Since the initial discovery of a pressor activity in kidney homogenates to its isolation and identification almost 100 years later, the protease renin constitutes a critical step in the activation of the renin-angiotensin (RAS) cascade leading to the generation of angiotensin II (Ang II) and stimulation of the ACE-Ang II-AT1 receptor (AT1R) axis. Renin is synthesized initially as a proenzyme (prorenin) in which the N-terminal region occupies the active site of the enzyme to maintain an inactive state. Processing of prorenin occurs in secretory vesicles within renal juxtaglomerular (JG) cells by an unidentified protease that cleaves the proenzyme to generate active renin. The JG cells release both prorenin and renin in essentially a fixed ratio as prorenin does not undergo additional proteolytic processing to its active form in the blood. Circulating levels of prorenin generally exceed that of renin and the levels may further increase under various conditions including pregnancy and diabetes. Nguyen et al.1 identified a key piece of the prorenin puzzle by demonstrating a cellular protein, termed the prorenin receptor (PRR) that binds prorenin and renin or (pro)renin at low nanomolar (nM) affinity. Binding of prorenin to the PRR induces a conformational change in the protease that fully activates renin. Importantly, the PRR does not cleave prorenin and the disassociation of prorenin from PRR would revert the enzyme to its original inactive state.
Apart from binding and inducing prorenin activity, the PRR may also serve as a functional receptor for the (pro)renin ligand. Binding of (pro)renin to PRR stimulates fibrotic and inflammatory signaling mediated by MAP kinase activation that may be independent of Ang II, although these cell signaling events by (pro)renin through the PRR appears to parallel the Ang II-AT1R axis 2. However, several aspects of the functional role of the PRR and its relationship to (pro)renin have remained controversial 2, 3. First, the PRR is not a unique protein, but is identical to the ATPase accessory protein 2 (ATP6ap2) of the hydrogen ion or vacuolar ATPase pump (V-ATPase) that is essential for lysosomal acidification, maintenance of cellular pH and other cell functions 2, 3. In this regard, the embryonic lethality of the PRR knockout models likely reflects the perturbation of V-ATPase assembly and activity rather than directly impacting the activation of prorenin. A second issue is that the majority of PRR appears to be localized intracellularly raising the question as to how extracellular (pro) renin accesses intracellular PRR 3. The apparent mismatch between circulating (pro)renin and the intracellular PPR may be reconciled by evidence of a secreted form termed the soluble PRR (sPRR) 4. The sPRR is present in blood, urine, and CSF, as well as shed from endothelial, proximal tubule and intercalating cells of the collecting duct 2, 4, 5. Although the sPRR retains the capacity to bind prorenin that may lead to the generation of Ang II, the receptor-like actions of the PRR mediated by (pro)renin are likely absent in the sPRR (Figure 1).
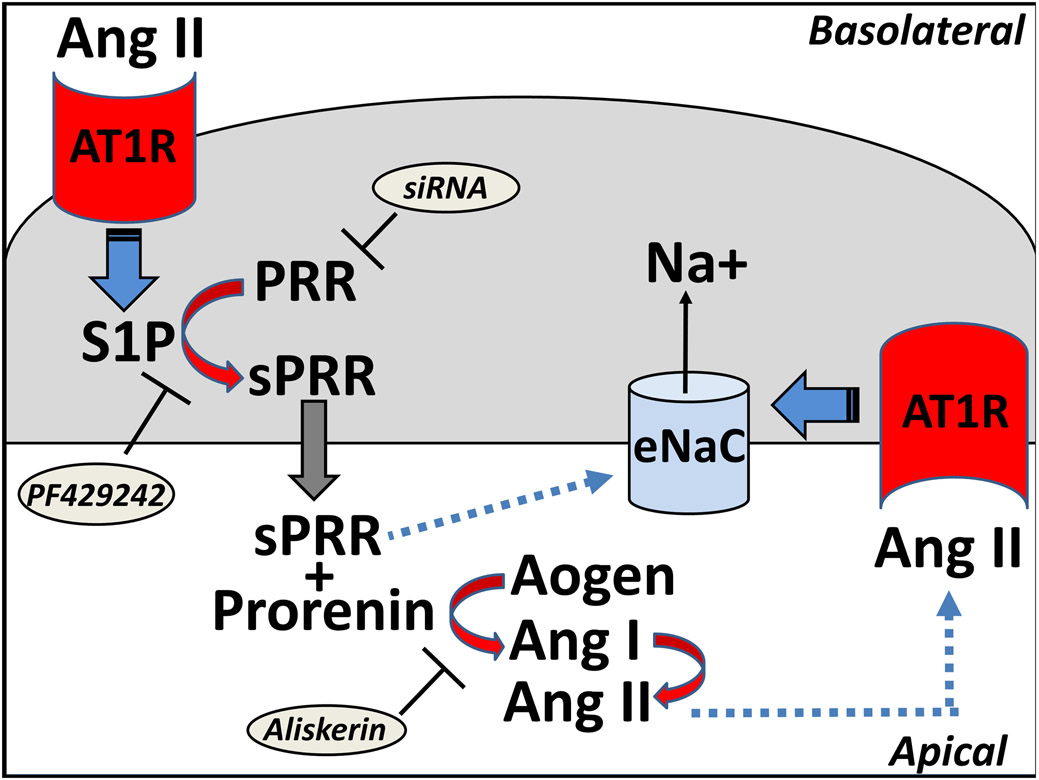
Figure 1.
Putative pathway for the site 1 protease (S1P)-mediated release of the soluble (pro)renin receptor (sPRR) and activation of the epithelial sodium channel (eNaC) in collecting duct cells. Angiotensin II (Ang II) infusion promotes activation of the basolateral AT1 receptors (AT1R) that stimulates the S1P to convert the PRR to the sPRR intracellularly. The sPRR is released into the tubular fluid to bind prorenin and stimulate conversion of angiotensinogen (Aogen) to Ang I and then Ang II by ACE. Increased levels of Ang II may bind the apical AT1R to stimulate eNaC and sodium reabsorption that facilitate an increase in blood pressure. The sPRR or sPRR-(pro)renin complex potentially may directly influence eNaC expression independent of, or in addition to the Ang II-AT1R pathway. The S1P inhibitor PF429242 blocks the release of sPRR, siRNA to PRR attenuates expression of PRR and sPRR, and the (pro)renin inhibitor aliskerin blocks conversion of Aogen to Ang I.
In the current issue of Hypertension, Feng et al.5 demonstrate a significant role of the renal sPRR in Ang II-dependent hypertension that is mediated by the site-1 protease (S1P) and the activation of the epithelial sodium channel (eNaC). This complex pathway is proposed in Figure 1 in which infused Ang II binds to the basolateral AT1R to stimulate S1P-dependent conversion to the sPRR and its apical release into the tubular fluid. The sPRR may associate with (pro)renin to increase local levels of Ang II that bind to the apical AT1R and stimulate eNaC to increase blood pressure. Moreover, the sPRR or sPRR-(pro)renin may potentially have direct effects to stimulate eNaC (Figure 1). This comprehensive study focuses on the role of S1P to mitigate the actions of sPRR.in Ang II-dependent hypertension using the selective S1P inhibitor PF-429242, siRNA against the PRR and the (pro)renin inhibitor aliskerin (Figure 1). Although the proteases furin and ADAM19 were originally reported to shed the sPRR, recent studies support a role for the intracellular protease S1P to release sPRR from proximal tubule and collecting duct cells of the kidney 2, 4, 5. The molecular forms of PRR and sPRR differ by ~10 kDa and are distinguished by Western blot with a PRR antibody while sPRR levels are quantified in the urine or the cell media by a PRR ELISA 5. The current study reveals that chronic administration of PF-429242 attenuates the Ang II-induced increase in mean arterial pressure assessed by telemetry, as well as the elevated levels of sPRR in the urine of male mice 5. The co-administration of recombinant sPRR reversed the decline in blood pressure by the S1P inhibitor supporting the specificity of the response. Although Ang II or the increase in blood pressure attenuates the release of prorenin and renin into the circulation, urinary levels prorenin and renin are increased reflecting positive feedback on the collecting duct that may in part contribute to the sustained increase in blood pressure by Ang II. Moreover, Ang II infusion increased the urinary levels of angiotensinogen suggesting that the required components of the RAS contribute to the local generation of Ang II. The present study finds that the S1P inhibitor also reduced both urinary angiotensinogen and renin and prorenin levels in the urine and renal medulla, but not the renal cortex 5. The study extends previous reports in that the administration of the S1P inhibitor reduced the Ang II-induced increase in both mRNA and protein levels of the α-subunit of ENaC in the renal medulla consistent with the chronic effects of Ang II on sodium transport 5. In the isolated collecting duct cells, both the S1P inhibitor and siRNA to PRR abolished the Ang II induced amiloride-sensitive current as evidence of sodium channel activity.
Of particular interest to the functional relationship of prorenin and the sPRR, the authors find that the S1P inhibitor was more effective than aliskerin to block increased eNaC activity to Ang II in the collecting duct cells 5. This discrepancy between the renin blockade and the S1P inhibitor was not addressed in this study. The SIP1 inhibitor also attenuated the increased cortical and urinary angiotensinogen levels to Ang II infusion, although the apparent requirement of (pro)renin activity for Ang II stimulation of its precursor from the proximal tubules was also not addressed. These latter results underscore the possibility that the S1P-mediated release of sPRR may exhibit additional actions apart from the activation of prorenin and the generation of Ang II (Figure 1). Indeed, a limitation of the present study was the failure to distinguish the Ang II-AT1R dependent effects and potentially the direct actions of the sPRR within the kidney. Since the PRR is an accessory protein of the V-ATPase complex, the extent that Ang II-induced release of the sPRR impacts the function of the V-ATPase is also unknown. As one example pertaining to this relationship, the PRR and the V-ATPase were shown to be essential to β-catenin/Wnt signaling which is also a key cellular pathway in the regulation of the RAS 6-8. As recently reviewed by Peters 3, other potential partners 7to the PRR include mTOR, cyclin D, cyclin E, c-myc and P21/P27, but whether these interactions are altered with the release of sPRR is presently unknown. Moreover, the potential clinical benefits of S1P inhibitors alone or in combination with RAS blockade for hypertension should be explored, although this approach may be mitigated by other cellular roles of the protease. In this regard, the SIP was recently shown to be essential for B-cell maturation and antibody secretion 9.
In conclusion, the present findings shed further light on the cardiovascular role of the sPRR and its processing protease S1P to mediate the actions of Ang II induced hypertension. This study extends previous findings by the authors and other investigators regarding the functional actions of the sPRR within the kidney, particularly the feed forward actions of Ang II to stimulate the sPRR 10. Not unexpectedly, the study raises additional questions on the functional actions or interactions of the sPRR that may not solely lead to the generation of Ang II and activation of the AT1R axis of the RAS. Finally, the efficacy of S1P inhibitor treatment in other forms of hypertension, as well as the relevance of the S1P-sPRR pathway in hypertensive females remains to be determined.
Acknowledgments
Sources of Funding
Support was provided by National Institutes of Health grants HD-084227, HL-146818, HL146818-S1, American Heart Association grants GRNT20480121 and TPA34170522, the Chronic Disease Research Fund and the Farley-Hudson Foundation (Jacksonville, NC).
Footnotes
DISCLOSURES
No conflicts of interest are declared by the authors.
REFERENCES
- 1.Nguyen G, Delarue F, Burckle C, Bouzhir L, Giller T, Sraer JD. Pivotal role of the renin/prorenin receptor in angiotensin II production and cellular responses to renin. J Clin Invest. 2002, 109:1417–1427. doi: 10.1172/JCI14276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ichihara A, Yatabe MS. The (pro)renin receptor in health and disease. Nat Rev Nephrol. 2019, 15:693–712. doi: 10.1038/s41581-019-0160-5.PMID: 31164719 [DOI] [PubMed] [Google Scholar]
- 3.Peters J. The (pro)renin receptor and its interaction partners. Eur J Physiol 2017, 469:1245–1256. doi: 10.1007/s00424-017-2005-z. PMID: 28620832 [DOI] [PubMed] [Google Scholar]
- 4.Cousin C, Bracquart D, Contrepas A, Corvol P, Muller L, Nguyen G. Soluble form of the (pro)renin receptor generated by intracellular cleavage by furin is secreted in plasma.Hypertension 2009,53:1077–1082 oi:101161/HYPERTENSIONAHA108.127258 [DOI] [PubMed] [Google Scholar]
- 5.Feng Y, Peng K, Luo R, Wang F, Yang T. Site-1 protease derived soluble (pro)renin receptor contributes to Angiotensin II-induced hypertension in mice. Hypertension 2020. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cruciat CM, Ohkawara B, Acebron SP, Karaulanov E, Reinhard C, Ingelfinger D, Boutros M, Niehrs C. Requirement of prorenin receptor and vacuolar H+-ATPase-mediated acidification for Wnt signaling. Science. 2010, 327:459–63. doi: 10.1126/science.1179802. [DOI] [PubMed] [Google Scholar]
- 7.Zhou L, Li Y, Hao S, Zhou D, Tan RJ, Nie J, Hou FF, Kahn M, Liu Y. Multiple genes of the renin-angiotensin system are novel targets of Wnt/β-catenin signaling. J Am Soc Nephrol. 2015, 26:107–120. doi: 10.1681/ASN.2014010085. PMID: 25012166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li Z, Zhou L, Wang Y, Miao J, Hong X, Hou FF, Liu Y. (Pro)renin receptor Is an amplifier of Wnt/β-Catenin signaling in kidney injury and fibrosis. J Am Soc Nephrol. 2017, 28:2393–2408. doi: 10.1681/ASN.2016070811. PMID: 28270411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Al-Maskari M, Car MA, Robinson E, Cocco M, Tooze RM, Doody GM. Site-1 protease function is essential for the generation of antibody secreting cells and reprogramming for secretory activity. Sci Reports. 2018; 8:: 14338–14347. doi: 10.1038/s41598-018-32705-7. PMCID: PMC6156501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gonzalez AA, Prieto MC. Renin and the (Pro)renin Receptor in the renal collecting duct: role in the pathogenesis of hypertension. Clin Exp Pharmacol Physiol. 2016, 42: 14–21. doi: 10.1111/1440-1681.12319. PMID: 25371190 [DOI] [PMC free article] [PubMed] [Google Scholar]