Objectives:
Gender-affirming hormone therapy (GHT) is utilized by people who are transgender to align their secondary sex characteristics with their gender identity. Data relating to cardiovascular outcomes in this population are limited. We aimed to review the impact of GHT on the blood pressure (BP) of transgender individuals.
Methods:
We searched PubMed/MEDLINE, SCOPUS and Cochrane Library databases for articles published relating to the BP of transgender adults commencing GHT. Methodological quality was assessed via the ‘Quality Assessment Tool for Before–After (Pre–Post) Studies with No Control Group’.
Results:
Six hundred articles were screened, of which 14 studies were included in this systematic review encompassing 1309 individuals (∼50% transgender men and women) treated with GHT between 1989 and 2019. These articles were all pre–post observational studies without control groups. Mean ages ranged between 23.0–36.7 years (transgender men) and 25.2–34.8 years (transgender women). Interventions were diverse and included oral, transdermal and injectable hormonal preparations with 4 months to 5 years follow-up. Most studies in transgender men did not demonstrate a change in BP, whereas transgender women on GHT demonstrated both increases and decreases in SBP. These studies were heterogenous with significant methodological limitations and only two were determined to have a good quality rating.
Conclusion:
There is currently insufficient data to advise the impact of GHT on BP in transgender individuals. Better quality research is essential to elucidate whether exogenous sex hormones modulate BP in transgender people and whether this putative alteration infers poorer cardiovascular outcomes.
Keywords: blood pressure, estrogen, testosterone, transgender
INTRODUCTION
Transgender people experience gender dysphoria due to incongruence between their gender identity and the sex they were assigned at birth [1]. Gender-affirming hormone therapy (GHT), including testosterone, estrogen, gonadotropin-releasing hormone (GnRH) analogues and antiandrogens, aims to align the characteristics of transgender people with their gender identity [1].
The recent substantive increases in population prevalence of transgender people requires the implementation of evidence-based guidance to better protect the health of this population [2–4]. However, due to a paucity of epidemiological and mechanistic data, there is considerable uncertainty surrounding the impact of GHT on the cardiovascular health of transgender individuals [5–7]. Existing data suggest that the use of estrogens in transgender women confers an increased risk of myocardial infarction and ischemic stroke. Conversely, transgender men receiving testosterone lack any consistent evidence of an increased risk of cardiovascular or cerebrovascular disease [7]. However, in the absence of randomized controlled trials or comprehensive prospective longitudinal studies, ambiguity remains regarding whether such risk exists, and by which interventions this risk can be ameliorated.
Consequently, international guidelines, set out by the Endocrine Society and World Professional Association for Transgender Health, have been cautious in their recommendations regarding modifiable risk factors for cardiovascular disease in this population [8,9]. Hypertension remains the most important modifiable risk factor for the development of cardiovascular disease. Therefore, these organizations have not unreasonably recommended that blood pressure (BP) is monitored in transgender people receiving GHT. However, these guidelines do not provide any indication of the effect size of this treatment on BP, treatment targets for this patient group, or the levels of evidence for this recommendation.
A comprehensive overview is therefore required to assess the impact of GHT on the BP. Our aim was to perform a systematic review of the relationship of exogenous sex hormones and SBP and DBP in transgender men and women commencing treatment.
METHODS
Eligibility criteria
We included randomized trials and observational studies of transgender individuals who used GHT regardless of gender-reassignment surgery status. Eligible studies included transgender men prescribed testosterone and transgender women prescribed estrogen, antiandrogens (cyproterone acetate, finasteride or spironolactone) or GnRH agonists. Studies were included regardless of dose of GHT. Studies were required to provide a comparison of BP before and after/during treatment for a minimum of 3 months. Articles with participants under the age of 16 were excluded in addition to review articles, commentaries and letters that did not contain original data. BP measurements could include manual, automated or ambulatory recordings. Many transgender individuals receiving GHT may be gender non-binary (e.g. their gender identity or expression does not confirm to typical binary gender), and may not consider themselves as simply men or women [3]. However, for the purposes of this systematic review, transgender people treated with estrogen (with or without antiandrogens and GnRH analogues) were considered transgender women, whereas transgender individuals treated with testosterone were considered transgender men.
Search strategy
The current systematic review adhered to the Preferred Reporting Items for Systematic Reviews guidelines [10]. PubMed/MEDLINE, SCOPUS and Cochrane Library databases were searched for articles published until 13 January 2020. The search was limited to English language articles.
The following medical subject headings were used in the search: ‘blood pressure’ AND ‘transgender’ OR ‘transgender person’ OR ‘person, transgender’ OR ‘persons, transgender’ OR ‘transgender persons’ OR ‘transmasculine’ OR ‘transfeminine’ OR ‘trans masculinization’ OR ‘trans feminization’ OR ‘gender incongruent’ OR ‘gender dysphoric’ OR ‘gender affirming’ OR ‘transsexual persons’ OR ‘person, transsexual’ OR ‘persons, transsexual’ OR ‘transsexual person’ OR ‘transgender male’ OR ‘transgender female’ OR ‘transgender man’ OR ‘transgender woman’ OR ‘trans man’ OR ‘trans woman’ OR ‘cross-sex’.
Data extraction, outcome and quality
The literature search, data extraction and quality assessment were conducted independently by two investigators (P.J.C. and A.C.). In the case of disagreement between the two reviewers, consensus was achieved after consulting with a third reviewer (C.D.). Duplicate records were removed and study titles and abstracts were screened to identify potentially eligible articles for inclusion in the full text review. The selected articles were read in full for confirmation of eligibility and data extraction. Data extracted from these articles included name of the first author, publication year, country, number of participants, duration of the follow-up, intervention (dose and therapy), and SBP and DBP before and after the introduction of GHT.
To determine the quality of the included studies the National Institute of Health Quality Assessment Tool for Before–After (Pre–Post) Studies with No Control Group was utilized [11]. This tool is comprised of 12 questions used to assess the risk of types of bias, such as selection, reporting or observer bias. The methodological quality of included studies was assessed by two authors (P.J.C. and A.C.) separately. Each study was defined as poor, fair or good based on the answers compiled from this tool. To determine the level of agreement between assessing authors, Cohen's kappa was calculated [12]. A score less than 0.4 is considered poor, between 0.4 and 0.8 fair to substantial, and above 0.8 as almost perfect interrater agreement. A meta-analysis of uncontrolled pre–post standardized mean differences in BP was not performed as they lack independence and fail to discern between intra-individual and interventional effects [13].
RESULTS
Study selection and characteristics
Our multidatabase literature search identified 600 nonduplicated potentially relevant records (Fig. 1). No records were identified through other resources. Following the screening of these records’ titles and abstracts, 84 full text articles were assessed for eligibility; however, 70 articles were excluded due to not being original investigations, not assessing relevant outcomes, not obtaining pre-GHT outcome measurements [14] or including adolescent populations [15–18].
FIGURE 1.
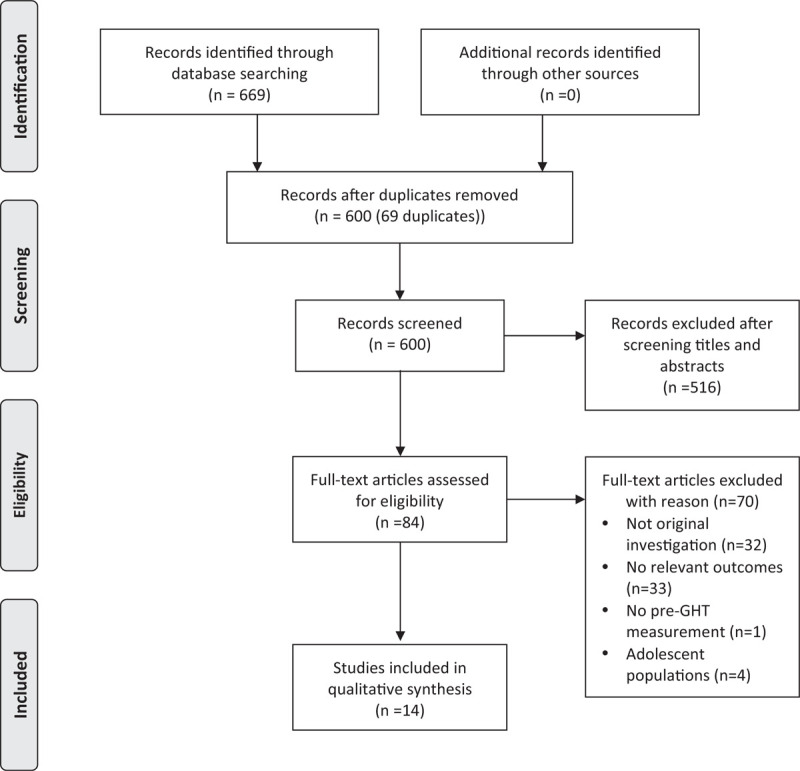
Flow diagram for study selection.
Consequently, 14 studies were included in this systematic review encompassing 1309 individuals (∼50% transgender men and women) treated with GHT between 1989 and 2019. Follow-up ranged between 4 months and 5 years. 11 of the included studies were undertaken in Europe and three in North America. Mean ages ranged between 23.0–36.7 years in transgender men and 25.2–34.8 years in transgender women.
Of the included studies, all studies were pre–post observational studies without control groups. Only one study provided the device used to measure BP (BP-8800; Colin Corporation, Hayashi, Japan) [19], the position of measurement was acknowledged in four studies [19–21], and the use of multiple BP measurements mentioned in three studies [19,20,22].
Transgender men
Between 2003 and 2019 a total of 13 studies measured the SBP and DBP of transgender men receiving GHT (Table 1). Mean follow-up time was 18.9 (SD 14.2) months (range 4–60 months). The average sample size was ∼50; however, three studies recruited less than 20 individuals [19,23,24].
TABLE 1.
Blood pressure in testosterone exposed transgender men
| Mean difference (mmHg) [95% CI] | |||||||
| Reference | Country | Follow-up (months) | Sample size, n | Mean age (years) (SD) | Intervention | SBP | DBP |
| Elbers et al.[19] | The Netherlands | 12 | 17 | 23 (5) | 250 mg IM testosterone esters/2 weeks | +1.0 [−5.1, 7.1] | −0.2 [−4.6, 4.2] |
| Giltay et al.[22] | The Netherlands | 4 | 81 | 36.7 (10) | 250 mg IM testosterone esters/2 weeks (n = 61) or oral testosterone undecanoate 160–240 mg/day (n = 20) | −4.6 [−8.3, −0.9] | −2.1 [−4.4, 0.2] |
| Mueller et al.[25] | Germany | 12 | 35 | 29.6 (8.9) | 1 g IM testosterone undecanoate/12 weeks | +4.3 [−1.5, 10.1] | +2.9 [−0.3, 6.1] |
| Mueller et al.[27] | Germany | 24 | 45 | 30.4 (9.1) | 1 g IM testosterone undecanoate/12 weeks | +5.2 [0.5, 9.9] | +2.8 [−0.0, 5.6] |
| Wierckx et al.[30] | Belgium, The Netherlands | 12 | 53 | 24.5 (7.0)a | 1 g IM testosterone undecanoate at baseline, then 6 weeks, then/12 weeks thereafter. If intolerant of testosterone undecanoate, 250 mg IM testosterone esters/2 weeks | +4.1 [−0.5, 8.7]a | +2.3 [−1.5, 6.1]a |
| Colizzi et al.[20] | Spain | 24 | 43 | 28.8 (5.6) | 250 mg IM testosterone ester/21.16 ± 3.17 days | +13.3 [9.6, 13.3] | +3.7 [0.24, 7.2] |
| Quirós et al.[31] | Spain | 24 | 97 | 28.6 (8.6) | 50 mg transdermal testosterone gel/day or 1 g IM testosterone undecanoate 1 g/12 weeks | +2.2 [−0.9, 5.3] | +1.5 [−1.1, 4.1] |
| Deutsch et al.[32] | USA | 6 | 34 | 27 (6.9) | 50–70 mg subcutaneous testosterone cypionate/week (n = 31), 5 g testosterone gel/day (n = 2) or 4 mg testosterone transdermal patch/day (n = 1) | +3.0 [−3.7, 9.7]b | −2 [−6.0, 2.0]b |
| Fernandez and Tannock [23] | USA | 18 | 19 | 27 (7) | ∼150 mg IM testosterone/2 weeks (type unspecified) | −2.0 [−12.6, 8.6] | −1 [−7.9, 5.9] |
| Vita et al.[24] | Italy | 25.5c | 11 | 25.1 (3.7) | IM testosterone enanthate (n = 10) or undecanoate (n = 1) (dose/frequency unspecified) | +6.2 [0.5, 11.8] | +3.1 [−1.9, 8.1] |
| Auer et al.[28] | Germany | 12 | 45 | 27.5 (1.3) | 1 g IM testosterone undecanoate/12 weeks | +7.2 [−3.1, 17.5] | +3.4 [−2.9, 9.8] |
| Gava et al.[26] | Italy | 60 | 50 | 30.1 (6.1)a | 1 g IM testosterone undecanoate at baseline, then 6 weeks, then/12 weeks thereafter (n = 25) or 250 mg IM testosterone enanthate/3–4 weeks (n = 25) | −3.0 [−6.6, 0.6]a | −1.5 [−4.6, 1.6]a |
| van Velzen et al.[21] | Belgium, The Netherlands | 12 | 118 | 26.4 (9.1) | 1 g IM testosterone undecanoate/12 weeks (n = 79) or 250 mg IM testosterone esters/2 weeks (n = 62) or 50 mg testosterone gel/day (n = 47) | +1.5 [−1.5, 4.5]d | +1.8 [−0.8, 4.4]d |
95% CI, 95% confidence interval; IM, intramuscular.
Pooled means ± SD.
Data extracted from median and IQR.
Mean follow-up of cohort.
Data derived from percentage change in baseline values after adjustment for age, SD derived from 95% CI.
Various formulations of testosterone were administered in these studies including intramuscular testosterone undecanoate (n = 8), intramuscular testosterone esters (n = 7), transdermal testosterone gel (n = 2) or patches (n = 1), oral testosterone undecanoate (n = 1), or subcutaneous testosterone cypionate (n = 1). One study did not specify the formulation of intramuscular testosterone administered [23].
As demonstrated in Table 1, the effect of testosterone GHT on BP varies considerably between these studies. The majority of these studies did not demonstrate a significant change in SBP (n = 9), whereas three studies reported a significant increase in SBP ranging between 4.3 and 13.3 mmHg [20,25,27] and only one study reported a significant decrease in SBP (4.6 mmHg) [22]. Most studies (n = 10) did not report a significant change in DBP.
Transgender women
Table 2 summarizes included studies (n = 10) of transgender women receiving estrogen therapy comprising of 661 individuals. These studies were undertaken between 1989 and 2019 with a mean follow-up of 16.4 (SD 7.9) months (range 6–31.7 months). The mean sample size of these studies was 66 individuals, with the largest being conducted by van Velzen et al.[21] of 242 individuals.
TABLE 2.
Blood pressure in estrogen exposed transgender women
| Mean difference (mmHg) [95% CI] | |||||||
| Reference | Country | Follow-up (months) | Sample size, n | Mean age (years) (SD) | Intervention | SBP | DBP |
| Prior et al.[29] | Canada | 12 | 23 | 30.7 (6.2) | 0.625 mg increasing to 2.5 mg conjugated estrogen twice daily for 3/4 weeks, followed by 10 mg medroxyprogesterone/day during weeks 3 and 4 or continuously if gonadotrophins increased, to aid in testosterone reduction or breast development, in addition 100–200 mg spironolactone/day | −7.3 [−15.3, 0.7]a | −2.6 [−9.3, 4.1]a |
| Elbers et al.[19] | The Netherlands | 12 | 20 | 26 (6) | 100 μg oral ethinyl estradiol and 100 mg cyproterone acetate/day | +7.2 [0.2, 14.6] | +5.7 [0.0, 11.4] |
| Wierckx et al.[30] | Belgium, The Netherlands | 12 | 53 | 30.3 (14.1)e | <45 Years (n = 40) 4 mg oral estradiol valerate and 50 mg cyproterone acetate/day. >45 Years (n = 13) 100 μg/24 h transdermal 17β estradiol patch. If intolerant of this, 2 mg transdermal 17β estradiol gel twice daily or 4 mg estradiol valerate per day | −5.4 [−10.8, 0.0]e | −0.1 [−4.0, 3.8]e |
| Colizzi et al.[20] | Spain | 24 | 79 | 30.2 (9.6) | 2.12 ± 0.57 mg transdermal estradiol gel/day and 100 mg cyproterone acetate/day | +17.8 [14.4, 21.2] | +3.2 [0.3, 6.1] |
| Quirós et al.[31] | Spain | 24 | 150 | 32.4 (10.1) | Oral estrogen (conjugated equine estrogens or estradiol valerate) and either cyproterone acetate or flutamide. >40 Years were recommended transdermal estrogens (dose/frequency/formulation unspecified) | +6.4 [3.5, 9.3] | +3.7 [1.4, 5.9] |
| Deutsch et al.[32] | USA | 6 | 16 | 29 (9.4) | 2 mg sublingual micronized 17β estradiol twice daily (n = 14) or 20 mg IM estradiol valerate/2 weeks (n = 1) or 100 μg estradiol via transdermal patch (n = 1) in addition to 50–100 mg spironolactone/day (n = 15) | −10.0 [−17.0, −2.9]b | −11 [−19.7, −2.3]b |
| Fernandez and Tannock [23] | USA | 18 | 33 | 31 (10) | 1.71 mg oral estrogen (type unspecified)/day (∼50%), transdermal estrogen (dose/type/frequency unspecified) (14%) or IM estrogen (36%) (dose/type/frequency unspecified), and 100 mg spironolactone/day | −6.0 [−13.9, 1.9] | −5.0 [−10.6, 0.6] |
| Vita et al.[24] | Italy | 31.7c | 21 | 25.2 (7) | 2–6 mg oral estradiol valerate/day and 50–100 mg cyproterone acetate/day (if not undergoing gender reassignment surgery). Patients (n = 4) receiving ethylestradiol were switched to estradiol valerate. Progesterone was also utilized (n = 3) (type/route/frequency unspecified) | −6.1 [−11.0, −1.2] | −4.0 [−9.1, 1.1] |
| Auer et al.[28] | Germany | 12 | 24 | 34.8 (1.4) | 2 mg oral estradiol valerate twice daily or 100 μg transdermal 17β estradiol patch/day (if >45 years) and 50 mg cyproterone acetate/day | −6.7 [−13.9, 0.6] | −1.7 [−6.1, 2.7] |
| van Velzen et al.[21] | Belgium, The Netherlands | 12 | 242 | 32.3 (12.6) | 2 mg oral estradiol valerate twice daily (n = 144) or 100 μg transdermal estradiol patch/day (if age >45 years, n = 98), and 50 mg cyproterone acetate/day | −3.4 [−5.9, −0.9]d | −1.8 [−4.1, 0.5]d |
95% CI, 95% confidence interval; GHT, gender-affirming hormone therapy; IM, intramuscular.
Data included on participants not previously exposed to GHT.
Data extracted from median and IQR.
Mean follow-up of cohort.
Data derived from percentage change in baseline values after adjustment for age, SD derived from 95% CI.
Pooled means ± SD.
The doses and formulations of GHT vary significantly between studies. Oral estradiol valerate (n = 6), estradiol patches (n = 6), estradiol gel (n = 2), oral ethynyl estradiol (n = 2), oral conjugated estrogen (n = 2), sublingual micronized 17β estradiol (n = 1), intramuscular estradiol valerate (n = 2) and unspecified oral estrogen (n = 1), were utilized. Four studies provided transdermal estrogen patches rather than oral estrogen to those over the age of 40–45 years [21,28,30,31]. The most common antiandrogen was cyproterone acetate (n = 7), whereas spironolactone (n = 3) and flutamide (n = 1) was also utilized. No study incorporated the use of GnRH analogues while two studies utilized progestogens.
The outcomes relating to these studies are also diverse with three studies demonstrating a rise in SBP between 7.2 and 17.8 mm Hg [19,20,31] and three studies demonstrating a fall in SBP between 3.4 and 10 mm Hg [21,24,32]. DBP was increased in two studies (3.2 to 5.7 mmHg) [19,20], with no significant change reported in seven studies.
The outcomes relating to these studies are also diverse with three studies demonstrating a rise in SBP between 7.2 and 17.8 mmHg [19,20,31] and three studies demonstrating a fall in SBP between 3.4 and 10 mmHg [21,24,32]. DBP was increased in two studies (3.2 to 5.7 mmHg) [19,20], with no significant change reported in seven studies.
Quality of evidence
Quality of methodology was assessed using the Quality Assessment Tool for Before–After Studies with no control group (Table 3). Inter-rater (P.J.C. and A.C.) agreement was 92.8% with an expected agreement of 69.4% and Cohen's kappa of 0.77 (standard error, 0.22), indicating substantial agreement. Only two studies were determined to have a good quality rating [20,21], however, as uncontrolled pre–post studies demonstrate weak evaluative designs, no study successfully fulfilled the entire quality criteria. Largely, studies did not provide power calculations or were underpowered to demonstrate significant changes in BP. Furthermore, studies did not report blinding, lacked multiple measurements before and after the introduction of GHT and comprised of significantly heterogenous groups receiving various doses, formulations and combinations of GHT treatments.
TABLE 3.
Quality assessment of studies assessing the effect of gender-affirming hormone therapy on blood pressure in transgender individuals
| Reference | Q1 | Q2 | Q3 | Q4 | Q5 | Q6 | Q7 | Q8 | Q9 | Q10 | Q11 | Q12 | Quality rating |
| Prior et al.[29] | Y | Y | Y | Y | NR | N | N | NR | Y | Y | N | NA | Fair |
| Elbers et al.[19] | Y | Y | Y | Y | NR | Y | Y | NR | Y | Y | N | NA | Fair |
| Giltay et al.[22] | Y | Y | Y | Y | N | N | Y | N | Y | Y | N | NA | Fair |
| Mueller et al.[25] | Y | Y | Y | Y | NR | Y | N | N | Y | Y | N | NA | Fair |
| Mueller et al.[27] | Y | Y | Y | Y | NR | Y | N | N | Y | Y | N | NA | Fair |
| Wierckx et al.[30] | Y | Y | Y | Y | NR | N | N | N | Y | Y | N | NA | Fair |
| Colizzi et al.[20] | Y | Y | Y | Y | Y | Y | Y | N | Y | Y | N | NA | Good |
| Quirós et al.[31] | Y | Y | Y | Y | Y | N | N | N | N | Y | N | NA | Fair |
| Deutsch et al.[32] | Y | Y | Y | Y | NR | N | Y | N | Y | Y | N | NA | Fair |
| Fernandez and Tannock [23] | Y | Y | Y | Y | NR | N | N | N | N | Y | N | NA | Poor |
| Vita et al.[24] | Y | Y | Y | Y | NR | N | Y | N | Y | Y | N | NA | Fair |
| Auer et al.[28] | Y | Y | Y | Y | NR | N | N | N | Y | Y | N | NA | Fair |
| Gava et al.[26] | Y | Y | Y | Y | NR | N | N | N | Y | Y | N | NA | Fair |
| van Velzen et al.[21] | Y | Y | Y | Y | Y | N | Y | N | N | Y | N | NA | Good |
N, no; NA, not applicable; NR, not reported; Y, yes.
DISCUSSION
The aim of this systematic review was to gain insights on the effects of GHT on BP of transgender men and women. This systematic review has examined the available evidence relating to BP alteration in this population. Overall, we have identified 14 studies relating to transgender people receiving GHT, who demonstrated pre and post GHT BP measurements.
Our findings demonstrate that current evidence is derived from uncontrolled pre–post studies, which are heterogenous in their recorded interventions and lack consistency in their outcomes. The majority of studies included in this systematic review demonstrate poor to fair quality, even after taking into account the limitations of uncontrolled study designs. Other common weaknesses include short follow-up durations, lack of standardization in delivery of GHT and small sample sizes, thereby limiting their power. Only one study provided information regarding the device used to measure BP (Colin, BP-8800) [19]. However, this device has been demonstrated to exhibit inconsistent aberration patterns, thereby invalidating its use in clinical research [33]. Lastly a meta-analysis is not possible as such results lack independence and cannot discern between intraindividual and GHT effects [13].
A limitation of this systematic review is that it did not include cross-sectional assessments of the development of hypertension. Asscheman et al.[34] observed an increase in the crude incidence of hypertension in transgender women but not men, and no difference in hypertension standardized incidence ratio (defined as >160/95 mmHg) in either groups when compared with cisgender populations. Furthermore, self-reported hypertension in the Behavioral Risk Factor Surveillance System survey has been found to be no higher than cisgender populations [35]. Results from these studies are also derived from relatively small populations and rely on outdated or self-reported hypertension definitions, thereby limiting their utility.
As many as 390 adults per 100 000 of the US population identify as transgender and this marginalized group suffer from significant health disparities [3,36]. To enact better care and evidence-based guidance for this expanding population, the collection and reporting of good quality data are imperative [1]. Randomized controlled trials reduce bias and permit the examination of the causal inference of an intervention, however, may not be permissible or ethical in this population [1]. However, the risk of bias inherent in the intrinsically weak evaluative quasiexperimental uncontrolled before–after design could be reduced by the introduction of cisgender controls (ideally both male and females per transgender individual) or an interrupted time series design, which would allow the intervention effect to be estimated accounting for the underlying trend [37]. Given the limited age groups that were assessed in these studies, research focusing on the interaction between advancing age and life-long GHT is also vital [38].
Furthermore, in line with recent guidance on BP assessment in clinic-based research, it is recommended that multiple BP readings be taken from a validated and calibrated BP device and averaged at each assessment [39]. Multiple visits with BP assessments are undertaken at baseline and during intervention follow-up, which would permit a simple interrupted time series design. The addition of ambulatory BP monitoring should be encouraged.
The majority of studies of transgender men receiving GHT did not demonstrate a significant change in BP, whereas both increases and decreases in SBP were observed in transgender women receiving hormone therapy. Mechanistically, estrogen promotes endothelium-dependent vasorelaxation via increasing nitric oxide bioavailability, expression and activation of endothelial nitric oxide synthase, increases in endothelium-derived hyperpolarizing factor and prostacyclin, and reductions in endothelin-1 [40–44]. Endothelium-independent vasodilation is achieved by modulation calcium flux in the vascular smooth muscle cells (VSMCs) [45]. Conversely, testosterone may facilitate either vasodilatation or vasoconstriction via endothelium-independent inhibition of voltage-operated calcium channels and activation of potassium channels in VSMCs, and enhancing thromboxane A2-mediated and endothelin-1-mediated vasoconstriction, respectively [46,47]. Importantly, sex hormones may also indirectly modulate vasodynamic pathways such as the renin–angiotensin–aldosterone system [48–50]. The putative relationship between estrogen and elevated BP in transgender women may highlight fundamental gaps in our understanding of the vasoconstrictive properties of this sex hormone.
However, due to the limited quality of the of the studies included in this review, there is insufficient data to determine meaningful conclusions regarding the effect of estrogen or testosterone-based GHT on the SBP or DBPs of transgender individuals or advise clinically on this matter. More robust research is required (Table 4) to determine whether GHT mediates alterations in BP of transgender individuals, whether this change is associated with increased cardiovascular risk, and if such risk exists, what interventions can be undertaken to improve the health of transgender people.
TABLE 4.
Gaps in evidence and recommendations for future research
| Research question | Recommendations for future research |
| Does GHT alter the blood pressure of transgender men and/or women? | Interrupted time series or controlled cohort study measuring blood pressure before and after the introduction of GHT using validated office or ideally ambulatory blood pressure recordings |
| Are alterations in blood pressure associated with increased cardiovascular risk in transgender men and women? | Prospective longitudinal observational cohort study of transgender people for cardiovascular disease in relation to components of blood pressure |
| Do blood pressure-lowering interventions reduce cardiovascular risk in transgender men and women? | Randomized controlled study of transgender individuals with SBP > 130 mmHg to SBP targets of <120 mmHg (intensive treatment) or less than 140 mmHg (standard treatment) with a primary composite outcome of myocardial infarction, other acute coronary syndromes, stroke, heart failure or death from cardiovascular causes |
GHT, gender-affirming hormone therapy.
ACKNOWLEDGEMENTS
Previous presentations: Neither the whole work nor part of the work presented in this article has been previously presented.
The current work was supported by the British Heart Foundation (Centre of Research Excellence Awards RE/13/5/30177 and RE/18/6/34217).
Conflicts of interest
There are no conflicts of interest.
Footnotes
Abbreviations: BP, blood pressure; GHT, gender-affirming hormone therapy; GnRH, gonadotropin-releasing hormone; VSMC, vascular smooth muscle cell
REFERENCES
- 1.T'Sjoen G, Arcelus J, Gooren L, Klink DT, Tangpricha V. Endocrinology of transgender medicine. Endocr Rev 2019; 40:97–117. [DOI] [PubMed] [Google Scholar]
- 2.Arcelus J, Bouman WP, Van Den Noortgate W, Claes L, Witcomb G, Fernandez-Aranda F. Systematic review and meta-analysis of prevalence studies in transsexualism. Eur Psychiatry 2015; 30:807–815. [DOI] [PubMed] [Google Scholar]
- 3.Meerwijk EL, Sevelius JM. Transgender population size in the United States: a meta-regression of population-based probability samples. Am J Public Health 2017; 107:e1–e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Thomson RM, Katikireddi SV. Improving the health of trans people: the need for good data. Lancet Public Health 2019; 4:e369–e370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Irwig MS. Cardiovascular health in transgender people. Rev Endocr Metab Disord 2018; 19:243–251. [DOI] [PubMed] [Google Scholar]
- 6.Streed CG, Harfouch O, Marvel F, Blumenthal RS, Martin SS, Mukherjee M. Cardiovascular disease among transgender adults receiving hormone therapy: a narrative review. Ann Intern Med 2017; 167:256–267. [DOI] [PubMed] [Google Scholar]
- 7.Connelly PJ, Marie Freel E, Perry C, Ewan J, Touyz RM, Currie G, et al. Gender-affirming hormone therapy, vascular health and cardiovascular disease in transgender adults. Hypertension 2019; 74:1266–1274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hembree WC, Cohen-Kettenis PT, Gooren L, Hannema SE, Meyer WJ, Murad MH, et al. Endocrine treatment of gender-dysphoric/gender-incongruent persons: an endocrine society clinical practice guideline. J Clin Endocrinol Metab 2017; 102:3869–3903. [DOI] [PubMed] [Google Scholar]
- 9.Coleman E, Bockting W, Botzer M, Cohen-Kettenis P, DeCuypere G, Feldman J, et al. Standards of care for the health of transsexual, transgender, and gender-nonconforming people, version 7. Int J Transgend 2012; 13:165–232. [Google Scholar]
- 10.Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred Reporting Items for Systematic Reviews and meta-analyses: the PRISMA statement. J Clin Epidemiol 2009; 62:1006–1012. [DOI] [PubMed] [Google Scholar]
- 11. NIH National Heart, Lung, and Blood Institute. Study quality assessment tools: quality assessment tool for before-after (pre-post) studies with no control group. https://www.nhlbi.nih.gov/health-topics/study-quality-assessment-tools. [Accessed 4 August 2020] [Google Scholar]
- 12.Cohen J. A coefficient of agreement for nominal scales. Educ Psychol Meas 1960; 20:37–46. [Google Scholar]
- 13.Cuijpers P, Weitz E, Cristea IA, Twisk J. Prepost effect sizes should be avoided in meta-analyses. Epidemiol Psychiatr Sci 2017; 26:364–368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Angus L, Leemaqz S, Ooi O, Cundill P, Silberstein N, Locke P, et al. Cyproterone acetate or spironolactone in lowering testosterone concentrations for transgender individuals receiving oestradiol therapy. Endocr Connect 2019; 8:935–940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stoffers IE, de Vries MC, Hannema SE. Physical changes, laboratory parameters, and bone mineral density during testosterone treatment in adolescents with gender dysphoria. J Sex Med 2019; 16:1459–1468. [DOI] [PubMed] [Google Scholar]
- 16.Olson-Kennedy J, Okonta V, Clark LF, Belzer M. Physiologic response to gender-affirming hormones among transgender youth. J Adolesc Health 2018; 62:397–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jarin J, Pine-Twaddell E, Trotman G, Stevens J, Conard LA, Tefera E, et al. Cross-sex hormones and metabolic parameters in adolescents with gender dysphoria. Pediatrics 2017; 139:e20163173. [DOI] [PubMed] [Google Scholar]
- 18.Hannema SE, Schagen SEE, Cohen-Kettenis PT, Delemarre-van de Waal HA. Efficacy and safety of pubertal induction using 17β-estradiol in transgirls. J Clin Endocrinol Metab 2017; 102:2356–2363. [DOI] [PubMed] [Google Scholar]
- 19.Elbers J, Giltay E, Teerlink T, Scheffer P, Asscheman H, Seidell J, et al. Effects of sex steroid on components of the insulin resistance syndrome in transsexual subjects. Clin Endocrinol 2003; 58:562–571. [DOI] [PubMed] [Google Scholar]
- 20.Colizzi M, Costa R, Scaramuzzi F, Palumbo C, Tyropani M, Pace V, et al. Concomitant psychiatric problems and hormonal treatment induced metabolic syndrome in gender dysphoria individuals: a 2 year follow-up study. J Psychosom Res 2015; 78:399–406. [DOI] [PubMed] [Google Scholar]
- 21.van Velzen DM, Paldino A, Klaver M, Nota NM, Defreyne J, Hovingh GK, et al. Cardiometabolic effects of testosterone in transmen and estrogen plus cyproterone acetate in transwomen. J Clin Endocrinol Metab 2019; 104:1937–1947. [DOI] [PubMed] [Google Scholar]
- 22.Giltay EJ, Toorians AW, Sarabjitsingh AR, de Vries NA, Gooren LJG. Established risk factors for coronary heart disease are unrelated to androgen-induced baldness in female-to-male transsexuals. J Endocrinol 2004; 180:107–112. [DOI] [PubMed] [Google Scholar]
- 23.Fernandez JD, Tannock LR. Metabolic effects of hormone therapy in transgender patients. Endocr Pract 2016; 22:383–388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vita R, Settineri S, Liotta M, Benvenga S, Trimarchi F. Changes in hormonal and metabolic parameters in transgender subjects on cross-sex hormone therapy: a cohort study. Maturitas 2018; 107:92–96. [DOI] [PubMed] [Google Scholar]
- 25.Mueller A, Kiesewetter F, Binder H, Beckmann MW, Dittrich R. Long-term administration of testosterone undecanoate every 3 months for testosterone supplementation in female-to-male transsexuals. J Clin Endocrinol Metab 2007; 92:3470–3475. [DOI] [PubMed] [Google Scholar]
- 26.Gava G, Mancini I, Cerpolini S, Baldassarre M, Seracchioli R, Meriggiola MC. Testosterone undecanoate and testosterone enanthate injections are both effective and safe in transmen over 5 years of administration. Clin Endocrinol (Oxf) 2018; 89:878–886. [DOI] [PubMed] [Google Scholar]
- 27.Mueller A, Haeberle L, Zollver H, Claassen T, Kronawitter D, Oppelt PG, et al. Effects of intramuscular testosterone undecanoate on body composition and bone mineral density in female-to-male transsexuals. J Sex Med 2010; 7:3190–3198. [DOI] [PubMed] [Google Scholar]
- 28.Auer MK, Ebert T, Pietzner M, Defreyne J, Fuss J, Stalla GK, et al. Effects of sex hormone treatment on the metabolic syndrome in transgender individuals: focus on metabolic cytokines. J Clin Endocrinol Metab 2018; 103:790–802. [DOI] [PubMed] [Google Scholar]
- 29.Prior JC, Vigna YM, Watson D. Spironolactone with physiological female steroids for presurgical therapy of male-to-female transsexualism. Arch Sex Behav 1989; 18:49–57. [DOI] [PubMed] [Google Scholar]
- 30.Wierckx K, Van Caenegem E, Schreiner T, Haraldsen I, Fisher A, Toye K, et al. Cross-sex hormone therapy in trans persons is safe and effective at short-time follow-up: results from the European network for the investigation of gender incongruence. J Sex Med 2014; 11:1999–2011. [DOI] [PubMed] [Google Scholar]
- 31.Quirós C, Patrascioiu I, Mora M, Aranda GB, Hanzu FA, Gómez-Gil E, et al. Effect of cross-sex hormone treatment on cardiovascular risk factors in transsexual individuals. Experience in a specialized unit in Catalonia. Endocrinol Nutr 2015; 62:210–216. [DOI] [PubMed] [Google Scholar]
- 32.Deutsch MB, Bhakri V, Kubicek K. Effects of cross-sex hormone treatment on transgender women and men. Obstet Gynecol 2015; 125:605–610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Naschitz JE, Gaitini L, Loewenstein L, Keren D, Zuckerman E, Tamir A, et al. In-field validation of automatic blood pressure measuring devices. J Hum Hypertens 2000; 14:37–42. [DOI] [PubMed] [Google Scholar]
- 34.Asscheman H, Gooren LJG, Eklund PLE. Mortality and morbidity in transsexual patients with cross-gender hormone treatment. Metabolism 1989; 38:869–873. [DOI] [PubMed] [Google Scholar]
- 35.Nokoff NJ, Scarbro S, Juarez-Colunga E, Moreau KL, Kempe A. Health and cardiometabolic disease in transgender adults in the U.S.: behavioral risk factor surveillance system 2015. J Endocr Soc 2018; 2:349–360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bradford J, Reisner SL, Honnold JA, Xavier J. Experiences of transgender-related discrimination and implications for health: results from the Virginia transgender health initiative study. Am J Public Health 2013; 103:1820–1829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Grimshaw J. Experimental and quasi-experimental designs for evaluating guideline implementation strategies. Fam Pract 2000; 17:11S–16S. [DOI] [PubMed] [Google Scholar]
- 38.Gooren LJ, T'Sjoen G. Endocrine treatment of aging transgender people. Rev Endocr Metab Disord 2018; 19:253–262. [DOI] [PubMed] [Google Scholar]
- 39.Muntner P, Einhorn PT, Cushman WC, Whelton PK, Bello NA, Drawz PE, et al. Blood pressure assessment in adults in clinical practice and clinic-based research: JACC Scientific Expert Panel. J Am Coll Cardiol 2019; 73:317–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sumi D, Ignarro LJ. Estrogen-related receptor α1 up-regulates endothelial nitric oxide synthase expression. Proc Natl Acad Sci U S A 2003; 100:14451–14456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chambliss KL, Yuhanna IS, Mineo C, Liu P, German Z, Sherman TS, et al. Estrogen receptor alpha and endothelial nitric oxide synthase are organized into a functional signaling module in caveolae. Circ Res 2000; 24:E44–E52. [DOI] [PubMed] [Google Scholar]
- 42.Liu MY, Hattori Y, Fukao M, Sato A, Sakuma I, Kanno M. Alterations in EDHF-mediated hyperpolarization and relaxation in mesenteric arteries of female rats in long-term deficiency of oestrogen and during oestrus cycle. Br J Pharmacol 2001; 132:1035–1046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Akishita M, Kozaki K, Eto M, Yoshizumi M, Ishikawa M, Toba K, et al. Estrogen attenuates endothelin-1 production by bovine endothelial cells via estrogen receptor. Biochem Biophys Res Commun 1998; 251:17–21. [DOI] [PubMed] [Google Scholar]
- 44.Sobrino A, Oviedo PJ, Novella S, Laguna-Fernandez A, Bueno C, García-Pérez MA, et al. Estradiol selectively stimulates endothelial prostacyclin production through estrogen receptor-α. J Mol Endocrinol 2010; 44:237–246. [DOI] [PubMed] [Google Scholar]
- 45.Han SZ, Karaki H, Ouchi Y, Akishita M, Orimo H. 17β-Estradiol inhibits Ca2+ influx and Ca2+ release induced by thromboxane A2 in porcine coronary artery. Circulation 1995; 91:2619–2626. [DOI] [PubMed] [Google Scholar]
- 46.Deenadayalu VP, White RE, Stallone JN, Gao X, Garcia AJ. Testosterone relaxes coronary arteries by opening the large-conductance, calcium-activated potassium channel. Am J Physiol Heart Circ Physiol 2001; 281:H1720–H1727. [DOI] [PubMed] [Google Scholar]
- 47.Ceballos G, Figueroa L, Rubio I, Gallo G, Garcia A, Martinez A, et al. Acute and nongenomic effects of testosterone on isolated and perfused rat heart. J Cardiovasc Pharmacol 1999; 33:691–697. [DOI] [PubMed] [Google Scholar]
- 48.Morselli E, Santos RS, Criollo A, Nelson MD, Palmer BF, Clegg DJ. The effects of oestrogens and their receptors on cardiometabolic health. Nat Rev Endocrinol 2017; 13:352–364. [DOI] [PubMed] [Google Scholar]
- 49.Lucas-Herald A, Alves-Lopes R, Montezano AC, Ahmed SF, Touyz RM. Genomic and nongenomic effects of androgens in the cardiovascular system: clinical implications. Clin Sci 2017; 131:1405–1418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Colafella KMM, Denton KM. Sex-specific differences in hypertension and associated cardiovascular disease. Nat Rev Nephrol 2018; 14:185–201. [DOI] [PubMed] [Google Scholar]


