Objective:
As uncertainties exist over underlying causes, we aimed to define the characteristics and prognostic significance of low blood pressure (BP) early after the onset of acute stroke.
Methods:
Post hoc analyzes of the international Head Positioning in acute Stroke Trial (HeadPoST), a pragmatic cluster-crossover randomized trial of lying flat versus sitting up in stroke patients from nine countries during 2015–2016. Associations of baseline BP and death or dependency [modified Rankin scale (mRS) scores 3–6] and serious adverse events (SAEs) at 90 days were assessed in generalized linear mixed models with adjustment for multiple confounders. SBP and DBP was analysed as continuous measures fitted with a cubic spline, and as categorical measures with low (<10th percentile) and high (≥140 and ≥90 mmHg, respectively) levels compared with a normal range (≥10th percentile; 120–139 and 70–89 mmHg, respectively).
Results:
Among 11 083 patients (mean age 68 years, 39.9% women) with baseline BP values, 7.2 and 11.7% had low SBP (<120 mmHg) and DBP (<70 mmHg), respectively. Patients with low SBP were more likely to have preexisting cardiac and ischemic stroke and functional impairment, and to present earlier with more severe neurological impairment than other patients. Nonlinear ‘J-shaped’ relationships of BP and poor outcome were apparent: compared with normal SBP, those with low SBP had worse functional outcome (adjusted odds ratio 1.27, 95% confidence interval 1.02–1.58) and more SAEs, particularly cardiac events, with adjustment for potential confounders to minimize reverse causation. The findings were consistent for DBP and were stronger for ischemic rather than hemorrhagic stroke.
Conclusion:
The prognostic significance of low BP on poor outcomes in acute stroke was not explained by reverse causality from preexisting cardiovascular disease, and propensity towards greater neurological deficits and cardiac events. These findings provide support for the hypothesis that low BP exacerbates cardiac and cerebral ischemia in acute ischemic stroke.
Keywords: acute stroke, blood pressure, hypotension, outcome, trial
INTRODUCTION
Blood pressure (BP) is often altered in acute stroke [1–3], typically as an acute hypertensive (physiological) response (SBP ≥140 mmHg and/or DBP ≥90 mmHg within 24 h of symptom onset), presumably related to cerebral ischemia and/or elevated intracranial pressure. Observational studies are consistent in showing that high BP is positively associated with adverse clinical outcomes [4,5]. However, randomized controlled trials of blood pressure lowering have produced mixed results [6,7], although any benefits on functional recovery appear more likely for intracerebral hemorrhage (ICH) [7] than acute ischemic stroke (AIS) [8]. Although the prognostic significance of the less frequent situation of low presentation BP (variously defined by SBP <120, <130, or <155 mmHg) in an acute stroke is well recognized [9–14], uncertainties exist over whether this represents a risk factor or risk marker in relation to premorbid factors in final few years of life [15] or critical cerebral injury from impaired perfusion pressure in acute stroke. Herein, we report post hoc secondary analyses of the Head Positioning in acute Stroke Trial (HeadPoST) dataset, where the systematic assessment of a large and broad range of relatively unselected patients provided us with an opportunity to determine relationships of low baseline BP and the patterns of functional recovery and serious adverse events (SAEs), overall and by major pathological subtype of acute stroke.
METHODS
Study design
HeadPoST was an international, pragmatic, multicenter, cluster crossover, clinical trial involving 11 093 adults (≥18 years) with acute stroke who were randomly allocated to the lying flat or sitting up head position at a median time of 14 h (interquartile range 5–35 h) after symptom onset at 114 hospitals in nine countries between March 2015 and November 2016 [16].
Standard protocol approvals, registrations, and patient consents
Relevant hospital ethics committees or institutional review boards approved the study, which included the use of a cluster guardian consent to allow implementation of the randomized intervention as a policy of usual service delivery for a predefined patient cluster; patients only provided written consent for use of their medical record data and release of personal information to allow centralized telephone interview for follow-up at 90 days. The study is registered at ClinicalTrials.gov (NCT02162017).
Procedures
Demographic information, clinical data, including the severity of neurological impairment according to the National Institutes of Health Stroke Scale (NIHSS). The NIHSS is a measure of neurological impairment caused by a stroke, composed of 11 items, each of which scores a specific ability between a 0 and 4. For each item, a score of 0 typically indicates normal function in that specific ability, while a higher score is indicative of some level of impairment. The individual scores from each item are summed in order to calculate a patient's total NIHSS score, ranging from 0 to 42. Baseline BP were collected at the time of presentation/admission to hospital. Trained and certified staff, blind to treatment allocation, contacted patients not known to have died by telephone to assess their functional status on the modified Rankin scale (mRS) at 90 days. The mRS is a global, seven-level assessment of disability, in which scores of 0 or 1 indicate good function without or with symptoms but no disability, scores of 2 indicates slight disability, 3 to 5 indicate increasing levels of disability (and dependency), and a score of 6 indicates death. The primary outcome for these analyses was death or dependency (mRS scores 3–6), and secondary outcomes were all-cause and cause-specific SAEs, as reported by site investigators and coded centrally according to standard definitions.
Statistical analysis
Descriptive statistics were reported across categories with ANOVA for normally distributed data, Kruskal--Wallis test for skewed continuous variables, and chi-squared test for categorical variables. Generalized linear mixed (GLM) models were built with initial adjustment for the study design of the fixed effects of head position (lying-flat versus sitting-up) and cross-over period, random effects of cluster, and random interaction effects between cluster and crossover period; and then adjusted for clinical risk factors with P less than 0.1 (listed in Table 1 and Supplemental Table e-1) to determine the estimates of low SBP and DBP on outcomes, respectively. Restricted cubic splines was used to visualize the relationships of baseline SBP and DBP as continuous variables and outcomes in the models, fitted with three to five knots with placement recommended by Harrell [17] and optimal knots selected according to the likelihood ratio test and Aikaike information classification (AIC). The point of lowest odds ratio (OR) was used as the optimal reference. To explore associations of categorical BP and clinical outcomes, the first decile of BP (121 mmHg for SBP and 68 mmHg for DBP) were used to define the ranges of low BP in the study population (<120 mmHg in SBP and <70 mmHg in DBP being considered for meaningful interpretability). High BP was defined as at least 140 and at least 90 mmHg in SBP and DBP, respectively, according to guidelines [18]. The primary analysis were according to the intention-to-treat population, with stratified analyses conducted by final diagnoses of AIS or ICH. Prespecified subgroup analyses were performed by ethnicity (Asian vs. non-Asian), use of intensive BP-lowering treatment, preuse of antihypertensive agents, and randomized head position. In view of the high proportion (12%) of missing 90-day mRS data, multiple imputation was conducted as a sensitivity analysis with all missing covariates and outcome variable imputed [19] in a mixed model for analysis of the main results (see Supplemental Appendix). Data are reported with OR and 95% confidence intervals (CI), with a two-sided P less than 0.05 considered statistically significant. All analyses were performed with SAS version 9.3 (SAS Institute, Cary, North Carolina, USA) and STATA version 15 (StataCorp, College Station, Texas, USA).
TABLE 1.
Baseline characteristics by categories of SBP
| SBP (mmHg) | |||||
| Variable | Overall | <120 | 120–139 | ≥140 | P value |
| Number of patients | 11083 | 795 (7.2) | 2393 (21.6) | 7895 (71.2) | |
| Age (years) | 68 (13.8) | 65 (15.5) | 66 (14.6) | 69 (13.2) | <0.0001 |
| Female | 4423 (39.9) | 300 (37.7) | 888 (37.1) | 3235 (41.0) | 0.0012 |
| Region | <0.0001 | ||||
| Australia and UK | 4754 (42.9) | 390 (49.1) | 971 (40.6) | 3393 (43.0) | |
| China and Taiwan | 4652 (42.0) | 245 (30.8) | 1056 (44.1) | 3351 (42.4) | |
| South America | 910 (8.2) | 83 (10.4) | 179 (7.5) | 645 (8.2) | |
| India and Sri Lanka | 770 (6.9) | 77 (9.7) | 187 (7.8) | 506 (6.4) | |
| Clinical features | |||||
| Final pathological type of stroke | <0.0001 | ||||
| AIS | 9467 (85.6) | 675 (85.1) | 2086 (87.4) | 6701 (85.1) | |
| ICH | 930 (8.4) | 39 (4.9) | 123 (5.1) | 768 (9.8) | |
| Uncertain | 696 (6.0) | 79 (10.0) | 178 (7.5) | 405 (5.1) | |
| Severity of neurological impairment, NIHSS scorea | 4 (2–8) | 5 (2–10) | 4 (2–8) | 4 (2–8) | 0.0002 |
| Score ≥15 | 1207 (11.1) | 112 (14.3) | 240 (10.2) | 855 (11.0) | 0.0079 |
| SBP (mmHg) | 151 (136–171) | 110 (105–116) | 130 (125–134) | 162 (150–180) | <0.0001 |
| DBP (mmHg) | 85 (76–97) | 70 (61–75) | 80 (70–84) | 90 (80–100) | <0.0001 |
| Time from onset (h) | 7.5 (2.3–26.8) | 6.4 (2.0–25.3) | 8.9 (2.5–31.8) | 7.2 (2.3–25.9) | <0.0001 |
| Medical history | |||||
| Hypertension | 7148 (64.7) | 382 (48.2) | 1310 (54.8) | 5456 (69.3) | <0.0001 |
| Current treatment | 5616 (50.7) | 322 (40.6) | 1055 (44.1) | 4238 (53.7) | 0.0007 |
| Number of antihypertensive agents | 1 (1–2) | 1 (1–2) | 1 (1–2) | 1 (1–2) | 0.0030 |
| Diabetes mellitus | 2650 (24.0) | 159 (20.1) | 546 (22.9) | 1945 (24.7) | 0.0054 |
| Atrial fibrillation | 1189 (10.8) | 112 (14.1) | 287 (12.0) | 790 (10.1) | 0.0002 |
| Coronary disease | 1540 (14.0) | 141 (17.8) | 361 (15.1) | 1038 (13.2) | 0.0003 |
| Heart failure | 413 (3.8) | 53 (6.7) | 105 (4.4) | 255 (3.3) | <0.0001 |
| Previous stroke | 2605 (23.6) | 189 (23.9) | 571 (23.9) | 1845 (23.4) | 0.8779 |
| Smoking | 2124 (19.4) | 177 (22.7) | 519 (21.9) | 1428 (18.3) | <0.0001 |
| High level of premorbid function (mRS 0–1)b | 8733 (78.9) | 603 (75.9) | 1860 (77.8) | 6270 (79.5) | 0.0205 |
| Hypercholesterolemia | 2731 (24.6) | 215 (27.3) | 591 (24.8) | 1925 (24.5) | 0.2175 |
| COPD/emphysema | 406 (3.7) | 37 (4.7) | 78 (3.3) | 291 (3.7) | 0.1809 |
| Medications | |||||
| Aspirin/other antiplatelet | 5398 (48.7) | 387 (48.7) | 1249 (52.2) | 3762 (47.7) | 0.0005 |
| Anticoagulation | 951 (8.6) | 87 (11.0) | 223 (9.3) | 641 (8.1) | 0.0088 |
| Statin/other lipid lowering | 2295 (20.7) | 188 (23.6) | 501 (20.9) | 1606 (20.3) | 0.2219 |
Data are mean (SD), median (IQR), and n (%). Analyses were ANOVA test for normally distributed variables, Kruskal--Wallis test for skewed continuous variables, and chi-squared test for categorical variables. AIS, acute ischemic stroke; COPD, chronic obstructive pulmonary disease; ICH, intracerebral hemorrhage; mRS, modified Rankin scale; NIHSS, National Institutes of Health Stroke Scale; UK, United Kingdom.
NIHSS is a measure of neurological impairment caused by a stroke, composed of 11 items, each of which scores a specific ability from 0 up to 4. For each item, a score of 0 typically indicates normal function in that specific ability, whereas a higher score is indicative of some level of impairment. The individual scores from each item are summed in order to calculate a patient's total NIHSS score, ranging from 0 to 42.
The mRS represents a global, seven-level assessment of disability, in which score of 0 or 1 indicate good function without or with symptoms but no disability, score of 2 indicates slight disability, scores of 3--5 indicate increasing levels of disability (and dependency), and a score of 6 indicates death.
Data availability
Individual de-identified participant data used in these analyses may be shared by request from any qualified investigator via the Research Office of The George Institute for Global Health, Australia.
RESULTS
A total of 11 083 patients (mean age 68 years, 39.9% women) had baseline BP recorded, of whom 64.7% had a history of hypertension and 85.6% had a final diagnosis of AIS (Table 1 and Table e-1). Overall, their mean baseline SBP and DBP were 155 and 87 mmHg, respectively, and 7.2% were defined as having low SBP (<120 mmHg). Compared with other patients, those with low SBP were younger, less likely to have history of hypertension, and more likely to have comorbid heart disease and functional impairment, and greater neurological impairment and strokes without brain imaging confirmation (Table 1). In multivariable analysis, history of atrial fibrillation, coronary disease, heart failure and current smoking were related to increased risk of low BP at presentation (Table e-2).
Low SBP and clinical outcomes
After adjustment for study design and various potential confounding baseline factors, restricted cubic splines regression curves show a nonlinear ‘J-shaped’ relationship between baseline SBP and death or dependency, with the odds increasing for SBP levels less than 130 mmHg (Fig. 1a) and in those with a final diagnosis of AIS rather than ICH (Fig. 2). Patients with low SBP had an increased risk of death or dependency in the main analysis (adjusted OR 1.27, 95% CI 1.02–1.58; Fig. 3), which was consistent after multiple imputation (adjusted OR 1.26, 95% CI 1.02–1.56; Fig. 3). Risks of death and any SAE were also higher in patients with low SBP, with the latter related to an increased risk of cardiac events (Table 2). Similar J-shaped relationships were found for SBP and SAEs, with adjusted OR increasing with SBP less than 140 mmHg (Fig. 1b). Compared with those with AIS, ICH patients were more likely to present with high SBP (≥140 mmHg; Table 1), which significantly increased the risk of SAEs compared with those with normal SBP (adjusted OR 2.31, 95% CI 1.16–4.60; Figure e-1A).
FIGURE 1.
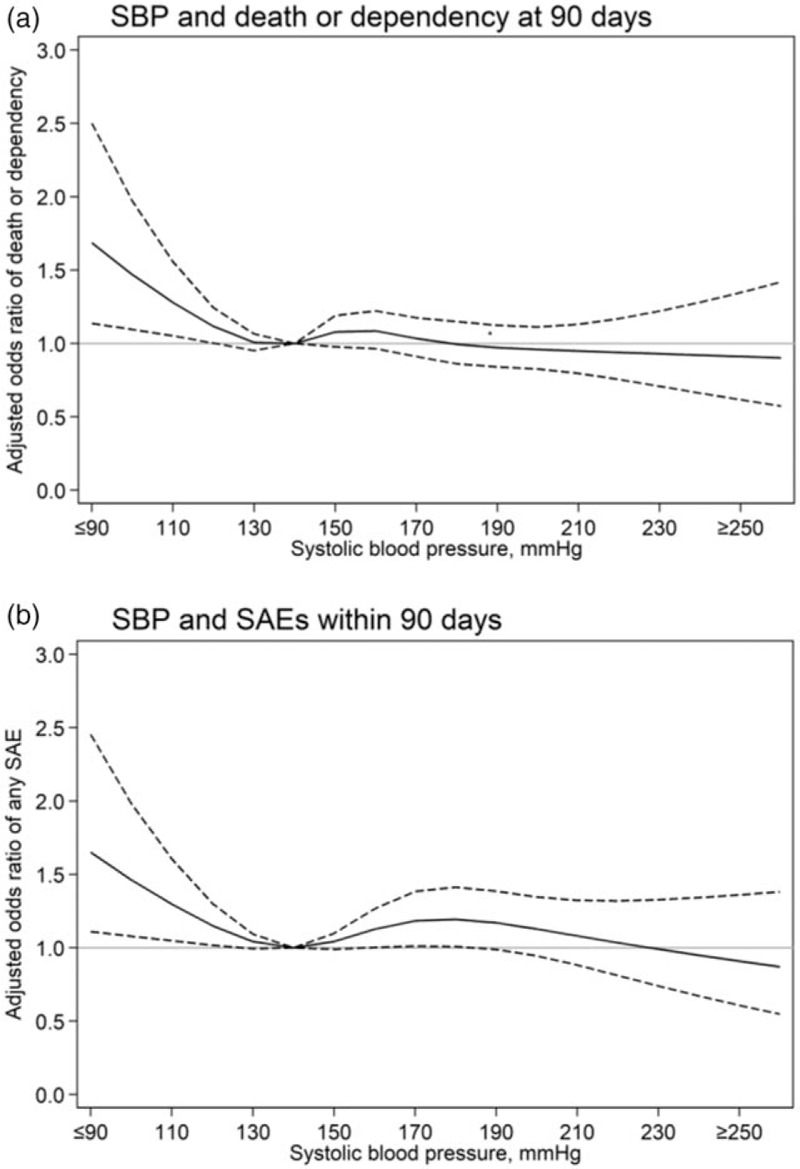
Restricted cubic spline of baseline SBP and clinical outcomes at 90 days. Generalized linear mixed models were used with adjustment for study design (fixed effects of head position and cross-over period, random effects of cluster, and random interaction effects between cluster and crossover period) and potential baseline confounders of age, sex, region, history of diabetes mellitus, hypertension, heart failure, atrial fibrillation, coronary heart disease, National Institutes of Health Stroke Scale score, pathological stroke type, premorbid score 0–1 on the modified Rankin scale, aspirin/other antiplatelet treatment, anticoagulant treatment, time from stroke onset to hospital arrival and current smoking. (a) Spline fitted with five knots (percentiles 5th, 27.5th, 50th, 72.5th, 95th) for SBP; (b) Spline fitted with four knots (percentiles 5th, 35th, 65th, 95th) for SBP. Reference SBP 140 mmHg. Solid line indicates odds ratios; dotted line indicates 95% confidence intervals. SAE, serious adverse events.
FIGURE 2.
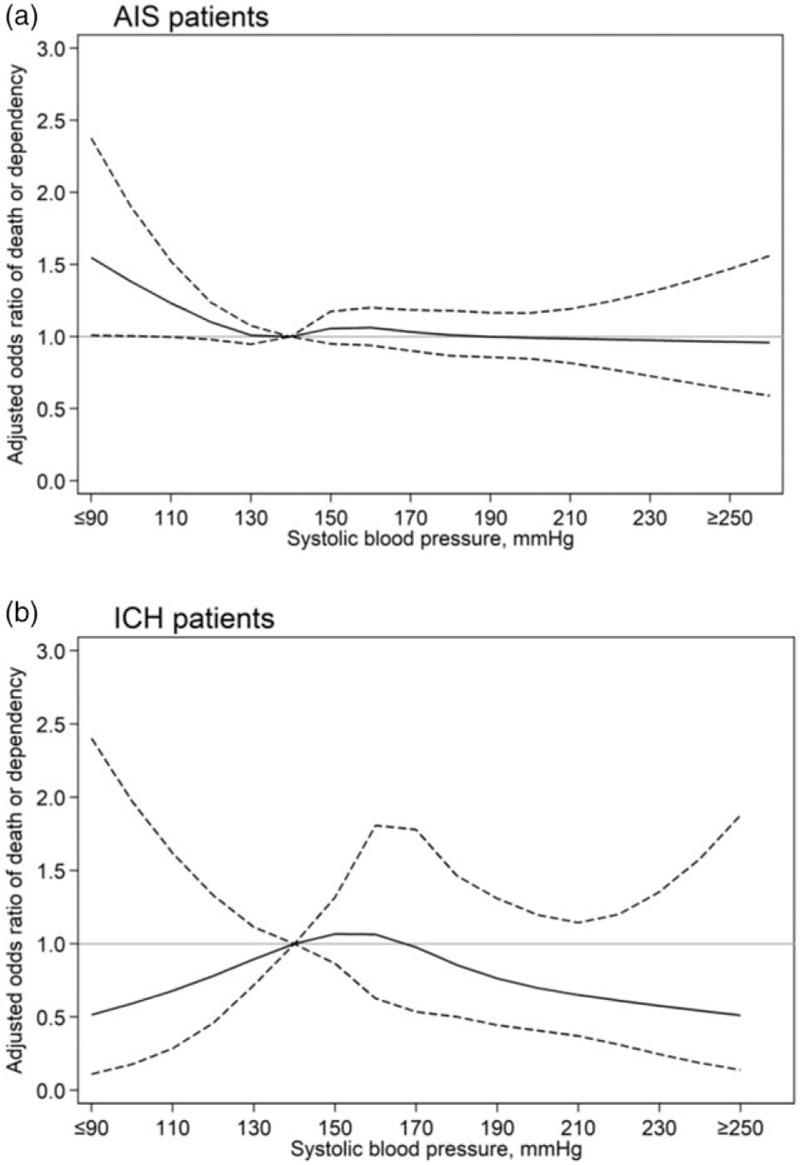
Restricted cubic spline of baseline SBP and 90-day death or dependency, by stroke subtype. Generalized linear mixed model fitted were used with adjustment for study design (fixed effects of head position and cross-over period, random effects of cluster, and random interaction effects between cluster and crossover period) and potential baseline confounders of age, sex, region, history of diabetes mellitus, hypertension, heart failure, atrial fibrillation, coronary heart disease, National Institutes of Health Stroke Scale score, pathological stroke type, premorbid score 0–1 on the modified Rankin scale, aspirin/other antiplatelet treatment, anticoagulant treatment, time from stroke onset to hospital arrival and current smoking. Splines fitted with five knots (percentiles 5th, 27.5th, 50th, 72.5th, 95th) for SBP. Reference SBP 140 mmHg. Solid line indicates odds ratios; dotted line indicates 95% confidence intervals. AIS, acute ischemic stroke; ICH, intracerebral hemorrhage.
FIGURE 3.
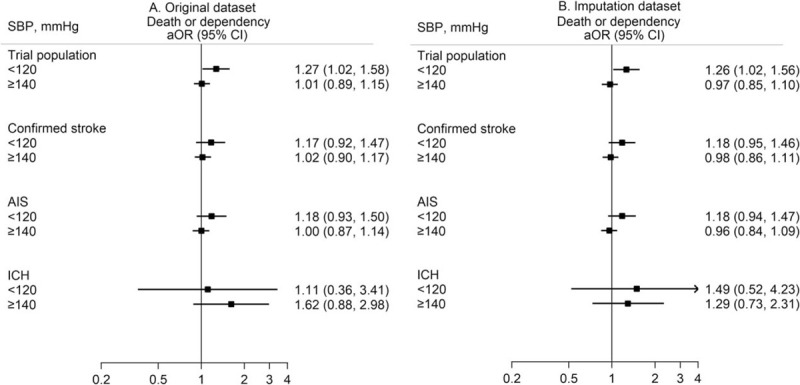
Association of categorical baseline SBP and 90-day death or dependency, by dataset. Generalized linear mixed model fitted were used with adjustment for study design (fixed effects of head position and cross-over period, random effects of cluster, and random interaction effects between cluster and crossover period) and potential baseline confounders of age, sex, region, history of diabetes mellitus, hypertension, heart failure, atrial fibrillation, coronary heart disease, National Institutes of Health Stroke Scale score, pathological stroke type, premorbid score 0–1 on the modified Rankin scale, aspirin/other antiplatelet treatment, anticoagulant treatment, time from stroke onset to hospital arrival and current smoking. Reference SBP 120–139 mmHg. Boxes indicate point estimate of odds ratios, solid line indicates 95% confidence intervals. AIS, acute ischemic stroke; aOR, adjusted odds ratio; CI, confidence interval; ICH, intracerebral hemorrhage; OR, odds ratio.
TABLE 2.
Outcomes at 90 days by baseline SBP
| SBP (mmHg) | ||||
| Outcome | <120 | 120–139 | ≥140 | P value |
| Death or dependency (mRSa 3–6) | 296/669b (44.2) | 778/2084b (37.3) | 2746/6986b (39.3) | 0.0060 |
| Disability (mRSa 3–5) | 228/669 (34.1) | 607/2084 (29.1) | 2189/6986b(31.3) | 0.0349 |
| Death | 68/669 (8.7) | 171/2084 (7.3) | 557/6986 (7.2) | 0.3308 |
| SAE | 138/795c (17.4) | 303/2393c (12.7) | 1098/7895c (13.9) | 0.0040 |
| Recurrent stroke | 43/795 (5.4) | 104/2393 (4.4) | 380/7895 (4.8) | 0.4289 |
| Cardiac or other vascular events | 26/795 (3.3) | 40/2393 (1.7) | 169/7895 (2.1) | 0.0247 |
| Pneumonia | 23/795 (2.9) | 54/2393 (2.3) | 215/7895 (2.7) | 0.4103 |
| Other infection | 11/795 (1.8) | 28/2393 (1.2) | 79/7895 (1.0) | 0.1326 |
| Other SAE | 28/795 (3.5) | 60/2393 (2.5) | 197/7895 (2.5) | 0.2134 |
Data are n/N (%); P value from chi-squared test. mRS, modified Rankin scale; SAE, serious adverse event.
The mRS represents a global, seven-level assessment of disability, in which scores of 0 or 1 indicate good function without or with symptoms but no disability, score of 2 indicates slight disability, scores of 3--5 indicate increasing levels of disability (and dependency), and a score of 6 indicates death.
N is according to total number of patients at 90-day follow-up.
N is according to total number of patients randomized.
Low DBP and clinical outcomes
A similar relationship applied for DBP, with odds of poor outcome increasing with DBP levels less than 90 mmHg, overall and in those with AIS and ICH (Figures e-2A and e-3). Similar associations were identified for low DBP and poor outcome, where patients with low DBP had an increased risk of death or dependency in the main analysis (adjusted OR 1.25, 95% CI 1.06–1.48), which was consistent after multiple imputation (adjusted OR 1.20, 95% CI 1.03–1.40) (Figure e-4). There was a nonlinear relationship between DBP and SAE (Figure e-2B), but this association was not significant when compared with a normal range (70–89 mmHg) of DBP (Figure e-1B).
Subgroup analysis
Heterogeneity was found in the association between low BP and outcome, comparing Asian (n = 4947) and non-Asian (n = 4428) patients, with the former having a significantly higher risk of poor outcome at 90 days (SBP, adjusted OR 1.53, 95% CI 1.11–2.11, Figure e-5; DBP, adjusted OR 1.38, 95% CI 1.05–1.81, Figure e-6). However, for non-Asian patients, high BP was associated with lower risk of poor outcome (SBP, adjusted OR 0.78, 95% CI 0.65–0.94, Figure e-5; DBP, adjusted OR 0.80, 95% CI 0.68–0.94, Figure e-6). No heterogeneity was found in subgroup analysis by use of intensive BP lowering during hospitalization, prior use of antihypertensive treatment, or randomized head positioning, and death or dependency (Figures e-5 and e-6).
DISCUSSION
In these secondary analyzes of a large and heterogenous international clinical cohort, low BP early after hospital presentation for acute stroke was associated with increased odds of poor clinical outcome after full adjustment for many potential confounding factors. The findings of consistency in the association for both SBP and DBP on functional recovery and cardiac SAEs, and being greater for AIS than ICH, provides support for the common concern for an interaction between low BP and cardiac and cerebral hypoperfusion and ischemia [1].
Most guidelines provide recommendations on the management of BP in AIS [20] and particularly ICH [20,21], principally as hypertension is a commonly observed phenomenon [22–24] with a well documented relation to increased adverse outcomes [25]. Although less frequently observed, low BP is also clearly not a benign phenomenon. Previous observational studies have found J-curve relationships between BP and adverse outcomes in relation to various heart diseases [26–28], and proposing ‘reverse causation’ as the basis for the progression of heart failure with premorbid comorbidities and frailty [29]. Similarly for stroke, one study has shown that low SBP (<120 mmHg) after recent AIS is related to increased recurrent stroke and vascular death [30], whereas another showed that diastolic hypotension increased the risks of death, myocardial infarction [31], and recurrent vascular events including stroke [32]. There is a strong argument that a reduction in systematic BP may worsen cerebral ischemia in relation to altered cerebral autoregulation in the vulnerable penumbral region of AIS [33], whereas comorbidity and greater neurological severity in those presenting with hypotension, may partly explain the increase in SAEs shown in previous acute stroke trials [34]. Although low BP in frail older patients might be a harbinger of preterminal decline [35], we found the increased risk of adverse events in stroke patients with low BP persisted after adjustment for premorbid disability and comorbid heart disease.
Our study also found that the prognostic significance of low BP was modified by ethnicity, with Asian patients having a worse outcome irrespective of BP than non-Asians, who had a comparably lower odds of death or dependency from low BP. Although some studies have also shown poorer functional outcome in Asian compared with European stroke patients [36], there are limited data specifically pertaining to ethnicity and low BP. Yet, ethnic disparities in cardiovascular risk factor control, as well as acute stroke care, and regional differences in rehabilitation, might explain some of this variation [37].
In addition to the large sample size and systematic recording of outcomes, key strengths of our study were the steps taken to adjust for many potential confounding factors and to minimize potential reverse causation. Moreover, the generalizability of the results is enhanced by the use of broad inclusion criteria applied to AIS and ICH patients recruited in multiple countries. However, as the HeadPoST study was a pragmatic clinical trial focused on different head positions after stroke, there were limited in-hospital BP measures recorded for the study or these were measured BP according to local protocols, although any effect of BP misclassification would be to attenuate the strength of associations. In addition, we did not have data concerning dose of prior antihypertensive therapy nor use of other cardiac medications, although the frequency of hypertension and number of antihypertensive agents were greater in those patients presenting with elevated BP. Other limitations related to these being secondary analyses with relatively small number of patients in subgroups, variable cut-points to define BP categories, and missing or misclassification of confounding covariates.
In conclusion, our study has shown that low presenting BP, both SBP and DBP, are associated with poor outcome after acute stroke, even after taking account of these patients being a particularly high-risk group from having preexisting cardiac or cerebral cardiovascular comorbidity and dependency/frailty; and of the severity of neurological injury. As well as alerting clinicians to the care of these vulnerable patients, there may be merit in further studies being undertaken to better define the mechanisms of low BP on the cerebral circulation, and the most appropriate management strategies for low BP.
ACKNOWLEDGEMENTS
The authors thank the HeadPoST investigators.
Author contributions: P.M.V., C.S.A., and M.O. contributed to the concept and rationale for the study. M.O. undertook statistical analyses with assistance from L.B. and X.W. M.O. wrote the first draft of manuscript with input from C.S.A., T.R., and P.M.V. All authors commented upon and approved the final version of the manuscript for publication.
Conflicts of interest
P.M.V. reports grants from The George Institute for Global Health and Clínica Alemana de Santiago, during the conduct of the study; and research grants from CONICYT, outside the submitted work. P.M.L. reports grants from The George Institute for Global Health and Clínica Alemana de Santiago, during the conduct of the study; and nonfinancial support from Boehringer Ingelheim, grants and personal fees from Bayer and AstraZeneca, and grants from CONICYT, outside the submitted work. X.W. receiving National Heart Foundation (102117) and New South Wales Health grants. M.L.H. holds a National Health and Medical Research Council of Australia (NHMRC) Career Development Fellowship. V.V.O. reports grants from The George Institute for Global Health and Clínica Alemana de Santiago, during the conduct of the study; and research grants from Boehringer Ingelheim and CONICYT outside the submitted work. A.B. reports grants from Clínica Alemana de Santiago. S.M. was a member of the NHMRC Research Committee during 2015–2018. O.M.P.N. received grants for the Brazilian Stroke Research Network by DECIT/MS and CNPQ (402388/2013–5) for conduct this study. C.L.W. is a National Institute for Health Research (NIHR) Senior Investigator (Emeritus), and holds a number of grants from NIHR. The views expressed in this article are not necessarily those of the NIHR or the Department of Health and Social Care. T.G.R. is a National Institutes for Health Research (NIHR) Senior Investigator, whose views expressed in this article are his and not necessarily those of the NIHR or the Department of Health and Social Care. C.S.A. holds an NHMRC Senior Investigator Fellowship, and reports honoraria and travel reimbursement, and grants, from Takeda China. The other authors have no disclosures to report.
Supplementary Material
Footnotes
Abbreviations: AIS, acute ischemic stroke; BP, blood pressure; CI, confidence interval; GLM, generalized linear mixed model; HeadPoST, Head Positioning in acute Stroke Trial; ICH, intracerebral hemorrhage; mRS, modified Rankin scale; NIHSS, National Institutes of Health Stroke Scale; OR, odds ratio; SAE, serious adverse events
REFERENCES
- 1.Tikhonoff V, Zhang H, Richart T, Staessen JA. Blood pressure as a prognostic factor after acute stroke. Lancet Neurol 2009; 8:938–948. [DOI] [PubMed] [Google Scholar]
- 2.Sare GM, Geeganage C, Bath PMW. High blood pressure in acute ischaemic stroke – broadening therapeutic horizons. Cerebrovasc Dis 2009; 27:156–161. [DOI] [PubMed] [Google Scholar]
- 3.Qureshi AI. Acute hypertensive response in patients with stroke: pathophysiology and management. Circulation 2008; 118:176–187. [DOI] [PubMed] [Google Scholar]
- 4.Willmot M, Leonardi-Bee J, Bath PM. High blood pressure in acute stroke and subsequent outcome: a systematic review. Hypertension 2004; 43:18–24. [DOI] [PubMed] [Google Scholar]
- 5.Miller J, Kinni H, Lewandowski C, Nowak R, Levy P. Management of hypertension in stroke. Ann Emerg Med 2014; 64:248. [DOI] [PubMed] [Google Scholar]
- 6.Sandset EC, Bath P, Boysen G, Jatuzis D, Korv J, Luders S, et al. SCAST Study Group. The angiotensin-receptor blocker candesartan for treatment of acute stroke (SCAST): a randomised, placebo-controlled, double-blind trial. Lancet 2011; 377:741–750. [DOI] [PubMed] [Google Scholar]
- 7.Moullaali TJ, Wang X, Martin RH, Shipes VB, Robinson TG, Chalmers J, et al. Blood pressure control and clinical outcomes in acute intracerebral haemorrhage: a preplanned pooled analysis of individual participant data. Lancet Neurol 2019; 18:857. [DOI] [PubMed] [Google Scholar]
- 8.Anderson CS, Huang Y, Lindley RI, Chen X, Arima H, Chen G, et al. ENCHANTED Investigators and Coordinators. Intensive blood pressure reduction with intravenous thrombolysis therapy for acute ischaemic stroke (enchanted): an international, randomised, open-label, blinded-endpoint, phase 3 trial. Lancet (London, England) 2019; 393:877–888. [DOI] [PubMed] [Google Scholar]
- 9.Vemmos KN, Tsivgoulis G, Spengos K, Zakopoulos N, Synetos A, Manios E, et al. U-shaped relationship between mortality and admission blood pressure in patients with acute stroke. J Intern Med 2004; 255:257–265. [DOI] [PubMed] [Google Scholar]
- 10.Stead GL, Gilmore MR, Decker WW, Weaver LA, Brown DR. Initial emergency department blood pressure as predictor of survival after acute ischemic stroke. Neurology 2005; 65:1179–1183. [DOI] [PubMed] [Google Scholar]
- 11.Wohlfahrt P, Redfield MM, Lopez-Jimenez F, Melenovsky V, Kane GC, Rodeheffer RJ, et al. Impact of general and central adiposity on ventricular-arterial aging in women and men. JACC Heart Fail 2014; 2:489–499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kim LJ, Gall RS, Nelson EM, Sharman GJ, Thrift GA. Lower systolic blood pressure is associated with poorer survival in long-term survivors of stroke. J Hypertens 2014; 32:904–911. [DOI] [PubMed] [Google Scholar]
- 13.Liu Y, Yang Y, Jin H, Fan C, Lv P, Sun W, et al. ChinaQUEST (Quality Evaluation of Stroke Care and Treatment) Investigators. Discrepant relationships between admission blood pressure and mortality in different stroke subtypes. J Neurol Sci 2017; 383:47–51. [DOI] [PubMed] [Google Scholar]
- 14.Verschoof MA, Groot AE, Vermeij J-D, Westendorp WF, van Den Berg SA, Nederkoorn PJ, et al. Association between low blood pressure and clinical outcomes in patients with acute ischemic stroke. Stroke 2020; 51:338–341. [DOI] [PubMed] [Google Scholar]
- 15.Ravindrarajah CR, Hazra HDN, Hamada CS, Charlton CJ, Jackson CS, Dregan CA, et al. Systolic blood pressure trajectory, frailty, and all-cause mortality >80 years of age: cohort study using electronic health records. Circulation 2017; 135:2357–2368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Anderson CS, Arima H, Lavados P, Billot L, Hackett ML, Olavarría VV, et al. HeadPoST Investigators and Coordinators. Cluster-randomized, crossover trial of head positioning in acute stroke. N Engl J Med 2017; 376:2437-2447. [DOI] [PubMed] [Google Scholar]
- 17.Harrell FE. Regression modeling strategies: with applications to linear models, logistic regression, and survival analysis. New York, New York: Springer; 2001. [Google Scholar]
- 18.Weber MA, Schiffrin EL, White WB, Mann S, Lindholm LH, Kenerson JG, et al. Clinical practice guidelines for the management of hypertension in the community: a statement by the american society of hypertension and the international society of hypertension. J Clin Hypertens (Greenwich) 2013; 16:14–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sterne JAC, White IR, Carlin JB, Spratt M, Royston P, Kenward MG, et al. Multiple imputation for missing data in epidemiological and clinical research: Potential and pitfalls. BMJ 2009; 338:b2393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Powers JW, Rabinstein AA, Ackerson MT, Adeoye CO, Bambakidis MN, Becker CK, et al. American Heart Association Stroke Council. 2018 guidelines for the early management of patients with acute ischemic stroke: a guideline for healthcare professionals from the american heart association/american stroke association. Stroke 2018; 49:e46–e110. [DOI] [PubMed] [Google Scholar]
- 21.Hemphill CJ, Greenberg MS, Anderson SC, Becker RK, Bendok LB, Cushman NM, et al. Guidelines for the management of spontaneous intracerebral hemorrhage: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2015; 46:2032–2060. [DOI] [PubMed] [Google Scholar]
- 22.Leonardi-Bee MWJ, Bath JP, Phillips AGS, Sandercock AGP. IST Collaborative Group. Blood pressure and clinical outcomes in the international stroke trial. Stroke 2002; 33:1315–1320. [DOI] [PubMed] [Google Scholar]
- 23.Bangalore S, Schwamm L, Smith EE, Hellkamp AS, Suter RE, Xian Y, et al. Get With the Guidelines-Stroke Steering Committee and Investigators. Blood pressure and in-hospital outcomes in patients presenting with ischaemic stroke. Eur Heart J 2017; 38:2827–2835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Appleton JP, Sprigg N, Bath PM. Blood pressure management in acute stroke. Stroke Vascul Neurol 2016; 1:72–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ahmed N, Wahlgren N, Brainin M, Castillo J, Ford GA, Kaste M, et al. Relationship of blood pressure, antihypertensive therapy, and outcome in ischemic stroke treated with intravenous thrombolysis: retrospective analysis from Safe Implementation of Thrombolysis in Stroke-international Stroke Thrombolysis Register (SITS-ISTR). Stroke 2009; 40:2442–2449. [DOI] [PubMed] [Google Scholar]
- 26.Lee SE, Lee H-Y, Cho H-J, Choe W-S, Kim H, Choi J-O, et al. Reverse j-curve relationship between on-treatment blood pressure and mortality in patients with heart failure. JACC Heart Fail 2017; 5:810. [DOI] [PubMed] [Google Scholar]
- 27.Vidal-Petiot E, Ford I, Greenlaw N, Ferrari R, Fox KM, Tardif J-C, et al. CLARIFY Investigators. Cardiovascular event rates and mortality according to achieved systolic and diastolic blood pressure in patients with stable coronary artery disease: an international cohort study. Lancet 2016; 388:2142–2152. [DOI] [PubMed] [Google Scholar]
- 28.Böhm M, Schumacher H, Teo KK, Lonn EM, Mahfoud F, Mann JFE, et al. Achieved blood pressure and cardiovascular outcomes in high-risk patients: results from ontarget and transcend trials. Lancet 2017; 389:2226–2237. [DOI] [PubMed] [Google Scholar]
- 29.Böhm M, Young R, Jhund PS, Solomon SD, Gong J, Lefkowitz MP, et al. Systolic blood pressure, cardiovascular outcomes and efficacy and safety of sacubitril/valsartan (lcz696) in patients with chronic heart failure and reduced ejection fraction: results from PARADIGM-HF. Eur Heart J 2017; 38:1132–1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ovbiagele B, Diener H-C, Yusuf S, Martin RH, Cotton D, Vinisko R, et al. PROFESS Investigators. Level of systolic blood pressure within the normal range and risk of recurrent stroke. JAMA 2011; 306:2137. [DOI] [PubMed] [Google Scholar]
- 31.Flint AC, Conell C, Ren X, Banki NM, Chan SL, Rao VA, et al. Effect of systolic and diastolic blood pressure on cardiovascular outcomes. N Engl J Med 2019; 381:243–251. [DOI] [PubMed] [Google Scholar]
- 32.Park JH, Ovbiagele B. Poststroke diastolic blood pressure and risk of recurrent vascular events. Eur J Neurol 2017; 24:1416–1423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Potter JF, Robinson TG, Ford GA, Mistri A, James M, Chernova J, et al. Controlling hypertension and hypotension immediately poststroke (CHHIPS): a randomised, placebo-controlled, double-blind pilot trial. Lancet Neurol 2009; 8:48. [DOI] [PubMed] [Google Scholar]
- 34.Hesse LK, Fulton HR, Abdul-Rahim RA, Lees RK. VISTA Collaborators. Characteristic adverse events and their incidence among patients participating in acute ischemic stroke trials. Stroke 2014; 45:2677–2682. [DOI] [PubMed] [Google Scholar]
- 35.Supiano MA, Pajewski NM, Williamson JD. Systolic blood pressure and mortality: role of reverse causation. J Am Geriatr Soc 2018; 66:205–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.McNaughton H, Feigin V, Kerse N, Barber PA, Weatherall M, Bennett D, et al. Auckland Regional Community Stroke Study Group. Ethnicity and functional outcome after stroke. Stroke 2011; 42:960–964. [DOI] [PubMed] [Google Scholar]
- 37.Tuhrim S. Ethnic disparities in stroke: Epidemiology, acute care, and postacute outcomes. Stroke 2005; 36:386–387. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Individual de-identified participant data used in these analyses may be shared by request from any qualified investigator via the Research Office of The George Institute for Global Health, Australia.


