Abstract
The COVID‐19 pandemic has caused tremendous suffering for patients with dementia and their caregivers. We conducted a survey to study the impact of the pandemic on patients with mild frontotemporal dementia (FTD). Our preliminary findings demonstrate that patients with FTD have significant worsening in behavior and social cognition, as well as suffer greater negative consequences from disruption to health‐care services compared to patients with AD. The reduced ability to cope with sudden changes to social environments places patients with FTD at increased vulnerability to COVID‐19 infection as well as to poorer clinical and social outcomes. Caregivers of FTD patients also demonstrate high burden during crisis situations. A proportion of patients with FTD benefitted from use of web‐based interactive platforms. In this article, we outline the priority areas for research as well as a roadmap for future collaborative research to ensure greatest benefit for patients with FTD and their caregivers.
Keywords: COVID‐19, frontotemporal dementia, research roadmap
1. INTRODUCTION
SARS‐CoV‐2 is a novel coronavirus that has rapidly spread to different parts of the world since its first report in December 2019. 1 , 2 This has led to the World Health Organization (WHO) declaring the coronavirus disease 2019 (COVID‐19) a pandemic in March 2020. As of July 18, 2020, >13 million confirmed cases and 591 000 confirmed deaths have been reported by the WHO. 3 In these unprecedented times, authorities around the world have instituted restrictions to curb the spread of COVID‐19. As such, these personal and social restrictions have significantly affected the lives of people all around the world.
However, the impact of the COVID‐19 pandemic is likely to be greater on vulnerable individuals, such as the elderly, people with chronic medical conditions, and persons with cognitive impairment and psychiatric diseases. For persons with dementia in general, concerns include increased risk of transmission of COVID‐19; higher likelihood of poor outcomes; and worsening of dementia due to lack of mental, social, and physical stimulation as a result of restrictions imposed by authorities worldwide. 4 , 5 , 6 , 7
2. FRONTOTEMPORAL DEMENTIA AND SPECIAL CONSIDERATIONS DURING THE COVID‐19 PANDEMIC
2.1. Spectrum of frontotemporal dementia
Frontotemporal dementia (FTD) is one of the most common causes of early‐onset dementia in people under the age of 65 years. 8 , 9 FTD is a phenotypically diverse disease and is classified into behavioral variant FTD (bvFTD) and language variant FTD (lvFTD) such as those with non‐fluent variant primary progressive aphasia and semantic variant primary progressive aphasia. 10 , 11 Patients with bvFTD display behavioral changes including disinhibition, loss of empathy, and apathy. In addition, they may also display perseverative and compulsive behaviors. 10 Patients with FTD display cognitive impairment including executive dysfunction, impaired social cognition, and progressive language difficulty such as agrammatism and loss of semantic knowledge. 12 , 13
2.2. Vulnerability of patients with FTD to COVID‐19
The clinical manifestations of FTD will likely make patients less able to comply with the social and personal distancing requirements imposed by the authorities, and infection control advice such as regular hand washing and wearing facemasks in the community. Perseverative, stereotyped, or compulsive behaviors may result in habits such as frequent touching of the nose or mouth or wandering out of the home environment, thus increasing risk of transmitting the infection. 14 Many of the behavioral and cognitive changes seen in FTD also increase the susceptibility of FTD patients to acquiring the COVID‐19 infection. Lack of regard to social distancing measures may put them into close proximity with infected individuals. Perseverative behaviors such as frequent touching of their faces may contribute to infections and improper use of facemasks.
RESEARCH IN CONTEXT
Systematic review: The COVID‐19 pandemic may have resulted in patients with frontotemporal dementia (FTD) suffering worsening of their clinical condition and their caregivers experiencing increased burden, given their wide‐ranging behavioral and cognitive deficits. We surveyed patients with mild FTD and mild Alzheimer's disease (AD) and share perspectives from specialists in the field.
Interpretation: Patients with FTD have significant worsening in behavior and social cognition and reacted adversely to disruption to health‐care services compared to AD. Their inability to cope with sudden changes to social environment increases their vulnerability to COVID‐19 infection, and poorer clinical and social outcomes. Caregivers of FTD demonstrate high burden during crisis situations. A proportion of patients with FTD benefitted from use of web based interactive platforms.
Future directions: We outline the priority areas for research as well as a roadmap for future collaborative research to ensure greatest benefit for patients with FTD and their caregivers.
2.3. Risk of worsening behavior and cognition
FTD is characterized by changes in behavior, including disinhibition, loss of empathy, apathy, and perseverative or compulsive behaviors. 10 Patients may also have repetitive behaviors. Some may also have psychotic symptoms including hallucinations and delusions. 15 These symptoms pose major challenges for patients and caregivers when having to live with the COVID‐19 pandemic. Disinhibition may result in patients confronting other members of the public who practice safe distancing measures and law enforcement officers or inappropriate and inconsiderate actions such as sneezing onto others. 16 Stereotypic behaviors may also result in over‐use of hand sanitizers and other cleaning substances. These stereotypic behaviors become incompatible with a highly restricted environment seen with the COVID‐19 pandemic. Anosognosia and accompanying lack of emotional concern may manifest as disregard of requirements for social distancing or use of facemasks.
In addition to behavioral changes, worsening of cognitive function such as deterioration in executive function and language further results in difficulty coping with social restrictions. 17 The frontal‐executive dysfunction and mental inflexibility characteristic of FTD patients significantly impairs their ability to adapt to changes in daily routine. Failure to learn and comply with new practices and rules mandated by pandemic control measures—be it in daily life, work, or leisure activities—may be interpreted as a decline in cognitive function by patients and caregivers. Semantic knowledge deficits in patients with primary progressive aphasia (PPA) may also reduce their ability to understand the concept of a pandemic and the rationales behind the various pandemic control measures. Language difficulties in PPA, such as a degraded word store in or agrammatism and apraxia of speech, may also impair patients’ ability to verbally communicate in novel circumstances. This combination of executive and language dysfunction may therefore lead to unique difficulties in performing instrumental activities of daily living (IADL). For example, executive dysfunction may not allow them to plan their requirements for essential supplies resulting in them leaving their home more than would be otherwise permitted. This can be further compounded by difficulty in communicating with enforcement personnel to express their special circumstances such as inability to queue up to obtain food or other necessities. These vulnerable patients, who may not have the mental and social flexibility to respond to the sudden change in environment, will find it particularly challenging to adhere to new social norms and COVID‐19–related regulatory measures. 18
Furthermore, by virtue of their behavioral changes and language impairment, patients with FTD are less likely to comply with restrictions and social regulations and thus they may inadvertently get into trouble with the law. Previous studies have demonstrated that between 33% and 37% of patients with bvFTD and 21% to 27% of patients with semantic variant FTD have committed law violations early in their course of the disease. 19 , 20 These acts of law violation are likely to increase in the setting of social and personal restrictions. Such behaviors are likely to be misinterpreted as flouting the law. Presence of language impairment such as difficulty with articulation or semantic deficits further compounds the problem.
2.4. Impact of COVID‐19 on caregivers of FTD
Among caregivers of people with various types of dementia, caregivers of patients with young onset dementia such as FTD have been shown to have the highest burden. 21 Caregivers of bvFTD patients report a higher presence and severity of neuropsychiatric symptoms and caregiver distress compared to caregivers of people with Alzheimer's disease (AD). 22 Caregivers of patients with young onset dementia such as FTD report 2.34 (95% confidence interval [CI]: 1.22–4.49: P = .010) higher odds of caregiver burden compared to late onset dementia. Risk factors for high caregiver burden in young onset dementia include family history of dementia and behavioral symptoms including disinhibited behavior, delusions, and apathy. 23 Cost of caring for patients with young onset dementia such as FTD is significantly higher than late onset dementia, which further compounds the burden of care in FTD. 24 , 25 These potential effects of COVID‐19 pandemic on FTD patients may place additional stress on caregivers who are already overburdened. 26
3. SURVEY OF PATIENTS WITH MILD FTD FOR PURPOSE OF HYPOTHESIS GENERATION
3.1. Survey methodology
To understand the extent to which patients with mild FTD and their caregivers were affected by the pandemic, we conducted a phone‐based survey using a structured questionnaire with 50 patients with mild FTD and 50 patients with mild AD dementia, matched for age and severity of cognitive impairment (see the Appendix). The aim of the survey was to identify major themes of particular concern to patient–caregiver dyads of FTD. The survey probed themes of behavior, cognition, disruption to health‐related services, coping mechanisms, and use of technology as an alternative platform to traditional caregiving. Findings from this preliminary survey were meant to allow for hypothesis generation for future studies in the field of FTD and COVID‐19. We additionally obtained opinions from international experts in FTD on strategies for further research into how FTD patients can be better prepared to meet challenges of future calamities.
3.2. Preliminary survey findings
Findings from the survey demonstrated that behavior symptoms significantly worsened in both FTD and AD with greater worsening in FTD (84% vs 58%, P = .008). The main behavioral issues in FTD included stereotypic behavior, apathy, and disinhibition (Figure 1). Patients also reported difficulty adapting to the social changes brought on by the pandemic such as planning their grocery shopping. Symptoms related to planning and judgment were observed in both FTD and AD patients and the difference was not statistically significant (84% vs 76%, P = ns). Patients with FTD had greater difficulty empathizing with the public when it came to measures such as when disabled individuals were allowed to join priority queues, suggesting that aspects of social cognition were significantly affected in FTD compared to AD (62% vs 16%, P < .001). Impaired social cognition has severe implications on ability of FTD patients to “care about” or “comply with” social personal restrictions. Thirty percent of FTD patients and 40% of AD patients in the survey were using web‐based platforms to stay connected to family, friends, and support groups (Figure 2). Web‐based platforms could be a strategy to help FTD and AD patients cope with future calamities. Patients with FTD reacted more adversely to disruption of care services compared to patients with AD (42% vs 20%, P = .012). Caregivers of both FTD and AD patients experienced severely increased caregiver burden with caregivers of FTD experiencing significantly higher burden (64% vs 42%, P = .023). As FTD mainly affects patients below 65 years, their caregivers (usually spouse) are thus also younger. Being young caregivers, often still in employment, adds an additional degree of stress to these caregivers. Thus, it is important that clinicians and public health authorities are made aware of the unique predicament that caregivers of patients of FTD find themselves in.
FIGURE 1.
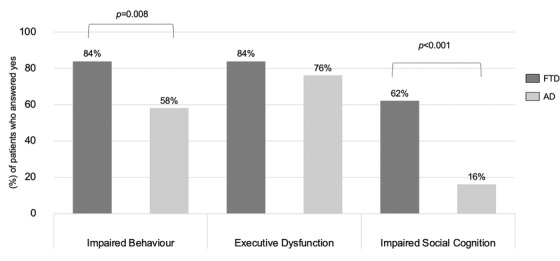
Response of FTD and AD patients to the COVID‐19 pandemic. Percentages of patients who answered yes to questions related to the categories of impaired behavior, executive dysfunction, and impaired social cognition. AD, Alzheimer's disease; FTD, frontotemporal dementia
FIGURE 2.
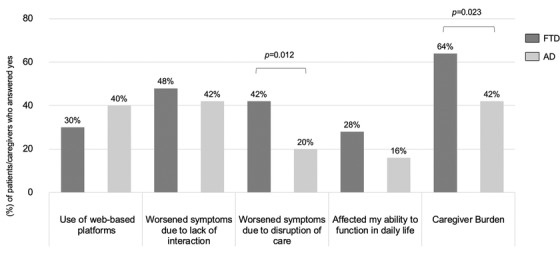
Impact of the COVID‐19 pandemic on patients and caregivers. Percentages of patients/caregivers who answered yes to questions related to the impact of COVID‐19 pandemic on their daily lives. AD, Alzheimer's disease; FTD, frontotemporal dementia
3.3. Limitation of the survey and remaining unanswered questions
The current survey is of relatively small sample size, limited to persons with mild dementia and as the FTD patients were recruited from a single center, lacks generalizability. The findings also suffer from limitations associated with phone surveys such as lack of objective measures and recall bias. However, the survey findings were meant to provide preliminary data for hypothesis generation for future prospective studies. Data on how FTD patients in other cultural and social settings respond to the pandemic is urgently needed. While the current findings demonstrate that behavior was severely affected, further research is required to understand which behavior subtypes worsened most, and if the worsening of behavior was due to worsening of specific pre‐existing behaviors or related to onset of new behaviors. Due to the relatively small sample size, we were not able to study if patients with bvFTD and lvFTD respond differentially to the pandemic and this should be addressed in a larger multicenter study. There is also need for data on FTD patients who were infected with COVID‐19 to clearly understand the consequences of COVID‐19 infection on clinical profile of FTD, general health, and mortality.
3.4. Patho‐anatomical and neurochemical basis for the preliminary findings
The finding of significantly greater worsening of behavior and social cognition in FTD compared to AD is likely related to the underlying pathobiology of FTD. Disinhibition, attributed to pathology in the right middle temporal and inferior frontal regions, may manifest as loss of manners, use of abusive language, and impulsivity. 27 Pathology in the prefrontal cortex among FTD patients has also been shown to result in significant lack of insight into their disease and additionally a lack of emotional concern about their behaviors. 28 , 29 , 30 In FTD, increased activity of dopaminergic neurotransmission and altered serotonergic modulation of dopaminergic neurotransmission is associated with agitated and aggressive behavior, respectively. 31 In bvFTD there is decreased dorsal salience network (DSN) connectivity, mainly involving the anterior cingulum, decreased ventral salience network connectivity in the basal ganglia, and divergent connectivity changes in the dorsolateral prefrontal cortex and precuneus within the right attention‐working memory network. 32 , 33
3.5. Implications of findings on care services, clinical management, and law‐enforcing authorities
The worsening behavior and cognition would result in caregivers being unable to care for their loved ones. This may necessitate the use of behavioral medications including anti‐psychotics with increased risk of side effects. There is also likely to be higher use of emergency medical services and hospital admissions, both of which are already overwhelmed by the pandemic. The inability to comply with pandemic‐related social and personal requirements could increase the risk of COVID‐19 infection, resulting in increased morbidity and mortality. The behavioral worsening may result in problems with the law such as not adhering to social distancing requirements and potentially criminal behavior.
4. RECOMMENDATIONS AND ROADMAP FOR THE FUTURE
4.1. New hypothesis and objectives
While the preliminary findings have provided a better understanding of the impact of COVID‐19 on FTD, further research is urgently needed to identify which subtype of FTD patients react more poorly to crisis situations. Studies would need to focus on FTD subtypes based on clinical phenotype as well as pathological subtypes. Research into neural mechanisms that result in a differential response between FTD and AD in a pandemic may allow for tailored interventional strategies for both FTD and AD. From a management perspective, studies examining the potential to reduce the adverse response of FTD patients to a crisis with the use of a multidisciplinary online platform that includes telehealth, virtual support groups, virtual mental health programs and virtual physical stimulation will be of high relevance. Similarly, studies looking into preparing caregivers of FTD patients to be “crisis ready” and resources for such a strategy will be needed.
4.2. Research strategy moving forward
There is a need for closer collaboration among clinicians worldwide as well as among clinicians, scientists, and public health authorities to better help patients with FTD and their caregivers cope with a crisis such as the COVID‐19 pandemic. With the threat of a second and third wave of infections, the need for these collaborations are urgent and relevant (Figure 3).
FIGURE 3.
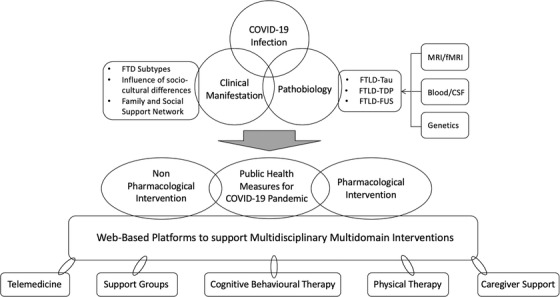
Roadmap toward multidisciplinary research on the impact of the COVID‐19 pandemic on frontotemporal dementia. A roadmap describing future research strategies toward closer collaboration among clinicians worldwide as well as among clinicians, scientists, and public health authorities to better help patients with FTD and their caregivers cope with a crisis such as the COVID‐19 pandemic. CSF, cerebrospinal fluid; fMRI, functional magnetic resonance imaging; FTD, frontotemporal dementia; FTLD, frontotemporal lobar degeneration; MRI, magnetic resonance imaging
These collaborative studies should focus on gathering large data at country, regional, or international settings. Focus on the impact of socio‐cultural differences on clinical manifestations during the pandemic will allow for better generalizability of findings. The use of state‐of‐the art fluid biomarkers, novel structural and functional neuroimaging, and artificial intelligence would allow for better insights into neural mechanisms accounting for the behavioral, cognitive, and social responses seen in FTD. With emerging data on cerebral complications post‐COVID‐19 including accelerated neuroinflammation, thrombosis, and damage to the blood brain barrier (BBB), longitudinal studies incorporating biomarkers for neuroinflammation (activated microglial, astrocytes, and cytokines), measures of BBB permeability (contrast enhanced imaging, cerebrospinal fluid immunoglobulin [Ig]G index) and vascular imaging, will shed more light on how COVID‐19 causes pathobiological changes in the central nervous system of patients with FTD. 34 Collaborative international efforts are needed to develop and study the role for a web‐based multidisciplinary platform that includes telehealth, virtual support groups, virtual mental health programs, and virtual physical stimulation. A concerted effort to bring together researchers in the field of medical technology under the auspices of an international organization such as the Alzheimer's Association or Consortium for Frontotemporal Dementia Research would enable development of a comprehensive digital platform for patients with FTD and other related dementias in various parts of the world in a rapid and comprehensive manner. Incorporating wearables and various home sensors for capturing a wide range of physiological parameters (for example: gait, sleep, blood pressure, pulse and heart rhythm, temperature, oxygen saturation), may also enable collection and analyses of big data and development of technologies to improve safety and rehabilitation for patients with FTD. 35 Concerted efforts by regional and international groups to analyze pooled FTD data would allow for development of a global resource pool for FTD patients worldwide.
5. CONCLUSIONS
Patients with FTD respond adversely to rapid changes to social environments with significant worsening in behavior and social cognition compared to patients with AD. Patients with FTD also suffer more when there is disruption to care services. Caregivers of patients with FTD experience greater caregiver burden compared to caregivers of patients with AD. Based on the preliminary findings, we outline the areas for future research and a roadmap to strategize research into FTD in the setting of a crisis. Such a concerted effort is likely to benefit FTD patients worldwide and their caregivers not only during the ongoing prolonged COVID‐19 pandemic but also for other similar crisis situations which the world is witnessing more frequently.
CONFLICT OF INTEREST
The authors report no conflicts of interest.
AUTHOR CONTRIBUTIONS
Kok Pin Ng: design of the study, analysis of data, drafting the manuscript and figures. Hui Jin Chiew: drafting the manuscript and figures. Shahul Hameed: drafting the manuscript and figures. Simon Kang Seng Ting: drafting the manuscript and figures. Adeline Ng: drafting the manuscript and figures. See Ann Soo: collection and analysis of data, drafting the manuscript and figures. Benjamin Wong: collection and analysis of data, drafting the manuscript and figures. Levinia Lim: collection and analysis of data, drafting the manuscript and figures. Alisa C. W. Yong: drafting the manuscript and figures. Vincent C.T. Mok: drafting the manuscript and figures. Pedro Rosa‐Neto: drafting the manuscript and figures. Jacqueline Dominguez: drafting the manuscript and figures. SangYun Kim: drafting the manuscript and figures. Robin G. Y. Hsiung: drafting the manuscript and figures. Manabu Ikeda: drafting the manuscript and figures. Bruce L. Miller: drafting the manuscript and figures. Serge Gauthier: drafting the manuscript and figures. Nagaendran Kandiah: conception and design of the study, drafting and critical review of the manuscript.
ACKNOWLEDGMENTS
We pay tribute to all health‐care staff, front‐line staff, and caregivers for their non‐wavering commitment to patients with dementia worldwide during the ongoing COVID‐19 pandemic. We also thank Dr Zaven Khachaturian and Dr Ara Khachaturian for guiding us on the scope of this article and Professor Martin Rossor for his contributions toward this manuscript.
APPENDIX 1.
METHODS
Study design and participants
Patients with mild FTD and mild AD dementia matched in age and severity of cognitive impairment were recruited from a tertiary neurology memory clinic. FTD diagnosis was made using the International consensus research criteria 10 , 11 while AD diagnosis was made using the Diagnostic and Statistical Manual of Mental Disorders 5th edition (DSM‐5) and National Institute on Aging‐Alzheimer's Assocation (NIA‐AA) criteria. 36 , 37 Patients with other concomitant severe systemic or neurological diseases and primary neuropsychiatric diseases were excluded. A structured questionnaire was administered to all patients and their caregivers via a phone interview by a team of psychologists (SAS, BW, LL).
Development of structured questionnaire
A structured questionnaire focusing on patient's cognitive and behavioral response to the COVID‐19 pandemic, and the impact of COVID‐19 pandemic on patients and their caregivers was developed. The questionnaire consisted of the following sections: General Information (eg, relationship between patient and caregiver, age of caregiver, disruption of medical care and treatment, worsening of dementia symptoms); Mood and Quality of Life (adapted from General Anxiety Disorder‐7 [GAD‐7] and Patient Health Questionnaire‐9 [PHQ‐9], and Quality of Life Enjoyment and Satisfaction Questionnaire Short Form [Q‐LES‐Q‐SF], respectively); and Compliance and Stressors Related to Authorities’ Regulations (eg, compliance to wearing a facemask or frequent hand washing; anxiety over the shortage of basic supplies). 38 , 39 , 40 To measure caregiving stress and burden, the team also incorporated questions from the Zarit Burden Interview into the questionnaire. 41 Finally, caregivers’ opinion toward certain topics was solicited (eg, online counseling and medical consultation services, home‐based exercise regimes). The original version of the questionnaire was administered to five patients and three experts (two neurologists and one psychologist) and further modified based on the feedback received. The final version of the questionnaire was then administered to the patients and caregivers.
During the interview, each patient–caregiver dyad was first asked to describe if there were changes to their dementia medications or if they had experienced any other major event (such as hospitalization) during the pandemic. The subsequent components of the questionnaire were only administered to patients who were on stable dosages of cognitive enhancers, behavioral medications, and not having experienced other significant events that may result in behavioral‐cognitive change. The question regarding the impact of COVID‐19 on behavior was presented so as to elicit if there was a change after COVID‐19–related measures were instituted compared to pre‐COVID‐19 social measures. Using the questionnaire, we evaluated the patient's subjective symptoms in the domains of memory, executive function, language, social cognition, mood, and behavior in response to the COVID‐19 pandemic. The questionnaire also evaluated whether the patient's symptoms worsened due to disruption of their usual health and social activities by the COVID‐19 pandemic, such as their interactions with family and friends, therapy sessions, medical appointments, and their means to perform their activities of daily living. We evaluated the patient's ability to adopt web‐based platforms to maintain their usual communications with family and friends in response to the personal and social restrictions. Last, the impact of COVID‐19 pandemic on caregivers was also assessed.
Input from domain experts on strategies to reduce impact on patients and caregivers
Specialists in the field of FTD who have ongoing collaborations with the National Neuroscience Institute, Singapore were invited to share their perspectives on what measures could be taken to reduce the impact of the COVID‐19 pandemic on FTD patients. Specialists were also asked to share potential strategies for concerted research toward finding solutions to assist patients with FTD cope with future crisis.
Statistical analyses
Statistical analyses were performed using Statistical Package for Social Sciences (SPSS) version 26 (SPSS, Inc.; Chicago, Illinois, USA). Descriptive statistics and frequency of baseline characteristics were summarized and compared between FTD and AD groups using two independent samples t‐test and chi‐square test for continuous and categorical variables respectively. Multiple binary logistic regression was performed to investigate the association of questionnaire responses with FTD and AD groups, controlling for age and years of education. Statistical significance was set at P < .05.
Standard protocol approvals, registrations, and patient consents
The study was approved by the Singhealth Centralized Review Board. Informed consent was obtained from each patient according to Declaration of Helsinki and local clinical research regulations.
RESULTS
One hundred patients (50 mild FTD and 50 mild AD) were included in this study and the baseline demographics of the study cohort and their caregivers are summarized in Table 1. The FTD patients included 37 with bvFTD and 13 with lvFTD. There were no statistical differences in the demographics, Mini‐Mental State Examination (MMSE) scores and disease duration between FTD and AD (Table 1).
TABLE 1.
Baseline demographics and sample characteristics
| Characteristics | FTD (n = 50) | AD (n = 50) | P value |
|---|---|---|---|
| Age in years, mean (SD) | 65.02 (5.86) | 66.57 (7.73) | .261 |
| Years of education, mean (SD) | 9.37 (4.24) | 9.36 (4.49) | .993 |
| Male, n (%) | 19 (38.0) | 24 (48.0) | .313 |
| MMSE, mean (SD) | 17.96 (6.54) | 19.26 (3.42) | .224 |
| Disease duration in years, mean (SD) | 4.60 (3.33) | 3.44 (1.99) | .037 |
| Spouse as main caregiver, n (%) | 30 (60.0) | 32 (65.3) | .549 |
| Age of caregiver in years, mean (SD) | 55.92 (14.11) | 54.57 (15.18) | .648 |
Abbreviations: AD, Alzheimer's dementia; FTD, frontotemporal dementia; MMSE, Mini‐Mental State Examination; SD, standard deviation.
In the section of the questionnaire that evaluated patient's cognitive and behavioral response to the COVID‐19 pandemic, a greater proportion of FTD patients compared to AD patients reported worsening of behavioral symptoms (84% vs 58%, P = .008) and worsening social cognition (62% vs 16%, P < .001), Figure 1. Both FTD and AD patients reported worsening of executive function, though the difference was not statistically significant (84% vs 76%, P = ns). More patients with AD reported worsening of memory compared to FTD, though the difference was not statistically significant (34% vs 24%, P = ns).
In the section of the questionnaire that evaluated the impact of the COVID‐19 pandemic on FTD and AD patients, a greater proportion of FTD patients compared to AD patients reported that their symptoms worsened due to disruption of their usual health care, such as medical appointments and therapy sessions (42% vs 20%, P = .012), Figure 2. A greater proportion of caregivers of FTD patients compared to caregivers of AD patients reported increased burden as a result of the COVID‐19 pandemic (64% vs 42%, P = .023). Thirty percent of FTD patients and 40% of AD patients reported that they were able to use web‐based platforms to maintain their usual communications with family, friends, and support services.
Ng KP, Chiew HJ, Hameed S, et al. Frontotemporal dementia and COVID‐19: Hypothesis generation and roadmap for future research. Alzheimer's Dement. 2020;6:e12085 10.1002/trc2.12085
REFERENCES
- 1. Zhu N, Zhang D, Wang W, et al. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 2020;382(8):727‐733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497‐506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. World Health Organization . Coronavirus disease (COVID‐19) pandemic. 2020. https://www.who.int/emergencies/diseases/novel-coronavirus-2019.
- 4. Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID‐19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395(10229):1054‐1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Williamson E, Walker AJ, Bhaskaran KJ, et al. OpenSAFELY: factors associated with COVID‐19‐related hospital death in the linked electronic health records of 17 million adult NHS patients. MedRxiv. 2020. [Google Scholar]
- 6. Aggarwal G, Lippi G, Michael Henry B. Cerebrovascular disease is associated with an increased disease severity in patients with Coronavirus Disease 2019 (COVID‐19): a pooled analysis of published literature. Int J Stroke. 2020;15(4):385‐389. [DOI] [PubMed] [Google Scholar]
- 7. Cesari M, Proietti M. COVID‐19 in Italy: ageism and decision making in a pandemic. J Am Med Dir Assoc. 2020;21(5):576‐577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Masellis M, Sherborn K, Neto PR, et al. Early‐onset dementias: diagnostic and etiological considerations. Alzheimer's Res Ther. 2013;5(suppl 1):S7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Rosso SM, Kaat LD, Baks T, et al. Frontotemporal dementia in The Netherlands: patient characteristics and prevalence estimates from a population‐based study. Brain. 2003;126(Pt 9):2016‐2022. [DOI] [PubMed] [Google Scholar]
- 10. Rascovsky K, Hodges JR, Knopman D, et al. Sensitivity of revised diagnostic criteria for the behavioural variant of frontotemporal dementia. Brain. 2011;134:2456‐2477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Gorno‐Tempini ML, Hillis AE, Weintraub S, et al. Classification of primary progressive aphasia and its variants. Neurology. 2011;76:1006‐1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Grossman M. The non‐fluent/agrammatic variant of primary progressive aphasia. Lancet Neurol. 2012;11:545‐555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Bonner MF, Ash S, Grossman M. The new classification of primary progressive aphasia into semantic, logopenic, or nonfluent/agrammatic variants. Curr Neurol Neurosci Rep. 2010;10(6):484‐490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Shigenobu K, Ikeda M, Fukuhara R, et al. The stereotypy rating inventory for frontotemporal lobar degeneration. Psychiatry Res. 2002;110(2):175‐187. [DOI] [PubMed] [Google Scholar]
- 15. Snowden JS, Rollinson S, Thompson JC, et al. Distinct clinical and pathological characteristics of frontotemporal dementia associated with C9ORF72 mutations. Brain. 2012;135(Pt 3):693‐708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Pressman PS, Miller BL. Diagnosis and management of behavioral variant frontotemporal dementia. Biol Psychiatry. 2014;75(7):574‐581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hornberger M, Piguet O, Kipps C, Hodges JR. Executive function in progressive and nonprogressive behavioral variant frontotemporal dementia. Neurology. 2008;71(19):1481‐1488. [DOI] [PubMed] [Google Scholar]
- 18. Eslinger PJ, Biddle KR. Prefrontal cortex and the maturation of executive functions, cognitive expertise, and social adaptation. Exec Funct Front Lobes A Lifesp Perspect. 2011. [Google Scholar]
- 19. Liljegren M, Naasan G, Temlett J, et al. Criminal behavior in frontotemporal dementia and Alzheimer disease. JAMA Neurol. 2015;72(3):295‐300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Shinagawa S, Shigenobu K, Tagai K, et al. Violation of laws in frontotemporal dementia: a multicenter study in Japan. J Alzheimer's Dis. 2017;57(4):1221‐1227. [DOI] [PubMed] [Google Scholar]
- 21. Liu S, Liu J, Wang XD, et al. Caregiver burden, sleep quality, depression, and anxiety in dementia caregivers: a comparison of frontotemporal lobar degeneration, dementia with Lewy bodies, and Alzheimer's disease. Int Psychogeriatrics. 2018;30(8):1131‐1138. [DOI] [PubMed] [Google Scholar]
- 22. Lima‐Silva TB, Bahia VS, Carvalho VA, et al. Neuropsychiatric symptoms, caregiver burden and distress in behavioral‐variant frontotemporal dementia and Alzheimer's disease. Dement Geriatr Cogn Disord. 2015;40(5‐6):268‐275. [DOI] [PubMed] [Google Scholar]
- 23. Lim L, Zhang A, Lim L, et al. High caregiver burden in young onset dementia: what factors need attention? J Alzheimer's Dis. 2017;61:537‐543. [DOI] [PubMed] [Google Scholar]
- 24. Kandiah N, Wang V, Lin X, et al. Cost related to dementia in the young and the impact of etiological subtype on cost. J Alzheimer's Dis. 2015;49:277‐285. [DOI] [PubMed] [Google Scholar]
- 25. Galvin JE, Howard DH, Denny SS, Dickinson S, Tatton N. The social and economic burden of frontotemporal degeneration. Neurology. 2017;89(20):2049‐2056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Koyama A, Hashimoto M, Fukuhara R, et al. Caregiver burden in semantic dementia with right‐and left‐sided predominant cerebral atrophy and in behavioral‐variant frontotemporal dementia. Dement Geriatr Cogn Dis Extra. 2018;8(1):128‐137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. O'Connor C, Landin‐Romero R, Clemson L, et al. Behavioral‐variant frontotemporal dementia: distinct phenotypes with unique functional profiles. Neurology. 2017;89(6):570‐577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Mendez MF, Shapira JS. Loss of emotional insight in behavioral variant frontotemporal dementia or “frontal anosodiaphoria”. Conscious Cogn. 2011;20(4):1690‐1696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Arroyo‐Anlló EM, Bouston AT, Fargeau MN, Orgaz Baz B, Gil R. Self‐consciousness in patients with behavioral variant frontotemporal dementia. J Alzheimer's Dis. 2016;49(4):1021‐1029. [DOI] [PubMed] [Google Scholar]
- 30. Baez S, Pinasco C, Roca M, et al. Brain structural correlates of executive and social cognition profiles in behavioral variant frontotemporal dementia and elderly bipolar disorder. Neuropsychologia. 2019;126:159‐169. [DOI] [PubMed] [Google Scholar]
- 31. Engelborghs S, Vloeberghs E, Le Bastard N, et al. The dopaminergic neurotransmitter system is associated with aggression and agitation in frontotemporal dementia. Neurochem Int. 2008;52(6):1052‐1060. [DOI] [PubMed] [Google Scholar]
- 32. Filippi M, Agosta F, Scola E, et al. Functional network connectivity in the behavioral variant of frontotemporal dementia. Cortex. 2013;49(9):2389‐2401. [DOI] [PubMed] [Google Scholar]
- 33. Agosta F, Sala S, Valsasina P, et al. Brain network connectivity assessed using graph theory in frontotemporal dementia. Neurology. 2013;81(2):134‐143. [DOI] [PubMed] [Google Scholar]
- 34. von Weyhern CH, Kaufmann I, Neff F, Kremer M. Early evidence of pronounced brain involvement in fatal COVID‐19 outcomes. Lancet. 2020;395(10241):e109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Bossen A, Kim H, Steinhoff A, Strieker M, Williams K. Emerging roles for telemedicine and smart technologies in dementia care. Smart Homecare Technol TeleHealth. 2015;3:49‐57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Diagnostic and Statistical Manual of Mental Disorders: DSM‐5. 5th ed., American Psychiatric Association, 2013.
- 37. McKhann GM, Knopman DS, Chertkow H, et al. The diagnosis of dementia due to Alzheimer's disease: recommendations from the National Institute on Aging‐Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimer's Dement. 2011;7:263‐269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Kroenke K, Spitzer RL, Williams JBW. The PHQ‐9: validity of a brief depression severity measure. J Gen Intern Med. 2001;16(9):606‐613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Spitzer RL, Kroenke K, Williams JBW, Löwe B. A brief measure for assessing generalized anxiety disorder: the GAD‐7. Arch Intern Med. 2006;166(10):1092‐1097. [DOI] [PubMed] [Google Scholar]
- 40. Stevanovic D. Quality of life enjoyment and satisfaction questionnaire ‐ short form for quality of life assessments in clinical practice: a psychometric study. J Psychiatr Ment Health Nurs. 2011;18(8):744‐750. [DOI] [PubMed] [Google Scholar]
- 41. Bédard M, Molloy DW, Squire L, Dubois S, Lever JA, O'Donnell M. The Zarit Burden Interview: a new short version and screening version. Gerontologist. 2001;41(5):652‐657. [DOI] [PubMed] [Google Scholar]


