compared to tetracycline (positive control) and non-inoculated medium (negative control) – a) the activity against S. marcescens and b) the activity against B. subtilis.
Characterization of B14 biosurfactant by TLC. Purified BS was separated on a silica gel plate using chloroform : methanol : acetic acid = 65 : 15 : 2 as an eluting solvent (Fig. 6). On the TLC plate, BS fractions did not show a positive reaction with the ninhydrin solution. It indicated the absence of amino acid groups in the BS molecule. The dark brown spot corresponding to the Rf value of 0.95, which appeared after spraying phenol-H2SO4 reagent, confirmed the presence of carbohydrate fraction. A yellow spot (Rf value = 0.92), which appeared after exposure to iodine vapor, was attributed to lipid moiety of the BS molecular structure. Our TLC results revealed that B14 BS tends to be a glycolipid.
Fig. 6.
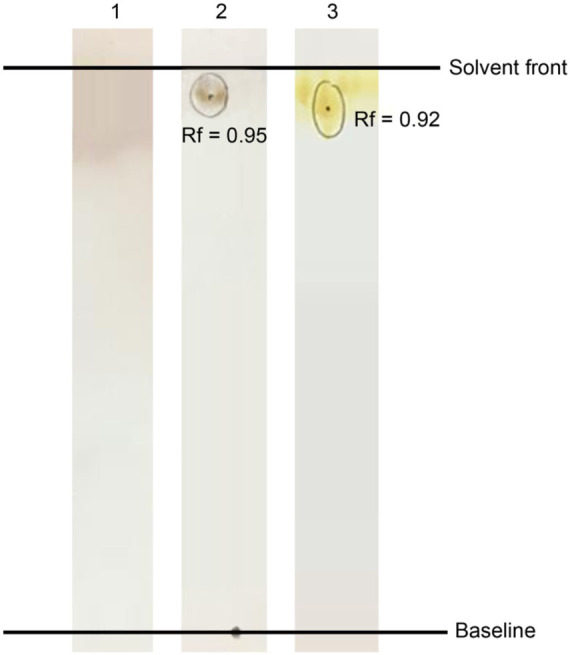
TLC analysis of the purified B14 BS obtained from an optimized culture. Samples on TLC plates were sprayed using ninhydrin solution (1); phenol-H2SO4 solution (2) and iodine vapor (3).
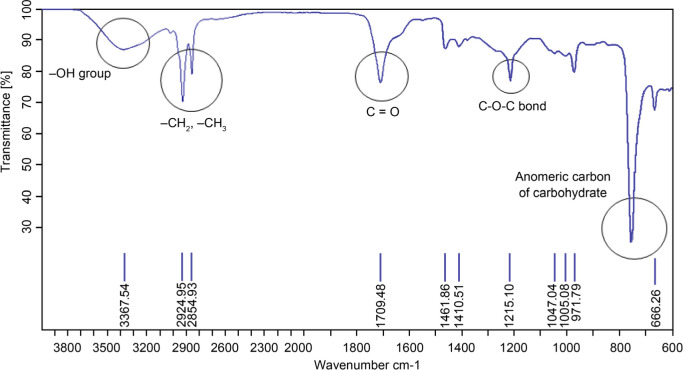
Characterization of B14 biosurfactant by using FTIR. Fig. 7 shows the FTIR spectrum of purified BS produced in maltose-yeast extract medium by E. cloacae B14. The spectrum illustrated a broad peak at 3,367.54 cm–1, indicating -OH stretching vibration of the hydroxyl group. The strong absorption spectrum at 2,924.95 and 2,854.93 cm–1 showed -CH2 and -CH3 bonds of hydrocarbon chains. A sharp peak at 1,709.48 cm–1 elucidates carbonyl groups of lipid moiety. A stretch signal appearing at 1,215.10 cm–1 indicates C-O-C bond (Jadhav et al. 2011). Finally, a characteristic peak appearing between 700 and 950 cm–1 was marked as anomeric carbon of the carbohydrate fingerprint (Fusconi et al. 2010). Similar absorption in FTIR spectra was reported as glycolipid-like BS (Derguine-Mecheri et al. 2018). Our FTIR analysis, combined with the TLC results, confirms that B14 BS is a glycolipid.
Fig. 7.
FTIR spectrum of the purified B14 biosurfactant.
Discussion
In our previous work (Ekprasert et al. 2019), we used spent engine oil as sole carbon and energy source for microbial growth and found the E. cloacae B14 BS activity to be 42.2%. In this recent work, the use of maltose and yeast extract as the carbon and nitrogen sources can increase the BS activity by up to 79% (Fig. 2), which is significantly higher than that of previous results. This activity was also higher than that of some other Enterobacter spp. such as strain SF-4 (40%) (Batool et al. 2017), strain LS1 (44%), strain CG101 (15%), and LS8 (50%) (Wong-Villarreal et al. 2016). The amount of BS obtained per gram cell dry weight was (39.8 ± 5.2) mg (Fig. 3). It suggested that water-soluble substrates were preferred to immiscible ones in strain B14, which is in agreement with some other hydrocarbon-utilizing bacteria. For example, in Pseudomonas putida SOL-10 isolated from an oil-contaminated soil, yeast extract was the preferable substrate for rhamnolipid production (Nwaguma et al. 2016). Likewise, Bacillus mojavensis A21 was capable of producing lipopeptides in higher concentrations of when grown in media containing glucose and yeast extract (Onwosi and Odibo 2012). However, some BS producers, such as Klebsiella pneumoniae IVN51, use dextrose and NH4NO3 as carbon and nitrogen sources (Nwaguma et al. 2016). The highest E24 obtained from strain IVN51 was still only 23.20 ± 1.41%, which is far lower than the E24 obtained from B14 BS in this study (79%). Additionally, rhamnolipid production in some Pseudomonas spp. was less when water-immiscible carbon sources like hydrocarbons, vegetable oil, and crude oil were used (Moya Ramírez et al. 2015; Varjani and Upasani 2016). A variety of BS producers, such as Pseudomonas nitroreducens (Onwosi and Odibo 2012), Candida lipolytica UCP0988 (Rufino et al. 2014), and Bacillus sp. (Joshi and Shekhawat 2014) showed a preference toward nitrate-based nitrogen sources, such as NH4NO3, for BS production. It is probably because the nitrogen content in this chemical was readily available for microbial growth and BS production. Note that, in our experiment, the high E24 was also obtained from NH4NO3 culture (Fig. 2), as in agreement with previous literature. Despite that, the best nitrogen source for B14 BS production was yeast extract, not NH4NO3, due to having the highest yield and better stability. Although in this work we only focused on effects of different types of C and N sources to cell growth and biosurfactant production, different concentrations of C or N sources will affect the microbial growth and the production of BS as well (e.g., Fontes et al. 2010), which is also worth investigating in the future.
The usefulness of BS in various fields depends on its stability under different temperatures and pH. Particularly, BS that can withstand a range of pH variation would be beneficial for antimicrobial coating agents because, for example, the pH of saliva varies depending on the patient’s diet (Sharma and Saharan 2016). We found that when pH was lower than 6, BS tends to precipitate due to its anionic nature, resulting in a decrease in emulsification activity. In this case, B14 BS became less stable under an acidic environment. Emulsion layers formed by B14 BS obtained when cells are grown on an optimum culture medium, were stable under a wide range of pH (2–10), and temperature (30–37°C). This result was in agreement with the glycolipid derived from Pseudomonas otitidis, isolated from a coal mine, whose E24 could still be detected when exposed to pH 3–11, and the temperature was as high as 80–100°C (Singh and Tiwary 2016). The stability studies on glycolipid BS derived from P. aeruginosa HAK01 indicated that this BS was able to function under a wide range of temperatures (40–121°C) and pH (4–10) (Khademolhosseini et al. 2019). Furthermore, an effective oil-degrading Rhodococcus erythropolis S67 produced glycolipid, which could function at a temperature as low as 10°C (Luong et al. 2018). Our findings suggested that glycolipid BS was able to withstand extreme pH, as it is consistent with other reports.
While there were many reports of E. cloacae strains producing lipopeptides (Mandal et al. 2013; Jemil et al. 2019), our TLC and FTIR results confirmed that B14 produced a glycolipid-type BS. It should be noted that there have been only a few cases of E. cloacae strains, which can produce glycolipid BS (Jadhav et al. 2011). In order to investigate our glycolipid potential application, antimicrobial activity of cell-free broth containing B14 BS derived from cultures grown on an optimized medium was tested. Although biosurfactant will typically be used in actual application in the form of pure compounds, the cell-free supernatant could be used as a pre-screening process for stability studies and antimicrobial tests (Singh and Tiwary 2016; Mouafo et al. 2018). At this stage, we then selected to use the cell-free supernatant from strain B14 for these studies, as it is also less time-consuming. We found that the broth could inhibit a wide range of bacterial pathogens. Higher activity was detected against Gram-positive versus Gram-negative ones. Notably, the cell-free broth could inhibit the growth of S. marcescens strain, which is not sensitive to tetracycline, a commonly used antibiotic. It also showed greater inhibition toward B. subtilis when compared to tetracycline. This biosurfactant’s possible antimicrobial mechanism is that it can bind to the phospholipid surface of the cytoplasmic membrane through electrostatic forces. With the help of water in the supernatant, biosurfactant then diffuses into the inner hydrophobic part of the membrane and weakens the lipid structure. It results in the leakage of essential molecules and the dissolution of the proton motive force (Sheppard et al. 1997; McDonnell et al. 1999). There was evidence that glycolipid tends to have higher specificity to inhibit the growth of Gram-positive pathogens. For example, B. cereus and S. aureus were sensitive to rhamnolipid (de Freitas Ferreira et al. 2019). We further discovered that the antimicrobial activity of B14 BS was not similar to that of some other E. cloacae strains. For example, the inhibitory activity of lipopeptide from E. cloacae C3 was more effective with Gram-negative than Gram-positive pathogens (Jemil et al. 2019). However, C3 lipopeptide did not inhibit the growth of B. cereus and E. coli; whereas, our BS has potent activity against them.
It should be noted that there were not many reports showing glycolipid BS that could inhibit the growth of antibiotic-resistant S. marcescens like what we found with the case of B14 BS. Our results suggested that a cell-free broth from B14 cultures can be used directly as an antimicrobial agent without purification of BS. It could significantly reduce the cost of BS production (Wong-Villarreal et al. 2016). This paper aimed to investigate emulsification activity because it is the major property that helps diminish biofilm formation by pathogens, and thus involves the antimicrobial activity of the compound. This work could be extended towards the study of the chemical structures of biosurfactant by, e.g., using nuclear magnetic resonance (NMR) and mass spectrometry (MS), so that the mode of action at molecular level could be determined.
Moreover, further investigation on the reduction of surface tension will provide us the information on critical micelle concentration (CMC) of the biosurfactant extracted of strain B14. A study on the synergistic effect of biosurfactant and broad-spectrum antibiotics would also be very interesting. All of which are planned for the future.
Conclusions
We investigated the effects of carbon and nitrogen sources for BS production by E. cloacae B14. It was found that E. cloacae B14 could grow well on various carbon and nitrogen sources. This finding suggests several advantages when maximizing BS production on an industrial scale. The optimum culture medium for producing the highest emulsification activity and yield of B14 BS was maltose-yeast extract medium. The produced BS was stable in a wide range of pH from 2–10 and a temperature range of 30–37°C. Antimicrobial activity assays suggested that cell-free broth containing B14 BS could inhibit various Gram-positive and Gram-negative pathogens. An inhibition effect of the broth against the growth of pathogens was more specific to Gram-positive than Gram-negative ones.
Interestingly, a tetracycline-resistant strain of Serratia marcescens was sensitive to B14 broth. The B14 cell-free broth can be used as an antimicrobial agent without purification steps, which could be economical for BS production in the industry. Our results then suggested that the B14 BS has the potential to apply not only for bioremediation but also for the production of antimicrobial products.
Acknowledgments
This research was partially supported by the Department of Microbiology, Faculty of Science, Khon Kaen University. We would like to thank Asst. Prof. Dr. Wiyada Mongkolthanarak for equipment support.
Footnotes
Conflict of interest
The authors do not report any financial or personal connections with other persons or organizations, which might negatively affect the contents of this publication and/or claim authorship rights to this publication.
ORCID
Jindarat Ekprasert https://orcid.org/0000-0002-9646-9078
Literature
- Aparna A, Srinikethan G, Smitha H. Production and characterization of biosurfactant produced by a novel Pseudomonas sp. 2B. Colloids Surf B Biointerfaces. 2012. June;95:23–29. 10.1016/j.colsurfb.2012.01.043 [DOI] [PubMed] [Google Scholar]
- Batool R, Ayub S, Akbar I. Isolation of biosurfactant producing bacteria from petroleum contaminated sites and their characterization. Soil Environ. 2017. May 28;36(01):35–44. 10.25252/SE/17/20992 [DOI] [Google Scholar]
- Bhadoriya SS, Madoriya N, Shakla K, Parihar MS. Biosurfactants: a new pharmaceutical additive for solubility enhancement and pharmaceutical development. Biochem Pharmacol (Los Angel). 2013;02(02):113 10.4172/2167-0501.1000113 [DOI] [Google Scholar]
- Cooper DG, Goldenberg BG. Surface-active agents from two bacillus species. Appl Environ Microbiol. 1987;53(2):224–229. 10.1128/AEM.53.2.224-229.1987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Freitas Ferreira J, Vieira EA, Nitschke M. The antibacterial activity of rhamnolipid biosurfactant is pH dependent. Food Res Int. 2019. February;116:737–744. 10.1016/j.foodres.2018.09.005 [DOI] [PubMed] [Google Scholar]
- Derguine-Mecheri L, Kebbouche-Gana S, Khemili-Talbi S, Djenane D. Screening and biosurfactant/bioemulsifier production from a high-salt-tolerant halophilic Cryptococcus strain YLF isolated from crude oil. J Petrol Sci Eng. 2018. March;162:712–724. 10.1016/j.petrol.2017.10.088 [DOI] [Google Scholar]
- Ekprasert J, Laopila K, Kanakai S. Biosurfactant production by a newly isolated Enterobacter cloacae B14 capable of utilizing spent engine oil. Pol J Environ Stud. 2019. April 9;28(4):2603–2610. 10.15244/pjoes/92120 [DOI] [Google Scholar]
- Fontes GC, Fonseca Amaral PF, Nele M, Zarur Coelho MA. Factorial design to optimize biosurfactant production by Yarrowia lipolytica. J Biomed Biotechnol. 2010;2010:1–8. 10.1155/2010/821306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fracchia L, Banat JJ, Cavallo M, Ceresa C, Banat IM. Potential therapeutic applications of microbial surface-active compounds. AIMS Bioeng. 2015;2(3):144–162. 10.3934/bioeng.2015.3.144 [DOI] [Google Scholar]
- Fusconi R, Maria Nascimento Assunção R, de Moura Guimarães R, Rodrigues Filho G, Eduardo da Hora Machado A. Exopolysaccharide produced by Gordonia polyisoprenivorans CCT 7137 in GYM commercial medium and sugarcane molasses alternative medium: FT-IR study and emulsifying activity. Carbohydr Polym. 2010. January;79(2):403–408. 10.1016/j.carbpol.2009.08.023 [DOI] [Google Scholar]
- Gharaei-Fathabad E. Biosurfactants in pharmaceutical industry (a mini-review). Am J Drug Discov Dev. 2011. January 1;1(1):58–69. 10.3923/ajdd.2011.58.69 [DOI] [Google Scholar]
- Gudiña EJ, Rangarajan V, Sen R, Rodrigues LR. Potential therapeutic applications of biosurfactants. Trends Pharmacol Sci. 2013. December;34(12):667–675. 10.1016/j.tips.2013.10.002 [DOI] [PubMed] [Google Scholar]
- Jadhav M, Kagalkar A, Jadhav S, Govindwar S. Isolation, characterization, and antifungal application of a biosurfactant produced by Enterobacter sp. MS16. Eur J Lipid Sci Technol. 2011. November; 113(11):1347–1356. 10.1002/ejlt.201100023 [DOI] [Google Scholar]
- Jahan R, Bodratti AM, Tsianou M, Alexandridis P. Biosurfactants, natural alternatives to synthetic surfactants: physicochemical properties and applications. Adv Colloid Interface Sci. 2020. January; 275:102061 10.1016/j.cis.2019.102061 [DOI] [PubMed] [Google Scholar]
- Jemil N, Hmidet N, Manresa A, Rabanal F, Nasri M. Isolation and characterization of kurstakin and surfactin isoforms produced by Enterobacter cloacae C3 strain. J Mass Spectrom. 2019. January;54(1):7–18. 10.1002/jms.4302 [DOI] [PubMed] [Google Scholar]
- Joshi PA, Shekhawat DB. Effect of carbon and nitrogen source on biosurfactant production by biosurfactant producing bacteria isolated from petroleum contaminated site. Adv Appl Sci Res. 2014; 5:159–164. [Google Scholar]
- Khademolhosseini R, Jafari A, Mousavi SM, Hajfarajollah H, Noghabi KA, Manteghian M. Physicochemical characterization and optimization of glycolipid biosurfactant production by a native strain of Pseudomonas aeruginosa HAK01 and its performance evaluation for the MEOR process. RSC Advances. 2019. March 11; 9(14):7932–7947. 10.1039/C8RA10087J [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khopade A, Biao R, Liu X, Mahadik K, Zhang L, Kokare C. Production and stability studies of the biosurfactant isolated from marine Nocardiopsis sp. B4. Desalination. 2012. January;285:198–204. 10.1016/j.desal.2011.10.002 [DOI] [Google Scholar]
- Li C, Fu X, Luo F, Huang Q. Effects of maltose on stability and rheological properties of orange oil-in-water emulsion formed by OSA modified starch. Food Hydrocoll. 2013. July;32(1):79–86. 10.1016/j.foodhyd.2012.12.004 [DOI] [Google Scholar]
- Luong TM, Ponamoreva ON, Nechaeva IA, Petrikov KV, Delegan YA, Surin AK, Linklater D, Filonov AE. Characterization of biosurfactants produced by the oil-degrading bacterium Rhodococcus erythropolis S67 at low temperature. World J Microbiol Biotechnol. 2018. February;34(2):20 10.1007/s11274-017-2401-8 [DOI] [PubMed] [Google Scholar]
- Mandal SM, Barbosa AEAD, Franco OL. Lipopeptides in microbial infection control: scope and reality for industry. Biotechnol Adv. 2013. March;31(2):338–345. 10.1016/j.biotechadv.2013.01.004 [DOI] [PubMed] [Google Scholar]
- McDonnell G, Russell AD. Antiseptics and disinfectants: activity, action, and resistance. Clin Microbiol Rev. 1999. January 01;12(1): 147–179. 10.1128/CMR.12.1.147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mouafo TH, Mbawala A, Ndjouenkeu R. Effect of different carbon sources on biosurfactants’ production by three strains of Lactobacillus spp. BioMed Res Int. 2018;2018:1–15. 10.1155/2018/5034783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moya Ramírez I, Tsaousi K, Rudden M, Marchant R, Jurado Alameda E, García Román M, Banat IM. Rhamnolipid and surfactin production from olive oil mill waste as sole carbon source. Bioresour Technol. 2015. December;198:231–236. 10.1016/j.biortech.2015.09.012 [DOI] [PubMed] [Google Scholar]
- Müller MM, Kügler JH, Henkel M, Gerlitzki M, Hörmann B, Pöhnlein M, Syldatk C, Hausmann R. Rhamnolipids – next generation surfactants? J Biotechnol. 2012. December;162(4):366–380. 10.1016/j.jbiotec.2012.05.022 [DOI] [PubMed] [Google Scholar]
- Nurfarahin A, Mohamed M, Phang L. Culture medium development for microbial-derived surfactants production – An overview. Molecules. 2018. May 01;23(5):1049 10.3390/molecules23051049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nwaguma IV, Chikere CB, Okpokwasili GC. Isolation, characterization, and application of biosurfactant by Klebsiella pneumoniae strain IVN51 isolated from hydrocarbon-polluted soil in Ogoniland, Nigeria. Bioresour Bioprocess. 2016. December;3(1):40 10.1186/s40643-016-0118-4 [DOI] [Google Scholar]
- Onwosi CO, Odibo FJC. Effects of carbon and nitrogen sources on rhamnolipid biosurfactant production by Pseudomonas nitroreducens isolated from soil. World J Microbiol Biotechnol. 2012. March;28(3):937–942. 10.1007/s11274-011-0891-3 [DOI] [PubMed] [Google Scholar]
- Qazi MA, Malik ZA, Qureshi GD, Hameed A, Ahmed S. Yeast extract as the most preferable substrate for optimized biosurfactant production by rhlB gene positive Pseudomonas putida SOL-10 isolate. J Bioremediat Biodegrad. 2013;4:204. [Google Scholar]
- Ranasalva N, Sunil R, Poovarasan G. Importance of biosurfactant in food industry. IOSR J Agric Vet Sci. 2014;7(5):06–09. 10.9790/2380-07540609 [DOI] [Google Scholar]
- Rane AN, Baikar VV, Ravi Kumar V, Deopurkar RL. Agro-industrial wastes for production of biosurfactant by Bacillus subtilis ANR 88 and its application in synthesis of silver and gold nanoparticles. Front Microbiol. 2017. March 24;8:492 10.3389/fmicb.2017.00492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rufino RD, de Luna JM, de Campos Takaki GM, Sarubbo LA. Characterization and properties of the biosurfactant produced by Candida lipolytica UCP 0988. Electron J Biotechnol. 2014. January;17(1):34–38. 10.1016/j.ejbt.2013.12.006 [DOI] [Google Scholar]
- Sekhon Randhawa KK, Rahman PKSM. Rhamnolipid biosurfactants – past, present, and future scenario of global market. Front Microbiol. 2014. September 02;5:454 10.3389/fmicb.2014.00454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma D, Saharan BS. Functional characterization of biomedical potential of biosurfactant produced by Lactobacillus helveticus. Biotechnol Rep (Amst). 2016. September;11:27–35. 10.1016/j.btre.2016.05.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheppard FC, Mason DJ, Bloomfield SF, Gant VA. Flow cytometric analysis of chlorhexidine action. FEMS Microbiol Lett. 1997. September;154(2):283–288. 10.1111/j.1574-6968.1997.tb12657.x [DOI] [PubMed] [Google Scholar]
- Shete AM, Wadhawa G, Banat IM, Chopade BA. Mapping of patents on bioemulsifier and biosurfactant: a review. J Sci Ind Res (India). 2006;65:91–115. [Google Scholar]
- Singh P, Tiwary BN. Isolation and characterization of glycolipid biosurfactant produced by a Pseudomonas otitidis strain isolated from Chirimiri coal mines, India. Bioresour Bioprocess. 2016. December; 3(1):42 10.1186/s40643-016-0119-3 [DOI] [Google Scholar]
- Sobrinho HBS, Luna JM, Rufino RD, Porto ALF, Sarubbo LA. Biosurfactants: classification, properties and environmental applications In: Govil JN. (editor). Recent development in biotechnology, vol.11 Houston (USA): Studium Press LLC; 2014. p. 303–330. [Google Scholar]
- Varjani SJ, Upasani VN. Carbon spectrum utilization by an indigenous strain of Pseudomonas aeruginosa NCIM 5514: Production, characterization and surface active properties of biosurfactant. Bioresour Technol. 2016. December;221:510–516. 10.1016/j.biortech.2016.09.080 [DOI] [PubMed] [Google Scholar]
- Walter V, Syldatk C, Hausmann R. Screening concepts for the isolation of biosurfactant producing microorganisms In: Sen R. (editor). Biosurfactants. Advances in Experimental Medicine and Biology, vol. 672 New York (USA): Springer; 2010. [DOI] [PubMed] [Google Scholar]
- Whittenbury R, Phillips KC, Wilkinson JF. Enrichment, isolation and some properties of methane-utilizing bacteria. J Gen Microbiol. 1970. May 01;61(2):205–218. 10.1099/00221287-61-2-205 [DOI] [PubMed] [Google Scholar]
- Wong-Villarreal A, Reyes-López L, Corzo-González H, Blanco-González C, Yáñez-Ocampo G. Characterization of bacteria isolation of bacteria from Pinyon rhizosphere producing biosurfactant from agro-industrial waste. Pol J Microbiol. 2016. June 7;65(2):183–189. 10.5604/17331331.1204478 [DOI] [PubMed] [Google Scholar]
- Wu JY, Yeh KL, Lu WB, Lin CL, Chang JS. Rhamnolipid production with indigenous Pseudomonas aeruginosa EM1 isolated from oil-contaminated site. Bioresour Technol. 2008. March;99(5):1157–1164. 10.1016/j.biortech.2007.02.026 [DOI] [PubMed] [Google Scholar]
- Zhang J, Xue Q, Gao H, Lai H, Wang P. Production of lipopeptide biosurfactants by Bacillus atrophaeus 5-2a and their potential use in microbial enhanced oil recovery. Microb Cell Fact. 2016. December;15(1):168 10.1186/s12934-016-0574-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang M, Yewe-Siang Lee Shee We M, Wu H. Direct emulsification of crude glycerol and bio-oil without addition of surfactant via ultrasound and mechanical agitation. Fuel. 2018. September;227:183–189. 10.1016/j.fuel.2018.04.099 [DOI] [Google Scholar]