Objective:
Ethnic minority populations (EMPs) are disproportionally affected by hypertension-mediated complications compared with European host populations (EHPs), which might be due to disparities in hypertension awareness, treatment and control. We conducted a systematic review and meta-analysis to compare awareness, treatment and control rates among EMPs with EHPs.
Methods:
MEDLINE, EMBASE and Web of Science were searched from inception to 29 January 2020. Critical appraisal was performed according to methods of Hoy et al. Pooled odds ratios with corresponding 95% confidence intervals were calculated for these rates, stratified by ethnic group, using either random or fixed effect meta-analysis based on I2-statistics. Study was registered in PROSPRO (CRD42020107897).
Results:
A total of 3532 records were screened of which 16 were included in the analysis with data on 26 800 EMP and 57 000 EHP individuals. Compared with EHPs, African origin populations were more likely to be aware (odds ratio 1.26, 95% confidence interval 1.02–1.56) and treated (1.49, 1.18–1.88) for hypertension, but were less likely to have their blood pressure controlled (0.56, 0.40–0.78), whereas South Asian populations were more likely to be aware (1.15, 1.02–1.30), but had similar treatment and control rates. In Moroccan populations, hypertension awareness (0.79, 0.62–1.00) and treatment levels (0.77, 0.60–0.97) were lower compared with EHPs, while in Turkish populations awareness was lower (0.81, 0.65–1.00).
Conclusion:
Levels of hypertension awareness, treatment and control differ between EMPs and EHPs. Effort should be made to improve these suboptimal rates in EMPs, aiming to reduce ethnic inequalities in hypertension-mediated complications.
Keywords: ethnic minority population, ethnicity, Europe, hypertension awareness, hypertension control, hypertension treatment
INTRODUCTION
Hypertension is the major modifiable risk factor for cardiovascular disease (CVD) morbidity and mortality. In Europe, over 50% of the CVD deaths is attributable to high SBP [1]. Mean blood pressure (BP) levels vary between ethnic minority and the European host population (EHP) [2], and hypertension-mediated CVD burden is not equally distributed between populations of different ethnic background. For instance, the prevalence of stroke is up to 2.1 times higher in sub-Saharan African (SSA) origin populations compared with the EHP [3], which is mainly attributable to the higher prevalence of hypertension [4].
BP-lowering therapy (i.e. medication, lifestyle modification) results in a considerable reduction in CVD morbidity and mortality [5] and early detection, adequate treatment and control of hypertension can lead to significant health and economic gains [6]. However, globally, less than half (47%) of the adults with hypertension is aware of their condition, 37% receives treatment, and 37% of those who are prescribed antihypertensive medication have their BP adequately controlled [7]. Studies from the USA show variation in these rates between populations of different ethnic origin [8]. Individual studies from Europe show similar patterns [9], however, systematically aggregated data comparing awareness, treatment and control levels among ethnic minority populations (EMPs) to the EHP are lacking. To reduce the ethnic inequalities in the CVD burden, improving hypertension control rates is vital. Whereas ethnic differences in hypertension prevalence are mainly determined by gene-environment interactions, hypertension awareness, treatment and control rates are principally determined by health behaviour (e.g. medication adherence) and healthcare-related factors (e.g. access to healthcare). Therefore, aggregated data on hypertension awareness, treatment and control, as well as on ethnic differences in these variables will help to optimize the allocation of healthcare resources and can guide culturally targeted preventive efforts, which is crucial for the ethnically diverse societies that Europe consists of today. Therefore, the aim of this systematic review and meta-analysis was to assess the levels of hypertension awareness, treatment and control among EMPs in Europe, compared with the EHP.
METHODS
Search strategy and selection criteria
For this systematic review and meta-analysis, all longitudinal or cross-sectional studies published in a peer-reviewed journal, that reported prevalence of hypertension awareness, treatment and/or control, conducted in an adult population (≥16 years), and compared one or more minority ethnic groups to the EHP, were eligible for inclusion. No language restriction was applied. Studies not defining the ethnic origin of the group under study, studies conducted outside Europe, non-population-based studies, studies defining hypertension based on a single BP reading or on self-report only, were excluded. To be able to draw general conclusions, studies or populations with a sample size of less than 100 participants were excluded. Search terms were developed by a medical librarian (J.G.D.) in collaboration with the research group, based on a scoping search to identify relevant search concepts. Reference list of primary studies were screened, tracked citations in GoogleScholar were checked, and trial registers searched to identify additional relevant studies. MEDLINE (OvidSP) (1946 to date), EMBASE (OvidSP) (1980 to date) and Web of Science (inception to date) were searched using (controlled) terms for the following relevant search concepts, adapted to the criteria of the specific database searched: ‘Hypertension’, ‘Europe’, ‘Minority ethnic population’, ‘Awareness’, ‘Treatment’, ‘Control’ (Supplementary Digital Content 1). The search terms were combined using Boolean terms AND and OR; NOT terms were identified based on bibliometric mapping of the MEDLINE and EMBASE search results using VOSviewer software (VOSviewer version 1.6.9. Leiden: Centre for Science and Technology Studies, 2018). No time limit was applied; studies conducted in animals were excluded. Search results were deduplicated before study selection.
Study selection was performed by one reviewer (E.L.v.d.L.), based on screening of title and abstract, using Rayyan application for systematic reviews (Rayyan Qatar Computing Research Institute). Hereafter, full-text screening was performed by two reviewers independently (E.L.v.d.L. and B.N.C.). Disagreements were solved through discussion and decision on inclusion was made upon the two author's opinion. Corresponding authors were contacted in case of unavailable full text or conference abstracts.
The systematic review and meta-analysis was performed in accordance to the Preferred Reporting Items for Systematic Reviews and Meta-Analysis guidelines [10], and was prospectively registered in PROSPERO (CRD42020107897).
Data analysis
For the included studies, a table format was used to extract data on study and population characteristics, and outcome measures [prevalence with corresponding 95% confidence interval (CI) of hypertension, and hypertension awareness, treatment and control], by both reviewers (E.L.v.d.L. and B.N.C.) independently. If results from the same study were published multiple times, the article reporting the most complete data were included. Corresponding authors were contacted by e-mail in case of incomplete outcome data.
Hypertension awareness was defined as the proportion of individuals with hypertension reporting any prior diagnosis of hypertension by a healthcare professional. Hypertension treatment was defined as the proportion of participants with hypertension being on treatment, with treatment defined by original authors as receiving prescribed BP-lowering medication or antihypertensive treatment not otherwise specified. Hypertension control was defined in two ways: first, as the proportion of participants receiving BP-lowering treatment with systolic BP (SBP) and diastolic (BP) below 140/90 or 160/95 mmHg and second, as the proportion of participants with hypertension reaching a SBP and DBP below 140/90 or 160/95 mmHg.
Risk of bias of the individual studies was assessed using the Risk of Bias Tool in population-based prevalence studies by Hoy et al.[11], including four items assessing internal and six items assessing external validity of the identified studies, classifying them into low, moderate or high risk of bias categories. Studies with low or moderate risk of bias were included in the meta-analysis. One study was excluded from meta-analysis of hypertension control rates, because it used a different case definition [12].
Unweighted pooled prevalence rates for hypertension awareness, treatment and control for the various populations were derived from the numbers reported by the original authors. To compare levels of hypertension awareness, treatment and control between EMPs and the EHP, data on proportions of hypertension awareness, treatment and control among those being treated were pooled, resulting in odds ratios (OR) with corresponding 95% CI, weighted for sample size according to the Mantel–Haenszel method [13]. Heterogeneity between studies was assessed using the I2 statistic. For the meta-analysis a random-effects model was used if the Q test for heterogeneity was significant (P < 0.05) and fixed-effects model in case of nonsignificance (P > 0.05). Meta-analysis was visualized in Forrest plots, using Review Manager (version 5.3; Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014) and R Statistics version 3.6.2 [R Core Team (2019). R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria]. For the meta-analysis, data were analysed separately for those studies using higher (SBP ≥ 160 mmHg and/or DBP ≥ 95 mmHg) and lower (≥140 mmHg SBP and/or ≥90 mmHg DBP) BP threshold to define hypertension, and results were stratified by sex and EMP. EMPs were grouped by their geographical region of origin as per categorization of the United Nations World Regions [14]. Pooled groups were African, South Asian, Moroccan, Turkish, Chinese origin populations; see Supplementary Digital Content three for specification on subpopulations included per pooled group. The African origin group consisted of African Caribbean and SSA populations. The South Asian origin population comprised of South Asian Surinamese and South Asian populations. These distinctions within the African and South Asian origin populations were made to allow comparison between groups with differences in migration history, that is those who migrated directly from SSA and South Asian to Europe, and those with SSA or South Asian ancestral origin who migrated to Europe via the Caribbean (including Suriname). The EHP was defined as the population of European origin living in one of the 27 members European Union (EU) member states, the United Kingdom, Norway or Switzerland, or specified by the original author as European, White, Caucasian, European Caucasian, native Dutch, native Portuguese or Italian.
RESULTS
Primary search was performed on 13 September 2018 and was updated on 29 January 2020, identifying 2002 studies in MEDLINE, 1600 studies in EMBASE and 2164 studies in Web of Science; six studies were identified through other sources (reference checking of primary studies and tracked citations in GoogleScholar). After removal of duplicates, 4160 records were screened, of which 4068 were excluded based on title and abstract. A total of 92 full-text articles were screened, and after exclusion of 76 studies, 16 studies were included for analysis (for flowchart, Supplementary Digital Content 3).
Included studies reported data of approximately 57 000 Europeans, 12 000 African origin (African Caribbean n = 6800, SSA n = 2310), 7600 South Asian origin (South Asian Surinamese n = 2500, South Asian n = 5000), 2700 Turkish, 2600 Moroccan and 1900 Chinese participants (Table 1), residing in the United Kingdom [12,15–24], the Netherlands [25–27], Portugal [28] or Italy [29]. 14 studies had a cross-sectional study design [15–23,25–29] and were conducted in urban areas; two studies [12,24] included nation-wide survey data. Ethnicity was mainly defined based on (self-reported) country of birth, (grand-) parental origin and/or self-reported ethnicity.
TABLE 1.
Characteristics of studies included in analysis
| Study design | Study characteristics | Population characteristics | |||||||
| Reference | a. Place b. Study period c. Study design d. Sampling method | Indicator of ethnicity | Ethnic groups | n (% women) | Response/Participation rate | Age range (years), mean (SD or CI) | BMI (kg/m2) or obesity (BMI > 30) | Fasting plasma glucose or diabetes mellitus | |
| Agyemang et al.[25] | a. South-East Amsterdam, The Netherlands b. 2001–2003 c. Cross-sectional d. Random sample from municipality register | Country of birth or parental country of birth, self-reported maternal ethnic origin | White Dutch: African Surinamese: SA Surinamese: | 508 (50.2) 581 (67.8) 294 (45.1) | 61% 60% (African + SA Surinamese) | Men White Dutch: African Surinamese: SA Surinamese: Women White Dutch: African Surinamese: SA Surinamese: | 35–60 48.1 (47.3–48.9) 44.1 (43.2–44.9) 44.3 (43.2–45.4) 47.4 (46.6–48.2) 43.3 (42.9–44.0) 45.0 (44.0–46.0) | Mean (CI) 26.2 (25.7–26.8) 26.4 (25.7–27.0) 26.4 (25.5–27.2) 26.1 (25.5–26.8) 29.4 (28.8–29.9) 27.5 (26.7–28.3) | n/a |
| Agyemang et al.[27] | a. Amsterdam, The Netherlands b. 2004 c. Cross-sectional d. Random sample from municipality register | Self-reported country of birth or parental country of birth | Native Dutch: Turkish: Moroccan: | 511 (58.5) 433 (53.6) 360 (45.8) | 46% 50% 39% | Men Native Dutch: Turkish: Moroccan: Women Dutch: Turkish: Moroccan: | ≥18 51.5 (49.5–53.6) 48.7 (46.9–50.5) 52.4 (50.5–54.2) 51.7 (49.9–53.5) 43.6 (41.8–45.4) 44.1 (41.9–6.3) | Mean (CI) 25.7 (25.2–26.3) 28.1 (27.5–28.7) 27.0 (26.5–27.6) 26.3 (25.8–26.9) 31.1 (30.3–31.9) 29.7 (28.7–30.7) | % 7.5 17.0 11.0 2.8 12.8 11.7 |
| Agyemang et al.[26] | a. Amsterdam, The Netherlands b. 2011–2014 c. Baseline data of prospective cohort d. Random sample from municipality register | Country of birth and parental country of birth | Dutch: Moroccan: Turkish: SA Surinamese: African Surinamese: Ghanaian: | 2142 (54.1) 2252 (60.7) 2277 (53.8) 2278 (55.3) 2184 (63.2) 1871 (59.5) | Overall response rate: 63% Participation rate: Dutch: 54% Moroccans: 35% Turkish: 36% Surinamese: 43% Ghanaians: 57% | Men Dutch: Moroccan: Turkish: SA Surinamese: African Surinamese: Ghanaian: Women Dutch: Moroccan Turkish: SA Surinamese: African Surinamese: Ghanaian: | 18–70 47.3 (46.4–48.2) 41.8 (40.9–42.7) 40.6 (39.9–41.3) 45.2 (44.4–46.0) 47.6 (46.7–48.5) 47.1 (46.3–47.9) 45.5 (44.7–46.4) 39.3 (38.6–39.9) 40.3 (39.6–40.9) 46.7 (45.8–47.3) 47.5 (46.8–48.2) 43.9 (43.2–44.5) | Mean (CI) 25.2 (25.0–25.4) 26.6 (26.3–26.9) 27.9 (27.6–28.2) 25.7 (25.5–26.0) 26.4 (26.1–26.7) 26.8 (26.5–27.1) 24.3 (24.1–24.5) 28.3 (28.0–28.6) 29.4 (29.0–29.8) 26.9 (26.6–27.2) 28.8 (28.5–29.1) 29.5 (29.2–29.8) | mmol/l 5.5 (5.4–5.5) 5.8 (5.7–5.9) 5.6 (5.5–5.7) 6.0 (5.9–6.1) 5.5 (5.4–5.7) 5.6 (5.5–5.7) 5.1 (5.0–5.2) 5.3 (5.2–5.4) 5.3 (5.2–5.4) 5.6 (5.5–5.7) 5.4 (5.3–5.5) 5.2 (5.1–5.3) |
| Cappuccio et al.[15] | a. South London, UK b. 1994–1996 c. Cross-sectional survey d. Patient list from 9 GP practices | Name analysis, verified at interview (country of birth, language, religion, migration history, parental country of birth) | White: African descent (SSA + African Caribbean): SA: | 524 (n/a) 549 (n/a) 505 (n/a) | Overall response rate 82%; participation rate 76% | Men + Women White: African descent: SA: Men White: African descent: SA: Women White: African descent: SA: | 49.8 (5.6) 51.1 (5.8) 49.4 (5.9) | % (CI) 14.8 (10.5–19.9) 14.8 (10.5–20.5) 8.4 (5.3–12.4) 18.8 (14.7–23.9) 39.8 (34.8–45.3) 19.7 (15.0–25.1) | n (%) 181 (6.7) 157 (17.9) 211 (25.4) 210 (5.2) 245 (14.9) 191 (20.5) |
| Chaturvedi et al.[16] | a. Inner London, UK b. n/a c. Cross-sectional d. Patient list from 9 GP practices | Assigned by interviewer based on appearance and parental origin | European: West African + Afro-Caribbean: | 585 (53.5) 581 (57.5) | Overall response rate 58% | Men European: Afro-Caribbean: Women European: Afro-Caribbean: | 40–64, n/a | Mean (CI) 26.4 (26.0–26.8) 26.0 (25.6–26.4) 26.0 (25.5–26.5) 29.1 (28.5–29.6) | % (CI) 6.5 (5.0–8.0) 12.9 (10.4–15.4) 4.0 (2.7–5.3) 17.7 (15.6–19.8) |
| Cruickshank et al.[18] | a. Birmingham, UK b. n/a c. Cross-sectional d. Health survey among employees of 12 factories | Assigned by observer based on appearance | White: Afro-Caribbean: Indian: | 603 (27.2) 274 (58.0) 172 (0) | Participation rate 80% | 16–64, n/a | n/a | n/a | |
| Cruickshank et al.[17] | a. North West London, UK b. n/a c. Cross-sectional d. Random sample from 2 GP practices | Grandparental origin | White: Afro-Caribbean: Gujarati Indian: | 101 (51.5) 106 (50.0) 107 (56.1) | Participation rate 77% | Men White: Afro-Caribbean: Gujarati Indian: Women White: Afro-Caribbean: Gujarati Indian: | 45–74 62.0 (7.0) 57.0 (5.0) 62.2 (5.0) 60.3 (7.0) 56.6 (6.0) 62.2 (5.0) | Mean (SD) 26.2 (4.0) 26.0 (4.0) 25.2 (3.0) 26.3 (5.0) 29.1 (5.0) 26.8 (5.0) | n (%) 2 (4.0) 22 (41.5) 15 (31.9) 1 (1.9) 9 (17.0) 17 (28.3) |
| Cruickshank et al.[19] | a. Inner city Manchester, UK b. 1992–1995 c. Cross-sectional d. Random sample from population registers of 4 health centres | Grandparental origin | European: African-Caribbean: | 558 (52.5) 480 (53.8) | European: n/a African-Caribbean: 66% | Men European: African-Caribbean: Women European: African-Caribbean: | 25–74 51.4 (49–53) 52.5 (50–54) 52.5 (50–54) 47.5 (45–59) | n/a | n/a |
| Haines et al.[20] | a. North West London, UK b. 1983–1986 c. Cross-sectional d. All patients from 1 GP practice | Self-reported ethnic group | Whites: Blacks of Caribbean origin: | 936 (51.9) 415 (54.0) | 61% | Men Whites: Blacks: Women Whites: Blacks: | 17–70 42.4 (15.6) 41.4 (15.6) 40.9 (15.5) 36.5 (13.8) | n/a | n/a |
| Knight et al.[21] | a. Bradford, UK b. n/a c. Cross-sectional d. Health survey among manual workers in 2 textile factories | Name analysis, self-reported origin | Asian (mainly Pakistani): Non-Asian (Europeans): | 128 (0) 160 (0) | Asian 83.4% Non-Asian 70.6% | Men Asian: Non-Asian: | 20–65, 41 | Mean (CI) 24.5 (24.0–25.1) 25.2 (24.7–25.7) | n (%) 14 (10.9) 7 (4.4) |
| Lemic-Stojcevic et al.[22] | a. Inner city South London, UK b. 1995 c. Cross-sectional d. Random sample from 6 GP practices | Self-reported ethnic group | White: Black Caribbean: Black African: | 291 (52.6) 310 (56.1) 102 (63.7) | Questionnaire: 45% Examination: White: 68% Black Caribbean: 73% Black African: 55% | White: Black Caribbean: Black African: | 45–74 58.7 (8.0) 60.3 (7.3) 54.0 (6.2) | n/a | n/a |
| Lopes et al.[28] | a. Lisbon region, Portugal b. 2010–2011 c. Cross-sectional d. Random sample from 52 GP practices with high proportion of immigrant patients | Country of birth and self-reported ethnic origin | Native Portuguese: Portuguese speaking African countries immigrants: | 449 (51.2) 337 (57.9) | Native Portuguese: 68% African immigrants: 75% | Portuguese: African Immigrants: | 40–80 64.2 (9.1) 57.1 (10.1) | n (%) 179 (47.6) 153 (51.0) | n (%) 120 (26.7) 92 (27.3) |
| Modesti et al.[29] | a. Prato, Tuscany, Italy b. 2014–2015 c. Baseline data from prospective cohort d. Chinese: Network sampling procedure through 4 community-based Chinese organizations; Italians: random sample from GP practices | Country of birth and grandparental country of birth | Italian: Chinese: | 291 (51.2) 1200 (52.9) | Italian: 67% Chinese: n/a | Italian: Chinese: | 35–59 47.6 (7.5) 46.2 (7.1) | Mean (SD) 25.4 (3.2) 23.7 (3.1) | mg/dl 103.6 (55.5) 118.3 (33.4) |
| Nazroo et al.[12] | a. England, UK b. 1998, 1999, 2003, 2004 c. Cross-sectional, National Health Survey d. Random sample from postcode sectors | Self-reported ethnic origin of family | White: Black Caribbean: Indian: Pakistani: Bangladeshi: Chinese: | 17 836 (n/a) 1067 (n/a) 1370 (n/a) 1041 (n/a) 712 (n/a) 701 (n/a) | Household response rate: General population: 74–76% Ethnic minority sample: 66–67% | White: Black Caribbean: Indian: Pakistani: Bangladeshi: Chinese: | 16–74 | n/a | n (%) 259 (3.7) 95 (10.3) 134 (10.9) 98 (11.5) 94 (16.9) 31 (5.9) |
| Patel et al.[23] | a. Inner city Sandwell, UK b. 2005–2006 c. Cross-sectional d. 10 Pubic screening events | Self-reported ethnicity | European: Gujarati Indian: Punjabi Indian: Bangladeshi: Other Indian (Sri Lankan, South Indian, Pakistani): | 276 (56.9) 79 (45.6) 163 (46.6) 160 (58.8) 68 (44.1) | Attendance rate: Indian: 80%; Bangladeshi, Pakistani: <50% | Men European Caucasian: Gujarati Indian: Punjabi Indian: Bangladeshi: Other Indian: Women European Caucasian: Gujarati Indian: Punjabi Indian: Bangladeshi: Other Indian: | 25–90, 47.7 (15.2) | BMI > 27 kg/m2 % (CI) 48.4 (39.4–57.4) 43.1 (28.0–58.3) 40.0 (29.7–50.3) 48.2 (36.1–60.2) 56.2 (39.7–72.6) 43.7 (35.8–51.5) 37.6 (21.4–53.9) 50.1 (38.8–61.3) 47.2 (37.1–57.3) 41.0 (0–109) | n (%) 4 (3.3) 8 (19.2) 14 (15.6) 23 (35.1) 2 (6.6) 3 (1.7) 8 (22.1) 5 (6.4) 25 (26.8) 3 (10.0) |
| Primatesta et al.[24] | a. England, UK b. 1991–1996 c. Cross-sectional, National Health Survey d. Multistage stratified probability sampling design | Self-reported ethnicity in predefined categories | White: Black (SSA + African Caribbean): South Asian: | 31 619 (53.7) 295 (53.9) 529 (45.9) | n/a | Men White: SSA + African Caribbean: SA: Women White: SSA + African Caribbean: SA: | ≥40, n/a | n (%) 2633 (18) 26 (19) 34 (12) 3568 (21) 78 (49) 56 (23) | n/a |
CI, confidence interval; GP, general practitioner; n/a, not available; SA, South Asian; SSA, sub-Saharan Africa.
Prevalence of hypertension awareness was reported in seven studies [12,15,23,25–27,29], treatment in 13 studies [15–22,24–27,29], and control in 10 studies [12,15,19,22,24–29]. Of the 16 included studies, eight used the higher BP cut-off [15–21,24] (Supplementary Digital Content 4) and eight used the lower BP cut-off to define hypertension [12,22,23,25–29] (Table 2). Generally, hypertension prevalence was higher among African origin populations compared with the EHP, but lower in Moroccan and Turkish origin populations.
TABLE 2.
Study results of included studies using the blood pressure cut-offs of systolic at least 140 and/or diastolic at least 90 mmHg to define hypertension
| Hypertension | |||||||||
| Reference | Diagnostic method | Definition | Population | Total n | Prevalence, n (%) | Awarenessa, n (%) | Treatmentb, n (%) | Control among treatedc, n (%) | Control among total prevalenced, n (%) |
| Agyemang et al.[25] | Automated digital BP device; mean of two BP readings on right arm | SBP ≥ 140 or DBP ≥ 90 mmHg or being on antihypertensive medication | Men White Dutch: African Surinamese: SA Surinamese: Women White Dutch: African Surinamese: SA Surinamese: | 253 187 135 255 394 159 | 104 (41.3) 88 (47.1) 56 (41.5) 66 (25.9) 182 (46.3) 68 (42.8) | 57 (54.8) 44 (50.0) 30 (53.6) 48 (72.7) 136 (74.7) 54 (79.4) | 35 (33.3) 21 (23.9) 16 (28.6) 20 (30.8) 54 (29.8) 30 (44.1) | 15 (42.1) 3 (14.3) 6 (37.5) 10 (50.0) 26 (48.1) 12 (40.0) | 15 (14.4) 3 (3.4) 6 (10.7) 10 (15.2) 26 (14.3) 12 (17.6) |
| Agyemang et al.[27] | Automated digital BP device; mean of two BP readings on left arm | SBP ≥ 140 or DBP ≥ 90 mmHg or being on antihypertensive medication | Men Dutch: Turkish: Moroccan: Women Dutch: Turkish: Moroccan: | 212 201 195 299 232 165 | 103 (48.8) 51 (25.6) 51 (26.1) 105 (35.0) 51 (22.1) 32 (19.6) | 38 (36.6) 14 (26.7) 15 (28.9) 56 (53.8) 27 (52.2) 9 (29.0) | 29 (28.6) 10 (20.5) 12 (22.6) 56 (53.5) 21 (41.2) 8 (25.9) | 9 (30.0) 3 (28.6) 3 (28.6) 9 (16.2) 6 (26.7) 1 (14.3) | 9 (8.7) 3 (5.9) 3 (5.9) 9 (5.6) 6 (11.8) 1 (3.1) |
| Agyemang et al.[26] | Automated digital BP device; mean of two BP readings on left arm | SBP ≥ 140 or DBP ≥ 90 mmHg or being on antihypertensive medication | Men Dutch: Moroccan: Turkish: SA Surinamese: African Surinamese: Ghanaian: Women Dutch: Moroccan: Turkish: SA Surinamese: African Surinamese: Ghanaian: | 983 885 1052 1018 803 758 1159 1367 1225 1260 1381 1113 | 331 (33.7) 207 (23.9) 323 (30.7) 435 (42.3) 378 (47.1) 449 (61.6) 219 (18.9) 216 (15.8) 279 (31.1) 461 (36.6) 635 (46.0) 567 (50.9) | 156 (47.3) 81 (40.1) 118 (37.2) 228 (52.3) 179 (47.9) 253 (57.1) 128 (58.8) 125 (58.1) 168 (60.4) 278 (61.4) 439 (69.6) 331 (59.1) | 118 (35.7) 62 (30.5) 97 (30.0) 225 (51.7) 156 (41.4) 204 (45.4) 118 (53.9) 112 (51.9) 180 (60.4) 228 (62.5) 390 (61.4) 321 (56.6) | 63 (53.4) 32 (51.6) 45 (46.6) 94 (41.8) 51 (32.7) 55 (27.0) 72 (61.0) 58 (51.8) 103 (57.2) 136 (47.2) 179 (45.9) 137 (42.7) | 63 (19.0) 32 (15.5) 45 (13.9) 94 (21.6) 51 (13.5) 55 (12.2) 72 (32.9) 58 (26.9) 103 (36.9) 136 (29.5) 179 (28.2) 137 (24.2) |
| Lemic-Stojcevic et al.[22] | Automated digital BP device; mean of three BP readings | SBP ≥ 140 or DBP ≥ 90 mmHg or being on antihypertensive treatment (treatment not specified) | White: Black Caribbean: Black African: | 291 310 102 | 158 (54.3) 246 (79.4) 73 (71.6) | n/a | 45 (15.5) 130 (41.9) 34 (33.3) | 15 (33.3) 18 (13.8) 9 (26.5) | 15 (9.5) 18 (7.3) 9 (12.3) |
| Lopes et al.[28] | Unknown device; mean of three BP readings | SBP ≥ 140 or DBP ≥ 90 mmHg and being on antihypertensive medication | Native Portuguese: African immigrant: | 449 337 | 449 (100) 337 (100) | n/a | n/a | 212 (47.2) 153 (45.4) | 212 (47.2) 153 (45.4) |
| Modesti et al.[29] | Semiautomatic device; three BP readings, mean of last two readings | SBP ≥ 140 or DBP ≥ 90 mmHg or being on antihypertensive medication | Italian: Chinese: | 291 1200 | 62 (21.3) 326 (27.2) | 30 (48.4) 187 (57.4) | 27 (43.5) 132 (40.5) | 12 (44.4) 56 (42.4) | 12 (19.4) 56 (17.2) |
| Nazroo et al.[12] | Automated digital BP device; 3 BP reading, mean of last two readings on right arm | SBP ≥ 140 or DBP ≥ 90 mmHg or previous diagnosis or being on antihypertensive medication | White: Black Caribbean: Indian: Pakistani: Bangladeshi: Chinese: | 17 836 1067 1370 1041 712 701 | 5868 (32.9)e,f 380 (35.6)e,f 393 (28.4)e,f 218 (20.9)e,f 137 (19.2)e,f 143 (20.4)e,f | 3621 (61.7)e,f 280 (73.6)e,f 259 (65.8)e,f 148 (67.9)e,f 67 (70.8)e,f 86 (59.8)e,f | n/a | 1892 (52.0)e,f,g 149 (53.1)e,f,g 152 (58.6)e,f,g 88 (59.6)e,f,g 41 (61.0)e,f,g 45 (52.5)e,f,g | 1892 (32.2)e,f 149 (39.2)e,f 152 (38.7)e,f 88 (40.4)e,f 41 (29.9)e,f 45 (31.5)e,f |
| Patel et al.[23] | Semiautomatic device; three BP readings, mean of last two readings | SBP > 140 or DBP > 90 mmHg or previous diagnosis or being on antihypertensive medication | Men European Caucasian: Gujarati Indian: Punjabi Indian: Bangladeshi: Other Indian: Women European Caucasian: Gujarati Indian: Punjabi Indian: Bangladeshi: Other Indian: | 119 43 87 66 37 157 36 76 94 31 | 54 (45.1)e 27 (62.4)e 43 (49.6)e 15 (23.1)e 23 (63.5)e 53 (33.8)e 15 (41.9)e 20 (26.5)e 32 (33.6)e 6 (19.5)e | 38 (71.0)e 18 (67.8)e 34 (78.0)e 14 (93.0)e 17 (75.7)e 42 (78.7)e 13 (85.0)e 18 (88.7)e 28 (86.4)e 5 (85.9)e | n/a | n/a | n/a |
BP, blood pressure; n/a, not available; SA, South Asian; SSA, sub-Saharan African.
In percentage of participants with hypertension.
In percentage of participants with hypertension.
In percentage of those on treatment for hypertension.
In percentage of participants with hypertension.
Age-adjusted.
Sex-adjusted.
In percentage of those being aware and/or treated for hypertension.
Hypertension awareness rates were highest in African, South Asian origin and EHPs, both in studies using the higher cut-off to determine hypertension, as well as in studies using the lower cut-off, whereas Turkish and Moroccan populations had lowest levels of awareness (Fig. 1a and c). This was reflected in the meta-analysis of the studies using the lower BP cut-off to define hypertension, showing higher odds of being aware for African (OR 1.26, 95% CI 1.02–1.56, I2 = 63%) and South Asian origin (OR 1.15, 1.02–1.30, I2 = 44%), but lower odds for Turkish (OR 0.81, 0.65–1.00, I2 = 41%) and Moroccan (OR 0.79, 0.62–1.00, I2 = 47%) populations compared with the EHP (Figs 2–5). OR for awareness did not differ between Chinese and European populations (Fig. 6).
FIGURE 1.
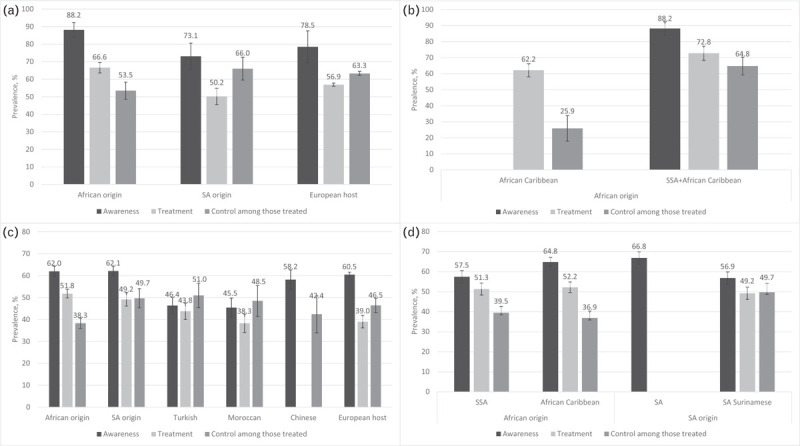
Unweighted pooled prevalence rates of hypertension awareness, treatment and control among those on treatment per ethnic population. Panel (a) shows the results of studies using blood pressure cut-offs of systolic at least 160 and/or diastolic at least 95 mmHg to define hypertension; panel (b) shows the pooled prevalence rates for the subpopulations of African origin that were pooled together in panel (a); panel (c) shows the results of studies using blood pressure cut-offs of systolic at least 140 and/or diastolic at least 90 mmHg to define hypertension; panel (d) shows the pooled prevalence rates for the subpopulations of African origin and South Asian origin that were pooled together in panel (c); error bars are 95% confidence intervals. SA, South Asian; SSA, sub-Saharan Africa.
FIGURE 2.
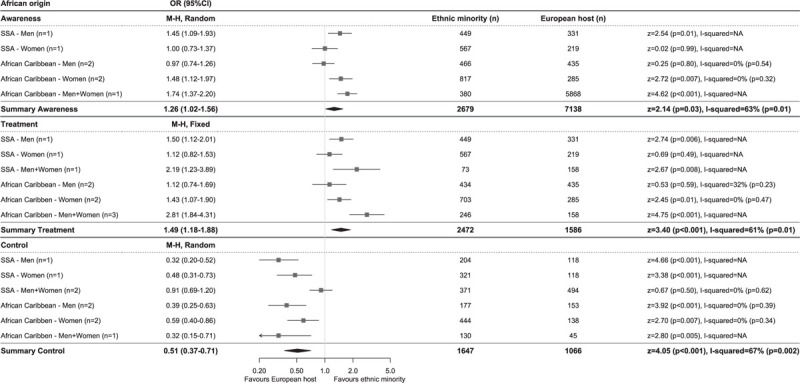
Comparison of prevalence of hypertension awareness, treatment and control among those on treatment between African origin and European host populations of studies using blood pressure cut-offs of systolic at least 140 and/or diastolic at least 90 mmHg to define hypertension. Diamonds denote the weighted pooled odds ratios with 95% confidence intervals. CI, confidence interval; M–H, Mantel–Haenszel; OR, odds ratio; SSA, sub-Saharan African, ‘n’ is the number of comparisons available for each subgroup.
FIGURE 5.
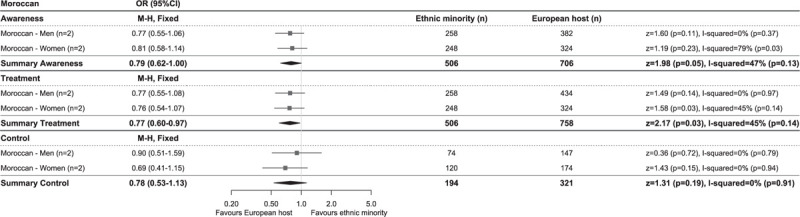
Comparison of prevalence of hypertension awareness, treatment and control among those on treatment between Moroccan and European host populations of studies using blood pressure cut-offs of systolic at least 140 and/or diastolic at least 90 mmHg to define hypertension. Diamonds denote the weighted pooled odds ratios with 95% confidence intervals. CI, confidence interval; M–H, Mantel–Haenszel; OR, odds ratio, ‘n’ is the number of comparisons available for each subgroup.
FIGURE 6.

Comparison of prevalence of hypertension awareness between Chinese and European host populations of studies using blood pressure cut-offs of systolic at least 140 and/or diastolic at least 90 mmHg to define hypertension. Diamonds denote the weighted pooled odds ratios with 95% confidence intervals. CI, confidence interval; M–H, Mantel–Haenszel; OR, odds ratio, ‘n’ is the number of comparisons available for each subgroup.
Hypertension treatment levels were generally higher in studies using the higher BP cut-off than in studies using the lower BP cut-off for hypertension. African origin populations had highest treatment levels, whereas Moroccan populations had lowest treatment levels (Fig. 1a and c). The meta-analysis of studies reporting on hypertension treatment showed higher OR in African origin than in the EHP, both in studies using the higher (OR 1.78, 1.44–2.19, I2 = 18%, Supplementary Digital Content 5) and lower (OR 1.49, 1.18–1.88, I2 = 61%) BP cut-off for hypertension (Fig. 2). No difference in odds of receiving antihypertensive treatment could be observed between South Asian origin (higher BP cut-off OR 0.96, 0.76–1.21, I2 = 0%; Supplementary Digital Content 6), lower BP cut-off OR 1.25, 0.72–2.17, I2 = 83% (Fig. 3), Turkish (lower BP cut-off OR 0.88, 0.54–1.41, I2 = 73%) and the EHPs, although a trend towards lower OR of being treated could be observed for Turkish men compared with their European counterparts (OR 0.75, 0.55–1.02, I2 = 0%) (Fig. 4). Moroccans were less likely to receive treatment compared with the EHP (OR 0.77, 0.60–0.97, I2 = 45%) (Fig. 5).
FIGURE 3.
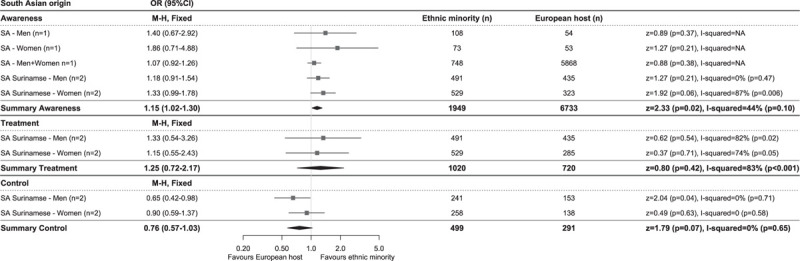
Comparison of prevalence of hypertension awareness, treatment and control among those on treatment between South Asian origin and European host populations of studies using blood pressure cut-offs of systolic at least 140 and/or diastolic at least 90 mmHg to define hypertension. Diamonds denote the weighted pooled odds ratios with 95% confidence intervals. CI, confidence interval; M–H, Mantel–Haenszel; OR, odds ratio; SA, South Asian, ‘n’ is the number of comparisons available for each subgroup.
FIGURE 4.
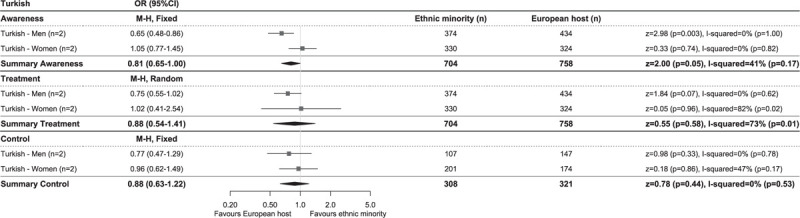
Comparison of prevalence of hypertension awareness, treatment and control among those on treatment between Turkish and European host populations of studies using blood pressure cut-offs of systolic at least 140 and/or diastolic at least 90 mmHg to define hypertension. Diamonds denote the weighted pooled odds ratios with 95% confidence intervals. CI, confidence interval; M–H, Mantel–Haenszel; OR, odds ratio, ‘n’ is the number of comparisons available for each subgroup.
Hypertension control rates among those on treatment were lowest in African origin populations, whereas South Asian origin populations most frequently reached adequate BP control (Fig. 1a and c). Similar trends could be observed for the prevalence of hypertension control as proportion of all hypertensive participants (Table 2). Compared with the EHP, African origin populations were less likely to have their BP controlled despite being on treatment (lower BP threshold, OR 0.51, 0.37–0.71, I2 = 67%), both in SSA and African Caribbean populations and in both men and women (Fig. 2). South Asian origin populations had a trend towards lower pooled odds of reaching BP control compared with the EHP (OR 0.76, 0.57–1.03, I2 = 0%), although this was only significant for South Asian Surinamese men compared with their Dutch counterparts (OR 0.65, 0.42–0.98, I2 = 0%) (Fig. 3). Turkish and Moroccan populations did not differ from the EHP in terms of BP control, although a trend towards lower odds could be observed in Turkish (OR 0.88, 0.63–1.22, I2 = 0%) and Moroccan populations (OR 0.78, 0.53–1.13, I2 = 0%) compared with the EHP (Figs. 4 and 5).
Of the 16 studies included for analysis, 10 had moderate risk of bias [16–22,24,27,28] and six studies had low risk of bias [12,15,23,25,26,29] (Supplementary Digital Content 7). With regard to the external validity of the studies, most studies had moderate risk of bias, because the target study population was not a close representation of the national population (e.g. study conducted in urban areas only), the sampling frame was not a close representation of the target population (e.g. sampling frame consisting of general practitioner practices) and/or there was a substantial risk of non-response bias (e.g. no comparison of characteristics between responders and nonresponders was reported). In general, the internal validity of the studies was good, although most older studies did not report on the calibration of the auscultatory BP measurement (standard or Hawksley random zero sphygmomanometer).
DISCUSSION
In the present systematic review and meta-analysis comparing 26 800 individuals of five different EMPs with 57 000 individuals from the EHP, we show that levels of hypertension awareness, treatment and control significantly differ between EMPs and the EHP. Compared with the EHP, African origin populations had higher rates of hypertension awareness and treatment, but a lower rate of hypertension control; South Asian origin populations had a higher rate of hypertension awareness, but no differences in the rates of hypertension treatment or control; Turkish populations had a lower rate of hypertension awareness, but no differences in treatment and control rates; Moroccan populations had lower rates of hypertension awareness and treatment, but no difference in control rate; Chinese populations did not differ in hypertension awareness rates.
The current findings of higher awareness and treatment in African and South Asian origin populations could be the result of health promotion campaigns specifically targeting these populations because of established higher risk of CVD, emphasizing the importance of regular BP checks and medication adherence [30]. In addition, alertness among healthcare providers might have increased, for instance by introduction of ethnic-specific hypertension treatment recommendations in CVD prevention guidelines [31]. The lower levels of awareness in Turkish and of awareness and treatment in Moroccan populations compared with the host population are of concern. Historically, these populations were considered as having a low CVD risk, because of their adherence to the Mediterranean diet and lower prevalence of hypertension compared with the EHP [27]. However, as trends in coronary heart disease incidence among Turkish and Moroccan populations are changing in an unfavourable direction [32], more BP screening activities should be directed towards these populations.
Remarkably, compared with the EHP, African origin populations had lower hypertension control levels, despite higher levels of hypertension awareness and treatment. In addition to lower levels of awareness and treatment, Turkish men and Moroccan women also tended to have lower hypertension control rates compared with their counterparts from the EHP. Compared the EHPs, included participants from EMPs seemed to be slightly younger, having higher BMI and higher fasting plasma glucose levels, potentially impacting the observed differences in hypertension control rates. Alternatively, differences in (determinants of) access to healthcare between ethnic minority and EHPs, might impact the hypertension control rates of these populations [33]. For instance, from the patient perspective, the ability to seek, to pay for, and to engage in hypertension care are influenced by culture, socioeconomic status and medication adherence, and these factors may differ between populations of different ethnic origin, resulting in differences in hypertension control rates. Results from studies conducted in the USA support this, showing differential contribution of factors to hypertension control between different ethnic groups [34]. In addition, from the healthcare provider perspective, factors like clinical inertia or non-adherence to European medical guidelines in terms of drug prescription could contribute to the ethnic differences in control rates among those patients being on BP-lowering treatment. For instance, despite ethnic-specific hypertension treatment recommendations for patients of African origin, physicians do not always implement this in their medication prescription practices [35]. To improve hypertension control rates, research is needed to identify key factors affecting hypertension control in EMPs of African, Turkish and Moroccan origin, on both patient's and healthcare provider's level, and based on that, targeted interventions should be developed and implemented.
The lower hypertension treatment and control rates in the studies using a low BP threshold (BP ≥ 140/90 mmHg) compared with the studies using a high BP cut-off (BP ≥ 160/95 mmHg) could be a result of introduction of cardiovascular risk management guidelines [36]. In the Netherlands and the United Kingdom, where most of the included studies originated from, the decision to start treatment for high BP not solely depends on BP cut-offs, but rather on a score based on multiple CVD risk factors [37,38]. Countries adhering to guidelines in which antihypertensive medication initiation does not necessarily depends on CVD risk scores, such as Canada and the USA, report higher rates of hypertension awareness, treatment and control rates than presented in this review [36]. The National Health and Nutrition Survey from the USA reported hypertension awareness, treatment and control rates (using BP cut-off of 140/90 mmHg to define hypertension) for those aged 45–65 years to be 86, 78 and 65% in African-American, and 84, 75 and 78% in White-Americans [8], which are considerably higher than the rates reported for African origin and EHP in this review.
The current review is the first to report aggregated data of population-based studies on hypertension awareness, treatment and control levels among EMPs in Europe, representing four of the five largest ethnic minority groups originating from outside Europe residing in the EU [39], and data from African origin populations.
Risk of bias mainly originated from limitations in the external validity of the included studies, as most of the studies were conducted in populations residing in urban settings, which might not be a close representation of the national population. Rural–urban differences in supply of healthcare services have been described [40], and hypertension prevalence and control rates are known to show intranational differences [41]. However, as migrant populations tend to congregate in cities [42], the data reported in this review are probably representative for the EMPs in these countries. In addition, external validity might be hampered as the sampling frame of most studies consisted of general practices or health centres, hereby excluding individuals that were not registered at these facilities. In the light of EMPs, this sampling frame could have resulted in excluding recently migrated or undocumented migrants, as in many European countries the access to healthcare service depends on the legal status of the immigrants, and is often limited for undocumented migrants to emergency care services only [43]. Therefore, more research is needed into hypertension status of these underserved populations.
Second, included studies were conducted in a limited number of European countries, with most studies originating from the United Kingdom and the Netherlands, hereby hampering the generalizability of our finding to the whole of Europe. To get a comprehensive overview of the hypertension status of EMPs in Europe, primary population-based studies conducted in more European countries are needed. Moreover, to identify and address inequalities in health, future research should take into consideration diversity within migrant populations originating from a shared geographical region (e.g. between African Caribbean and SSA populations), for instance by taking migration history into account and defining ethnicity in a more detailed and consistent way [44].
Third, the results of this review should be interpreted in the light of heterogeneity between studies. For instance, studies used different indicators of ethnicity, included participants from different age ranges, or used a slightly different definition for hypertension control. The limited number of included studies did not allow for sensitivity analysis stratifying for these differences.
Lastly, the included studies diagnosed hypertension based on multiple BP readings taken on a single occasion. Therefore, as BP is highly variable, it is possible that the reported prevalence of hypertension was overestimated, hereby underestimating the levels of hypertension awareness and treatment. However, as these limitations apply both to the minority ethnic groups as well as to the EHPs included in this study, the estimated differences in awareness, treatment and control rates between the minority and the host population remain valid.
The findings from this systematic and meta-analysis show clear differences between ethnic minority and EHPs in terms of hypertension awareness, treatment and control rates. To improve hypertension awareness, treatment and control rates and diminish differences between populations, determinants of these rates should be identified and ethnic-specific intervention strategies should be designed and implemented, ultimately aiming to reduce ethnic inequalities in hypertension-related complications.
ACKNOWLEDGEMENTS
C.A. was funded by the European Research Council under the EU Framework Programme for Research and Innovation (grant number 772244). All other authors did not receive funding for this work.
Authors’ contributions: E.L.v.d.L., B.-J.H.v.d.B. and C.A. conceived the study. J.G.D. designed the literature search strategy; E.L.v.d.L. and B.N.C. conducted the literature search and data extraction. E.L.v.d.L. performed the statistical analysis and wrote the article, in close collaboration with E.J.A.J.B, B.-J.H.v.d.B and C.A. All authors approved the final version of the article.
Conflicts of interest
There are no conflicts of interest.
Supplementary Material
Footnotes
Abbreviations: BP, blood pressure; CI, confidence interval; CVD, cardiovascular disease; EHP, European host population; EMP, ethnic minority population; EU, European Union; OR, odds ratio; SSA, sub-Saharan Africa
REFERENCES
- 1.Wilkins E, Wilson R, Wickramasinghe K, Bhatnagar P, Leal J, Luengo-Fernandez R, et al. European cardiovascular disease statistics 2017. Brussels: European Heart Network AISBL; 2017. [Google Scholar]
- 2.Modesti PA, Reboldi G, Cappuccio FP, Agyemang C, Remuzzi G, Rapi S, et al. Panethnic differences in blood pressure in Europe: a systematic review and meta-analysis. PLoS One 2016; 11:e0147601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Agyemang C, van Oeffelen AA, Norredam M, Kappelle LJ, Klijn CJ, Bots ML, et al. Ethnic disparities in ischemic stroke, intracerebral hemorrhage, and subarachnoid hemorrhage incidence in the Netherlands. Stroke 2014; 45:3236–3242. [DOI] [PubMed] [Google Scholar]
- 4.Agyemang C, Addo J, Bhopal R, Aikins Ade G, Stronks K. Cardiovascular disease, diabetes and established risk factors among populations of sub-Saharan African descent in Europe: a literature review. Global Health 2009; 5:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ettehad D, Emdin CA, Kiran A, Anderson SG, Callender T, Emberson J, et al. Blood pressure lowering for prevention of cardiovascular disease and death: a systematic review and meta-analysis. Lancet 2016; 387:957–967. [DOI] [PubMed] [Google Scholar]
- 6.World Health Organization. A global brief on hypertension. Geneva, Switzerland: World Health Organization; 2013. [Google Scholar]
- 7.Mills KT, Bundy JD, Kelly TN, Reed JE, Kearney PM, Reynolds K, et al. Global disparities of hypertension prevalence and control: a systematic analysis of population-based studies from 90 countries. Circulation 2016; 134:441–450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Foti K, Wang D, Appel LJ, Selvin E. Hypertension awareness, treatment, and control in US adults: trends in the hypertension control cascade by population subgroup (National Health and Nutrition Examination Survey, 1999–2016). Am J Epidemiol 2019; 188:2165–2174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lane DA, Lip GY. Ethnic differences in hypertension and blood pressure control in the UK. QJM 2001; 94:391–396. [DOI] [PubMed] [Google Scholar]
- 10.Moher D, Liberati A, Tetzlaff J, Altman DG, Group P. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ 2009; 339:b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hoy D, Brooks P, Woolf A, Blyth F, March L, Bain C, et al. Assessing risk of bias in prevalence studies: modification of an existing tool and evidence of interrater agreement. J Clin Epidemiol 2012; 65:934–939. [DOI] [PubMed] [Google Scholar]
- 12.Nazroo JY, Falaschetti E, Pierce M, Primatesta P. Ethnic inequalities in access to and outcomes of healthcare: analysis of the Health Survey for England. J Epidemiol Community Health 2009; 63:1022–1027. [DOI] [PubMed] [Google Scholar]
- 13.Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al. Cochrane handbook for systematic reviews of interventions version 6.0: Cochrane. 2019; Available from: www.training.cochrane.org/handbook. [Accessed 13 December 2019]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.United Nations Statistics Division. Standard country or area codes for statistical use (M49). New York: United Nations Statistics Division; 1999. [Google Scholar]
- 15.Cappuccio FP, Cook DG, Atkinson RW, Strazzullo P. Prevalence, detection, and management of cardiovascular risk factors in different ethnic groups in south London. Heart 1997; 78:555–563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chaturvedi N, McKeigue PM, Marmot MG. Resting and ambulatory blood pressure differences in Afro-Caribbeans and Europeans. Hypertension 1993; 22:90–96. [DOI] [PubMed] [Google Scholar]
- 17.Cruickshank JK, Cooper J, Burnett M, MacDuff J, Drubra U. Ethnic differences in fasting plasma C-peptide and insulin in relation to glucose tolerance and blood pressure. Lancet 1991; 338:842–847. [DOI] [PubMed] [Google Scholar]
- 18.Cruickshank JK, Jackson SH, Bannan LT, Beevers DG, Beevers M, Osbourne VL. Blood pressure in black, white and Asian factory workers in Birmingham. Postgrad Med J 1983; 59:622–626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cruickshank JK, Mbanya JC, Wilks R, Balkau B, Forrester T, Anderson SG, et al. Hypertension in four African-origin populations: current ‘Rule of Halves’, quality of blood pressure control and attributable risk of cardiovascular disease. J Hypertens 2001; 19:41–46. [DOI] [PubMed] [Google Scholar]
- 20.Haines AP, Booroff A, Goldenberg E, Morgan P, Singh M, Wallace P. Blood pressure, smoking, obesity and alcohol consumption in black and white patients in general practice. J Hum Hypertens 1987; 1:39–46. [PubMed] [Google Scholar]
- 21.Knight T, Smith Z, Lockton JA, Sahota P, Bedford A, Toop M, et al. Ethnic differences in risk markers for heart disease in Bradford and implications for preventive strategies. J Epidemiol Community Health 1993; 47:89–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lemic-Stojcevic N, Dundas R, Jenkins S, Rudd A, Wolfe C. Preventable risk factors for coronary heart disease and stroke amongst ethnic groups in London. Ethn Health 2001; 6:87–94. [DOI] [PubMed] [Google Scholar]
- 23.Patel JV, Gunarathne A, Lane D, Lim HS, Tracey I, Panja NC, et al. Widening access to cardiovascular healthcare: community screening among ethnic minorities in inner-city Britain – the Healthy Hearts Project. BMC Health Serv Res 2007; 7:192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Primatesta P, Bost L, Poulter NR. Blood pressure levels and hypertension status among ethnic groups in England. J Hum Hypertens 2000; 14:143–148. [DOI] [PubMed] [Google Scholar]
- 25.Agyemang C, Bindraban N, Mairuhu G, Montfrans G, Koopmans R, Stronks K, et al. Prevalence, awareness, treatment, and control of hypertension among Black Surinamese, South Asian Surinamese and White Dutch in Amsterdam, The Netherlands: the SUNSET study. J Hypertens 2005; 23:1971–1977. [DOI] [PubMed] [Google Scholar]
- 26.Agyemang C, Kieft S, Snijder MB, Beune EJ, van den Born BJ, Brewster LM, et al. Hypertension control in a large multiethnic cohort in Amsterdam, The Netherlands: the HELIUS study. Int J Cardiol 2015; 183:180–189. [DOI] [PubMed] [Google Scholar]
- 27.Agyemang C, Ujcic-Voortman J, Uitenbroek D, Foets M, Droomers M. Prevalence and management of hypertension among Turkish, Moroccan and native Dutch ethnic groups in Amsterdam, the Netherlands: the Amsterdam Health Monitor Survey. J Hypertens 2006; 24:2169–2176. [DOI] [PubMed] [Google Scholar]
- 28.Lopes E, Alarcao V, Simoes R, Fernandes M, Gomez V, Souto D, et al. Hypertension control at the primary healthcare: a comparison among Portuguese natives and Portuguese speaking African countries immigrants. Acta Med Port 2016; 29:193–204. [DOI] [PubMed] [Google Scholar]
- 29.Modesti PA, Calabrese M, Marzotti I, Bing H, Malandrino D, Boddi M, et al. Prevalence, awareness, treatment, and control of hypertension among Chinese first-generation migrants and Italians in Prato, Italy: the CHIP Study. Int J Hypertens 2017; 2017:6402085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Agyemang C, Meeks K, Boateng R, Beune E. Your health is your wealth: faith-based community action on the health of African migrant communities in Amsterdam. J Epidemiol Community Health 2018; 72:409–412. [DOI] [PubMed] [Google Scholar]
- 31.Barrera L, Leaper C, Pape UJ, Majeed A, Blangiardo M, Millett C. Impact of ethnic-specific guidelines for antihypertensive prescribing in primary care in England: a longitudinal study. BMC Health Serv Res 2014; 14:87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.van Oeffelen AA, Agyemang C, Stronks K, Bots ML, Vaartjes I. Incidence of first acute myocardial infarction over time specific for age, sex, and country of birth. Neth J Med 2014; 72:20–27. [PubMed] [Google Scholar]
- 33.Levesque JF, Harris MF, Russell G. Patient-centred access to healthcare: conceptualising access at the interface of health systems and populations. Int J Equity Health 2013; 12:18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gu A, Yue Y, Desai RP, Argulian E. Racial and ethnic differences in antihypertensive medication use and blood pressure control among US adults with hypertension: the National Health and Nutrition Examination Survey, 2003 to 2012. Circ Cardiovasc Qual Outcomes 2017; 10:e003166. [DOI] [PubMed] [Google Scholar]
- 35.Agyemang C, Nyaaba G, Beune E, Meeks K, Owusu-Dabo E, Addo J, et al. Variations in hypertension awareness, treatment, and control among Ghanaian migrants living in Amsterdam, Berlin, London, and nonmigrant Ghanaians living in rural and urban Ghana – the RODAM study. J Hypertens 2018; 36:169–177. [DOI] [PubMed] [Google Scholar]
- 36.Collaboration NCDRF. Long-term and recent trends in hypertension awareness, treatment, and control in 12 high-income countries: an analysis of 123 nationally representative surveys. Lancet 2019; 394:639–651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.National Institute for Health and Care Excellence, National Institute for Health and Care Excellence. Hypertension in adults: diagnosis and management. NICE guideline. 2019. [Google Scholar]
- 38.Nederlands Huisartsen Genootschap, Nederlands Huisartsen Genootschap. NHG-standaard cardiovasculair risicomanagement. 2019; Available from: https://richtlijnen.nhg.org/standaarden/cardiovasculair-risicomanagement. [Accessed 14 July 2020]. [Google Scholar]
- 39.Eurostat. People in the EU: who are we and how do we live? 2015; Luxembourg: Publications Office of the European Union, 90. [Google Scholar]
- 40.Baeten R, Spasova S, Vanhercke B, Coster S. Inequalities in access to healthcare. Brussels, Belgium: European Social Policy Network; 2018. [Google Scholar]
- 41.Diederichs C, Neuhauser H. Regional variations in hypertension prevalence and management in Germany: results from the German Health Interview and Examination Survey (DEGS1). J Hypertens 2014; 32:1405–1413. discussion 14. [DOI] [PubMed] [Google Scholar]
- 42.European Commission, UN-Habitat. The state of European cities 2016. Luxembourg: Publications Office of the European Union; 2016. [Google Scholar]
- 43.Suess A, Ruiz Perez I, Ruiz Azarola A, March Cerda JC. The right of access to healthcare for undocumented migrants: a revision of comparative analysis in the European context. Eur J Public Health 2014; 24:712–720. [DOI] [PubMed] [Google Scholar]
- 44.Villarroel N, Davidson E, Pereyra-Zamora P, Krasnik A, Bhopal RS. Heterogeneity/granularity in ethnicity classifications project: the need for refining assessment of health status. Eur J Public Health 2019; 29:260–266. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


