Abstract
Mind-wandering is a cognitive process in which people spontaneously have thoughts that are unrelated to their current activities. The types of mind-wandering thoughts that people have when affected by a negative mood resemble thoughts associated with mood disorders (e.g., negative thoughts about the past). Transcranial direct current stimulation (tDCS) is a form of noninvasive brain stimulation that can modulate cognition and affect in healthy and clinical populations. Ninety participants received either excitatory, inhibitory, or sham tDCS to bilateral inferior parietal lobe (IPL) nodes of the default mode network (DMN) to assess changes in maladaptive mind-wandering following criticism. tDCS did not change mind-wandering frequency after hearing criticism, but it did change what people mind-wandered about. Specifically, cathodal stimulation decreased the frequency of negative mind-wandering thoughts about the past. Future studies could investigate tDCS of DMN regions as an intervention for patients with mood disorders who suffer from negative, past-oriented cognitions.
Keywords: Mind-wandering, Default Mode Network, Inferior Parietal Lobe, Neuromodulation, transcranial Direct Current Stimulation
Introduction
When people are left to their own thoughts, they have a tendency to think about plans for the future and think about themselves in relation to others (Andrews-Hanna, Reidler, Huang, & Buckner, 2010; Buckner, Andrews-Hanna, & Schacter, 2008). This ongoing “stream of consciousness” (James, 1890) is also known as mind-wandering. Daydreaming, stimulus-independent thought, or task-unrelated thought have also been used to describe this phenomenon. It is a relatively common mental process; people report mind-wandering up to 50% of the time (Killingsworth & Gilbert, 2010; Klinger & Cox, 1987). People still mind-wander even when it is increasingly important or beneficial for them to not do so (e.g., when a task becomes progressively more difficult or when better performance on a task leads to more money; Antrobus, 1968; Antrobus, Singer, Goldstein, & Fortgang, 1970; Antrobus, Singer, & Greenberg, 1966).
Mind-wandering has been linked with activity in the default mode network (DMN), a network of brain regions that are functionally and structurally connected (Andrews-Hanna, Reidler, Sepulcre, Poulin, & Buckner, 2010; Christoff, Gordon, Smallwood, Smith, & Schooler, 2009; Mason et al., 2007). The DMN consists of two core hubs, the posterior cingulate cortex and anterior medial prefrontal cortex, and at least two subsystems, the dorsal medial prefrontal cortex subsystem and the medial temporal lobe (MTL) subsystem (Andrews-Hanna, Reidler, Sepulcre, et al., 2010; Buckner et al., 2008). In particular, the MTL subsystem appears to support mind-wandering about the past and the future (Andrews-Hanna, Reidler, Sepulcre, et al., 2010; Buckner & Carroll, 2007; Cabeza & St Jacques, 2007; Maguire, 2001; Okuda et al., 2003; Schacter, Addis, & Buckner, 2007, 2008; Svoboda, McKinnon, & Levine, 2006; Szpunar, Watson, & McDermott, 2007).
Maladaptive Forms of Mind-Wandering
While some researchers have claimed that any form of mind-wandering has negative consequences (Epel et al., 2013; Killingsworth & Gilbert, 2010), others have suggested that the context and the content of the mind-wandering behavior matter when determining whether mind-wandering is maladaptive (McMillan, Kaufman, & Singer, 2013; Smallwood & Andrews-Hanna, 2013). In terms of context, mind-wandering during a cognitively demanding situation (e.g., during a complicated task) or when in a negative mood is potentially more maladaptive (Antrobus, 1968; Antrobus et al., 1966). In terms of content, the tendency to engage in negative mind-wandering thoughts about the past is thought to be more maladaptive. In particular, repetitive negative and passive thoughts (i.e. rumination) and negative thoughts about the past in general are associated with the development and maintenance of depressive symptoms (Beck, 1967; Nolen-Hoeksema, 2000). Numerous psychological treatments for depression, including cognitive behavioral therapy, focus on changing negative thoughts about the past or behaviors in response to these thoughts (Beck, 1970; Hayes, Strosahl, & Wilson, 1999; Kabat-Zinn, 2003); these treatments are associated with medium to large effect sizes in decreasing psychiatric symptoms (Butler, Chapman, Forman, & Beck, 2006; Hofmann, Asnaani, Vonk, Sawyer, & Fang, 2012; Hofmann, Sawyer, Witt, & Oh, 2010). Taken together, there is sufficient evidence to consider mind-wandering about the past, especially when in a negative mood and/or during a cognitively demanding task, a therapeutic target for mood and anxiety disorders, leading to the hypothesis that modulating its brain substrate to reduce its frequency or content could result in improvement of clinical outcomes.
Transcranial Direct Current Stimulation
In the present study, we used transcranial Direct Current Stimulation (tDCS) as the method of noninvasive brain stimulation to potentially modulate maladaptive mind-wandering thoughts. tDCS has been used to safely modulate cognition, behavior and affect in healthy and clinical populations (Antal et al., 2017; Lefaucheur et al., 2017; Sarkis, Kaur, & Camprodon, 2014; Stagg & Nitsche, 2011). Compared with other noninvasive brain stimulation modalities such as transcranial magnetic stimulation (TMS), tDCS is simpler, cheaper, safer and portable, and therefore has potential as a home-based treatment. In tDCS, electrodes are applied on the scalp to transmit direct electrical currents at low amplitudes (Peterchev et al., 2012). Its effects are specific to the selected target of stimulation and the stimulation parameters, and its safety has been well tested in both animal and human studies (Antal et al., 2017; Bikson et al., 2016). Current models hypothesize that the electrical current increases or decreases the chances of neuronal firing (i.e., changing the resting synaptic membrane potential). More specifically, neurons under cathode electrodes are thought to be primarily hyperpolarized (inhibited) while those under anode electrodes are thought to be primarily depolarized (facilitated). In other words, cathodal tDCS is thought to have “inhibitory” effects whereas anodal tDCS is thought to have “excitatory” effects. Although the effects of a single tDCS session last up to 1 hour (Nitsche & Paulus, 2000, 2001) until the network goes back to its homeostatic equilibrium point, the effect of repeated daily sessions of tDCS can have longer-lasting effects, which supports its therapeutic potential (Nitsche et al., 2008).
Another advantage of tDCS is its capacity to modulate not one but multiple nodes at a time. Traditionally, tDCS has used bipolar montages with one cathode and one anode. Novel applications have developed smaller electrodes that allow greater current density and focality, and stimulators able to distribute the current across more than two electrodes, e.g. high definition tDCS (HD-tDCS). This hardware allows the simultaneous use of multiple cathodes and anodes, generating complex electric field topographies that can facilitate and inhibit multiple regions at a time, modulating not one but different nodes of a given network (Ruffini, Fox, Ripolles, Miranda, & Pascual-Leone, 2014). Multiple researcher teams have used HD-tDCS to induce behavioral and physiological changes (e.g., Borckardt et al., 2012; Edwards et al., 2013; Kuo et al., 2013; Nikolin, Loo, Bai, Dokos, & Martin, 2015; Villamar et al., 2013). Importantly, HD-tDCS has been linked with longer lasting changes in cortical excitability (Kuo et al., 2013) and may be better tolerated (Gbadeyan, Steinhauser, McMahon, & Meinzer, 2016) compared with conventional tDCS. The present study made use of this technology and approach to develop a novel montage that simultaneously modulated bilateral nodes of the DMN aiming to affect maladaptive mind-wandering behavior.
Neuromodulation of Mind-Wandering Behavior
tDCS has been previously used to change mind-wandering behavior (Axelrod, Rees, Lavidor, & Bar, 2015; Axelrod, Zhu, & Qiu, 2018; Bertossi, Peccenini, Solmi, Avenanti, & Ciaramelli, 2017; Kajimura, Kochiyama, Nakai, Abe, & Nomura, 2016; Kajimura & Nomura, 2015). tDCS of prefrontal regions has been linked with both increased mind-wandering (anode on dorsolateral prefrontal cortex; Axelrod et al., 2015; Axelrod et al., 2018) and decreased mind-wandering (cathode on the medial prefrontal cortex; Bertossi et al., 2017). Anodal tDCS of parietal regions such as the inferior parietal lobule has also been associated with decreases in mind-wandering (Kajimura et al., 2016; Kajimura & Nomura, 2015). The majority of these studies only investigated changes in the frequency of mind-wandering behavior. It is important to note that the type of mind-wandering that was modulated may not have been a maladaptive form of mind-wandering. As previously mentioned, the context and the content of the mind-wandering behavior can differentiate maladaptive from more adaptive forms of mind-wandering. Participants may have had the cognitive resources to mind-wander and complete low cognitive demand computer tasks (such as the Sustained Attention to Response Task [Robertson, Manly, Andrade, Baddeley, & Yiend, 1997], a simple go/no go task used in the studies by Axelrod and colleagues). Similarly, the majority of these investigators did not assess the content of participants’ mind-wandering thoughts (i.e., whether they were having negative thoughts). Therefore, it remains unclear if tDCS can be used to change maladaptive and more clinically relevant forms of mind-wandering.
Neuromodulation of the DMN
TMS and tDCS have been previously used to change activity within the DMN, particularly the prefrontal and parietal regions (Lou et al., 2004; Rossi et al., 2006). The inferior parietal lobule (IPL) is functionally and structurally connected to other DMN regions, and is one of the brain regions in the MTL subsystem hypothesized to support mind-wandering about the past and the future (Andrews-Hanna, Reidler, Sepulcre, et al., 2010; Buckner et al., 2008). It is the most accessible component of this network for noninvasive neuromodulation techniques given its short distance to the surface of the scalp. Stimulating the left posterior IPL (pIPL) using TMS has been linked with parameter-dependent changes in activity within the MTL subsystem and the overall DMN (Eldaief, Halko, Buckner, & Pascual-Leone, 2011). Specifically, high-frequency (20 Hz) excitatory repetitive TMS (rTMS) of the 1pIPL decreased its resting-state functional connectivity with the MPFC and the right pIPL. Low-frequency (1 Hz) rTMS increased the left pIPL’s resting-state functional connectivity with the left hippocampal formation. Similarly, anodal tDCS of the right IPL has been linked with decreased connectivity between the IPL and the posterior cingulate cortex (PCC; Kajimura et al., 2016). In other words, excitatory neuromodulation with TMS or tDCS decreased connectivity within DMN regions and inhibitory stimulation increased connectivity within the MTL subsystem. Noninvasive brain stimulation of the pIPL can therefore modulate activity and connectivity within the DMN and the MTL subsystem in a parameter-dependent manner.
Present Study
Based on the existing literature, we formed hypotheses about the effects of anodal and cathodal tDCS on the DMN and mind-wandering behavior. For anodal tDCS, previous tDCS studies (Kajimura et al., 2016; Kajimura & Nomura, 2015) found that anodal stimulation of the IPL was associated with decreases in mind-wandering. We therefore investigated whether we could replicate this finding. For cathodal tDCS, we based our hypotheses on a combination of findings from TMS and neuroimaging studies. A previous TMS study (Eldaief et al., 2011) found that low-frequency inhibitory stimulation of the pIPL was linked with increased connectivity within the MTL subsystem; the MTL subsystem is associated with mind-wandering about the past (Andrews-Hanna, Reidler, Sepulcre, et al., 2010; Buckner & Carroll, 2007; Cabeza & St Jacques, 2007; Maguire, 2001; Okuda et al., 2003; Schacter et al., 2007, 2008; Svoboda et al., 2006; Szpunar et al., 2007). We therefore hypothesized in the current study that cathodal tDCS would be associated with increased mind-wandering thoughts about the past, potentially through increased MTL connectivity (although changes in MTL connectivity could not be directly evaluated in the current study). The present study was designed to investigate these hypotheses using a novel multifocal HD-tDCS montage which simultaneously targeted the left and right IPL and did so with greater focality than traditional bipolar tDCS strategies. Overall, we investigated whether we could modulate mind-wandering behavior, and more specifically, maladaptive mind-wandering behavior (i.e., having negative mind-wandering thoughts while in a negative mood and during a cognitively demanding computer task).
Methods
Participants
Following approval by Massachusetts General Hospital’s Institutional Review Board, The Partners Human Research Committee, ninety participants were recruited from the community. Based on previous studies on mind-wandering and mood (Smallwood & O’Connor, 2011), an average effect size of 0.41 was determined. A statistical power analysis revealed that an approximate sample of ninety participants would be needed to achieve 80% power using an F-test with alpha at 0.05. Exclusion criteria included a history of epilepsy, metallic implants in the head or neck, brain stimulators, vagus nerve stimulators, VP shunts, pacemakers, pregnant or nursing females, neurological conditions, current or past psychiatric disorders, or current use of psychotropic medications. All study procedures were carried out according to the Declaration of Helsinki.
Overall Study Design
After participants provided informed consent, in a parallel study design (see Figure 1 for overall study design), participants practiced the computer task (see Multi-Source Interference Task section) and then rated their mood (see Mood Ratings section). They were then exposed to critical comments (see Emotional Stimuli section) and rated their mood again. Participants then completed the computer task and rated their mood once more. Next, they received a single session of either cathodal (n = 30), anodal (n = 30), or sham (n = 30) stimulation of the IPL bilaterally (see tDCS Parameters section for information on the specific montage, randomization and double-blind procedure). Following stimulation, they rated their mood again, were exposed to another set of critical comments, rated their mood, and then completed the computer task again. They then rated their mood for a final time.
Figure 1.
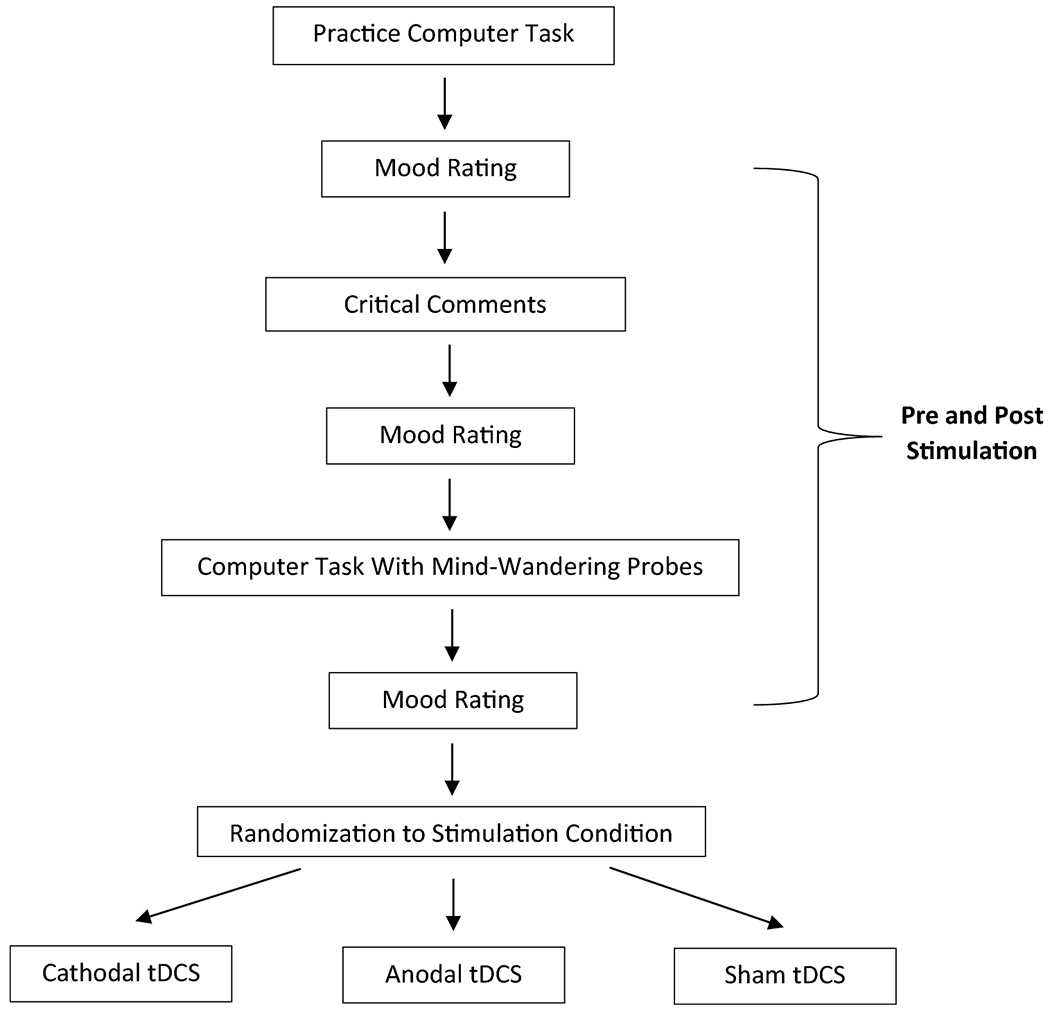
Participants were randomized to receive either cathodal, anodal, or sham transcranial direct current stimulation (tDCS). They heard criticism, rated their mood, and completed the computer task with mind-wandering probes before and after stimulation.
tDCS Parameters
We used a new multifocal HD-tDCS montage with round Ag/AgCl electrodes (3.14 cm2), consisting of a double 3x1 configuration (one in each hemisphere). According to the international 10-20 EEG system, we placed the primary electrodes over P4 (right IPL) and P3 (left IPL) with 3 corresponding return electrodes on each side (P8, CP2, O2 on the right and P7, CP1, O1 on the left, Figure 2). The anodal stimulation condition used 2 anodes (excitatory) over P4 and P3 each and all other return electrodes were cathodes, while the cathodal condition (inhibitory) used the exact opposite pattern. Current intensity was 1 mA each over P3 and P4 (2 mA total), and each return received one third of that (0.33 mA each). Duration of stimulation was 30 minutes, including a 15 second ramp up and ramp down to minimize skin discomfort. Sham stimulation used the same electrode distribution but only applied the 15 second ramp up and ramp down currents to emulate the skin tingling sensation at the beginning and end of the session, hence blinding subjects but without real neuromodulation. The experiment was conducted with the tDCS Starstim® system (Neuroelectrics, USA) and used its integrated double-blinding software option. The principal investigator created the stimulation protocols which were then assigned the generic names of “Protocol 1,” “Protocol 2,” and “Protocol 3.” Before any participants were enrolled in the study, the principal investigator then created a list which included random assignment of these 3 protocols for 90 different subjects (this was done while ensuring equal sample sizes for each stimulation group). This list was then used by a research assistant to determine which stimulation protocol to use for each participant during the study visit. The research assistant did not have access to the specific parameters for each stimulation protocol, thus remaining blinded.
Figure 2.
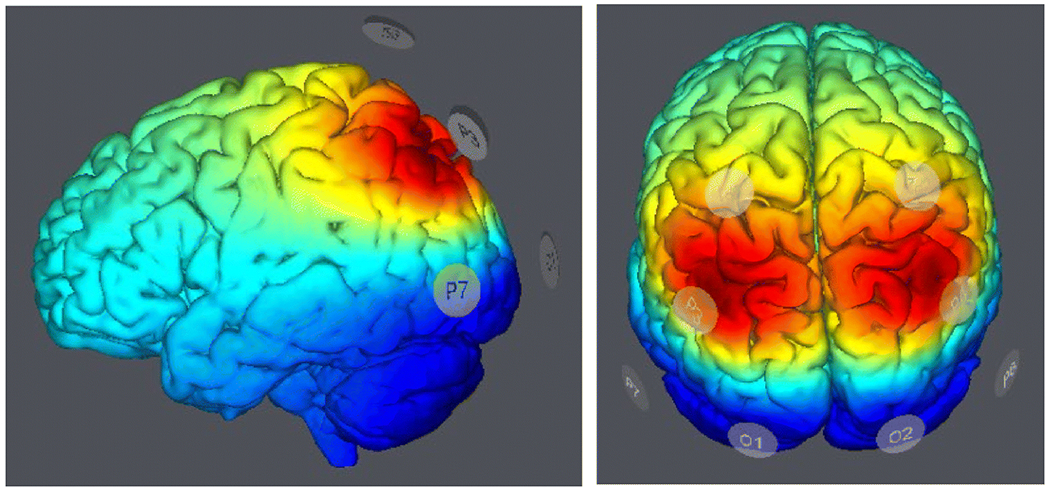
A bilateral 3x1 electrode montage was used to perform high definition transcranial direct current stimulation (tDCS) targeting the posterior inferior parietal lobule. Anodes or cathodes (depending on the condition) were surrounded by 3 return electrodes on each side.
Mood Ratings
Participants rated their current negative mood using a 0-100 Visual Analogue Scale (0 = not at all, 100 = completely). This mood rating scale was administered at baseline, before and after the emotional stimuli, before and after the computer task, and before and after stimulation to assess any changes in negative mood throughout the study (see Figure 1 for overall study design).
Emotional Stimuli
Participants heard a total of four 30-second critical comments directed at them (they heard two comments before stimulation and two comments after stimulation). These comments have been developed, standardized and used in several other studies (e.g., Hooley et al., 2010; Nook, Dodell-Feder, Hooley, DeLisi, & Hooker, 2018). The critical comments began with “One of the things that really bothers me about you is that you … (e.g., always have to get your own way. You have a hard time taking no for an answer and you can get really resentful when you don’t get what you want”). The order of the comments was counterbalanced.
Multi-Source Interference Task
Participants completed a modified version of the Multi-Source Interference Task (MSIT; Bush, Shin, Holmes, Rosen, & Vogt, 2003; the modification was the random thought probes which are described below). The MSIT is a measure of cognitive control and attention. It requires participants to identify a target number in a three-digit number that is different from the other two numbers (the digits can be 1, 2, or 3). On control trials, the target number and its location in the sequence are the same. For example, if the participant sees “122” the correct answer is “1” and the participant would press “1” on the button box. On interference trials, the identity of the target number is not the same as its position in the sequence (e.g., “221”) requiring the participant to suppress the urge to press “3” (which reflects the position of the number) and instead press “1” on the button box to identify the correct target number. The MSIT does not have practice effects (Bush et al., 2003), so the task can be repeatedly administered without significant changes in task performance over time.
Prior to the modified MSIT, participants were trained to recognize and categorize mind-wandering thoughts. They were given 5 sample thoughts to categorize. For example, for the sample thought “I can’t wait to hang out with my best friend later,” participants would press “Not Task,” “future,” and “positive” keys to indicate that this thought was unrelated to the task, was about the future, and was positive. Participants continued with the real experiment only after they had correctly categorized all 5 of these sample thoughts.
During the real version of the modified MSIT, participants were randomly interrupted 8 times. Instead of seeing a digit on the screen, the following question appeared: “Right before you saw these words, were you thinking about the task or something else?” Participants responded by pressing one key if they were having task-related thoughts and another key for task-unrelated thoughts. If they reported having task-unrelated thoughts they then saw another screen with the question “Were your thoughts about the past, present, or future?” This was followed by a final screen with a question that asked, “Were your thoughts positive, neutral, or negative?” After completion of these questions, participants resumed seeing digits on the screen.
Imaginal Processes Inventory
Previous tDCS studies have shown that baseline individual differences in the study construct can significantly change the possible effects of tDCS (e.g., Shen et al., 2016). All participants therefore completed the Imaginal Processes Inventory (IPI; Singer & Antrobus, 1972), a two-part self-report questionnaire, to assess and control for any individual differences in mind-wandering behavior in daily life. The first part contains 24 items assessing how frequently people daydream in real life (e.g., “When I am at a meeting or show that is not very interesting, I daydream rather than pay attention”). For the second part, items from three subscales were used (Huba, Aneshensel, & Singer, 1981). These 45 items assess how often people engage in guilty and fear of failure daydreams, (e.g., “I imagine myself failing those I love”) positive-constructive daydreams, (e.g., “My daydreams are often stimulating and rewarding”), and if people have poor attentional control in general (e.g., “No matter how hard I try to concentrate, thoughts unrelated to my work always creep in”). The IPI has adequate levels of internal consistency with alpha coefficients of at least .7 (Huba et al., 1981).
Data Analyses
We used one-way ANOVAs and chi-square tests to investigate whether the three stimulation (cathodal, anodal, or sham) groups differed on demographic characteristics, negative mood changes, and attention (as assessed by computer task accuracy and reaction time). Follow-up independent samples t-tests were used to examine significant between group differences. Paired samples t-tests were also conducted to test overall changes in negative mood ratings from baseline to after hearing criticism.
To examine whether receiving cathodal, anodal, or sham tDCS affected how often people mind-wandered during the computer task, we conducted a one-way ANOVA. The dependent variable was the change in percentage of mind-wandering reported during the computer task when interrupted (e.g., if they reported mind-wandering 4 out of the 8 times [50%] they were randomly interrupted during the computer task before stimulation, and then reported mind-wandering 6 out of the 8 times [75%] during the computer task after stimulation, then the change score would be an increase of 25%). Post-hoc t-tests were conducted to explore any significant findings. We also used one-way ANOVAs to test whether receiving cathodal, anodal, or sham tDCS changed the frequency of negative mind-wandering thoughts about the past. For our significant findings, we calculated η2 effect sizes for ANOVA analyses and Cohen’s d for t-tests.
Results
Demographics
The sample was 43.30% female and the average age was 30.07 years (SD = 8.66, range = 18 to 48 years). The average years of education was 15.47 (SD =3.01, range = 8 to 30 years). Participants were Caucasian (46.7%), Asian (14.4%), African American (14.4%), Hispanic (6.7%), Native American (1.1%), and Multiracial (16.7%). Participants in the three stimulation groups did not differ in age, gender, years of education, ethnicity, general daydreaming frequency in real life (as measured by the IPI), tendency to engage in positive-constructive or guilty-fear of failure daydreams. However, the groups differed in general inattention in real life as measured by the IPI poor attentional control subscale (F(2,89) = 3.58, p = 0.03, partial η2 = 0.08). Follow-up t-tests revealed that participants in the cathodal group had higher levels of inattention in real life (M = 48.20, SD = 7.88) than participants in the anodal group (M = 42.53, SD = 9.77; t(58) = 2.47, p = 0.02, Cohen’s d = 0.64). Participants in the sham group (M = 46.47, SD = 7.37) were not significantly different from participants in the anodal (t(58) = 1.16, p > 0.05) or cathodal (t(58) = −0.88, p > 0.05) groups. For all subsequent analyses, general inattention in real life was entered as a covariate to control for group differences.
Mood Ratings
Participants across the three groups felt more negative after hearing criticism (baseline M = 11.34 on a 0-100 scale, SD = 16.13; after criticism M = 19.34, SD = 22.38; t(89) = 5.05, p < 0.001); there were no significant group differences in this baseline (i.e. pre-tDCS) negative mood shift (F(2,90) = 0.44, p > 0.05). There was also no significant effect of stimulation condition on the post-tDCS change in negative mood ratings after criticism; that is, receiving tDCS did not change how negative people felt after hearing criticism (F(2,90) = 1.62, p > 0.05). Regarding absolute negative mood ratings, there were no significant group differences at baseline (F(2,87) = 0.69, p > 0.05) or after stimulation (F(2,87) = 1.30, p > 0.05).
Computer Task Performance
There was no significant effect of stimulation condition on computer task accuracy (F(2,86) = 0.16, p > 0.05) or reaction time (F(2,86) = 0.88, p > 0.05).
Mind-Wandering Frequency
There was no significant effect of stimulation condition on the change from pre to post stimulation in frequency of mind-wandering reported when participants were probed during the computer task (pre M = 26.25%, SD = 22.95%, post M = 28.89%, SD = 26.87%; F(2,28.26) = 1.03, p > 0.05), after controlling for the effect of general inattention in real life.
Mind-Wandering Thought Content
Before stimulation, there was no significant difference between the three groups in the frequency of negative mind-wandering thoughts about the past (F(2,87) = 1.04, p > 0.05). Stimulation condition had a significant effect on the change from pre to post stimulation in the frequency of negative mind-wandering thoughts about the past (F(2,86) = 3.72, p = 0.03, partial η2 = 0.08), even after controlling for the effect of general inattention in real life. This significant main effect was explored via post-hoc t-tests; Participants who received cathodal stimulation had a significant decrease in negative past-oriented mind-wandering thoughts (before stimulation M = 12.50%, SD = 31.31%; after stimulation M = 1.11%, SD = 6.09%) compared with participants who received sham stimulation who had an increase in negative past-oriented mind-wandering thoughts (before stimulation M = 3.06%, SD = 12.85; after stimulation M = 15.00%, SD = 35.11%; t(58) = −2.64, p = 0.01, Cohen’s d = 0.68; Figure 3). Participants who received anodal stimulation also had a decrease in negative mind-wandering thoughts about the past (before stimulation M = 10.00%, SD = 30.51%; after stimulation M = 3.33%, SD = 18.26%) relative to participants who received sham stimulation, but this difference was trending in significance (t(58) = −1.95, p = 0.06, Cohen’s d = 0.50).
Figure 3.

Participants who received cathodal stimulation had a significant decrease in negative past-oriented mind-wandering thoughts compared with participants who received sham stimulation (who had an increase in negative past-oriented mind-wandering thoughts). Participants who received anodal stimulation also had a decrease in negative mind-wandering thoughts about the past relative to participants who received sham stimulation, but this difference was trending in significance. Note: NS = not significant, *Two-tailed p < 0.05
Discussion
We investigated whether we could use a HD-tDCS montage targeting the inferior parietal DMN nodes bilaterally (1mA per hemisphere, 2mA total) using 8 combined electrodes to modulate maladaptive mind-wandering behavior. tDCS of the pIPL did not change the likelihood that participants would mind-wander during the computer task. In other words, tDCS did not change the frequency of overall mind-wandering. This was contrary to our hypotheses that anodal stimulation would lead to decreased DMN activity via the pIPL, and that, with decreased DMN activity, there would be a decrease in mind-wandering. Regardless of whether participants received cathodal or anodal stimulation, their mind-wandering frequency was comparable to participants who received sham stimulation.
The fact that tDCS of DMN parietal nodes did not change the frequency of mind-wandering in the present study is in contrast with previous studies by Kajimura and colleagues (Kajimura et al., 2016; Kajimura & Nomura, 2015) in which they found decreases in mind-wandering thoughts. However, their stimulation montage consisted of an electrode over the right IPL (not bilateral) and an electrode over the left LPFC. This montage may have resulted in opposite effects in multiple networks such as the executive control network (ECN) and the DMN (i.e. it may have been inhibiting one network with cathodal modulation while simultaneously activating the other networks with anodal). Also, electrodes were larger (5x7cm) and less focal, and although the overall applied current was lower (1.5 mA in their study versus 2 mA in ours) the current applied to each IPL target was lower in our study (1 mA for each hemisphere).
Beyond the studies by Kajimura and colleagues, other studies that have changed overall mind-wandering frequency with tDCS also targeted lateral prefrontal regions (Axelrod et al., 2015; Axelrod et al., 2018; Bertossi et al., 2017). The IPL and lateral prefrontal regions are both part of a fronto-parietal cognitive control network (Vincent, Kahn, Snyder, Raichle, & Buckner, 2008); it is possible that tDCS of the prefrontal nodes, rather than parietal, regions is required to regulate the general frequency of mind-wandering. At the same time, the DLPFC is in a network of brain regions that are “anticorrelated” (i.e., negatively correlated) with activity in the DMN; as activity increases in task-positive network regions such as the DLPFC, activity decreases in DMN regions (Fox et al., 2005). Therefore, excitatory tDCS of the DLPFC should affect, and likely reduce, activity in the DMN as well via trans-synaptic network effects. In other words, changing DMN activity should have also changed DLPFC activity, and therefore, mind-wandering behavior. However, our present null findings are more consistent with emerging evidence that the relationship between DMN activity and the occurrence of mind-wandering is more complex, such that functional coupling between the medial prefrontal cortex (MPFC) and the left IPL is associated with more deliberate forms of mind-wandering whereas weak patterns of functional coupling between the MPFC and the PCC are associated with more spontaneous, off-task forms of mind-wandering (Wang et al., 2017). In our study, we could not determine if tDCS of the pIPL actually changed its functional connectivity with the MPFC or PCC, and thus having a possible effect on deliberate or spontaneous mind-wandering. Future studies need to include the concurrent use of fMRI during tDCS and also test the modulation of other DMN brain regions such as the MPFC to understand the DMN’s involvement in the occurrence of mind-wandering.
Although tDCS of the pIPL had no effect on mind-wandering frequency, tDCS of the pIPL did change the content of people’s mind-wandering thoughts. Specifically, participants who received cathodal stimulation of the pIPL had a significant reduction in the frequency of negative mind-wandering thoughts about the past (Figure 3). This finding is in line with the fact that the pIPL is primarily coupled with other regions within the DMN’s MTL subsystem, which is associated with the remembering the past and the future (Andrews-Hanna, Reidler, Sepulcre, et al., 2010). However, we had hypothesized that cathodal tDCS would actually increase, rather than decrease, mind-wandering thoughts about the past based on Eldaief et al.’s (2011) study showing that inhibitory low frequency rTMS to the left pIPL increased connectivity within the MTL subsystem. Other researchers have also found that participants who report having no mind-wandering thoughts about the past and future show the lowest levels of MTL functional correlations (Andrews-Hanna, Reidler, Huang, et al., 2010). Luture studies will need to investigate tDCS concurrently with fMRI to identify whether cathodal tDCS of the pIPL actually increases or decreases activity within the MTL, and if this change in activity corresponds with changes in mind-wandering thought content.
Interestingly, although anodal stimulation did not cause a significant change (trending p = 0.06), it was still associated with a decrease in negative mind-wandering thoughts about the past (Figure 3). Anodal stimulation is typically thought to increase cortical excitability. However, it is important to note that the idea of anodal tDCS being “excitatory” and cathodal tDCS being “inhibitory” was conceived from studies targeting motor areas of the brain (e.g., Fregni et al., 2006; Nitsche & Paulus, 2000; Stagg et al., 2009). In fact, a meta-analysis revealed that the majority of tDCS studies targeting cognitive areas of the brain did not show a cathodal “inhibitory” and anodal “excitatory” effect (Jacobson, Koslowsky, & Lavidor, 2012). Multiple studies have also found that anodal and cathodal stimulation can have the same effect (in the same direction; e.g., Marshall, Mölle, Siebner, & Born, 2005; Sparing et al., 2009). Therefore, our findings are consistent with the non-motor area tDCS literature. Luture tDCS studies involving concurrent fMRI would clarify if anodal tDCS of the pIPL was actually decreasing activation in the pIPL and therefore inhibitory in nature.
Limitations and Future Directions
It is possible that our findings were due to random chance and/or an unidentified variable was driving our effects. Therefore, replication is needed and future studies should include control conditions such as including an active control stimulation group with individuals receiving active stimulation at a different brain region to clarify the specificity of the effects to tDCS of the pIPL. Further, we intentionally investigated mind-wandering behavior occurring during a negative mood due to its associations with maladaptive mind-wandering thoughts. The emotional stimuli used in this study were also standardized comments. In our study, listening to these comments resulted in an average increase of 8 points in negative mood ratings. Other studies have similarly found that these comments are associated with a small yet significant effect on mood ratings (Hooley et al., 2009; Hooley, Gruber, Scott, Hiller, & Yurgelun-Todd, 2005; Nook et al., 2018). Although the change in our study might appear small, this change translates to a 70.55% increase in negative mood. This is considered a large effect, particularly in the context of clinical trials often using the criterion of a 50% decrease in depressive symptoms as an indicator of treatment response (e.g., Frank et al., 1991). Nonetheless, future studies could investigate whether tDCS can be used to change negative thoughts that are induced by customized, highly self-relevant negative feedback (which would presumably be linked with greater increases in negative mood).
Additional research should examine whether tDCS of the pIPL can change the content of people’s mind-wandering thoughts during a positive or neutral mood. Since healthy individuals in positive and neutral moods tend to have more thoughts about the future rather than the past (Andrews-Hanna, Reidler, Huang, et al., 2010; Andrews-Hanna, Smallwood, & Spreng, 2014), it would be important to investigate whether tDCS of the pIPL in these individuals would change thoughts about the past and/or future.
Although the overall current used in this study was 2 mA, it was divided equally across hemispheres (1 mA per hemisphere). It is possible that stronger parietal currents may be needed to change the overall frequency of mind-wandering (as reported by others; e.g, Kajimura & Nomura, 2015) and could have stronger effects on the emotional content and temporal focus of mind wandering; dose-response study designs would be critical to address this question.
Another important limitation of this study was that it is possible that our approach to target definition using the 10-20 EEG coordinate system may not have been sufficiently specific in targeting the DMN representation within the IPL for each individual subject. tDCS in conjunction with MRI would first allow us to localize each participant’s pIPL for tDCS. Second, we could use fMRI to assess tDCS-related changes in DMN activation and connectivity. This approach would be the valuable next step to explore whether mind-wandering changes are specifically due to changes in DMN activity.
Conclusions
In this study, a novel multifocal high-definition tDCS montage targeting bilateral pIPL changed the content of people’s mind-wandering thoughts while in a negative mood. Given the clinical relevance of negative past-oriented thinking in individuals with depression, a natural extension of this work will be to investigate if bilateral cathodal tDCS of the pIPL will similarly help reduce this type of cognition in depressed individuals, and if these changes can be maintained over time. If so, tDCS could be further studied as a potential intervention or as an augmentation to cognitive therapies.
Acknowledgments
Funding
This study was funded by a training grant from the National Institutes of Health Blueprint for Neuroscience Research (Grant Numbers T90DA022759, R90DA023427) to T. Chou; National Institutes of Health (Grant Numbers RO1 MH112737, R21 DA042271, R21 AG056958, and R21 MH113018) to J.A. Camprodon.
Footnotes
Publisher's Disclaimer: This Author Accepted Manuscript is a PDF file of an unedited peer-reviewed manuscript that has been accepted for publication but has not been copyedited or corrected. The official version of record that is published in the journal is kept up to date and so may therefore differ from this version.
Conflict of Interest
T. Chou and J.M. Hooley declare that they have no conflict of interest. J.A. Camprodon is on the scientific advisory board for Apex Neuroscience.
Ethical Approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of Massachusetts General Hospital’s Institutional Review Board (Partners Human Research Committee; IRB protocol # 2015P000407) and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. This article does not contain any studies with animals performed by any of the authors.
Informed Consent
Informed consent was obtained from all individual participants included in the study.
References
- Andrews-Hanna JR, Reidler JS, Huang C, & Buckner RL (2010). Evidence for the default network’s role in spontaneous cognition. J Neurophysiol, 104(1), 322–335. doi: 10.1152/jn.00830.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews-Hanna JR, Reidler JS, Sepulcre J, Poulin R, & Buckner RL (2010). Functional-anatomic fractionation of the brain’s default network. Neuron, 65(4), 550–562. doi: 10.1016/j.neuron.2010.02.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews-Hanna JR, Smallwood J, & Spreng RN (2014). The default network and self-generated thought: component processes, dynamic control, and clinical relevance. Ann N Y Acad Sci, 1316, 29–52. doi: 10.1111/nyas.12360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antal A, Alekseichuk I, Bikson M, Brockmoller J, Brunoni AR, Chen R, … Paulus W. (2017). Low intensity transcranial electric stimulation: Safety, ethical, legal regulatory and application guidelines. Clin Neurophysiol, 128(9), 1774–1809. doi: 10.1016/j.clinph.2017.06.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antrobus JS (1968). Information theory and stimulus-independent thought. British Journal of Psychology, 59(4), 423–430. [Google Scholar]
- Antrobus JS, Singer JL, Goldstein S, & Fortgang M (1970). Mindwandering and cognitive structure. Trans N Y Acad Sci, 32(2), 242–252. [DOI] [PubMed] [Google Scholar]
- Antrobus JS, Singer JL, & Greenberg S (1966). Studies in the stream of consciousness: Experimental enhancement and suppression of spontaneous cognitive processes. Percept Mot Skills, 23(2), 399–417. [Google Scholar]
- Axelrod V, Rees G, Lavidor M, & Bar M (2015). Increasing propensity to mind-wander with transcranial direct current stimulation. Proceedings of the National Academy of Sciences, 112(11), 3314–3319. doi: 10.1073/pnas.1421435112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Axelrod V, Zhu X, & Qiu J (2018). Transcranial stimulation of the frontal lobes increases propensity of mind-wandering without changing meta-awareness. Scientific Reports, 5(1), 15975. doi: 10.1038/s41598-018-34098-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck AT (1967). Depression: Clinical, Experimental, and Theoretical Aspects. New York: Harper & Row. [Google Scholar]
- Beck AT (1970). Cognitive therapy: Nature and relation to behavior therapy. Behavior Therapy, 1(2), 184–200. doi: 10.1016/S0005-7894(70)80030-2 [DOI] [Google Scholar]
- Bertossi E, Peccenini L, Solmi A, Avenanti A, & Ciaramelli E (2017). Transcranial direct current stimulation of the medial prefrontal cortex dampens mind-wandering in men. Scientific Reports, 7(1), 16962. doi: 10.1038/s41598-017-17267-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bikson M, Grossman P, Thomas C, Zannou AL, Jiang J, Adnan T, … Woods AJ (2016). Safety of Transcranial Direct Current Stimulation: Evidence Based Update 2016. Brain Stimul, 9(5), 641–661. doi: 10.1016/j.brs.2016.06.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borckardt JJ, Bikson M, Frohman H, Reeves ST, Datta A, Bansal V, … George MS (2012). A Pilot Study of the Tolerability and Effects of High-Definition Transcranial Direct Current Stimulation (HD-tDCS) on Pain Perception. The Journal of Pain, 13(2), 112–120. doi: 10.1016/j.jpain.2011.07.001 [DOI] [PubMed] [Google Scholar]
- Buckner RL, Andrews-Hanna JR, & Schacter DL (2008). The brain’s default network: anatomy, function, and relevance to disease. Ann N Y Acad Sci, 1124, 1–38. doi: 10.1196/annals.1440.011 [DOI] [PubMed] [Google Scholar]
- Buckner RL, & Carroll DC (2007). Self-projection and the brain. Trends Cogn Sci, 11(2), 49–57. doi: 10.1016/j.tics.2006.11.004 [DOI] [PubMed] [Google Scholar]
- Bush G, Shin LM, Holmes J, Rosen BR, & Vogt BA (2003). The Multi-Source Interference Task: validation study with fMRI in individual subjects. Molecular Psychiatry, 8, 60. doi: 10.1038/sj.mp.4001217 [DOI] [PubMed] [Google Scholar]
- Butler AC, Chapman JE, Forman EM, & Beck AT (2006). The empirical status of cognitive-behavioral therapy: A review of meta-analyses. Clinical psychology review, 26(1), 17–31. doi: 10.1016/j.cpr.2005.07.003 [DOI] [PubMed] [Google Scholar]
- Cabeza R, & St Jacques P (2007). Functional neuroimaging of autobiographical memory. Trends Cogn Sci, 11(5), 219–227. doi: 10.1016/j.tics.2007.02.005 [DOI] [PubMed] [Google Scholar]
- Christoff K, Gordon AM, Smallwood J, Smith R, & Schooler JW (2009). Experience sampling during fMRI reveals default network and executive system contributions to mind wandering. Proc Natl Acad Sci U S A, 106(21), 8719–8724. doi: 10.1073/pnas.0900234106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards D, Cortes M, Datta A, Minhas P, Wassermann EM, & Bikson M (2013). Physiological and modeling evidence for focal transcranial electrical brain stimulation in humans: A basis for high-definition tDCS. Neuroimage, 74, 266–275. doi: 10.1016/j.neuroimage.2013.01.042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eldaief MC, Halko MA, Buckner RL, & Pascual-Leone A (2011). Transcranial magnetic stimulation modulates the brain’s intrinsic activity in a frequency-dependent manner. Proc Natl Acad Sci U S A, 108(52), 21229–21234. doi: 10.1073/pnas.1113103109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epel ES, Puterman E, Lin J, Blackburn E, Lazaro A, & Mendes WB (2013). Wandering minds and aging cells. Clinical Psychological Science, 1, 75–83. [Google Scholar]
- Fox MD, Snyder AZ, Vincent JL, Corbetta M, Van Essen DC, & Raichle ME (2005). The human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proc Natl Acad Sci U S A, 102(27), 9673–9678. doi: 10.1073/pnas.0504136102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank E, Prien RF, Jarrett RB, Keller MB, Kupfer DJ, Lavori PW, … Weissman MM (1991). Conceptualization and rationale for consensus definitions of terms in major depressive disorder. Remission, recovery, relapse, and recurrence. Arch Gen Psychiatry, 48(9), 851–855. [DOI] [PubMed] [Google Scholar]
- Fregni F, Boggio PS, Santos MC, Lima M, Vieira AL, Rigonatti SP, … Pascual-Leone A (2006). Noninvasive cortical stimulation with transcranial direct current stimulation in Parkinson’s disease. Mov Disord, 21(10), 1693–1702. doi: 10.1002/mds.21012 [DOI] [PubMed] [Google Scholar]
- Gbadeyan O, Steinhauser M, McMahon K, & Meinzer M (2016). Safety, Tolerability, Blinding Efficacy and Behavioural Effects of a Novel MRI-Compatible, High-Definition tDCS Set-Up. Brain Stimul, 9(4), 545–552. doi: 10.1016/j.brs.2016.03.018 [DOI] [PubMed] [Google Scholar]
- Hayes SC, Strosahl K, & Wilson KG (1999). Acceptance and Commitment Therapy: An experiential approach to behavior change. . New York, NY: Guilford Press. [Google Scholar]
- Hofmann SG, Asnaani A, Vonk IJJ, Sawyer AT, & Fang A (2012). The Efficacy of Cognitive Behavioral Therapy: A Review of Meta-analyses. Cognitive therapy and research, 36(5), 427–440. doi: 10.1007/s10608-012-9476-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofmann SG, Sawyer AT, Witt AA, & Oh D (2010). The effect of mindfulness-based therapy on anxiety and depression: A meta-analytic review. J Consult Clin Psychol, 78(2), 169–183. doi: 10.1037/a0018555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hooley JM, Gruber SA, Parker HA, Guillaumot J, Rogowska J, & Yurgelun-Todd DA (2009). Cortico-limbic response to personally challenging emotional stimuli after complete recovery from depression. Psychiatry Res, 172(1), 83–91. [DOI] [PubMed] [Google Scholar]
- Hooley JM, Gruber SA, Parker HA, Guillaumot J, Rogowska J, & Yurgelun-Todd DA (2010). Neural processing of emotional overinvolvement in borderline personality disorder. J Clin Psychiatry, 71(8), 1017–1024. doi: 10.4088/JCP.07m03465blu [DOI] [PubMed] [Google Scholar]
- Hooley JM, Gruber SA, Scott LA, Hiller JB, & Yurgelun-Todd DA (2005). Activation in dorsolateral prefrontal cortex in response to maternal criticism and praise in recovered depressed and healthy control participants. Biol Psychiatry, 57(7), 809–812. doi: 10.1016/j.biopsych.2005.01.012 [DOI] [PubMed] [Google Scholar]
- Huba GJ, Aneshensel CS, & Singer JL (1981). Development Of Scales For Three Second-Order Factors Of Inner Experience. Multivariate Behav Res, 16(2), 181–206. doi: 10.1207/s15327906mbr1602_4 [DOI] [PubMed] [Google Scholar]
- Jacobson L, Koslowsky M, & Lavidor M (2012). tDCS polarity effects in motor and cognitive domains: a meta-analytical review. Experimental Brain Research, 216(1), 1–10. doi: 10.1007/s00221-011-2891-9 [DOI] [PubMed] [Google Scholar]
- James W (1890). The stream of consciousness Principles of psychology. (Vol. 1, pp. 224–290). New York: NY: Dover Publications. [Google Scholar]
- Kabat-Zinn J (2003). Mindfulness-Based Interventions in Context: Past, Present, and Future. Clinical Psychology: Science and Practice, 10(2), 144–156. doi: 10.1093/clipsy.bpg016 [DOI] [Google Scholar]
- Kajimura S, Kochiyama T, Nakai R, Abe N, & Nomura M (2016). Causal relationship between effective connectivity within the default mode network and mind-wandering regulation and facilitation. Neuroimage, 133, 21–30. doi: 10.1016/j.neuroimage.2016.03.009 [DOI] [PubMed] [Google Scholar]
- Kajimura S, & Nomura M (2015). Decreasing propensity to mind-wander with transcranial direct current stimulation. Neuropsychologia, 75, 533–537. doi: 10.1016/j.neuropsychologia.2015.07.013 [DOI] [PubMed] [Google Scholar]
- Killingsworth MA, & Gilbert DT (2010). A wandering mind is an unhappy mind. Science, 330(6006), 932. doi: 10.1126/science.1192439 [DOI] [PubMed] [Google Scholar]
- Klinger E, & Cox WM (1987). Dimensions of thought flow in everyday life. Imagination, Cognition and Personality, 7(2), 105–128. [Google Scholar]
- Kuo H-I, Bikson M, Datta A, Minhas P, Paulus W, Kuo M-F, & Nitsche MA (2013). Comparing Cortical Plasticity Induced by Conventional and High-Definition 4 × 1 Ring tDCS: A Neurophysiological Study. Brain Stimul, 6(4), 644–648. doi: 10.1016/j.brs.2012.09.010 [DOI] [PubMed] [Google Scholar]
- Lefaucheur J-P, Antal A, Ayache SS, Benninger DH, Brunelin J, Cogiamanian F, … Paulus W (2017). Evidence-based guidelines on the therapeutic use of transcranial direct current stimulation (tDCS). Clinical Neurophysiology, 128(1), 56–92. doi: 10.1016/j.clinph.2016.10.087 [DOI] [PubMed] [Google Scholar]
- Lou HC, Luber B, Crupain M, Keenan JP, Nowak M, Kjaer TW, … Lisanby SH (2004). Parietal cortex and representation of the mental Self. Proc Natl Acad Sci U S A, 101(17), 6827–6832. doi: 10.1073/pnas.0400049101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maguire EA (2001). Neuroimaging studies of autobiographical event memory. Philosophical Transactions of the Royal Society of London. Series B, 356(1413), 1441–1451. doi: 10.1098/rstb.2001.0944 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall L, Mölle M, Siebner HR, & Born J (2005). Bifrontal transcranial direct current stimulation slows reaction time in a working memory task. BMC neuroscience, 6, 23–23. doi: 10.1186/1471-2202-6-23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason MF, Norton MI, Van Horn JD, Wegner DM, Grafton ST, & Macrae CN (2007). Wandering minds: the default network and stimulus-independent thought. Science, 315(5810), 393–395. doi: 10.1126/science.1131295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMillan R, Kaufman S, & Singer J (2013). Ode to positive constructive daydreaming. Front Psychol, 4(626). doi: 10.3389/fpsyg.2013.00626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikolin S, Loo CK, Bai S, Dokos S, & Martin DM (2015). Focalised stimulation using high definition transcranial direct current stimulation (HD-tDCS) to investigate declarative verbal learning and memory functioning. Neuroimage, 117, 11–19. doi: 10.1016/j.neuroimage.2015.05.019 [DOI] [PubMed] [Google Scholar]
- Nitsche MA, Cohen LG, Wassermann EM, Priori A, Lang N, Antal A, … Pascual-Leone A (2008). Transcranial direct current stimulation: State of the art 2008. Brain Stimul, 1(3), 206–223. doi: 10.1016/j.brs.2008.06.004 [DOI] [PubMed] [Google Scholar]
- Nitsche MA, & Paulus W (2000). Excitability changes induced in the human motor cortex by weak transcranial direct current stimulation. The Journal of Physiology, 527(3), 633–639. doi: 10.1111/j.1469-7793.2000.t01-1-00633.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nitsche MA, & Paulus W (2001). Sustained excitability elevations induced by transcranial DC motor cortex stimulation in humans. Neurology, 57(10), 1899–1901. [DOI] [PubMed] [Google Scholar]
- Nolen-Hoeksema S (2000). The role of rumination in depressive disorders and mixed anxiety/depressive symptoms. Journal of abnormal psychology, 109(3), 504–511. doi: 10.1037/0021-843X.109.3.504 [DOI] [PubMed] [Google Scholar]
- Nook EC, Dodell-Feder D, Hooley JM, DeLisi LE, & Hooker CI (2018). Weak dorsolateral prefrontal response to social criticism predicts worsened mood and symptoms following social conflict in people at familial risk for schizophrenia. NeuroImage: Clinical, 18, 40–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okuda J, Fujii T, Ohtake H, Tsukiura T, Tanji K, Suzuki K, … Yamadori A (2003). Thinking of the future and past: the roles of the frontal pole and the medial temporal lobes. Neuroimage, 19(4), 1369–1380. [DOI] [PubMed] [Google Scholar]
- Peterchev AV, Wagner TA, Miranda PC, Nitsche MA, Paulus W, Lisanby SH, … Bikson M (2012). Fundamentals of transcranial electric and magnetic stimulation dose: definition, selection, and reporting practices. Brain Stimul, 5(4), 435–453. doi: 10.1016/j.brs.2011.10.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson IH, Manly T, Andrade J, Baddeley BT, & Yiend J (1997). ‘Oops!’: performance correlates of everyday attentional failures in traumatic brain injured and normal subjects. Neuropsychologia, 55(6), 747–758. [DOI] [PubMed] [Google Scholar]
- Rossi S, Pasqualetti P, Zito G, Vecchio F, Cappa SF, Miniussi C, … Rossini PM (2006). Prefrontal and parietal cortex in human episodic memory: an interference study by repetitive transcranial magnetic stimulation. European Journal of Neuroscience, 25(3), 793–800. doi: doi: 10.1111/j.1460-9568.2006.04600.x [DOI] [PubMed] [Google Scholar]
- Ruffini G, Fox MD, Ripolles O, Miranda PC, & Pascual-Leone A (2014). Optimization of multifocal transcranial current stimulation for weighted cortical pattern targeting from realistic modeling of electric fields. Neuroimage, 89, 216–225. doi: 10.1016/j.neuroimage.2013.12.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarkis RA, Kaur N, & Camprodon JA (2014). Transcranial Direct Current Stimulation (tDCS): Modulation of Executive Function in Health and Disease. Current Behavioral Neuroscience Reports, 1(2), 74–85. doi: 10.1007/s40473-014-0009-y [DOI] [Google Scholar]
- Schacter DL, Addis DR, & Buckner RL (2007). Remembering the past to imagine the future: the prospective brain. Nat Rev Neurosci, 8(9), 657–661. doi: 10.1038/nrn2213 [DOI] [PubMed] [Google Scholar]
- Schacter DL, Addis DR, & Buckner RL (2008). Episodic simulation of future events: concepts, data, and applications. Ann N Y Acad Sci, 1124, 39–60. doi: 10.1196/annals.1440.001 [DOI] [PubMed] [Google Scholar]
- Shen B, Yin Y, Wang J, Zhou X, McClure SM, & Li J (2016). High-definition tDCS alters impulsivity in a baseline-dependent manner. Neuroimage, 143, 343–352. doi: 10.1016/j.neuroimage.2016.09.006 [DOI] [PubMed] [Google Scholar]
- Singer JL, & Antrobus JS (1972). Daydreaming, imaginal processes, and personality: A normative study In Sheehan PW (Ed.), The Function and Nature of Imagery. New York and London: Academic Press. [Google Scholar]
- Smallwood J, & Andrews-Hanna J (2013). Not all minds that wander are lost: the importance of a balanced perspective on the mind-wandering state. Front Psychol, 4, 441. doi: 10.3389/fpsyg.2013.00441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smallwood J, & O’Connor RC (2011). Imprisoned by the past: unhappy moods lead to a retrospective bias to mind wandering. Cogn Emot, 25(8), 1481–1490. doi: 10.1080/02699931.2010.545263 [DOI] [PubMed] [Google Scholar]
- Sparing R, Thimm M, Hesse MD, Kust J, Karbe H, & Fink GR (2009). Bidirectional alterations of interhemispheric parietal balance by non-invasive cortical stimulation. Brain, 132(Pt 11), 3011–3020. doi: 10.1093/brain/awp154 [DOI] [PubMed] [Google Scholar]
- Stagg CJ, & Nitsche MA (2011). Physiological basis of transcranial direct current stimulation. Neuroscientist, 17(1), 37–53. doi: 10.1177/1073858410386614 [DOI] [PubMed] [Google Scholar]
- Stagg CJ, O’Shea J, Kincses ZT, Woolrich M, Matthews PM, & Johansen-Berg H (2009). Modulation of movement-associated cortical activation by transcranial direct current stimulation. Eur J Neurosci, 30(7), 1412–1423. doi: 10.1111/j.1460-9568.2009.06937.x [DOI] [PubMed] [Google Scholar]
- Svoboda E, McKinnon MC, & Levine B (2006). The functional neuroanatomy of autobiographical memory: a meta-analysis. Neuropsychologia, 44(12), 2189–2208. doi: 10.1016/j.neuropsychologia.2006.05.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szpunar KK, Watson JM, & McDermott KB (2007). Neural substrates of envisioning the future. Proc Natl Acad Sci U S A, 104(2), 642–647. doi: 10.1073/pnas.0610082104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villamar MF, Wivatvongvana P, Patumanond J, Bikson M, Truong DQ, Datta A, & Fregni F (2013). Focal Modulation of the Primary Motor Cortex in Fibromyalgia Using 4×1-Ring High-Definition Transcranial Direct Current Stimulation (HD-tDCS): Immediate and Delayed Analgesic Effects of Cathodal and Anodal Stimulation. The Journal of Pain, 14(4), 371–383. doi: 10.1016/i.ipain.2012.12.007 [DOI] [PubMed] [Google Scholar]
- Vincent JL, Kahn I, Snyder AZ, Raichle ME, & Buckner RL (2008). Evidence for a frontoparietal control system revealed by intrinsic functional connectivity. J Neurophysiol, 100(6), 3328–3342. doi: 10.1152/jn.90355.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang HT, Poerio G, Murphy C, Bzdok D, Jefferies E, & Smallwood J (2017). Dimensions of Experience: Exploring the Heterogeneity of the Wandering Mind. Psychol Sci, 956797617728727. doi: 10.1177/0956797617728727 [DOI] [PMC free article] [PubMed] [Google Scholar]


