Abstract
Chronic lymphocytic leukemia/small lymphocytic lymphoma (CLL/SLL) accounts for approximately 1% of all lymphomas in our department. In this article, we describe the differential diagnosis of CLL/SLL from other indolent lymphomas, with special reference to follicular lymphoma, marginal zone B-cell lymphoma, lymphoplasmacytic lymphoma, and mantle cell lymphoma, although the latter is considered to be aggressive. CLL/SLL often exhibits proliferation centers, similar to follicular lymphoma. Immunohistological examination can easily distinguish these two lymphomas. The most important characteristic of CLL/SLL is CD5 and CD23 positivity. Mantle cell lymphoma is also CD5-positive and there are some CD23-positive cases. Such cases should be carefully distinguished from CLL/SLL. Some marginal zone lymphomas are also positive for CD5 and such cases are often disseminated. Lymphoplasmacytic lymphoma should also be a differential diagnosis for CLL/SLL. It frequently demonstrates MYD88 L265P, which is a key differential finding. By immunohistological examination, the expression of lymphoid enhancer-binding factor 1 is specific for CLL/SLL and can be a good marker in the differential diagnosis.
Keywords: chronic lymphocytic leukemia/small lymphocytic lymphoma, differential diagnosis, indolent lymphoma
PATHOLOGICAL CHARACTERISTICS OF CHRONIC LYMPHOCYTIC LEUKEMIA/SMALL LYMPHOCYTIC LYMPHOMA
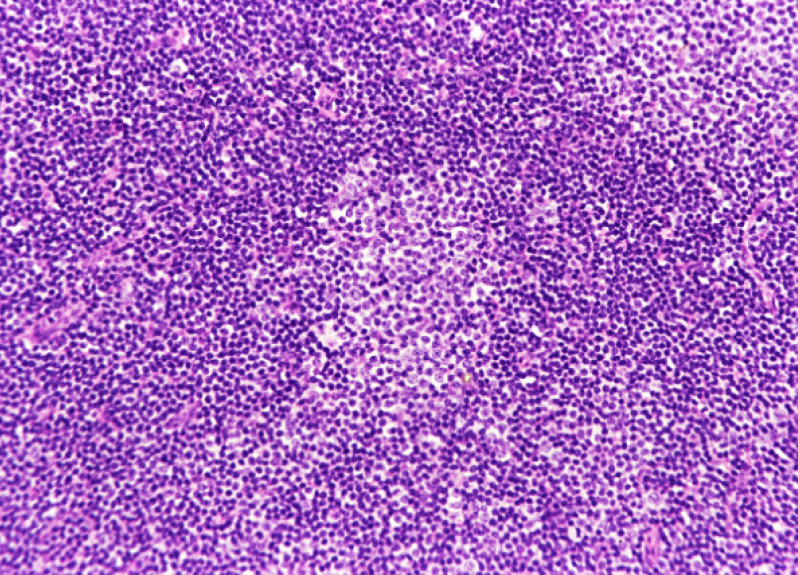
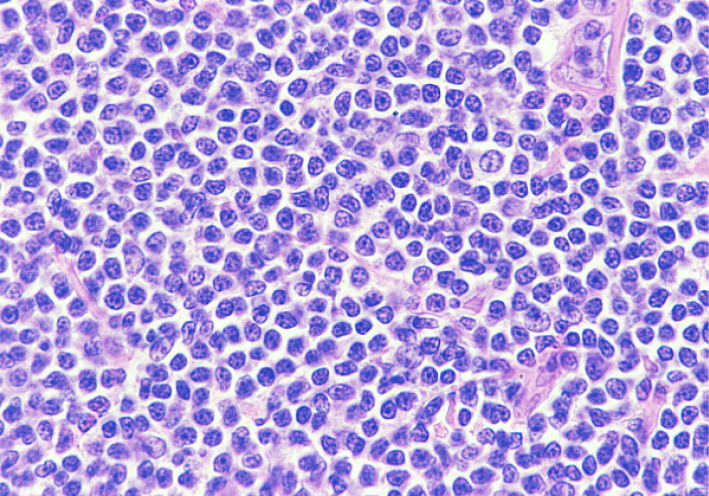
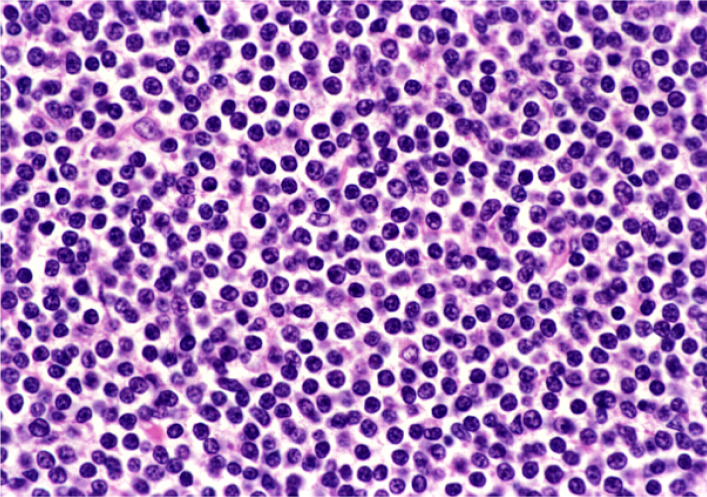
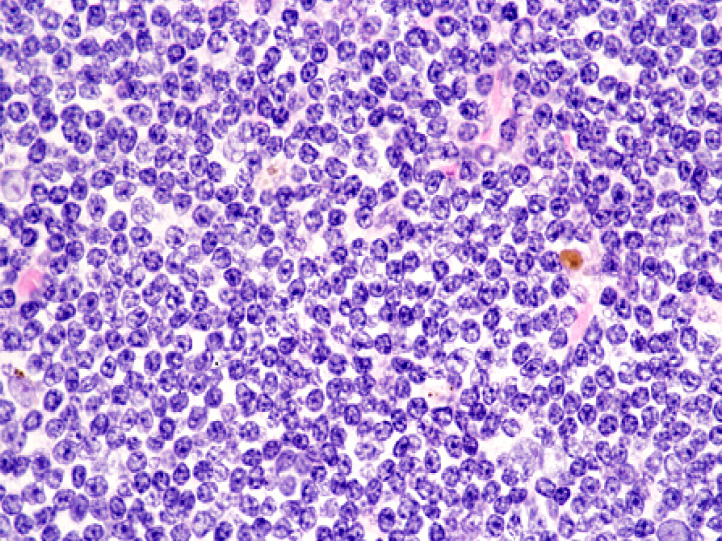
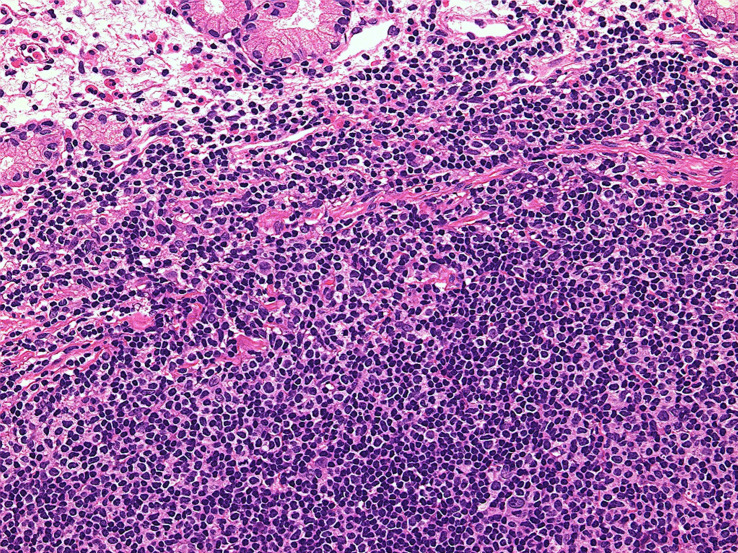
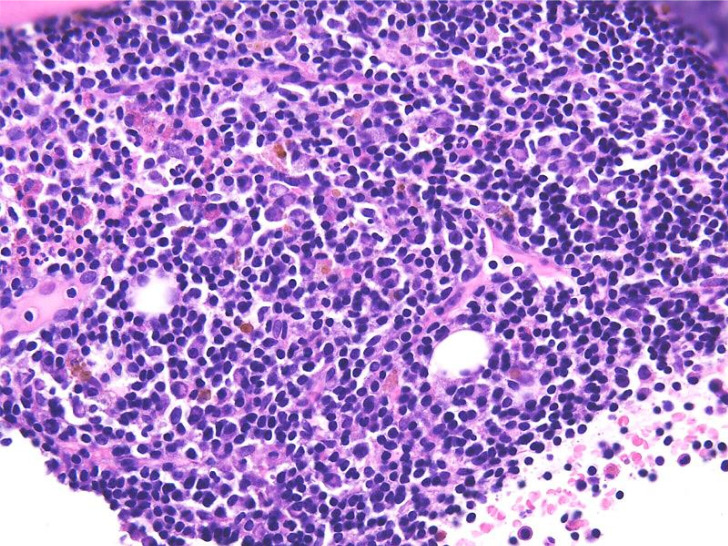
Chronic lymphocytic leukemia/small lymphocytic lymphoma (CLL/SLL) usually exhibits heterogeneous features such as ill-defined bright nodules, termed proliferation centers, with chromatin-rich background cells (Figure 1). The proliferation centers are composed of paraimmunoblasts, which are medium-sized cells, and the Ki67 index of these cells is higher than that of ordinary CLL/SLL cells. On the contrary, the majority of CLL/SLL cells are usually “small” lymphoma cells, but they are slightly larger than normal lymphocytes (Figure 2). In some cases, paraimmunoblasts are prominent and such cases should be differentiated from follicular lymphomas. By lymphoma-cell morphology and immunostaining, this differential diagnosis is simple. CLL/SLL cases may exhibit prominent paraimmunoblasts (Figure 3). Such cases should not be diagnosed as Richter syndrome because it features large blast cells; one feature of diffuse large B-cell lymphoma is the monotonous proliferation of large cells (the nucleus is more than twice the size of that of normal lymphocytes with varying cytoplasm sizes) (Figure 4).
Fig. 1.
Typical features of CLL/SLL. There are two proliferation centers in this field. If the proliferation centers are prominent, follicular lymphoma is to be differentiated.
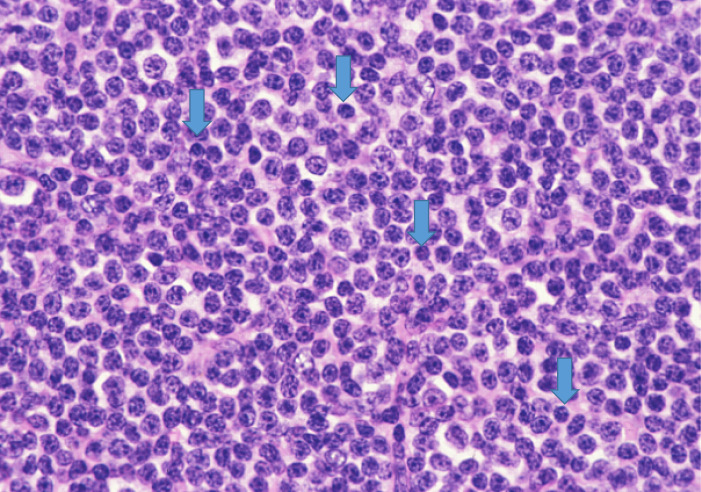
Fig. 2.
“Small-cell” component of CLL/SLL. In comparison with non-neoplastic lymphocytes (arrows), lymphoma cells are slightly larger than “true” non-neoplastic small lymphocytes.
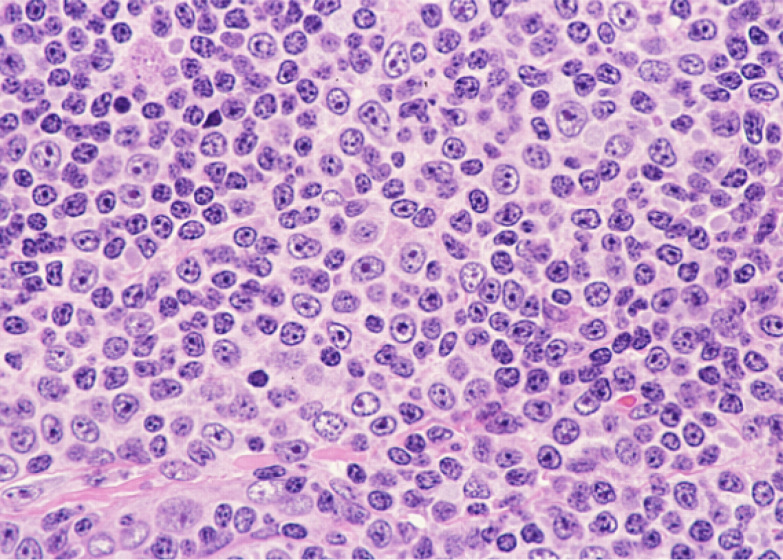
Fig. 3.
Paraimmunoblasts. These cells have a prominent nucleoli, and are approximately 1.5-times larger than non-neoplastic lymphocytes.
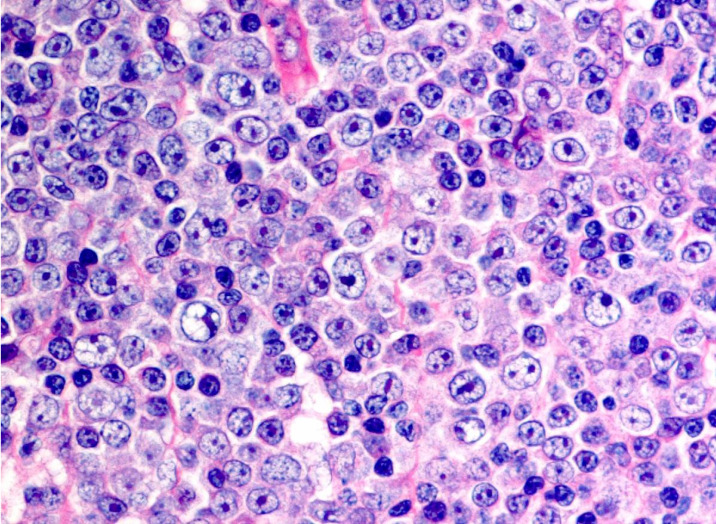
Fig. 4.
Richter’s syndrome. The proliferated cells have a large nucleus that is more than twice the size of that of small lymphocytes.
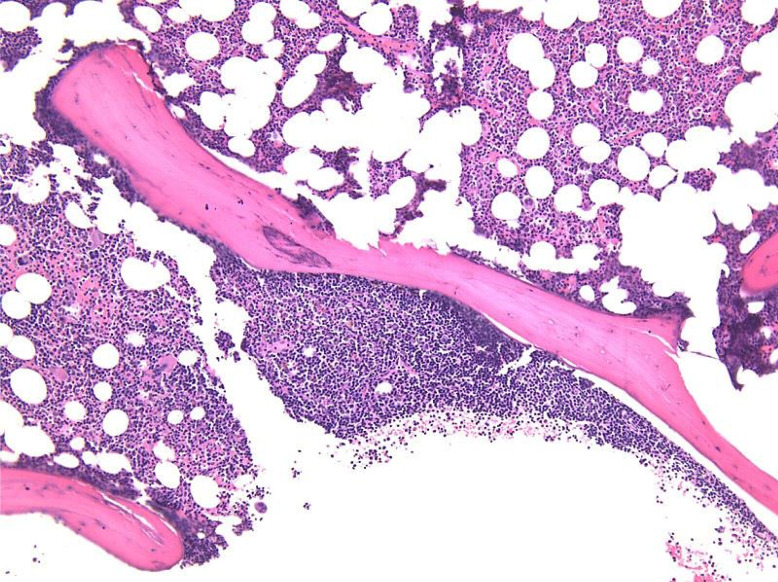
Bone marrow biopsy demonstrates many features. Some cases exhibit a nodular pattern of infiltration, or interstitial or mixed nodular and interstitial pattern, whereas others have diffuse involvement, which usually suggests more advanced disease.1
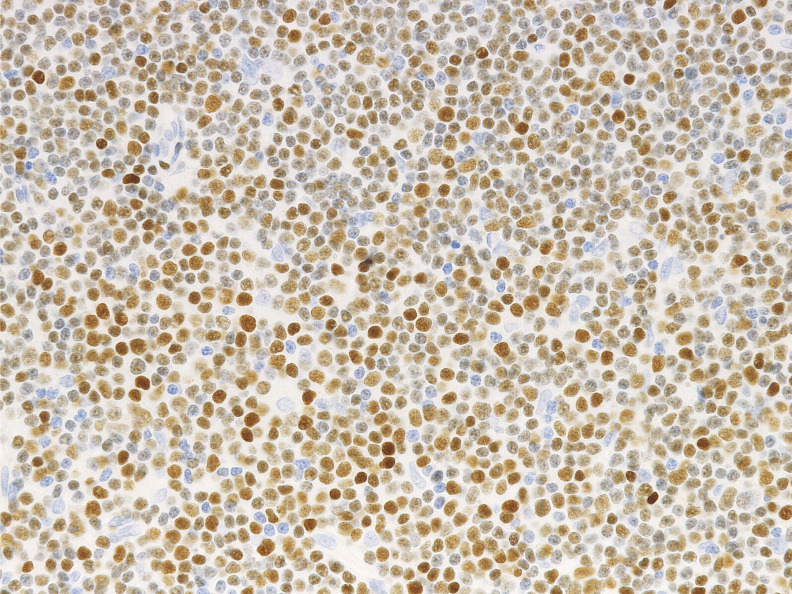
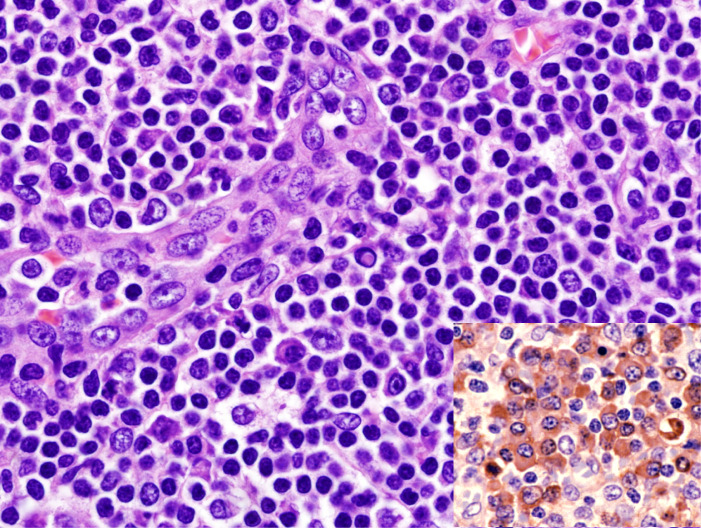
Immunohistologically, circulating leukemic B cells express CD19, and weak surface CD20, CD22, and CD79b. They are positive for CD5, and CD43, and strongly positive for CD23 and CD200. They are negative for CD10, FMC7 (usually), and cyclinD1, although cyclinD1 may be positive at proliferation centers.1 Lymphoid enhancer-binding factor 1 (LEF1) is specific to CLL/SLL and is a good marker in the differential diagnosis. Menter T et al. reported that 77/80 of CLL cases were positive for LEF1 (Figure 5), whereas only one of 38 follicular lymphoma and two of 33 marginal zone B-cell lymphoma cases were positive. The sensitivity of LEF1 for CLL is 0.96 and the specificity is 0.93.2 O’Malley DP et al. reported that only 4-9% of MCL cases express LEF1.3 The differential diagnosis by immunostaining and flow cytometry analysis is summarized in Table 1.
Fig. 5.
LEF1 expression in CLL/SLL. The lymphoma cells are highly positive for LEF1.
Table 1. Immunophenotype of indolent lymphomas, including mantle cell lymphoma*.
| CD19 | CD20 | CD22 | CD79b | CD5 | CD10 | CD23 | CD200 | cyclinD1 | BCL2 | LEF1 | IRTA1 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CLL/SLL | + | +weak | +weak | +weak | + | - | +strong | +strong | - | + | + | - |
| Mantle cell lymphoma | + | + | + | + | + | - | - | - | + | + | - | - |
| Follicular lymphoma | + | + | + | + | - | + | - | - | - | ++ | - | - |
| Marginal zone B-cell lymphoma | + | + | + | + | - | - | - | - | - | + | - | + |
| Lymphoplasmacytic lymphoma | + | + | + | + | - | - | - | - | - | + | - | - |
*: The presented immunophenotype of each lymphoma is the most common. There are rare exceptional findings, which are described in the text.
Although CLL/SLL has no specific genetic markers, most (80-90%) cases have cytogenetic abnormalities. The most common alternations are deletions in 13q14.3 and trisomy 12 or partial trisomy 12q13. Deletion in 11q22-23, 17q13, or 6q21 are less common. Deletion in 11q (ATM and BIRC3) and 17p (TP53) leads to a poorer clinical outcome, whereas isolated deletion in 13q14 is associated with a more favorable clinical course.1
CLL, SLL, AND MONOCLONAL B-CELL LYMPHOCYTOSIS
In the 2017 WHO classification,1 monoclonal B-cell lymphocytosis (MBL) is defined as a monoclonal B-cell count less than 5X109/L in peripheral blood of subjects who have no associated lymphadenopathy, organomegaly, or other extramedullary involvement. MBL is classified into three categories: (1) CLL-type, (2) atypical CLL type, and (3) non-CLL type. The CLL type is the most common (75% of all cases), and it is characterized by B-cell markers (CD19, CD20 (weak)), CD23, and CD5, and the B-cells exhibit light chain restriction or lack surface immunoglobulin. The estimated incidence of CLL-type MBL is 3.5% to 12% in healthy individuals. Although all CLLs are preceded by MBL, low-count (lower than 0.5X109L) MBL does not progress to CLL, whereas high-count (0.5X109L or higher) MBL has features identical to low-stage CLL and progresses to therapy-required CLL at a rate of 1-2%/year. MBL cells usually have mutated IGHV genes.
MBL has an atypical CLL phenotype positive for CD19, CD20 (bright), CD5, and surface immunoglobulin. CD23 may be negative, and such cases should be carefully excluded from those of mantle cell lymphoma and other B-cell lymphomas. MBL with a non-CLL phenotype is characterized by negative or weak CD5 expression, and CD19 and CD20-positive B-cells. Some cases exhibit transient clonal expansion and are self-limited. Additional phenotypic and cytogenetic studies are needed to exclude a specific lymphoid neoplasm.
SLL includes cases with a circulating CLL cell count of less than 5X109/L and documented nodal, splenic, or other extramedullary involvement. SLL should be differentiated from CLL-type MBL. Nodal infiltration by CLL-type cells without notable proliferation centers in individuals without lymphoadenopathy (more than 1.5 cm across) may constitute a nodal equivalent of MBL rather than SLL.1
DIFFERENTIAL DIAGNOSIS: MANTLE CELL LYMPHOMA
Mantle cell lymphoma (MCL) is also positive for CD5. Most mantle cell lymphomas are composed of intermediate lymphocytes and centrocytes exhibiting irregular nuclear contours, which is an important differential point (Figure 6). However, it may be composed of small-sized cells (Figure 7) that resemble CLL/SLL. Saksena A et al.4 reported that 103 (13%) MCL cases were weakly positive for CD23. CD23 expression in CLL/SLL is usually high. CD23-positive MCL lymphoma has an increased leukocyte count, bone marrow involvement, and leukemic presentation. Of note, CD23-positive MCL is more often associated with CD200 positivity and weak SOX11 expression. Although patients with CD23-positive MCL have a leukemic presentation similar to CLL, their prognosis is better than that of CD23-negative patients. The expression of CD23 is closely associated with CD200 expression. Ye H et al.5 reported similar findings and referred to such cases as smoldering mantle cell lymphoma. CD200 is an important marker of CLL/SLL in the differential diagnosis from other CD5-positive indolent lymphomas. However, Hu Z et al.6 reported that approximately 4% of MCL (25 cases in their series) is positive for CD200, and most of these cases (76%) are positive for CD23. Moreover, only 24% of CD200-positive MCL cases express SOX11, 39% (9 cases) exhibit round nuclear contours, similar to CLL, and 44% of (11 cases) cases are a non-nodal leukemic variant of MCL, which is closely related to IGHV-mutated cases. CLL/SLL can be subdivided into two groups: IGHV-unmutated and IGHV-mutated, and the patient prognosis of the former group is poorer than that of the latter group. Of MCL cases, only a small subset is IGHV-mutated, which is frequently associated with a non-nodal leukemic presentation. In conclusion, CD200-positive MCL is highly similar to CLL/SLL, and IgH-cylinD1 rearrangement is needed for the differential diagnosis.
Fig. 6.
Mantle cell lymphoma with typical features. The lymphoma cells are intermediate between small lymphocytes and medium-sized centrocytes with irregular nuclei contours.
Fig. 7.
Mantle cell lymphoma. This case is composed of lymphoma cells, which are almost the same size as small lymphocytes, and was difficult to distinguish from small lymphocytic lymphoma without immunohistological examination.
CD200 belongs to a type I immunoglobulin gene superfamily composed of a light-chain-like structure with extracellular variable and constant-like domains, and a cytoplasmic tail. CD23 is also an important marker of CLL/SLL. It is a low-affinity IgE receptor, and contains a C-terminal lectin-like domain, which resembles C-type carbohydrate-recognition domains.7 CD23 has two isotypes, CD23a and CD23b.8 As described above, some MCL cases express low-level CD23, which is closely related to the expression of CD200. To our knowledge, the relationship between CD200 and CD23 has not been clarified.
OTHER CD5-POSITIVE LYMPHOMAS
CD5 is another important marker of CLL/SLL. CD5 was first found in mice, and CD5-positive B-cells (B-1 cells) are distinct from CD5-negative B-cells (B-2 cells).9 B-1 cells are responsible for natural antibody production and rapid immune responses. B-1 cells in humans are abundant in cord blood and the fetal spleen. CLL is thought to originate from B-1 cells. Of note, CLL is characterized not only by CD5 expression, but also by an abnormal BCR repertoire encoding autoreactive and/or poly-reactive antibodies. Indeed, an increase in CD5-positive cells is observed in patients with autoimmune diseases. B-1 cells may proliferate greatly with age and eventually develop into CLL.9
As described above, CD5 is usually observed in CLL and MCL, and the incidence of CLL and MCL in Japan is 1% and 2%, respectively. Their incidences in Western countries are higher, accounting for 6% of all lymphomas. This strongly suggests that Japanese (and Asian) people infrequently develop B-1 cell-related lymphomas.
Marginal zone B-cell lymphoma (MZL) is composed of mucosa-associated lymphoid tissue (MALT) lymphoma, nodal MZL, and splenic MZL. CD5 is rarely positive in MALT lymphoma. Jaso J et al.10 described 14 cases of CD5-positive MALT lymphoma: 4 in the salivary glands, 2 in the nasopharynx, 1 each in the conjunctiva, thyroid gland, stomach, colon, skin, lung, kidney, and retroperitoneum. Approximately 60% of MALT lymphoma cases originate from the stomach, but CD5-positive MALT lymphoma is rare in the stomach, making CD5-positive cases rare. CD5-positive MALT lymphoma was reported to commonly present with disseminated disease, although the prognosis is fair.
MALT lymphoma may comprise small lymphoma cells such as ocular adnexal MALT lymphoma (Figure 8) and gastric MALT lymphoma with t(11;18) (Figure 9).
Fig. 8.
Ocular-adnexal MALT lymphoma. Lymphoma cells are uniform and similar to those in SLL.
Fig. 9.
Gastric MALT lymphoma with t(11;18). The lymphoma cells are uniform, and nuclear size is similar to that in SLL.
Jaso JM et al.11 compared 7 patients with CD5-positive nodal MZL with 66 with CD5-negative nodal MZL. Six of 7 CD5-positive nodal MZL patients exhibited wide-spread lymphoadenopathy and bone marrow involvement. They concluded that CD5-positive nodal MZL often presents dissemination, but the patients have an indolent clinical course.
Kojima M et al.12 reported 11 patients with CD5-positive splenic MZL with leukemic manifestation. They found that less than 20% of splenic MZL patients are CD5 positive. The clinical characteristics of the examined patients did not differ from those of CD5-negative patients. IRTA-1 is specific for marginal zone B-cell lymphoma and is useful for diagnosis.13
Li Y et al.14 reported 88 patients with CD5-positive follicular lymphoma. For MZL, CD5-positive patients often exhibit dissemination, bone marrow involvement, and/or leukemic state. In contrast, patients with CD5-positive follicular lymphoma more commonly have a high international prognostic index, often develop diffuse large B-cell lymphoma, and have a shorter median progression-free survival. These findings suggest that CD5 expression in follicular lymphoma is closely related to aggressiveness, and the role of CD5 varies among lymphomas.
LYMPHOPLASMACYTIC LYMPHOMA (LPL)
Lymphoplasmacytic lymphoma is closely associated with Waldenstrom macroglobulinemia. It is composed of small lymphomacytes intermingled with plasmacytic cells (Figures 10 and 11). The typical morphology is not difficult to differentiate from that of CLL/SLL; however, some cases demonstrate lymphoplasmacytoid features of a small nucleus with heterochromatin in a characteristic cartwheel or clock face arrangement and narrow cytoplasm with occasional Dutcher bodies (Figure 12). Such cases resemble CLL/SLL. Lymphoplasmacytic lymphoma is highly positive for CD5, but not CD23. The most important characteristic of lymphoplasmacytic (including lymphoplasmacytoid) lymphoma is MYD88 mutation.15 Lymphoplasmacytic lymphoma involving extranodal organs is often difficult to differentiate from MALT lymphoma. Both lymphoma types are associated with plasma cells with Dutcher bodies. Treon SP et al. reported that most (approximately 90%) LPL cases have MYD88 L265P.15 Similar reports have been published, and although examination of MYD88 mutation is useful to diagnose LPL, MYD88 L265P is not specific to LPL.16 Approximately 10% of marginal zone B-cell lymphomas exhibit this mutation. CLL/SLL also has this mutation, although rarely. Regarding diffuse large B-cell lymphoma (DLBCL), CNS, testicular, and leg-type lymphomas highly frequently exhibit MYD88 mutation,16 and CNS and testicular patients have a poor prognosis, with most cases being the activated B-cell type. We previously reported that 59% of breast DLBCL cases have MYD88 L265P, leading to a poor prognosis,17 and only 6% of gastrointestinal DLBCL has MYD88 L265P. Most cases are the ABC type, but the prognosis is fair.18 These findings strongly suggest that MYD88 L265P plays a key role in the lymphomagenesis of LPL and DLBCL, and is related to the prognosis.
Fig. 10.
Bone marrow involvement of lymphoplasmacytic lymphoma. The lymphoma cells attach to the trabecular bone, which is thought to be specific to follicular lymphoma, but lymphoplasmacytic cells show a similar feature.
Fig. 11.
Lymphoplasmacytic lymphoma with MYD88 L265P. Small-sized lymphoma cells are intermingled with plasmacytic cells. Cases with plasmacytoid cells are highly similar to small lymphocytic lymphoma.
Fig. 12.
Lymphoplasmacytic lymphoma. Lymphoma cells are uniform and have Dutcher bodies. Inset is immunostaining of IgM.
CONCLUSION
CLL/SLL is a rare type of leukemia/lymphoma in Japan. Therefore, differential diagnosis is difficult for pathologists. Moreover, CLL cases in Japan are not uniform and some exhibit “atypical” features. In this article, we did not describe atypical cases. However, CLL/SLL should be differentiated from other indolent types (small-sized lymphoma cells), including mantle cell lymphoma. A precise diagnosis is needed to select the most effective therapy, and although the exact role of each molecule described in this article has not been fully clarified, the molecular pathogenesis will be clarified in the near future. Newly developed drugs are expected to be used for lymphoma treatment.
ACKNOWLEDGMENT
We sincerely thank Ms. Misa Sakamoto for immunohistological examination.
Footnotes
CONFLICT OF INTEREST
The authors declare no conflict of interest.
REFERENCES
- 1.Campo E, Chia P, Montserrat E, et al. Chronic lymphocytic leukaemia / small lymphocytic lymphoma. In: Swerdlow SH, Campo E, Harris NL et al. (eds). WHO Classification of Tumours. 4th ed, Lyon, IARC Press. 2017; pp. 216-221. [Google Scholar]
- 2.Menter T, Trivedi P, Ahmad R, et al. Diagnostic utility of lymphoid enhancer binding factor 1 immunohistochemistry in small B-cell lymphomas. Am J Clin Pathol. 2017; 147: 292-300. [DOI] [PubMed] [Google Scholar]
- 3.O’Malley DP, Lee JP, Bellizzi AM. Expression of LEF1 in mantle cell lymphoma. Ann Diagn Pathol. 2017; 26: 57-59. [DOI] [PubMed] [Google Scholar]
- 4.Saksena A, Yin CC, Xu J, et al. CD23 expression in mantle cell lymphoma is associated with CD200 expression, leukemic non-nodal form, and a better prognosis. Hum Pathol. 2019; 89: 71-80. [DOI] [PubMed] [Google Scholar]
- 5.Ye H, Desai A, Zeng D, et al. Smoldering mantle cell lymphoma. J Exp Clin Cancer Res. 2017; 36: 185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hu Z, Sun Y, Schlette EJ, et al. CD200 expression in mantle cell lymphoma identifies a unique subgroup of patients with frequent IGHV mutations, absence of SOX11 expression, and an indolent clinical course. Mod Pathol. 2018; 31: 327-336. [DOI] [PubMed] [Google Scholar]
- 7.Jégouzo SAF, Feinberg H, Morrison AG, et al. CD23 is a glycan-binding receptor in some mammalian species. J Biol Chem. 2019; 294: 14845-14859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kriston C, Bödör C, Szenthe K, et al. Low CD23 expression correlates with high CD38 expression and the presence of trisomy 12 in CLL. Hematol Oncol. 2017; 35: 58-63. [DOI] [PubMed] [Google Scholar]
- 9.Baumgarth N. A hard(y) look at B-1 cell development and function. J Immunol. 2017; 199: 3387-3394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jaso J, Chen L, Li S, et al. CD5-positive mucosa-associated lymphoid tissue (MALT) lymphoma: a clinicopathologic study of 14 cases. Hum Pathol. 2012; 43: 1436-1443. [DOI] [PubMed] [Google Scholar]
- 11.Jaso JM, Yin CC, Wang SA, et al. Clinicopathologic features of CD5-positive nodal marginal zone lymphoma. Am J Clin Pathol. 2013; 140: 693-700. [DOI] [PubMed] [Google Scholar]
- 12.Kojima M, Sato E, Oshimi K, et al. Characteristics of CD5-positive splenic marginal zone lymphoma with leukemic manifestation; clinical, flow cytometry, and histopathological findings of 11 cases. J Clin Exp Hematop. 2010; 50: 107-112. [DOI] [PubMed] [Google Scholar]
- 13.Falini B, Agostinelli C, Bigerna B, et al. IRTA1 is selectively expressed in nodal and extranodal marginal zone lymphomas. Histopathology. 2012; 61: 930-941. [DOI] [PubMed] [Google Scholar]
- 14.Li Y, Hu S, Zuo Z, et al. CD5-positive follicular lymphoma: clinicopathologic correlations and outcome in 88 cases. Mod Pathol. 2015; 28: 787-798. [DOI] [PubMed] [Google Scholar]
- 15.Treon SP, Xu L, Yang G, et al. MYD88 L265P somatic mutation in Waldenström’s macroglobulinemia. N Engl J Med. 2012; 367: 826-833. [DOI] [PubMed] [Google Scholar]
- 16.Yu X, Li W, Deng Q, et al. MYD88 L265P mutation in lymphoid malignancies. Cancer Res. 2018; 78: 2457-2462. [DOI] [PubMed] [Google Scholar]
- 17.Taniguchi K, Takata K, Chuang SS, et al. Frequent MYD88 L265P and CD79B mutations in primary breast diffuse large B-Cell lymphoma. Am J Surg Pathol. 2016; 40: 324-334. [DOI] [PubMed] [Google Scholar]
- 18.Nagakita K, Takata K, Taniguchi K, et al. Clinicopathological features of 49 primary gastrointestinal diffuse large B-cell lymphoma cases; comparison with location, cell-of-origin, and frequency of MYD88 L265P. Pathol Int. 2016; 66: 444-452. [DOI] [PubMed] [Google Scholar]