Abstract
Natural killer (NK) cells are cytotoxic innate lymphoid cells that protect the host from infection andme-diate anti-tumor responses. Classically considered part of the innate immune system, NK cells were previously thought to not possess the specificity or enhanced recall responses associated with adaptive T and B lymphocytes. However, a large body of work has transformed these long-held divisions between innate and adaptive immunity; NK cell memory and memory-like responses are clearly established after hapten exposure, viral infection, and combined cytokine activation. These advances come with opportunities to translate innate NK cell recall responses into the clinic as cancer immunotherapy. Here, we review our current understanding of the heterogeneity of memory and memory-like NK cell responses, with distinct formation, molecular biology, and memory type functions. We elaborate on cytokine-induced memory-like NK cells and highlight their application as adoptive immunotherapy for cancer, and as a platformfor engineering optimal NK cell anti-tumor responses.
Keywords: Natural killer cell, Innate memory, Cancer immunotherapy, Cytokine, Cytokine receptor, Cytokine-induced memory-like
Introduction
Natural killer (NK) cells are cytotoxic innate lymphoid cells that protect against viral infection and mediate anti-tumor responses [1–3]. NK cells use 2 primary functions to protect the host after recognizing diseased cells: (1) cytokine production to communicate and orchestrate an immune response and (2) direct target cell killing. NK cells secrete proinflammatory cytokines (eg, Interferon (IFN)- γ and tumor necrosis factor [TNF]) and chemokines (eg, CCL3–5), which stimulate macrophages for phagocytosis and lysis, upregulate Major Histocompatibility Complex (MHC) class I expression on antigen presenting cells, recruit additional immune effectors, and promote cytotoxicity [2]. NK cells also mediate contact-dependent killing of target cells through exocytosis of preformed cytotoxic granules containing perforin and granzymes, as well as via death receptors ligands [4].
Human NK cells are identified phenotypically by the absence of the T- and B-cell receptors (eg, surface CD3, TCR, BCR, and CD19) and presence of NK cell markers and receptors (eg, CD56 and NKp46). First described in the 1970s, early experiments observed a lymphoid cell subset that was capable of killing tumor cell lines without prior antigen sensitization, distinguishing their activity from T cells [5,6]. This capacity for “natural killing” has since been further elaborated to our modern concept of NK cells, with evidence of their importance arising from a multitude of studies, including a prospective study identifying that individuals with low NK cell killing activity had a higher risk of developing cancer [7]. In humans, primary NK cell immunodeficiency presents with severe recurrent infections with herpes and papilloma viruses [8]. These findings highlight the importance of NK cells for host defense against pathogens and their potential as immunotherapy for cancer and infection.
Human NK cells arise from bone marrow precursors and mature in secondary lymphoid tissue, with distinct developmental stages and functional subsets that can overlap with innate lymphoid cell precursors [9–12]. CD56dim CD16bright NK cells are the major mature population in peripheral blood, are primarily cytotoxic, and preferentially respond through activating NK cell receptor trigger-ing. In contrast, while representing a minor population in the peripheral blood, CD56bright CD16−/low NK cells are the major NK cell population in secondary lymphoid tissue and respond preferentially via cytokine receptors. Notably, recent work has shown that priming with interleukin (IL)-15 can induce potent CD56bright anti-tumor responses [12]. NK cells recognize targets via families of activating and inhibitory receptors encoded in genomic DNA [13] and may be stimulated via cytokine receptors [14]. NK cells distinguish between healthy and diseased cells through the integration of activating and inhibitor receptor signals [15]. In humans, examples of NK cell activation receptors include NK gene 2D (NKG2D) and natural cytotoxicity triggering receptor 1 (NKp46), that recognize ligands upregulated on stressed, infected, or transformed cells, as well as CD16 (FcγRIIIa), which binds antibody opsonized targets [16]. Inhibitory receptors are from 2 main structural classes: killer cell immunoglobulin-like receptors (KIR) and C-type lectin receptors (ie, CD94/NKG2A). These inhibitory receptors recognize self-HLA/MHC class I or class I-like molecules that may be down regulated in virus-infected or tumor-transformed targets [15,16]. In addition to functionally recognizing targets, inhibitory receptor interactions with at least one self-MHC molecule “license” or “ed-ucate” an NK cell during development and maturation [17,18]. The diverse sets of activating and inhibitory receptors present on the surface of NK cells allow for tolerance against healthy cells expressing self-MHC class I molecules, while recognizing virus-infected or malignant cells in a nonantigen-specific manner. NK cells also constitutively express a number of cytokine receptors that impact the threshold of NK cell activation, and can activate NK cells to produce cytokine and chemokines in the absence ofNK cell receptor engagement [14].
Initially classified as innate immune cells, NK cells were thought to be incapable of an antigen-specific response and to lack any memory of prior activation. Paradigm shifting experiments performed over the last decade have clearly identified that NK cells remember their prior experience with an enhanced response upon restimulation, following an initial activation event. Here, we provide an overview of the multiple types of innate NK cell memory described to date, but focus on human cytokine-induced memory-like (ML, aka CIML) NK cell biology. We describe the successful clinical translation of these cells for the adoptive immunotherapy of hematologic malignancies and new approaches to engineer these cells as a broad cancer immunotherapy.
Innate memory and memory-like responses: not all recall is the same
Defined as the ability to rapidly recognize and respond more robustly to previously encountered pathogens, immunologic memory provides long-lasting and enhanced antigen-specific immunity [19]. Memory T and B cell formation has been well characterized and is divided into 3 stages: (1) naïve cells clonally expand following activation with antigen and co-stimulation and cytokine receptor stimulation; (2) the majority (> 90%) of cells rapidly undergo activation-induced apoptosis in the contraction phase; and (3) the remaining cells undergo memory differentiation [20]. The resultant pool of memory cells are long-lived and have enhanced proliferation, cytokine production, and cytotoxicity during recall responses to the individual antigen [15,21]. The protective immune memory response can persist for many years after initial antigen exposure, with repeated exposures boosting the adaptive immune responses [20], as with vaccination [22].
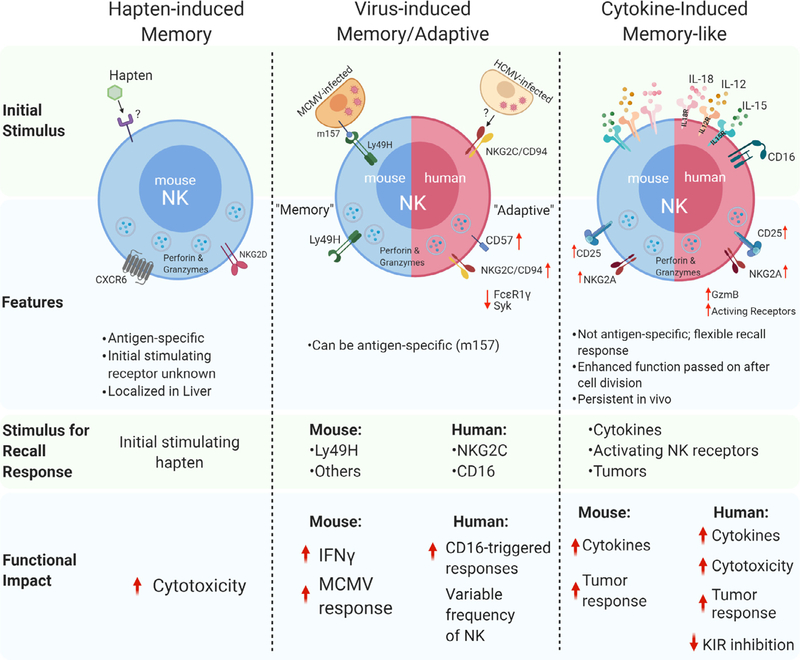
Innate memory or memory-like responses have been defined following exposure of NK cells to specific haptens [23], viral infection [24], or combined cytokine activation with IL-12, IL-15, and IL-18 [25]. Generally, innate memory is functionally defined by an activation event, followed by a return to baseline function during differentiation, and an enhanced response upon subsequent restimulation. However, these types of innate NK cell memory are distinct, variably specific to the inciting activation stimulus, and have unique functional and molecular biology [26]. Thus, current evidence suggests that there is not one NK cell memory program, and it is important to consider each type, as well as the species being evaluated (Fig. 1), when considering the biology and translational potential.
Fig. 1.
Summary of different types of innate memory and memory-like responses of NK cells.
Hapten-specific NK cell memory
The ability for NK cells to acquire antigen-specific memory was initially described in the setting of contact hypersensitivity (CHS) responses to chemical haptens [23]. Hapten-induced CHS is a classic example of adaptive immunity in which the epithelium is exposed to small molecule irritants that bind and modify proteins, triggering formation of hapten-specific T cell memory. O’Leary and colleagues found that mice lacking T and B cells, but not NK cells, were able to exhibit hapten-induced CHS responses and that these recall responses not only discriminated between different haptens (DNFB [2,4-dinitro-1-fluorobenzene] and OXA [oxazolone]) but also persisted for at least 4 weeks with re-challenge. Furthermore, adoptive transfer of hepatic NK cells, but not splenic NK cells, from DNFB-sensitized donors resulted in CHS responses in the recipients upon re-exposure to the same hapten [23]. These observations were the first to conclude that NK cells were capable of mediating long-lived, antigen-specific adaptive recall responses. Subsequent experiments demonstrated that NK cell memory responses to haptens were dependent on expression of CXCR6, a chemokine receptor critical for persistence and homeostasis of hepatic NK cells [27]. Additional studies have further defined this hepatic-specific subset of NK cells, with CXCR6+ NK cells demonstrating an immature, tissue resident phenotype that are T-betloEomeshi [28,29]. However, the molecular mechanisms that explain the ability for NK cells to discriminate between distinct haptens in the absence of RAG-mediated receptor rearrangement remain poorly understood, leading to challenges in clinical application.
MCMV memory and adaptive NK cells
It was later discovered by Sun and Lanier that murine NK cells exhibit a specific adaptive response following murine cytomegalovirus (MCMV) infection [24]. Following MCMV infection, NK cells bearing the activating receptor Ly49H, which binds the MHC class I-like glycoprotein m157 encoded by MCMV, exhibit expansion for about one week following infection. While this expanded Ly49H+ NK population declines after initial expansion, there remains a persistent expression of Ly49H [30]. Sun and colleagues used this model to identify a “memory” pool of NK cells 4 weeks after infection. Isolation and ex vivo restimulation of these memory NK cells showed heightened functional responses to Ly49H stimulation, measured by increased IFN-γ and degranulation compared to MCMV-naïve NK cells [24]. Adoptive transfer of memory NK cells following MCMV infection protected neo-natal mice from lethal MCMV infection, while naïve NK cells were not as effective. Further studies revealed that during later phase of MCMV infections, Ly49H+ NK cells selectively undergo a Ly49H-depended 2- to 3- fold expansion in the spleen and 10-fold increase in the liver [30]. Additionally, when naïve Ly49H+ NK cells were adoptively transferred into Ly49H-deficient mice, MCMV infection dramatically skewed the NK cell population toward Ly49H+ NK cells [24]. Thus, analogous to the formation of T cell memory against pathogens, these experiments uncovered a unique and dis-crete NK cell population that underwent phases of expansion, contraction, and memory differentiation in the context of a specific activating NK cell receptor–ligand interaction. Later studies have implicated the pro-apoptotic factor Bim and proinflammatory cytokines IL-12 and IL-18 as important components of NK cell expansion, contraction, and memory differentiation to generate a pool of mature, MCMV-specific memory NK cells [31–33]. O’Sullivan and Sun further demonstrated that the enhancement of BNIP3-and BNIP3L-mediated autophagic pathways removed dysfunctional mitochondria during the contraction-to-memory phase transition for virus-specific NK cells. BNIP3- and BNIP3L were essential for the persistence of memory NK cells after viral infection [34]. Tran-scriptional analysis of NK cells during MCMV-specific activation reveal that a number of genes are differentially and dynamically expressed between naïve and Ly49H+ NK cells, including indicators of proliferation (ie, Il2ra, FoxmI, and Klf13) and effector function (eg, Ifng and GzmB) [35]. Interestingly, there is a significant overlap in the transcriptional profile of memory NK cells and CD8+ memory T cells, including upregulation of Ly6C and CD49a [35].
Human cytomegalovirus-adapted NK cells
While NK cell memory responses to viruses have been most clearly delineated in mouse models, human studies revealed an impact of prior cytomegalovirus infection in humans. Infection with CMV [36–38], hantavirus [39], or chikungunya virus [40] results in an imprinted NK cell receptor repertoire with an increased frequency of CD94/NKG2C+ NK cells. In the setting of hematopoietic stem cell transplantation (HCT), the CD94/NKG2C+ population demonstrated adaptive memory responses to a secondary CMV event [37]. This in vivo NKG2C+ NK cell population expanded and persisted long after CMV clearance, preferentially expressing KIR for self, CD57 (a marker of NK maturity), and lacking the inhibitory receptor NKG2A [37]. Thus, similar to murine Ly49H+ memory NK cells after MCMV, NKG2C+ NK cells selectively expand following CMV reactivation in recipients of adult donor allogeneic HCT, albeit to a highly variable degree between different individuals. In a study by Cichocki and Miller, adaptive NK cells isolated from human CMV-seropositive donors were observed to have heightened glycolysis and oxidative phosphorylation compared to conventional NK cellsfrom CMV-seronegative donors, suggesting 8 a model in which increased cellular metabolism supports adaptive NK cell expansion and survival [41]. Two studies identified epigenetic mechanisms contributing to the alteration in CMV-adapted human NK cells, which included down regulation of Syk and FcεR1γ, among other changes [38,42]. Despite initial descriptions of NKG2C being a phenotypic marker of CMV-adaptive NK cells, individuals that ge-netically lack this receptor also have an adaptive-type NK cell compartment, suggesting that NKG2C is not necessary for their generation [43]. Functionally, CMV-adaptive NK cells appear special-ized for enhanced responses through Fcγ RIIIa/CD16a, thus, pairing adaptive NK cells with therapeutic antibodies may be an ideal translational application [44]. CMV-adapted NK cells have been successfully generated in a co-culture model with HCMV-infected fibroblasts and IL-12, identifying one approach to differentiate and expand these cells ex vivo [45]. Notably, mechanisms that are clearly operative in human CMV-adaptive NK cells were not observed in MCMV-induced Ly49H+ murine memory NK cells, suggesting a lack of mechanistic parallels between species [42].
Cytokine-induced memory-like NK cells
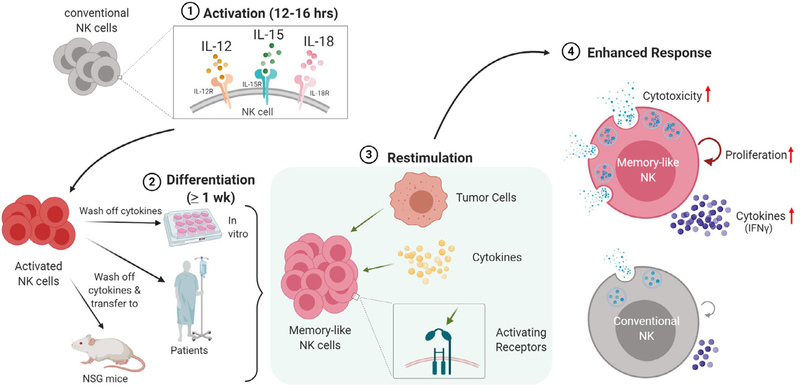
Reported simultaneously with MCMV-induced memory NK cells, Cooper and Yokoyama discovered that murine NK cells activated with a potent cytokine combination exhibit memory-like properties, named as such to distinguish the differences between T/B cell memory and memory-like NK cells [25]. Starting with a cytokine combination of IL-12, IL-15, and IL-18 that induced nearly all NK cells to produce abundant IFN-γ [46,47], they hypothesized that this prior activation would result in an enhanced recall response to subsequent cytokine stimulation [25]. Murine NK cells activated with IL-12, IL-15, and IL-18 or NK cells supported with IL-15 alone (required to maintain survival) were adoptively transferred into syngeneic mice and tracked using CFSE and congenic markers. Cytokine-induced memory-like (ML and aka CIML) NK cells proliferated in vivo, but had returned to a resting state 1 week after adoptive transfer. These ML NK cells exhibited enhanced IFN-γ response upon restimulation, compared to adoptive transferred control NK cells, or the endogenous host NK cells [25,47]. In addition, this enhanced ability to produce IFN-γ was cell-intrinsic, independent of proliferative capacity, and persisted following cell division [48].
Similar to murine NK cells, human NK cells exhibit cytokine-induced memory-like differentiation after activation with IL-12, IL-15, and IL-18 (Fig. 2). Human NK cells activated overnight with combined IL-12, IL-15, and IL-18, and then allowed to differentiate in vitro for 1–6 weeks, had significantly enhanced IFN-γ production after restimulation with cytokines (IL-12 + IL-15 or IL-12 + IL-18) or K562 leukemia targets [49]. In addition, both CD56bright and CD56dim NK cell subsets exhibited memory-like IFN-γ production after cytokine restimulation. Human ML NK cells also had extensive proliferation measured by CFSE dilution, with enhanced recall responses maintained even after several rounds of cell division. In this first human ML NK cell report [49], stimulation via activating receptors (FcγRIIIa) combined with cytokines to further enhance ML NK cell formation in vitro. Thus, both human and murine NK cells exhibit enhanced recall function to multiple stimuli after IL-12, IL-15, and IL-18 activation followed by differentiation into ML NK cells, distinguishing this type of memory from hapten and virus-induced memory [44].
Fig. 2.
Schematic summary of human memory-like NK cell generation and enhanced response. Conventional (cNK) NK cells from the blood are activated with IL-12, IL-15, and IL-18 for 12–16 hours, and then cytokines are removed by washing. Memory-like NK cells differentiate either in vitro (with IL-15 support for survival), or in vivo in NSG mice or human patients. The resultant ML NK cells exhibit flexible, enhanced responses to a variety of NK cell stimuli, including tumor targets.
Closely following these initial reports, Ni and Cerwenka demonstrated that adoptively transferred murine IL-12, IL-15, and IL-18 activated NK cells display enhanced effector functions against established tumors in vivo [50]. A single injection of murine ML NK cells, but not control NK cells, combined with radiation therapy was able to substantially reduce growth of established MHC class I-deficient RMA-S lymphoma or B16-Rae1ε melanoma tumorsin mice. Furthermore, transcriptional analysis revealed that ML NK cells had increased STAT5 phosphorylation, which may play a role in binding to promoter regions to enhance IFN-γ production. Epigenetic imprinting of the Ifng CNS1 region has been detected in ML NK cells and has been hypothesized to contribute to the stable phenotype observed in vivo [51].
Studies have also shown that adoptive transfer of murine ML NK cells suppresses graft-versus-host disease (GVHD), but not graft-versus-leukemia [52,53]. Using a mouse model of fully mismatched HCT and lymphoma, Hüber et al demonstrated that ML NK cells strongly suppressed acute GVHD and improved overall survival in comparison to IL-2-only activated NK cells. Interestingly, while IL-2-treated NK cells became exhausted and lost expression of Eomesodermin (Eomes) and T-bet upon adoptive transfer into the GVHD mouse model, ML NK cells still had high expression of Eomes and T-bet [52]. This Eomes and T-bet upregulation may also explain the observed high proliferative capacity of ML NK cells in vivo. Collectively, these studies show that murine NK cells activated with IL-12, IL-15, and IL-18 exhibit antigen-independent enhanced functionality following adoptive transfer.
Preclinical studies of human cytokine-induced memory-like NK Cells
Unlike T cell memory populations, ML NK cells cannot be defined by minimal surface markers, but instead require multidimensional analysis for consistent tracking. Initial flow cytometric analyses identified that combined cytokine activation resulted in increased surface expression of CD94, NKG2A, NKp46, and CD69 in vitro [49]. Further analysis with mass cytometry has allowed for defining a large number of parameters on single ML NK cells, which has revealed a distinct multidimensional phenotype of ML NK cells [54]. High-dimensional CyTOF analysis of human ML NK cells differentiated in vitro confirmed phenotypic markers previously measured by flow cytometry, including increased expression of CD94, NKG2A, NKp46, and CD25. Further expression changes included increases in NKp30, NKp44, CD62L, TRAIL, perforin and granzyme B, and decreases in NKp80 compared to controls.
CD25 (IL2Rα) is a component of the high-affinity IL-2R, expressed constitutively on a subset of CD56bright NK cells or tran-siently following activation [9,55]. Robust CD25 (IL2Rα) expression on the surface of ML NK cells, prolonged compared to IL-15-activated control NK cells, was also identified [56], which persisted for ≥ 1 week in vitro. Notably, the majority of NK cells constitutively express IL-2Rβ and γ c, which form the intermediate-affinity heterodimer IL-2/15Rβγ, but have low expression of CD25, which is required for the high-affinity heterotrimer IL-2Rαβγ [55,57]. This stable and prolonged CD25 surface expression (and subsequent formation of functional high-affinity IL-2 receptors) in ML NK cells suggests its responsiveness to low concentrations of IL-2, with downstream effects on enhanced IFN-γ production and cy-totoxicity. Picomolar concentrations of IL-2 increased ML NK cell STAT5 phosphorylation and proliferation compared to controls in vivo in NOD/SCID/γc (NSG) xenograft models [56]. These studies set the stage for using low dose rhIL-2 as a supporting cytokine after adoptive transfer in clinical trials.
Based on enhanced recall function to leukemia cell lines in vitro [49], studies quickly focused on establishing whether ML NK cells exhibited enhanced responses to myeloid leukemia targets in vitro and in vivo. Our group performed an in depth analysis of whether ML NK cells had enhanced anti-leukemic functionality against AML cell line and primary leukemia blasts, and whether these responses were impacted by specific NK receptor-ligand interactions. ML NK cells were highly functional against several myeloid tumor cell lines, suggesting the potential for enhanced responses to AML [54]. Indeed, ML NK cells had enhanced functional responses when stimulated in vitro with primary AML blasts, exhibiting superior effector cytokine production as well as cytotoxicity, compared to control conventional NK (cNK) cells. This was also tested in vivo using a NSG mouse xenograft models, where ML NK cells demonstrated improved control of leukemia burden by bioluminescent imaging and prolonged the survival of leukemia-engrafted hosts [54]. In vitro studies also demonstrated that ML NK cell exhibited enhanced single cell responses to AML, with evidence of recruitment of additional NK cell effectors compared to cNK cells. Using a functional mass cytometry approach, single cNK and ML NK cells specificity was tracked at the level of inhibitory KIR (iKIR, ie, KIR2DL1, KIR2DL2/3, KIR3DL1), allowing the distinction between responding NK cells as inhibitory KIR to KIR ligand matched (inhibition present) or mismatched (no inhibition present). These studies revealed that the increased functional response of ML NK cells to primary AML blasts was not inhibited by iKIR-ligand interactions. ML NK cell differentiation increased IFN-γ production and cell surface expression of CD107a, TNF, and macrophage inflammatory protein-1α (MIP-1α) in response to primary AML blasts, regardless of whether ML NK cells expressed inhibitory KIR (iKIR) to a ligand present on target AML blasts [54]. Moreover, ML NK cell differentiation was shown to rescue normally hypofunctional “unlicensed” or “uneducated” NK cells to respond robustly to AML [58]. These studies suggest that MLNK cells have an increased pool of alloreactive NK cells, which points to one potential mechanism underlying the enhanced response to AML, in addition to enhanced activating NK receptor expression and effector molecules.
While able to overcome KIR inhibition, ML NK cells exhibit increased NKG2A expression and recent work has implicated NKG2A as an important checkpoint in ML NK cell functional responses [49,54]. NKG2A is a C-type lectin receptor that heterodimerizes with CD94 to generate an NK cell surface inhibitory receptor that binds to its ligand, HLA-E. In contrast to iKIR–ligand interactions, NKG2A is not a predominant inhibitor of cNK cells. In the phase 1 clinical trial utilizing ML NK cells in rel/ref AML, we utilized mass cytometry to assess donor ML NK cell phenotypes and markers of functionality and observed that increased NKG2A expression was significantly correlated with treatment failure [59,60]. These findings suggested that CD94/NKG2A-HLA-E interactions limited ML NK cell tumor responses. This was confirmed with mechanistic in vitro studies, where either antibody blockade of NKG2A or CRISPR/Cas9 deletion of NKG2A resulted in enhanced ML NK cell responses against HLA-E+ targets. The importance of NKG2A as an immune checkpoint in regulating ML NK cell activity has key clinical applications, including the ability to enhance adoptive ML NK anti-tumor functionality in vivo with specific anti-NKG2A blockade.
These early successes with ML NK cells in preclinical laboratory studies against leukemia targets provided the framework for using adoptively transferred ML NK cells in clinical trials. Subsequent preclinical studies have identified ML NK cells as exciting potential effector cells against other malignancies—including acute lymphoblastic leukemia (ALL) [61], hepatocellular carcinoma [62], ovarian cancers [63], and melanoma [64]. ML NK cells had enhanced IFN-γ and TNF production in vitro against a variety of ovarian cancer cell lines (including MA148, A1847, OVCAR5, andSKOV3)and also were capable of decreasing tumor burden in vivo in a xenogeneic mouse model of ovarian cancer [63]. In addition, NK cells isolated from the ascites of advanced stage high-grade serous ovarian cancer patients (ASC NK) that underwent cytokine-induced ML differentiation demonstrated enhanced IFN-γ against ovarian cancer cell lines compared to IL-15 treated controls; however, these cells were also noted to have consistently lower functional responses compared to NK cells from healthy donors, suggesting long-term impacts of the immunosuppressive tumor environment [63]. To evaluate if memory-like differentiation was capable of surmounting the soluble immunosuppressive environment in vitro, healthy donor ML NK cells were incubated for a week with donor ascites supernatant samples and then evaluated for IFN-γ production, CD107a expression, and proliferation. Notably, ML NK cells had enhanced IFN-γ and proliferation compared to conventional NK cells exposed to the same ascites supernatant, suggesting that ML NK cells retain their durable, enhanced functional activity, even in an immunosuppressive tumor microenvironment. These studies indicate the advantage ML NK cells may have in the solid tumor NK cell immunotherapy setting.
Clinical trails of memory-like NK cells
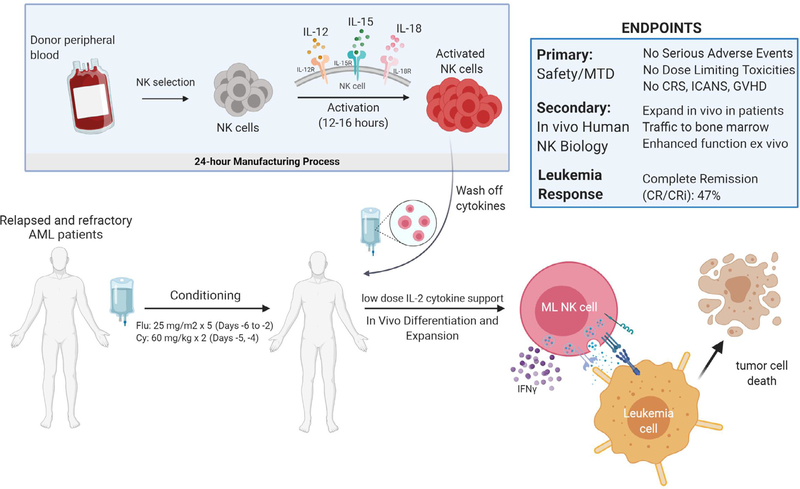
NK cell adoptive transfer is an emerging cellular immunotherapy for AML [65–67]. NK cells have been recognized as a cellular therapy for myeloid malignancies, with MHC-haploidentical NK cells exhibiting anti-leukemia responses without causing GVHD after HCT [68,69] or adoptive transfer [70–72]. Based on the preclinical data showing superior responses of ML NK cells compared to cNK cells, the application of ML NK cells presented a logical next stepin enhancing the anti-tumor activity of adoptive NK cellular immunotherapy. We performed the first-in-human clinical trial of adoptively transferred allogeneic ML NK cells in patients with relapsed or refractory (rel/ref) AML (ClinicalTrials.gov, NCT01898793) [54]. In this study, rel/ref AML patients received fludarabine and cyclophosphamide (flu/cy) lymphodepletion, and then were infused with a single ML NK cell dose generated from a related MHC-haploidentical donor (Fig. 3). After ML NK cell adoptive transfer, patients received low dose rhIL-2 for 2 weeks to support NK cell survival, expansion and function. Correlative immunology was employed using flow and mass cytometry to track allogeneic donor NK cells, leveraging this opportunity to study human ML NK cells in vivo in patients. Donor ML NK cells peaked after 7–14 days, comprising >90% of the patient’s NK cell compartment, tracked by donor- or recipient-specific HLA mAbs. These ML NK cells also localized to the bone marrow and exhibited macro-chimerism for up to 21 days. Functional studies revealed that ML NK cells from the blood and BM exhibited enhanced functional responses when re-stimulated with leukemia targets, supporting the concept of innate memory-like responses in vivo in humans [54]. Clinical results were promising, with 47% of the final 15 evaluable patients achieving a complete remission (CR/CRi) [59]. These data support the use of allogeneic ML NK cells as a “salvage” therapy for AML, allowing patients to proceed with potentially curative HCT. Collectively, this first-in-human study demonstrated that ML NK cells differentiate in vivo in patients, identified a maximal tested dose of upto 1 × 107/kg ML NK cells, which did not result in cytokine release syndrome (CRS), immune cell associated neurotoxicity, or GVHD. Based on the promising results with initial ML NK cell clinical trial, a phase II study is now underway in patients with rel/ref AML utilizing the maximal tested cell dose as a bridge to HCT.
Fig. 3.
Summary of the first-in-human ML NK cell clinical study. Relapsed or refractory AML patients were lymphodepleted with flu/cy, and then received allogeneic donor NK cells that were activated with IL-12, IL-15, and IL-18. Following transfer, memory-like differentiation and expansion occurs in vivo in the patient, providing a 2–3 week window of opportunity to attack and eliminate AML.
However, as an allogeneic lymphocyte therapy, eventually the recipient’s adaptive immune system recovers from flu/cy lym-phodepletion and eliminated the allogeneic donor NK cells, typically after 2–3 weeks. Thus, the long-term ML NK cell persistence could not be evaluated in the allogeneic adoptive transfer clinical setting. To investigate the concept that ML NK cells may expand and persistent in vivo in patients if administered in an immune compatible setting, we are performing a second clinical trial (ClinicalTrials.gov, NCT02782546). In this trial, rel/ref AML patients undergo a standard of care MHC-haploidentical peripheral blood HCT after reduced intensity conditioning with post-transplantation cyclophosphamide for GVHD prophylaxis. ML NK cells are generated from a second, same-donor leukapheresis, and administered on day 7, after the post-transplantation cyclophosphamide has been cleared. Preliminary data demonstrate that ML NK cells used to augment MHC-haploidentical HCT are safe, and result in complete remissions in >90% of AML patients at day 28. Strikingly, ML NK cell expansion exceeds normal blood NK cell numbers and persist for > 2 months [73]. Thus, this same-donor immune compatible HCT and ML NK cell adoptive transfer provides a clinical platform whereby a single dose of ML NK cells expand and persist with macro-chimerism for months in patients.
In patients who relapse after allogeneic HCT, donor lymphocyte infusion (DLI) with a set dose of donor T cells following salvage chemotherapy is a widely used treatment, however, current clinical outcomes remain poor and there remains an unmet need to develop more effective treatments for this patient population [74,75]. In a third clinical trial, same-donor ML NK cells are being testedas a therapy for AML relapse after HCT, used in concert with standard of care salvage chemotherapy followed by DLI (ClinicalTrials.gov, NCT03068819). By combining ML NK cells with DLI approach, we hypothesize that these adoptively transferred cells may persist and improve complete remission and long-term survival in these patients. This rationale was supported by murine studies, showing synergistic interactions between T cells and ML NK cells in vivo [51]. This trial is ongoing, with one exceptional responder with > 1 year leukemia-free-survival and remarkable ML NK cell macro chimerism reported [76].
The future: improving ML NK cell responses through combination immunotherapy and engineering
Combining ML NK cells with tumor targeting bi-specific NK cell engagers
ML NK cells have a number of properties that make them ideal for combined immunotherapy, and thereby address some of the existing barriers in the NK cell therapeutics field. Many cancers are not perfectly recognized by NK cells through endogenous activating receptors, and instead rely on combination with antibodies for an effective NK cell response. ML NK cells have preserved CD16 expression, and have been shown to exhibit enhanced FcγRIIIa/CD16 triggered responses [58]. Thus, one approach to provide an enhanced spectrum of recognition is to include tumor-targeting mAbs or bi-specific proteins, such as the anti-CD30 and anti-CD16a bi-specific AFM13. ML NK cells are effectively triggered by AFM13, establishing an approach to make ML NK cell antigen- specific in a highly flexible fashion through combination with therapeutic mAbs or bi-specific NK cell engagers [77].
Targeting NKG2A, the dominant "checkpoint" on ML NK cells
NK cells are distinct from T cells in their expression of constitutive and induced inhibitory receptors, for example KIRs and NKG2A. In contrast to cNK cells, ML NK cells can ignore inhibitory KIR signals, and blockade of KIR had no impact on responses to AML, as noted above. Instead, recent data indicate that NKG2A is the predominant “checkpoint” on ML NK cells and prior work demonstrates blocking NKG2A in vitro enhances ML NK cell anti-tumor functionality against tumor targets. Thus, combinations of signals that reduce NKG2A-based signals will be necessary to maximize ML NK cell functional responses in vivo, analogous to PD1/PDL1/L2 interaction with T cells.
CAR engineering expands the cellular immunotherapy potential of ML NK cells
The inclusion of CD19-targeted chimeric antigen receptors (CAR) on effector T-cells has revolutionized immunotherapy for refractory B cell malignancies. However, this has been associated with life-threatening side effects including CRS and neurotoxicity. Since ML NK cells do not cause these side effects while expanding and inducing remission in myeloid malignancies [54], they are an attractive effector to modify with CAR, thereby increasing their specificity. Additional advantages include combined activation with endogenous NK cell activating receptors, potentially reducing antigen escape, and the possibility of dual-targeting cancers via FcγRIIIa/CD16.
There have been multiple preclinical studies of CAR-transduced NK cells, and recently the first report of using CAR-NK to treat CD19-expressing cancer in patients has been published [78,79]. In a phase I study, Liu and Rezvani demonstrated that adoptive therapy with IL-15-secreting, cord-blood-derived CAR-NK cells resulted in minimal adverse events and that these CAR-NK cells can persist for up to a year postinfusion [79]. Synergizing CAR expression and ML differentiation is thus a promising strategy to further enhance NK function while increasing specificity.
Recently, we developed a proof-of-principle approach to engineer ML NK cells with a CAR (initially using 41BB and CD3ζ signals) [80]. These CAR ML NK cells exhibited enhanced degran-ulation, IFN-γ production, and cytotoxicity against NK-resistant targets. Notably, ML differentiation and CAR modification were synergistic, and superior to cNK cells engineered with CAR. CD19-CAR-ML NK cells exhibited potent response to B cell malignant targets, including primary lymphoma cells. In addition, CD19-CAR-ML NK cells preferentially expanded in NSG mice engrafted with CD19+ lymphoma targets, and controlled tumor burden in vivo. This initial preclinical study of CAR ML NK cells suggests that they have advantages over CAR cNK cells, and thus sets the stage for further engineering of ML NK cells that would facilitate the broad application of ML NK cells against a variety of cancer types.
Conclusions
NK cells are now clearly established to remember their prior activation events, with distinct forms of innate memory and memory-like responses following hapten exposure, virus infection, and combined IL-12, IL-15, and IL-18 activation. Moreover, ML NK cells exemplify a highly translational approach for enhancing NK cell responses against cancer. From their discovery in mice in 2009 and humans in 2012, this innate cellular therapy was rapidly trans-latedto theclinic,with thefirst AML patient treated withMLNK cells in 2014. ML NK cells are safe and induce complete remission in patients with AML, in several clinical contexts, without CRS or immune cell associated neurotoxicity. In an immune compatible setting, ML NK cells expand and persist for months after a single cell dose. Furthermore, ML NK cells have been clinically shown to be limited by NKG2A, providing a clear checkpoint for blockade to enhance ML NK cell responses. In addition, ML NK cells may be engineered with CAR, enhancing their spectrum of anti-tumor activity. Thus, cytokine-induced memory-like NK cells represent a promising NK cell therapeutic approach for cancer immunotherapy.
Acknowledgments
We thank all members of the Fehniger laboratory for insightful discussion. We apologize to all authors whose work we could not cite due to space limitations. Figures created with Biorender.com.
Funding: This work was supported by the Howard Hughes Medical Institute (Medical Fellow Award). National Institutes of Health (NIH): T32HL00708843, K12CA167540, SPORE in Leukemia P50CA171963, R01CA205239. Additional funding from the Siteman Cancer Center P30CA091842, V Foundation for Cancer Research, the Children’s Discovery Institute (CDI) at WUSM, and the Jamie Erin Follicular Lymphoma Research Fund.
Abbreviations:
- ML
memory-like
- CIML
cytokine-induced memory-like
- cNK
conventional NK cell
- ILC
innate lymphoid cell
Footnotes
Disclosure Statement
TAF and MMBE are consultants from Wugen, and are inventors on patents and pending patents licensed by Wugen from Washington University. TAF has received research support from Immunity-Bio, Compass Therapeutics, and HCW Biologics, and advises Kiadis, Nkarta, Indapta, and Orca Biosystems.
References
- [1].Yokoyama WM, Kim S, French AR. The dynamic life of natural killer cells. Annu Rev Immunol 2004;22:405–29. 10.1146/annurev.immunol.22.012703.104711. [DOI] [PubMed] [Google Scholar]
- [2].Caligiuri MA. Human natural killer cells. Blood 2008;112:461–9. 10.1182/blood-2007-09-077438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Vivier E, Tomasello E, Baratin M, Walzer T, Ugolini S. Functions of natural killer cells. Nat Immunol 2008;9:503–10. 10.1038/ni1582. [DOI] [PubMed] [Google Scholar]
- [4].Orange JS. Formation and function of the lytic NK-cell immunological synapse. Nat Rev Immunol 2008;8:713–25. 10.1038/nri2381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Kiessling R, Klein E, Pross H, Wigzell H. "Natural killer cells in the mouse. 2. Cytotoxic cells with specificity for mouse Moloney leukemia cells. Characteris-tics of the killer cell. Eur J Immunol 1975;5:117–21. 10.1002/eji.1830050209. [DOI] [PubMed] [Google Scholar]
- [6].Herberman RBRRB, Nunn MEM, Holden HTH, Lavrin. Natural cytotoxic reactivity of mouse lymphoid cells against syngeneic and allogeneic tumors. II. Characterization of effector cells. Int J Cancer 1975;16:230–9. 10.1002/ijc.2910160205. [DOI] [PubMed] [Google Scholar]
- [7].Imai K, Matsuyama S, Miyake S, Suga K, Yu H, Nakachi K. Natural cytotoxic activity of peripheral-blood lymphocytes and cancer incidence: an 11-year follow-up study of a general population. Lancet 2000;356:1795–9. 10.1016/S0140-6736(00)03231-1. [DOI] [PubMed] [Google Scholar]
- [8].Miller JS. The biology of natural killer cells in cancer, infection, and pregnancy. Exp Hematol 2001;29:1157–68. 10.1016/S0301-472X(01)00696-8. [DOI] [PubMed] [Google Scholar]
- [9].Cooper MA, Fehniger TA, Caligiuri MA. The biology of human natural killer-cell subsets. Trends Immunol 2001;22:633–40. http://www.ncbi.nlm.nih.gov/pubmed/11698225. [DOI] [PubMed] [Google Scholar]
- [10].Freud AG, Mundy-Bosse BL, Yu J, Caligiuri MA. The broad spectrum of human natural killer cell diversity. Immunity 2017;47:820–33. 10.1016/j.immuni.2017.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Freud AG, Yu J, A Caligiuri M. Human natural killer cell development in secondary lymphoid tissues. Semin Immunol 2014;26:132–7. 10.1016/j.smim.2014.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Wagner JA, A, Rosario M, Romee R, et al. CD56bright NK cells exhibit potent antitumor responses following IL-15 priming. J Clin Invest 2017;127:4042–58. 10.1172/JCI90387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Hackett J, Bosma GC, Bosma MJ, Bennett M, Kumar V. Transplantable progenitors of natural killer cells are distinct from those of T and B lymphocytes. Proc Natl Acad Sci U S A 1986;83:3427–31. 10.1073/pnas.83.10.3427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Romee R, Leong JW, Fehniger TA. Utilizing cytokines to function-enable human NK cells for the immunotherapy of cancer. Scientifica (Cairo) 2014. 10.1155/2014/205796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Long EO, Sik Kim H, Liu D, Peterson ME, Rajagopalan S. Controlling natural killer cell responses: integration of signals for activation and inhibition. Annu Rev Immunol 2013;31:227–58. 10.1146/annurev-immunol-020711-075005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Lanier LL. Up on the tightrope: natural killer cell activation and inhibition. Nat Immunol 2008;9:495–502. 10.1038/ni1581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Di Vito C, Mikulak J, Mavilio D. On the way to become a natural killer cell. Front Immunol 2019:10 10.3389/fimmu.2019.01812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Goodridge JP, Önfelt B, Malmberg KJ. Newtonian cell interactions shape natural killer cell education. Immunol Rev 2015;267:197–213. 10.1111/imr.12325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Vitetta ES, Berton MT, Burger C, et al. Annu Rev Immunol 1991;9:193–217. 10.1146/annurev.iy.09.040191.001205. [DOI] [PubMed] [Google Scholar]
- [20].Gourley TS, Wherry EJ, Masopust D, Ahmed R. Generation and maintenance of immunological memory. Semin Immunol 2004;16:323–33. 10.1016/j.smim.2004.08.013. [DOI] [PubMed] [Google Scholar]
- [21].Farber DL, Yudanin NA, Restifo NP. Human memory T cells: generation, compartmentalization and homeostasis.. Nat Rev Immunol 2014;14:24–35. 10.1038/nri3567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Sallusto F, Lanzavecchia A, Araki K, Ahmed R. From vaccines to memory and back. Immunity 2010;33:451–63. 10.1016/j.immuni.2010.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].O’Leary JG, Goodarzi M, Drayton DL, von Andrian UH. T cell– and B cell– independent adaptive immunity mediated by natural killer cells. Nat Immunol 2006;7:507–16. 10.1038/ni1332. [DOI] [PubMed] [Google Scholar]
- [24].Sun JC, Beilke JN, Lanier LL. Adaptive immune features of natural killer cells. Nature 2009;457:557–61. 10.1038/nature07665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Cooper MA, Elliott JM, Keyel PA, Yang L, Carrero JA, Yokoyama WM. Cytokine-induced memory-like natural killer cells. Proc Natl Acad Sci. U S A 2009;106:1915–19. 10.1073/pnas.0813192106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Min-Oo G, Kamimura Y, Hendricks DW, Nabekura T, Lanier LL. Natural killer cells: walking three paths down memory lane. Trends Immunol 2013;34:251–8. 10.1016/j.it.2013.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Paust S, Gill HS, Wang B-ZZ, et al. Critical role for the chemokine receptor CXCR6 in NK cell-mediated antigen-specific memory of haptens and viruses. Nat Immunol 2010;11:1127–35. 10.1038/ni.1953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Hydes T, Abuhilal M, Armstrong T, Primrose J, Takhar A, Khakoo S. Natural killer cell maturation markers in the human liver and expansion of an NKG2C+KIR+ population. Lancet 2015;385:S45 10.1016/s0140-6736(15)60360-9. [DOI] [PubMed] [Google Scholar]
- [29].Stegmann KA, Robertson F, Hansi N, et al. CXCR6 marks a novel subset of T-bet lo Eomes hi natural killer cells residing in human liver. Sci Rep 2016:6 10.1038/srep26157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Dokun AO, Kim S, Smith HR, Kang HS, Chu DT, Yokoyama WM. Specific and nonspecific NK cell activation during virus infection. Nat Immunol 2001;2:951–6. 10.1038/ni714. [DOI] [PubMed] [Google Scholar]
- [31].Min-Oo G, Bezman NA, Madera S, Sun JC, Lanier LL. Proapoptotic BIM regulates antigen-specific NK cell contraction and the generation of the memory NK cell pool after cytomegalovirus infection. J Exp Med 2014;211:1289–96. 10.1084/jem.20132459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Madera S, Sun JC, Edge Cutting. Stage-Specific Requirement of IL-18 for Antiviral NK Cell Expansion. J Immunol 2015;194:1408–12. 10.4049/jimmunol.1402001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Sun JC, Madera S, Bezman NA, Beilke JN, Kaplan MH, Lanier LL. Proinflammatory cytokine signaling required for the generation of natural killer cell memory. J Exp Med 2012;209:947–54. 10.1084/jem.20111760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].O’Sullivan TE, Johnson L, Kang H, Sun J. BNIP3 and BNIP3L-mediatedmi-tophagy promotes the generation of natural killer cell memory. Immunity 2015;43(2):331–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Bezman NA, Kim CC, Sun JC, et al. Molecular definition of the identity and activation of natural killer cells. Nat Immunol 2012;13:10 0 0–8. 10.1038/ni.2395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Gumá M, Angulo A, Vilches C, et al. Imprint of human cytomegalovirus infection on the NK cell receptor repertoire. Blood 2004;104:3664–71. 10.1182/blood-2004-05-2058. [DOI] [PubMed] [Google Scholar]
- [37].Foley B, Cooley S, Verneris MR, et al. Human cytomegalovirus (CMV)-induced memory-like NKG2C(+) NK cells are transplantable and expand in vivo in response to recipient CMV antigen.. J Immunol 2012;189:5082–8. 10.4049/jimmunol.1201964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Schlums H, Cichocki F, Tesi B, et al. cytomegalovirus infection drives adaptive epigenetic diversification of NK cells with altered signaling and effector function. Immunity 2015;42:443–56. 10.1016/j.immuni.2015.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Björkström NK, Lindgren T, Stoltz M, et al. Rapid expansion and long-term persistence of elevated NK cell numbers in humans infected with hantavirus. J Exp Med 2011;208:13–21. 10.1084/jem.20100762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Petitdemange C, Becquart P, Wauquier N, et al. Unconventional repertoire profile is imprinted during acute chikungunya infection for natural killer cells polarization toward cytotoxicity. PLoS Pathog 2011;7:e1002268 10.1371/journal.ppat.1002268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Cichocki F, Wu CY, Zhang B, et al. ARID5B regulates metabolic programming in human adaptive NK cells. J. Exp. Med. 2018;215:2379–95. 10.1084/jem.20172168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Lee J, Zhang T, Hwang I, et al. Epigenetic modification and antibody-dependent expansion of memory-like NK cells in human cytomegalovirus-infected individuals. Immunity 2015;42:431–42. 10.1016/j.immuni.2015.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Lisa LL,Landskron J, Ask EH, et al. Beziat,CriticalroleofCD2co-stimulation in adaptive NK cell responses revealed in NKG2C-deficient humans. Cell Rep 2016;15:1088–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Fehniger TA, Cooper MA. Harnessing NK cell memory for cancer immunotherapy. Trends Immunol 2016;2 Epub Oct 10.1016/j.it.2016.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Rölle A, Pollmann J, Ewen E, et al. IL-12 – producing monocytes and HLA-E control HCMV-driven NKG2C + NK cell expansion. J Clin Invest 2014;124:5305–16. 10.1172/JCI77440.determinants. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Fehniger TA, Shah MH, Turner MJ, et al. Differential cytokine and chemokine gene expression by human NK cells following activation with IL-18 or IL-15 in combination with IL-12: implications for the innate immune response. J Immumol 1999;162:4511–20. http://www.ncbi.nlm.nih.gov/pubmed/10201989. [PubMed] [Google Scholar]
- [47].Keppel MMP, Yang L, Cooper MMA. Murine NK cell intrinsic cytokine-induced memory-like responses are maintained following homeostatic proliferation. J Immumol 2013;190:4754–62. 10.4049/jimmunol.1201742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Cooper MA, Colonna M, Yokoyama WM. Hidden talents of natural killers: NK cells in innate and adaptive immunity. EMBO Rep 2009;10:1103–10. 10.1038/embor.2009.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Romee R, Schneider SE, Leong JW, et al. Cytokine activation induces human memory-like NK cells. Blood 2012;120:4751–60. 10.1182/blood-2012-04-419283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Ni J, Miller M, Stojanovic A, Garbi N, Cerwenka A. Sustained effector function of IL-12/15/18-preactivated NK cells against established tumors. J Exp Med 2012;209:2351–65. 10.1084/jem.20120944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Ni J, Hölsken O, Miller M, et al. Adoptively transferred natural killer cells maintain long-term antitumor activity by epigenetic imprinting and CD4+ T cell help. Oncoimmunology 2016;5 10.1080/2162402X.2016.1219009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Hüber CM, Doisne JM, Colucci F. IL-12/15/18-preactivated NK cells suppress GvHD in a mouse model of mismatched hematopoietic cell transplantation. Eur. J. Immunol. 2015;45:1727–35. 10.1002/eji.201445200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Song Y, Hu B, Liu Y, et al. IL-12/IL-18-preactivated donor NK cells enhance GVL effects and mitigate GvHD after allogeneic hematopoietic stem cell transplantation. Eur J Immunol 2018;48:670–82. 10.1002/eji.201747177. [DOI] [PubMed] [Google Scholar]
- [54].Romee R, Rosario M, Berrien-Elliott MM, et al. Cytokine-induced memory-like natural killer cells exhibit enhanced responses against myeloid leukemia. Sci Transl Med 2016;8 357ra123–357ra123. doi: 10.1126/scitranslmed.aaf2341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Fehniger TA, Cooper MA, Caligiuri Ma. Interleukin-2 and interleukin-15: immunotherapy for cancer.. Cytokine Growth Factor Rev 2002;13:169–83. 10.1016/S1359-6101(01)00021-1. [DOI] [PubMed] [Google Scholar]
- [56].Leong JW, Chase JM, Romee R, et al. Preactivation with IL-12, IL-15, and IL-18 induces CD25 and a functional high-affinity IL-2 receptor on human cytokine-induced memory-like natural killer cells. Biol Blood Marrow Transpl 2014;20:463–73. 10.1016/j.bbmt.2014.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Waldmann TA. The biology of interleukin-2 and interleukin-15: implications for cancer therapy and vaccine design. Nat Rev Immunol 2006;6:595–601. 10.1038/nri1901. [DOI] [PubMed] [Google Scholar]
- [58].Wagner JA, Berrien-Elliott MM, Rosario M, et al. Cytokine-induced memory-like differentiation enhances unlicensed NK cell anti-leukemia and FcγRIIIa-triggered responses. Biol Blood Marrow Transplant 2016;23:398–404. 10.1016/j.bbmt.2016.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Berrien-Elliott MM, Wagner JA, Romee R, et al. Abstract 5704: Mass cytometry identifies the expansion, persistence, and immune checkpoints of adoptively transferred memory-like NK cells in patients with leukemia. In: in: American Association for Cancer Research (AACR; 2018. 5704–5704 10.1158/1538-7445.am2018-5704. [DOI] [Google Scholar]
- [60].Berrien-Elliott MM, Pamela W, Neal C, et al. Primary human NK cell gene-editing reveals a critical role for NKG2A in cytokine-induced memory-like NK cell responses. Blood 2019;134 3237–3237 10.1182/blood-2019-129162. [DOI] [Google Scholar]
- [61].Boieri M, Ulvmoen A, Sudworth A, et al. IL-12, IL-15, and IL-18 pre-activated NK cells target resistant T cell acute lymphoblastic leukemia and delay leukemia development in vivo. Oncoimmunology 2017:6 10.1080/2162402X.2016.1274478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Zhuang L, Fulton RJ, Rettman P, et al. Activity of IL-12/15/18 primed natural killer cells against hepatocellular carcinoma. Hepatol. Int. 2019;13:75–83. 10.1007/s12072-018-9909-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Uppendahl LD, Felices M, Bendzick L, et al. Cytokine-induced memory-like natural killer cells have enhanced function, proliferation, and in vivo expansion against ovarian cancer cells. Gynecol Oncol 2019;153:149–57. 10.1016/j.ygyno.2019.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Marin-Agudelo NM, Krasnick B, Becker-Hapak M, et al. Cytokine-induced memory-like NK cells exhibit enhanced autologous responses to metastatic melanoma.. Soc Nat Immun 2019:69. [Google Scholar]
- [65].Lee DA, Denman CJ, Rondon G, et al. Haploidentical natural killer cells infused before allogeneic stem cell transplantation for myeloid malignancies: a phase I trial. Biol Blood Marrow Transplant 2016;22:1290–8. 10.1016/j.bbmt.2016.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Curti A, Ruggeri L, Parisi S, et al. Larger size of donor alloreactive NK cell repertoire correlates with better response to NK cell immunotherapy in elderly acute myeloid leukemia patients. Clin Cancer Res 2016;22:1914–21. 10.1158/1078-0432.CCR-15-1604. [DOI] [PubMed] [Google Scholar]
- [67].Shaffer BC, Le Luduec J-BB, Forlenza C, et al. Phase II study of haploidentical natural killer cell infusion for treatment of relapsed or persistent myeloid malignancies following allogeneic hematopoietic cell transplantation. Biol Blood Marrow Transplant 2016;22:705–9. 10.1016/j.bbmt.2015.12.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Ruggeri L, Capanni M, Urbani E, et al. Effectiveness of donor natural killer cell alloreactivity in mismatched hematopoietic transplants. Science 2002;295:2097–100. 10.1126/science.1068440. [DOI] [PubMed] [Google Scholar]
- [69].Velardi A, Ruggeri L, Mancusi A, Aversa F, Christiansen FT. Natural killer cell allorecognition of missing self in allogeneic hematopoietic transplantation: a tool for immunotherapy of leukemia. Curr Opin Immunol 2009;21:525–30. 10.1016/j.coi.2009.07.015. [DOI] [PubMed] [Google Scholar]
- [70].Miller JS, Soignier Y, Panoskaltsis-Mortari A, et al. Successful adoptive transfer and in vivo expansion of human haploidentical NK cells in patients with cancer. Blood 2005;105:3051–7. 10.1182/blood-2004-07-2974. [DOI] [PubMed] [Google Scholar]
- [71].Rubnitz JE, Inaba H, Ribeiro RC, et al. NKAML: a pilot study to determine the safety and feasibility of haploidentical natural killer cell transplantation in childhood acute myeloid leukemia. J Clin Oncol 2010;28:955–9. 10.1200/JCO.2009.24.4590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Curti A, Ruggeri L, D’Addio A, et al. Successful transfer of alloreactive haploidentical KIR ligand-mismatched natural killer cells after infusion in elderly high-risk acute myeloid leukemia patients. Blood 2011;118:3273–9. 10.1182/blood-2011-01-329508. [DOI] [PubMed] [Google Scholar]
- [73].Foltz JA, Berrien-Elliott MM, Neal C, et al. Cytokine-induced memory-like (ML) NK cells persist for & GT; 2 months following adoptive transfer into leukemia patients with a MHC-compatible hematopoietic cell transplant (HCT). Blood 2019;134 1954–1954 10.1182/blood-2019-126004. [DOI] [Google Scholar]
- [74].Levine JE, Braun T, Penza SL, et al. Prospective trial of chemotherapy and donor leukocyte infusions for relapse of advanced myeloid malignancies after allogeneic stem-cell transplantation. J Clin Oncol 2002;20:405–12. 10.1200/JCO.20.2.405. [DOI] [PubMed] [Google Scholar]
- [75].Schmid C, Labopin M, Nagler A, et al. EBMT acute leukemia working party, donor lymphocyte infusion in the treatment of first hematological relapse after allogeneic stem-cell transplantation in adults with acute myeloid leukemia: a retrospective risk factors analysis and comparison with other strategies by the EBMT acute leukemia. J Clin Oncol 2007;25:4938–45. 10.1200/JCO.2007.11.6053. [DOI] [PubMed] [Google Scholar]
- [76].Bednarski JJ, Zimmerman C, Cashen AF, et al. Adoptively transferred donor-derived cytokine induced memory-like NK cells persist and induce remission in pediatric patient with relapsed acute myeloid leukemia after hematopoietic cell transplantation. Blood 2019;134 3307–3307 10.1182/blood-2019-126982. [DOI] [Google Scholar]
- [77].Marin N, Becker-Hapak M, Koch J, et al. The CD30/CD16A bispecific innate immune cell engager AFM13 elicits heterogeneous single-cell NK cell responses and effectively triggers memory-like (ML) NK cells, in: Immunology. Am Assoc Cancer Res 2019. 1546–1546 10.1158/1538-7445.AM2019-1546. [DOI] [Google Scholar]
- [78].Gang M, Marin ND, Wong P, et al. CAR-modified memory-like NK cells exhibit potent responses to NK-resistant lymphomas. Blood 2020. 10.1182/blood.2020006619. doi: 10.1182/blood.2020006619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Liu E, Marin D, Banerjee P, et al. Use of CAR-transduced natural killer cells in CD19-positive lymphoid tumors.. N Engl J Med 2020;382:545–53. 10.1056/NEJMoa1910607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Gang M, Marin-Agudelo NM, Wong P, et al. CAR-modified memory– like NK cells exhibit potent responses to NK-resistant lymphomas. Blood 2020;136(20):2308–18. [DOI] [PMC free article] [PubMed] [Google Scholar]