Abstract
Introduction
In recent years, there has been significant growth of ambulatory oncology pharmacy, yet there is a paucity of published studies on the clinical activities and impact of ambulatory oncology clinical pharmacists. At Duke Cancer Center, dedicated pharmacist services are embedded in specialized outpatient oncology areas. Pharmacists document their clinical and administrative activities in the electronic health record. The primary objective of this study is to quantify and assess ambulatory oncology pharmacist interventions in clinics in a large academic comprehensive cancer center.
Methods
For the purposes of this single-center, retrospective, descriptive study, pharmacist interventions were collected, quantified, and described over a 6-month period from July 1 to December 31, 2015. The study evaluated the perceived contribution and impact of a pharmacist on patient care in ambulatory oncology clinics via a survey that was distributed to providers and nurses.
Results
In the 6-month time period, there were 5,091 interventions spanning 3,967 patient encounters between nine ambulatory oncology clinic pharmacists. The average time per encounter in the 6-month time frame was 22.4 minutes. There were 92 respondents to the survey (61.7% response rate). Overall, responses showed that the clinical pharmacists add value to patient care and are integral members of the team.
Conclusions
Although previous studies have described pharmacist activities in outpatient oncology clinics, this study showed a larger number and variety of clinical pharmacist activities in outpatient cancer clinics to improve patient care. Future directions include conducting prospective, controlled studies to link pharmacist activities to tangible outcomes.
Currently, there is a paucity of published data on the clinical activities and impact of ambulatory oncology clinical pharmacists. In 1999, Wong and Gray evaluated the impact of a clinical pharmacist in ambulatory hematology/oncology clinics. In the 36-day study time period, 211 pharmacy interventions were documented, with the most common being patient education and therapeutic recommendations (Wong & Gray, 1999). In 2006, Shah and colleagues published an evaluation from the Veterans Affairs (VA) North Texas Health Care System that examined 423 patient visits for chemotherapy follow-up or disease management in a 1-year period. They quantified the visits and interventions and found that the major drug-specific pharmacist interventions were for drug addition (41%), discontinuation (23%), and/or adjustment (21%). The pharmacist(s) addressed 342 supportive care issues including anemia (18%), pain (10%), constipation/diarrhea (6%), and nausea/vomiting (6%; Shah, Dowell, & Greene, 2006). In 2010, Ruder and colleagues published a retrospective descriptive analysis of clinical interventions of a single ambulatory oncology pharmacist in a 2-year period. They measured the number of interventions (583) and reported that the average time per intervention was 10 minutes. Drug-related interventions (35% of measured interventions) included obtaining a medication history, providing drug information, and drug calculations. The remaining 65% were consultative interventions including patient education, patient visits, and follow-up, and the provision of drug information (Ruder, Smith, Madsen, & Kass, 2011). Gatwood and colleagues published a review article of the impact of clinical pharmacists in outpatient oncology practices, which summarizes eight former publications, mostly retrospective chart reviews and descriptive analyses. Some studies reported cost avoidance, patient drug savings, prescription referral revenue, and patient satisfaction associated with pharmacist-related services (Gatwood, Gatwood, Gabre, & Alexander, 2017).
The Duke University Hospital is a 957-bed academic medical center and is the flagship hospital of Duke University Health System. In 2012, Duke University Hospital opened a new comprehensive ambulatory cancer center where all necessary services are provided in one location including clinics, radiology, infusion, lab, pharmacy, and counseling services. The cancer center has pharmacists in disease-based clinics, an infusion pharmacy, and a specialty retail pharmacy. At the time of the study, the Duke Cancer Center had 9 (7.5 FTEs) ambulatory oncology clinical pharmacists (AOCPs) who worked with providers throughout 11 specialized oncology clinics.
The aim of this study is to quantify the pharmacologic interventions and support that these pharmacists provide in the ambulatory oncology setting. An intervention is referred to as the interaction between pharmacists and patients or pharmacists and other health-care providers. Interventions are documented through the use of iVents in the electronic health record (EHR), which was implemented in 2013. This includes but is not limited to patient education on chemotherapy regimens, drug information provided to health-care providers, modifications in chemotherapy treatment plans, and activities related to financial review and assisting in the prior authorization process for medications.
This study will (1) showcase the variety of interventions made on a daily basis, (2) highlight the perceived impact they have on patient care, and (3) contribute to the literature in the regard of ambulatory oncology pharmacy practice.
METHODS
In this single-center, observational, descriptive analysis, all documented oncology clinic–based pharmacist interventions between July 1, 2015, and December 31, 2015, were included. Interventions, called “iVents,” were collected via the EHR iVent tool. The iVents were reviewed both at a global, qualitative view, and then reviewed more quantitatively at the details of each pharmacist intervention.
To gather the data of the overall interventions made, pharmacist iVents were searched using their last name in the 6-month time period via monthly reports exported into Microsoft Excel from EHR for analysis. The information provided in the Excel file included the type of intervention (category), any subtype(s), time spent in the encounter, and the pharmacist name that created the intervention.
To gather more details and quantitative data about the interventions, iVents were searched using the last name of the pharmacist and manually collected into a Qualtrics data collection tool. The specific iVents collected for further analysis included pharmacotherapy intervention, oral chemotherapy management, pharmacy administration, and patient education. The data for these iVents were collected over a 3-month period from July 1, 2015, to September 30, 2015. Some encounters included multiple types of iVents; thus, one encounter could be categorized and counted under multiple categories of interventions. Data collected on these iVents included type of intervention (category), time spent in intervention (minutes), clinic area in which the intervention was performed, if there was a prescription or medication ordered, and a blank text box to describe the encounter.
Table 1 describes the different specialized oncology clinics within Duke Cancer Center, with associated pharmacist participation. The gynecologic oncology and melanoma clinics did not have a dedicated pharmacist in clinic, but were covered by the clinical pharmacist group on an as-needed basis. Contact information for pharmacy staff is readily available and utilized by providers in these clinics.
Table 1. Pharmacist Time Spent in Clinic at the Time of Study.
| Primary clinic | Pharmacist time in clinic |
|---|---|
| Adult BMT | Full time |
| Brain tumor | Full time |
| Breast | Full time |
| Gastrointestinal | Full time |
| Genitourinary | Full time for 33% of study period |
| Gynecologic oncology | No dedicated pharmacist in clinic |
| Hematologic malignancy | Full time |
| Melanoma | No dedicated pharmacist in clinic |
| Pediatric BMT | 2 days per week |
| Sarcoma | 2 days per week |
| Thoracic | 2.5 days per week |
Note. BMT = bone marrow transplant.
The primary endpoint was to quantify the number of pharmacologic interventions made by pharmacists in the ambulatory oncology setting. Secondary endpoints included evaluating, categorizing, and describing the different types of interventions performed by ambulatory oncology pharmacists and evaluating the perceived contribution and impact on patient care of a pharmacist in ambulatory oncology clinics via a provider and nursing survey.
Providers and nurses who worked in any of the outpatient oncology clinics at the Duke Cancer Center were provided with a survey assessing pharmacist services in clinic. An e-mail describing the study with a link to the survey was sent to 149 providers and nurses. Questions were asked using the Likert-type ranking scale, where participants answered questions regarding pharmacy services on the scale from “strongly agree” to “strongly disagree.”
Statistical Analysis
This study used descriptive statistics to evaluate the number of pharmacologic interventions made by pharmacists as well as to categorize the different types of interventions performed by ambulatory oncology pharmacists. The descriptive statistics for categorical variables are reported as counts and percentages.
RESULTS
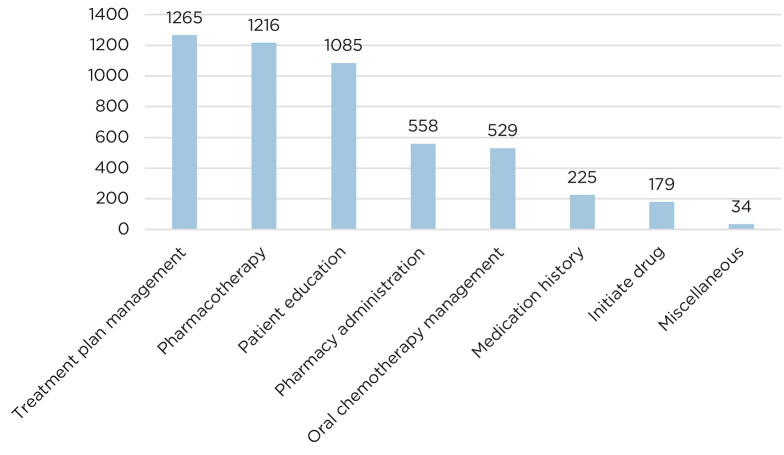
In the 6-month time period, there were 5,091 iVents spanning 3,967 patient encounters (Figure 1) between 9 AOCPs.
Figure 1.
Number of iVents collected per category in 6-month study period.
One quarter (25%) of the 5,091 interventions were documented as the “treatment plan management” iVent. This category included pharmacists entering and writing chemotherapy orders/plans, writing and sending prescriptions, and adjusting regimens based on labs, vitals, and side effects. The 3-month in-depth analysis revealed that there were over 400 orders and prescriptions written by clinic pharmacists.
The second largest category of interventions was “pharmacotherapy interventions” (1,216; 24%), in which pharmacists provided medication regimen or dosing changes (31%), symptom management (24%), drug information (19%), adverse drug event or drug-drug interaction support (14%), or miscellaneous interventions including antibiotic management, stress ulcer prophylaxis, and total parenteral nutrition recommendations (3.6%). Within the 3-month data analysis, there were 166 interventions regarding symptom management. Of these, nausea/vomiting (67; 40%), constipation/diarrhea (43; 27%), pain (19; 11%), and other symptoms such as hypertension, mucositis, rash, headache, and sleep (37, 22%) were the primary reasons for pharmacist involvement.
Patient education was another common intervention category, accounting for 21% (1,085 of 5,091) of total interventions over 6 months. The in-depth analysis with quantitative data revealed that there were 535 direct patient education sessions. Of these, 80% were for chemotherapy education, and the remaining 20% were for nonchemotherapy education including antiepileptic medications, steroids, antiemetics, and anticoagulants. Chemotherapy education by a pharmacist includes talking with the patient and family about how chemotherapy drugs are given, frequency of the regimen, possible side effects, and how to manage these. Additionally, pharmacists review current medications and evaluate if there will be any concerns related to drug interactions, and determine supportive care prescriptions that are needed.
In the 6-month time period, 11% (558) of interventions were related to “pharmacy administration.” All of these interventions are of the subtype “financial review,” in which pharmacists primarily perform prior authorizations and refer patients to a patient assistance program as needed.
Oral chemotherapy management comprised 529 (10%) of the 5,091 interventions in the 6-month study period. The subtypes in this intervention include patient education, symptom management, financial review, and other. “Other” was most frequently inclusive of a follow-up phone call regarding oral chemotherapy. In the 3-month quantitative data analysis, there were 173 interventions categorized as “oral chemotherapy management.” Of the oral chemotherapy management iVents, financial review (29%; 51), other (42%; 72), patient education (24%; 41), and symptom management (5%; 9) were the types of activities included.
In the 6-month time period, there were a total of 5,091 interventions documented in which pharmacists spent a total of 1,485 hours of time. The average time per iVent was 17.5 minutes, while the average time per encounter between the pharmacist and patient, which in some cases included multiple interventions, was 22.4 minutes.
Provider and Nurse Survey Regarding Pharmacist Impact in Clinic
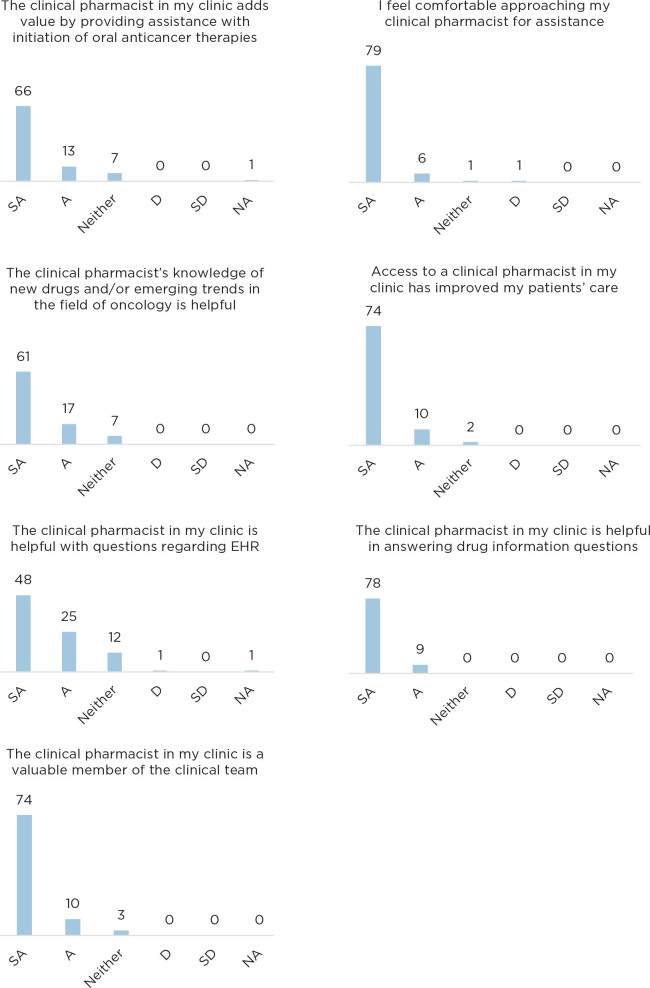
A provider and nurse survey was circulated to assess the perceived impact of the pharmacist in the ambulatory oncology clinics. The survey was sent to 149 providers and nurses; there were 92 responses (61.7%) in the 2-week survey window. Respondents included 40% (37) providers and 60% (55) nurses. The majority, 93%, strongly agreed or agreed that they routinely work with the clinical pharmacist in their clinic. The results to each survey question can be seen in Figure 2.
Figure 2.
Responses to provider and nursing survey. SA = strongly agree; A = agree; D = disagree; SD = strongly disagree; NA = not applicable.
At the end of the survey, participants were provided a free text box to respond and leave any additional feedback about clinical pharmacy services in clinic. Forty of the respondents left comments. An example of a comment is: “The access to our clinical pharmacist has not only added to the quality of care provided to the patients but to the standard of practice of the nurses and providers. The pharmacist presence in clinic has proven to be invaluable.” Many of the responses included strongly positive words such as invaluable, knowledgeable, outstanding, approachable, fantastic, reliable, and helpful. Many participants commented that they would support further expanding with additional pharmacists in clinic to adequately cover all of their needs.
DISCUSSION
This study included nine clinical oncology pharmacists who were actively involved in direct patient care at eleven specialized oncology clinics. In 6 months, pharmacists participated in over 5,000 direct patient care activities with patients.
When pharmacists assist with treatment plan management, it includes placing chemotherapy orders for patients, adjusting orders for patients based on new information (such as dose reductions), writing prescriptions, and ordering labs. The roles of oncology Clinical Pharmacy Practitioners (CPPs) at Duke Cancer Center has been described in the literature (Sessions, Valgus, Barbour, & Iacovelli, 2010). Clinical Pharmacy Practitioners form collaborative practice agreements with physicians in a specific disease state(s) and have privileges that include ordering, changing, substituting therapies, or ordering laboratory tests. In our study, of the nine ambulatory oncology clinical pharmacists, the majority were credentialed CPPs. Allowing pharmacists to practice at the top of their license and participate in direct patient care saves providers’ time and allows providers to focus on treating the primary disease. In a 3-month period, the pharmacists placed over 400 orders and prescriptions for medications. Pharmacists are highly knowledgeable in the computerized physician order entry (CPOE) system and can efficiently enter orders and prescriptions, especially in the context of complex chemotherapy regimens. In states without a CPP credentialing process, licensed pharmacists can participate in all activities described in this study outside of placing prescription orders. Further, with a collaborative practice agreement in place that details specific patient care responsibilities, pharmacists may be able to further participate in pharmacotherapy interventions, oral chemotherapy management, as well as treatment plan management.
In addition to treatment plan management, in which pharmacists adjust orders and send prescriptions, pharmacists are also frequently consulted to develop medication regimens. Over 6 months, there were 374 “medication regimen or dosing change” subtype iVents under the “pharmacotherapy intervention” category. Pharmacists are most frequently consulted to proactively adjust insulin, supplement electrolytes, taper steroids, provide antibiotic dosing and adjustments, and adjust formulations of medications to meet the needs of the patient. Occasionally, pharmacists are sent messages from patients via e-mail through the EHR’s messaging system regarding over-the-counter supplement safety, among other medication-related topics, to which they respond and provide recommendations. This can help alleviate some of the workload of primary providers; moreover, pharmacists are knowledgeable about supplements and medications and are credentialed to answer patient questions.
Another subtype of “pharmacotherapy intervention” is pharmacists providing supportive care management, documented under “symptom management.” In 6 months, there were 295 symptom management interventions. When investigated further in a 3-month time period, of the 148 interventions, the majority were for management of nausea and vomiting (47%). Pharmacists recommend initiation of an alternative antiemetic regimen and communicate the regimens to the patient, either in person or via telephone. Pharmacists also counsel patients and adjust regimens for patients’ symptoms during chemotherapy, including constipation, diarrhea, hypertension, mucositis, rash, headache, and sleep.
Pharmacists frequently provide patient education with almost every encounter, but there were 21% of total interventions dedicated to patient education. When clinic pharmacists provide chemotherapy education, they address many aspects of the regimen, including potential side effects and how to manage those effects. Pharmacists provide patients with treatment calendars of chemotherapy and supportive care management. For example, the brain tumor clinical pharmacist educates all patients on their steroid dosing and taper schedule, and provides them with a detailed calendar of how to taper. The time that pharmacists spend with patients is valuable to increase patient adherence and understanding of their medications and to decrease medication errors such as taking a medication incorrectly. Patients are proactively taught how to manage their side effects, which can decrease the potential for urgent care or emergency department visits and hospitalizations.
In addition to clinical activities, Duke Cancer Center pharmacists assist in the financial review of patients’ medications and streamline the process for patients. Pharmacists frequently support the completion of third-party prior authorizations for medications and proactively alert the Duke Cancer Center Specialty Pharmacy and the patient to communicate that medications have been approved by insurance and to begin processing the prescription. Pharmacists file appeals with insurance companies and assist in referring patients to patient assistance programs and financial care counselors. An area for future studies is to compare differences in time to treatment initiation with pharmacist involvement in the prescription process from the beginning compared with no pharmacist involvement. Additionally, financial burden and outcomes in these patients should also be studied. There were over 550 encounters in which a pharmacist assisted in the financial review process, which we view as a significant aid to patients, providers, and the overall process of procuring specialty medications in a timely manner.
Many patients are currently being treated with oral chemotherapy agents. There are numerous new oral chemotherapy drugs on the market today, and in 2008, the National Comprehensive Cancer Network estimated that a quarter of the 400 antineoplastic drugs in the pipeline were planned as oral agents (Weingart et al., 2008). “Oral chemotherapy management” (10% of total interventions) was also captured as an intervention category. This category had three subtypes, including patient education, symptom management, and financial review, in which pharmacists performed the same type of activities as they did in other categories. In addition, pharmacists provide follow-up phone calls to patients recently started on oral chemotherapy to assess for adherence and toxicity. This iVent category is important to capture the benefit of an AOCP regarding oral chemotherapy management and follow-up.
In 6 months, AOCPs at Duke Cancer Center spent over 1,400 hours working on patient-related activities. The average time per intervention was 17.5 minutes, while the average time per encounter was 22.4 minutes. Multiple interventions often occur during a single encounter.
Limitations
The main limitation of this study includes potential underdocumentation of interventions or iVents. All AOCPs were briefed about the study, including standardizing how to document iVents for consistency of the data prior to the study period. Documenting iVents was not mandatory for all AOCPs. While this limitation may have affected the total number of interventions and encounters, it did not inhibit the study because there was still ample data to fully understand and describe pharmacists’ contribution to patient care in clinics. In future studies that tie interventions to outcomes, it is prudent that all pharmacists document iVents consistently.
Additionally, it should be noted that the differences in total monthly iVents and encounters do not take into account pharmacists’ time outside of clinic, including paid time off, meetings, and other administrative activities in which pharmacists participate. Of note, the pharmacists in this study do not verify chemotherapy infusion orders, and thus this study was a way to highlight their contribution and productivity in the clinic setting. Regardless of these limitations, the study adequately described pharmacists’ activities, roles, and perceived impact in ambulatory oncology clinics.
Future Directions and Studies
This study describing pharmacists’ activities in clinics provides a backbone to future controlled studies that will link pharmacists’ activities to patient outcomes. For example, a future study related to the activities surrounding “pharmacy administration” iVent activities can include studying the difference in time to treatment initiation, patient out-of-pocket cost, overall costs of therapy, and outcomes when patients have pharmacist involvement in their care on the financial side compared with no pharmacist involvement. When studied, this level of service for patients can prove the impact of pharmacist involvement on patients overall care and out-of-pocket costs of therapy. Randolph and colleagues conducted a prospective study in an ambulatory cancer center over 4 weeks in which a pharmacy resident and centralized pharmacist documented and assigned their interventions to cost avoidance values based on previous literature. In 1 month, the 962 interventions made by the two pharmacists estimated to approximately $40,478 in cost avoidance, and when extrapolated to a 40-hour work week, the combined cost avoidance for 1 year was $565,482 (Randolph, Walker, Nguyen, & Zachariah, 2016). Future studies in the Duke Cancer Clinics can associate pharmacist interventions to validated cost saving or cost avoidance activities, which will further validate the clinical pharmacist role in an ambulatory oncology clinic.
Another potential controlled study on the activities of pharmacists could evaluate adherence and understanding of chemotherapy regimens, the benefits of treatment calendars, and overall outcomes with treatment in those patients who received chemotherapy education from a pharmacist compared with those who did not. Similarly, in regards to supportive care education and follow-up by pharmacists, one could study patient adherence to supportive care regimens, impact of pharmacist follow-ups (via phone or EHR messaging), and the difference in urgent care/emergency department visits or hospitalizations.
Finally, a major intervention for pharmacists in this study was activities related to “treatment plan management” and “medication regimen or dosing change” (subtype under pharmacotherapy intervention). Future controlled studies could compare provider time saved by utilizing a pharmacist to enter orders or treatment plans or the difference in medication errors and adverse events related to ordering/prescribing medications with pharmacist involvement compared with no pharmacist involvement.
CONCLUSION
Previous studies have described pharmacist activities in outpatient oncology clinics. This study showed a larger number and breadth of clinical pharmacist activities in outpatient cancer clinics with embedded pharmacists to improve and streamline patient care. It was shown that pharmacists are highly valued members of the clinical team. Future directions include conducting prospective, controlled studies to link the pharmacist activities to tangible outcomes to provide data to bolster the presence of oncology pharmacists in ambulatory clinics.
References
- Gatwood G., Gatwood K., Gabre E., & Alexander M. (2017). Impact of clinical pharmacists in outpatient oncology practices: A review. American Journal of Health-System Pharmacy, 74(19), 1549–1557. 10.2146/ajhp160475 [DOI] [PubMed] [Google Scholar]
- Randolph L. A., Walker C. K., Nguyen A. T., & Zachariah S. R. (2016). Impact of pharmacist interventions on cost avoidance in an ambulatory cancer center. Journal of Oncology Pharmacy Practice, 24(1), 3–8. 10.1177/1078155216671189 [DOI] [PubMed] [Google Scholar]
- Ruder A. D., Smith D. L., Madsen M. T., & Kass F. H. (2011). Is there a benefit to having a clinical oncology pharmacist on staff at a community oncology clinic? Journal of Oncology Pharmacy Practice, 17(4), 425–432. 10.1177/1078155210389216 [DOI] [PubMed] [Google Scholar]
- Sessions J. K., Valgus J. M., Barbour S. Y., & Iacovelli L. (2010). Role of oncology clinical pharmacists in light of the oncology workforce study. Journal of Oncology Practice, 6(5), 270–272. 10.1200/JOP.000037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah S., Dowell J., & Greene S. (2006). Evaluation of clinical pharmacy services in a hematology/oncology outpatient setting. Annals of Pharmacotherapy, 40(9), 1527–1533. 10.1345/aph.1H162 [DOI] [PubMed] [Google Scholar]
- Weingart S. N., Brown E., Bach P. B., Eng K., Johnson S. A., Kuzel T. M.,…Walters R. S. (2008). NCCN Task Force report: Oral chemotherapy. Journal of the National Comprehensive Cancer Network, 6(suppl 3), S1–S14. [PubMed] [Google Scholar]
- Wong S. W., & Gray E. S. (1999). Clinical pharmacy services in oncology clinics. Journal of Oncology Pharmacy Practice, 5(1), 49–54. 10.1177/107815529900500104 [DOI] [Google Scholar]