Abstract
In this literature review, we present the main scientific findings on the antifungal activity of essential oils (EOs) applicable for a new drug formulation to treat oral candidiasis. Seven literature databases were systematically searched for eligible in vitro and clinical trials. Selected articles were screened for biological activity, botanical species, phytochemical composition, study design, and methodological quality. A total of 26 articles were included in the review, of which 21 were in vitro studies and 5 clinical trials. The most promising EOs were obtained from Allium tubeorosum, Cinnamomum cassia, Cinnamomum zeylanicum, and Coriandrum sativum L. Among the phytochemicals, citral and thymol were the most active. Clinical trials indicated that the EOs from Pelargonium graveolens and Zataria multiflora are potentially effective to treat oral candidiasis. Further nonclinical and clinical studies with these EO are warranted to determine their potential use and safety for the treatment of oral candidiasis.
1. Introduction
Oral candidiasis is an infection caused by Candida spp. which manifests clinically as erythematous, ulcerated, sensitive white lesions, with soft consistency and easy removal, commonly affecting the palate, oral mucosa, tongue, or oropharynx [1]. Due to the opportunistic pathogenicity of yeasts, oral candidiasis is more prevalent in immunocompromised individuals [2].
Yeast colonization of complete dentures occurs mainly due to the strong adhesion of yeast cells to acrylic resin of base materials [1]. Direct adhesion of yeasts onto dental surfaces is a critical pathogenic factor for the onset of dental stomatitis. Yeast cells can co-aggregate with various bacterial species from the oral plaque and integrated into a robust biofilm pellicle on the surface of dentures. Oral biofilms can be considered microbial reservoirs and significantly affect the oral and systemic health of denture users [1, 3].
Polyene and azole drugs, such as nystatin and miconazole, respectively, have been commonly prescribed for the treatment of oral candidiasis [2]. However, recent years have seen failures in antifungal therapy due to increasing microbial resistance rates and high drug toxicity, which have altogether contributed to rise the prevalence of morbidity and mortality indicators related to fungal infections [4].
This scenario has encouraged the search for novel substances capable of controlling and treating yeast infections while having low toxicity to the host. Some naturally occurring products are considered an important source of new molecules with biological properties, displaying antifungal efficacy comparable or stronger than that of drugs currently available for clinical use. Essential oils (EOs) are a class of natural products with pharmacological properties, which include antimicrobial, antiseptic, anti-inflammatory, and antioxidant activities [5–9]. These compounds are described as a mixture of volatile constituents produced as secondary metabolites by aromatic plants. With the chemical characteristic of lipophilicity, EOs have the ability to interact with fungal cell membranes and lipid structures. Among their mechanisms of action, EO can disrupt the activity of enzymes involved in ergosterol synthesis or complex with membrane ergosterol, thereby creating pores in the membrane and disrupting permeability [10–12]. In addition, EO can affect cell wall biosynthesis, interfere with protein synthesis or cell division, and stimulate the production of reactive oxygen species, causing growth inhibition or cell death [13, 14]. In this literature review, we present the main scientific findings on the antifungal activity of EO and their isolated phytochemicals on Candida spp. commonly responsible for oral infections. In vitro and clinical (controlled clinical trials in humans) studies were selected and are further discussed in this review.
2. Materials and Methods
2.1. Study Question
This literature review was conducted to address the specific question: “Is there scientific evidence to support the use of EO and/or their isolated constituents for the treatment of oral candidiasis or to warrant further nonclinical and clinical research?”
2.2. Search Strategy and Study Selection
The PRISMA guidelines (Transparent Reporting of Systematic Reviews and Meta-Analyses) [15] were followed. Seven databases were systematically searched for studies of experimental oral candidiasis and randomized controlled clinical trials published up to 1 March 2020 (Table 1).
Table 1.
Search strategy and bibliographic databases used to retrieve the articles for this systematic review.
| Primary bibliographic sources | Search strategy (descriptors and combinations with Boolean operators) |
|---|---|
| SciVerse Scopus (since 1995) | (i) (Essential oil AND oral candidiasis) |
| (ii) (Oils, volatile, OR essential oil) AND (oral candidiasis) | |
| (iii) (Oils, volatile, OR essential oil) AND (denture stomatitis) | |
|
| |
| Web of Science (since 1990) | Filters; article or review and language |
| (i) (Essential oil AND oral candidiasis) | |
| (ii) (Oils, volatile, OR essential oil) AND (oral candidiasis) | |
| (iii) (Oils, volatile OR essential oil) AND (denture stomatitis) | |
|
| |
| Medline via Pubmed (since 1966) | (i) (Essential oil AND oral candidiasis) |
| (ii) (Oils, volatile OR essential oil) AND (oral candidiasis) | |
| (iii) (Oils, volatile OR essential oil) AND (denture stomatitis) | |
|
| |
| SciELO (Scientific Electronic Library Online) (since 1998), LILACS (Latin American and Caribbean Health sciences Literature) (since 1982), and Cochrane Library | (i) (Essential oil AND oral candidiasis) |
| (ii) (Oils, volatile OR essential oil) AND (oral candidiasis) | |
| (iii) (Oils, volatile OR essential oil) AND (denture stomatitis) | |
| (iv) (Aceite esencial y candidiasis oral) | |
| (v) (EO e candidíase oral) | |
| (vi) (EO e estomatite protética) | |
|
| |
| Google Scholar | (i) Manual searches according to the reference lists of the articles |
Search strategy and bibliographic databases and keywords.
2.3. Eligibility Criteria
A systematic screening of the articles was performed by two independent examiners according to the following inclusion criteria:
Biological activity: clinical effects of an EO-containing formulation on denture stomatitis or oral candidiasis in in vitro or clinical trials. Primary outcome of interest: antifungal activity of the EO and/or isolated constituent based on their MIC (minimum inhibitory concentration). Secondary outcome of interest: reduction in CFUs (colony-forming units) after treatment with the EO-containing formulation leading to remission or cure of infection. Tertiary outcome of interest: cure or reduction in the size and number of erythematous lesions upon treatment with the EO-containing formulation.
Plant material and chemical elucidation: chemically characterized EO and/or their isolated constituents from aromatic plants.
Study design: in vitro studies and phases I, II, III or IV clinical trials. Sample size and study power (at least 80%) should be adequate to determine accurate statistical inferences.
Methodological quality: accuracy of methods and outcomes; internal and external validity; for clinical trials—high quality standards.
Language: articles written in English, Spanish, or Portuguese. Examiners agreed that in cases of inconsistence, the final verdict on which articles should be included would be reached by consensus.
2.4. Data Analysis
For in vitro studies, a range of MIC values was used as a parameter to determine the extent of antifungal activity for interstudy comparisons (adapted from [16]). The established scoring criteria for MIC values are shown in Table 2.
Table 2.
Established parameters based on minimum inhibitory concentrations of essential oils or related chemical constituents.
| MIC range (μg/mL) | Intensity of antifungal activity | Score |
|---|---|---|
| <100 | Very strong activity | ++++ |
| 101–500 | Strong activity | +++ |
| 501–1000 | Moderate activity | ++ |
| 1000–2000 | Weak activity | + |
| >2001 | No activity | − |
Source: adapted from Freires et al. [16].
Randomized controlled trials of herbal interventions were analyzed based on the CONSORT guidelines [17]. The Jadad scale [18] was used to check study validity and methodological quality (randomization, blinding, and loss of follow-up). Based on these requirements, clinical studies were assigned scores ranging from 0 to 5, in which a score <3 was indicative of poor quality.
3. Results
3.1. Search Strategy
Using a previously defined strategy, bibliographic searches were carried out using specific keyword combinations. A total of 395 articles were retrieved, of which 26 were considered eligible and included in the final review (Figure 1). Twenty-one studies with in vitro design and five clinical trials were included and are further discussed.
Figure 1.
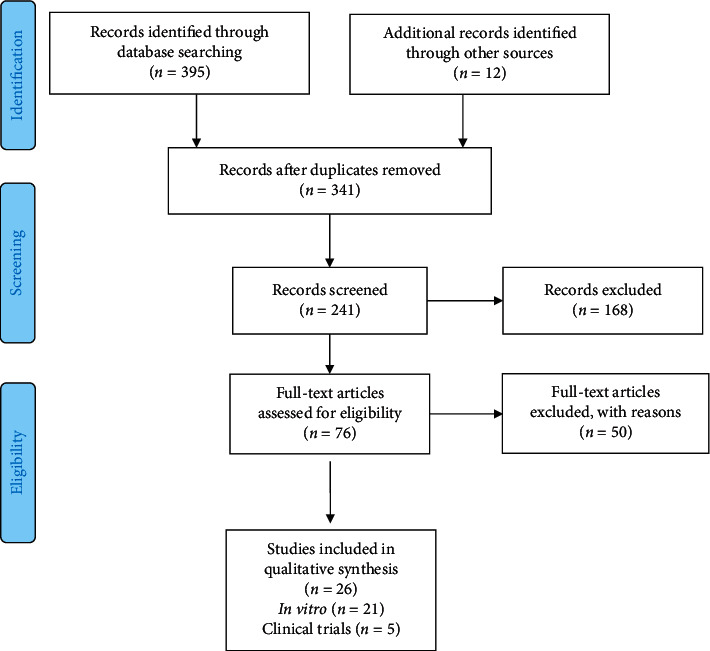
Flow diagram of the search strategy comprising the identification of potentially relevant material, preliminary screening (based on PRISMA guidelines) (main categories by which the articles were excluded from the study).
3.2. In Vitro Antifungal Activity
The antifungal activity of thirty-one EO and four phytochemicals against Candida spp. strains (clinical isolates and reference strains) was analyzed. As shown in Tables 3 and 4, the most promising EOs were obtained from Allium tuberosum, Cinnamomum cassia, Cinnamomum zeylanicum, and Coriandrum sativum. Citral and Thymol were the most active isolated constituents, with MIC values lower than 100 μg/mL, indicating very strong antifungal activity (Table 5)[.
Table 3.
In vitro antifungal activity of essential oils against Candida albicans strains.
| Plant species | Source | Microorganism | MIC50% (μg/mL) | Score MIC | Ref |
|---|---|---|---|---|---|
| Achillea millefolium | Leaves | C. albicans clinical strain | 625 | ++ | [3] |
| Allium tuberosum Rottl. ex Spreng | Commercial Source | C. albicans CBS 562 | 500 | +++ | [19] |
| C. albicans clinical strain | 500 | +++ | |||
| Anethum graveolens | Seeds | C. albicans ATCC (62342) | >2001 | − | [20] |
| Candida albicans clinical strain | >2001 | − | |||
| Bursera morelensis | Leaves | C. albicans clinical strain | 125 | +++ | [21] |
| Cinnamomum burmannii | Commercial source | C. albicans ATCC 28366 | 1000 | ++ | [22] |
| Cinnamomum cassia | Bark | C. albicans ATCC 76485 | 64 | ++++ | [23] |
| C. albicans C01-C11 | 64 | ++++ | |||
| Cinnamomum zeylanicum | Commercial source | C. albicans ATCC 76845 | 312.5 | +++ | [24] |
| Cinnamomum zeylanicum | Commercial source | C. albicans ATCC 76485 | 312.5 | +++ | [25] |
| C. albicans ATCC 76645 | 312.5 | +++ | |||
| C. albicans clinical strain | 625 | ++ | |||
| Cinnamomum zeylanicum Blume | Commercial source | C. albicans ATCC 40277 | 312.5 | +++ | [14] |
| C. albicans clinical strain | 312.5 | +++ | |||
| Cinnamomum zeylanicum Blume | Commercial source | C. albicans CBS 562 | 250 | +++ | [26] |
| C. albicans ATCC 60193 | 250 | +++ | |||
| C. albicans ATCC 90029 | 125 | +++ | |||
| C. albicans LM01 | 250 | +++ | |||
| C. albicans LM03 | 250 | +++ | |||
| C. albicans LM04 | 500 | +++ | |||
| Coriandrum sativum | Commercial source | C. albicans CBS 562 | 15.0 | ++++ | [19] |
| C. albicans clinical strain 3 A5 | 31.0 | ++++ | |||
| Coriandrum sativum L. | Leaves | C. albicans CBS 562 | 15.6 | ++++ | [27] |
| Cuminum cyminum | Seeds | C. albicans ATCC62342 | >2001 | − | [20] |
| C. albicans clinical strain | >2001 | − | |||
| Curcuma longa L. | Rhizomes | C. albicans Clinical strain | 625 | ++ | [3] |
| Laurus nobilis L. | Commercial source | C. albicans ATCC 60193 | 250 | +++ | [28] |
| Melaleuca alternifólia | Commercial source | C. albicans ATCC 289065 | >2001 | − | [29] |
| C. albicans ATCC 40277 | >2001 | − | |||
| Melaleuca alternifolia | Commercial source | C. albicans ATCC 18804 | 1950 | + | [30] |
| Melaleuca alternifolia | Commercial source | C. albicans clinical strain | >2001 | − | [31] |
| Pimpinella anisum | Seeds | C. albicans ATCC 62342 | >2001 | − | [20] |
| Candida albicans clinical strain | >2001 | − | |||
| Ocotea odorifera | Commercial source | C. albicans ATCC-90028 | >2001 | − | [24] |
| C. albicans ATCC-76615 | >2001 | − | |||
| C. albicans ATCC-76645 | >2001 | − | |||
| C. albicans ATCC-76485 | >2001 | − | |||
| C. albicans clinical strain | >2001 | − | |||
| Rosmarinus officinalis | Commercial source | C. albicans, ATCC 289065 | 562,5 | ++ | [29] |
| C. albicans ATCC 40277 | <2001 | − | |||
| Rosmarinus officinalis L. | Commercial source | C. albicans ATCC-90028 | >2001 | − | [24] |
| C. albicans ATCC-76615 | >2001 | − | |||
| C. albicans ATCC-76645 | >2001 | − | |||
| C. albicans ATCC-76485 | >2001 | − | |||
| C. albicans clinical strain | >2001 | − | |||
| Santolina chamaecyparissus | Commercial source | C. albicans CBS 562 | 1000 | ++ | [19] |
| C. albicans clinical strain | >2001 | − | |||
| Satureja hortensis L. | Leaves | C. albicans Clinical strain | 200 | +++ | [32] |
nt (not tested); comparative MIC values (μg/mL): (++++) <100; (+++) 100 to 500; (++) 501 to 1000; (+) >1001 to 2000; (−) >2001.
Table 4.
In vitro antifungal activity of essential oils against non-albicans Candida strains.
| Plant species | Source | Microorg. | C. dubliniensis 1 | C. glabrata 2 | C. krusei 3 | C. parapsilosis 4 | C. tropicalis 5 | Ref. | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MIC50% (μg/mL) | Score MIC | MIC50% (μg/mL) | Score MIC | MIC50% (μg/mL) | Score MIC | MIC50% (μg/mL) | Score MIC | MIC50% (μg/mL) | Score MIC | ||||
| Ageratum conyzoides L | Leaves | ATCC 138035 | nt | − | nt | − | nt | − | nt | − | 1000 | ++ | [33] |
| Clinical strain5 | nt | − | nt | − | nt | − | nt | − | 1000 | ++ | |||
|
| |||||||||||||
| Allium tuberosum Rottl. Ex Spreng | Commercial source | CBS 6044 | <2001 | − | nt | − | nt | − | 15 | ++++ | nt | − | [19] |
| CBS 79871 | Nt | − | nt | − | nt | − | nt | − | nt | − | |||
| Clinical strain3 | nt | − | nt | − | <2001 | − | nt | − | nt | − | |||
| Clinical strain4 | nt | − | nt | − | nt | − | 15 | − | nt | − | |||
| Clinical strain1 | <2001 | − | nt | − | nt | − | nt | − | nt | − | |||
|
| |||||||||||||
| Anethum graveolens | Seeds | Clinical strain2,3,4 | nt | − | >2001 | − | >2001 | − | >2001 | − | Nt | − | [20] |
|
| |||||||||||||
| Artemisia absinthum L | Leaves | ATCC 138035 | nt | − | nt | − | nt | − | nt | − | 500 | +++ | [33] |
| Clinical strain5 | nt | − | nt | − | nt | − | nt | − | 500 | +++ | |||
|
| |||||||||||||
| Artemisia camphorata L | Leaves | ATCC 138035 | nt | − | nt | − | nt | − | nt | − | 1000 | ++ | [33] |
| Clinical strain5 | nt | − | nt | − | nt | − | nt | − | 1000 | ++ | |||
|
| |||||||||||||
| Bidens sulphurea | Leaves | ATCC 138035 | nt | − | nt | − | nt | − | nt | − | 500 | +++ | [33] |
| Clinical strain5 | nt | − | nt | − | nt | − | nt | − | 500 | +++ | |||
|
| |||||||||||||
| Chenopodium ambrosioides L | Leaves | ATCC 138035 | nt | − | nt | − | nt | − | nt | − | 500 | +++ | [33] |
| Clinical strain5 | nt | − | nt | − | nt | − | nt | − | 500 | +++ | |||
|
| |||||||||||||
| Cinnamomum zeylanicum | Commercial source | ATCC 138035 | nt | − | nt | − | nt | − | nt | − | 625 | ++ | [25] |
| Clinical strain5 | nt | − | nt | − | nt | − | nt | − | 625 | ++ | |||
|
| |||||||||||||
| Cinnamomum zeylanicum Blume | Commercial source | ATCC 400425 | nt | − | nt | − | nt | − | nt | − | 312.5 | +++ | [14] |
| Clinical strain5 | nt | − | nt | − | nt | − | nt | − | 312.5 | +++ | |||
| ATCC 401473 | nt | − | nt | − | 312.5 | +++ | nt | − | nt | − | |||
| Clinical strain3 | nt | − | nt | − | 312.5 | +++ | nt | − | nt | − | |||
|
| |||||||||||||
| Cinnamomum zeylanicum Blume | Commercial source | ATCC 34133 | nt | − | nt | − | 1000 | ++ | nt | − | nt | − | [26] |
| CBS 945 | nt | − | nt | − | nt | − | nt | − | 25 | +++ | |||
| ATCC 7505 | nt | − | nt | − | nt | − | nt | − | 250 | +++ | |||
| Clinical strain5 | nt | − | nt | − | nt | − | nt | − | 250 | +++ | |||
| — | nt | − | nt | − | nt | − | nt | − | 250 | +++ | |||
| — | nt | − | nt | − | nt | − | nt | − | 625 | ++++ | |||
|
| |||||||||||||
| Citrus reticulata Blanco | Leaves | ATCC 138035 | nt | − | nt | − | nt | − | nt | − | 500 | +++ | [33] |
| Clinical strain5 | nt | − | nt | − | nt | − | nt | − | 500 | +++ | |||
|
| |||||||||||||
| Coreopsis lanceolata L. | Leaves | ATCC 138035 | nt | − | nt | − | nt | − | nt | − | 500 | +++ | [33] |
| Clinical strain5 | nt | − | nt | − | nt | − | nt | − | 1000 | ++ | |||
|
| |||||||||||||
| Coriandrum sativum | Commercial source | CBS 5733 | nt | − | nt | − | 15 | ++++ | nt | − | nt | − | [19] |
| CBS 6044 | nt | − | nt | − | nt | − | 125 | +++ | nt | − | |||
| CBS 79871 | 7 | ++++ | nt | − | nt | − | nt | − | nt | − | |||
| CBS 945 | nt | − | nt | − | nt | − | nt | − | 125 | +++ | |||
| Clinical strain3 | nt | − | nt | − | 7 | ++++ | nt | − | nt | − | |||
| Clinical strain4 | nt | − | nt | − | nt | − | 7 | ++++ | nt | − | |||
| Clinical strain1 | 7 | ++++ | nt | − | nt | − | nt | − | nt | − | |||
| Clinical strain5 | nt | − | nt | − | nt | − | nt | − | 63 | ++++ | |||
|
| |||||||||||||
| Coriandrum sativum L. | Leaves | CBS 945 | nt | − | nt | − | nt | − | nt | − | 312 | ++++ | [27] |
| CBS 5733 | nt | − | nt | − | 156 | ++++ | nt | − | nt | − | |||
| CBS 79871 | 31.2 | ++++ | nt | − | nt | − | nt | − | nt | − | |||
|
| |||||||||||||
| Cuminum cyminum | Seeds | Clinical strain2,3,4 | nt | − | >2001 | − | >2001 | − | >2001 | − | Nt | − | [20] |
|
| |||||||||||||
| Foeniculum vulgare | Leaves | Clinical strain2,3,4 | nt | − | >2001 | − | >2001 | − | >2001 | − | Nt | − | [20] |
|
| |||||||||||||
| Laurus nobilis L. | Commercial source | ATCC 7505 | nt | − | nt | − | nt | − | nt | − | 250 | +++ | [28] |
| ATCC 34133 | nt | − | nt | − | 500 | +++ | nt | − | nt | − | |||
| CBS 945 | nt | − | nt | − | nt | − | nt | − | 500 | +++ | |||
| CBS 5733 | nt | − | nt | − | 500 | +++ | nt | − | nt | − | |||
| — | nt | − | 500 | +++ | nt | − | nt | − | nt | − | |||
|
| |||||||||||||
| Lavandula officinalis L. | Leaves | ATCC 138035 | nt | − | nt | − | nt | − | nt | − | 500 | +++ | [33] |
| Clinical strain5 | nt | − | nt | − | nt | − | nt | − | 500 | +++ | |||
|
| |||||||||||||
| Melaleuca alternifólia | Commercial source | ATCC 401473 | nt | − | nt | − | >2001 | − | nt | − | nt | − | [29] |
| ATCC 400425 | nt | − | nt | − | nt | − | nt | − | 562.5 | ++ | |||
| ATCC 138035 | nt | − | nt | − | nt | − | nt | − | >2001 | − | |||
|
| |||||||||||||
| Ocimum gratissimum L | Leaves | ATCC 138035 | nt | − | nt | − | nt | − | nt | − | 500 | +++ | [33] |
| Clinical strain5 | nt | − | nt | − | nt | − | nt | − | 500 | +++ | |||
|
| |||||||||||||
| Ocotea odorifera | Commercial source | ATCC-138035 | nt | − | nt | − | nt | − | nt | − | <2000 | − | [24] |
| Clinical strain5 | nt | − | nt | − | nt | − | nt | − | <2000 | − | |||
|
| |||||||||||||
| Pelargonium graveolens L'H'er | Leaves | ATCC 138035 | nt | − | nt | − | nt | − | nt | − | 125 | +++ | [33] |
| Clinical strain5 | nt | − | nt | − | nt | − | nt | − | 125 | +++ | |||
|
| |||||||||||||
| Pimpinella anisum | Seeds | Clinical strain | nt | − | >2001 | − | >2001 | − | >2001 | − | — | — | [20] |
|
| |||||||||||||
| Plectranthus neochilus Schl | Leaves | ATCC 138035 | nt | − | nt | − | nt | − | nt | − | 1000 | ++ | [33] |
| Clinical strain5 | nt | − | nt | − | nt | − | nt | − | 1000 | ++ | |||
|
| |||||||||||||
| Rosmarinus officinalis | Commercial source | ATCC 401473 | nt | − | nt | − | 1125 | ++ | nt | − | nt | − | [29] |
| ATCC 400425 | nt | − | nt | − | nt | − | nt | − | 562.5 | ++ | |||
| ATCC 138035 | nt | − | nt | − | nt | − | nt | − | 1125 | + | |||
|
| |||||||||||||
| Rosmarinus officinalis L. | Commercial source | ATCC-138035 | nt | − | nt | − | nt | − | nt | − | <2001 | − | [24] |
| Clinical strain5 | nt | − | nt | − | nt | − | nt | − | <2001 | − | |||
|
| |||||||||||||
| Santolina chamaecyparis sus | Commercial source | CBS 5733 | nt | − | nt | − | 500 | +++ | nt | − | nt | − | [19] |
| CBS 6044 | nt | − | nt | − | nt | − | >2001 | − | nt | − | |||
| CBS 79871 | 63 | ++++ | nt | − | nt | − | nt | − | nt | − | |||
| CBS 945 | nt | − | nt | − | nt | − | nt | − | >2001 | − | |||
| Clinical strain3 | 500 | +++ | nt | − | nt | − | nt | − | nt | − | |||
| Clinical strain4 | nt | − | nt | − | nt | − | 500 | +++ | nt | − | |||
| Clinical strain1 | nt | − | nt | − | nt | − | nt | − | nt | − | |||
| Clinical strain5 | nt | − | nt | − | nt | − | nt | − | >2001 | − | |||
|
| |||||||||||||
| Syzigium aromaticum | Leaves | ATCC 138035 | nt | − | nt | − | nt | − | nt | − | 500 | +++ | [33] |
| Clinical strain5 | nt | − | nt | − | nt | − | nt | − | 500 | +++ | |||
|
| |||||||||||||
| Tagetes erecta L. | Leaves | ATCC 138035 | nt | − | nt | − | nt | − | nt | − | 500 | +++ | [33] |
| Clinical strain5 | nt | − | nt | − | nt | − | nt | − | 500 | +++ | |||
|
| |||||||||||||
| Tetradenia Riparia (Hochst.) Codd | Leaves | ATCC 138035 | nt | − | nt | − | nt | − | nt | − | 250 | +++ | [33] |
| Clinical strain5 | nt | − | nt | − | nt | − | nt | − | 250 | +++ | |||
nt (not tested); comparative MIC values (μg/mL): (++++) <100; (+++) 100 to 500; (++) 501 to 1000; (+) >1001 to 2000; (−) >2001. Strain of C. dubliniensis = C. dubliniensis; strain of C. glabrata = C. glabrata2; strain of C. krusei = C. krusei3; strain of C. parapsilosis = C. parapsilosis4; strain of C. tropicalis = C. tropicalis5.
Table 5.
In vitro antifungal activity of phytoconstituents isolated from essential oils against Candida spp. strains.
| Plant species | Source | Microorganism | MIC (μg/mL) | MFC (μg/mL) | Score MIC | Ref. |
|---|---|---|---|---|---|---|
| Citral | Commercial source | Candida albicans ATCC 76645 | 32 | 32 | ++++ | [34] |
| Clinical isolates Candida albicans | 32–64 | 32–64 | ++++ | |||
|
| ||||||
| Linalol | Commercial source | C. albicans CA 032 | 2000 | 2000 | + | [35] |
| Candida albicans 051 | 1000 | 2000 | + | |||
| Clinical isolate Candida tropicalis | 500 | 500 | +++ | |||
| Clinical isolates Candida krusei | 2000 | 2000 | + | |||
|
| ||||||
| α-Pinene | Leaves | Candida albicans clinical strain | 500 | >2001 | ++ | [21] |
|
| ||||||
| Terpinen-4-ol | Commercial source | Clinical isolates C. albicans | >2001 | nt | − | [31] |
|
| ||||||
| γ-Terpinene | Leaves | Candida albicans clinical strain | >2001 | >2001 | − | [21] |
|
| ||||||
| Thymol | Commercial source | C. albicans CBS 562 | 39 | 39 | ++++ | [36] |
| C. tropicalis CBS 94 | 78 | 78 | ++++ | |||
| C. krusei CBS 573 | 39 | 39 | ++++ | |||
Note: comparative MIC values (μg/mL): (++++) <100; (+++) 100 to 500; (++) 501 to 1000; (+) >1001 to 2000; (−) >2001.
3.3. Randomized Clinical Trials
3.3.1. Effects of Intervention
According to the pre-established criteria, five clinical studies were included in this review: Pelargonium graveolens, Zataria multiflora, and Melaleuca alternifolia in three formulations. The main methodological characteristics and outcomes of selected studies are shown in Table 6 and Figure 2. An experimental gel containing Pelargonium graveolens EO healed completely (34%) or partially (56%) patients with prosthetic stomatitis as compared to those who received only the gel with a placebo. In addition, the gel was effective in reducing the fungal load as well as in decreasing erythema in patients with prosthetic stomatitis as compared to those treated with the placebo. Another experimental gel containing Zataria multiflora EO was also effective in reducing the fungal load in participants' saliva and denture samples as well as n reducing local inflammation.
Table 6.
Drug formulation from essential oils, study design, and outcomes of the randomized clinical trials included in this literature review.
| Plant species | Essential oilformulation | Study design | Sample size | Country | Age (mean ± SD)/gender (Fem)† | Sample loss/reasons | Control group | Dosing protocol | Assessment check points | Assessment instruments of interest | Outcome | Ref. |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pelargonium graveole ns | Gel | Phase II, randomized, double-blind | 80 patients (40 treated with Pelargonium gel and 40 treated with placebo) | Iran | 38 to 78 years (61.39 ± 9.038)/(51 women and 29 men) | − | Treated with placebo (base gel 1% geranium essence) | Application of the gel twice a day (morning and night) for 14 days | 2 weeks | Collection and culture of mycological samples from the palatal mucosa at each visit and colony count | + | [37] |
|
| ||||||||||||
| Zataria multiflora | 0.1% gel | Phase II double-blind, open randomized and controlled | 24 patients (12 treated with miconazole gel and 12 treated with Zataria multiflora gel) | Iran | 24 (15 women and 9 men) aged 45 to 83 years (average 60.83) years | − | Miconazole gel (2%) | The gel was applied to the base of the denture four times a day for 4 weeks | 4 weeks | Colony counting of samples from the palatal mucosa, erythematous lesion on the palatal surface and from the surface of the denture | +/+ | [38] |
|
| ||||||||||||
| Melaleuca alternifolia | Alcoholic and nonalcoholic solutions | Phase II randomized, single-center open clinical trial | 27 patients (13 treated with the alcoholic solution and 14 treated with the nonalcoholic solution) | USA | Men and women aged 18 to 65 years | 5 (two did not return to receive the study medication and three received the medication but never returned for follow-up) | − | Group I: rinse using 15 mL of the solution for 30–60 s four times a day for 14 days; Group II: rinse using 5 mL of solution for 30–60 s, four times a day for 14 days | 2 and 4 weeks | Assessment of signs and symptoms (thrush and erythema), extent of lesions, assessment of cure, improvement, change or worsening of oropharyngeal candidiasis | + | [39] |
|
| ||||||||||||
| Melaleuca alternifólia | Cream | Phase II, randomized clinical trial | 27 patients (3 groups of 9: control group, Melaleuca artenifolia group and , nystatin group) | Chile | 50 to 77 years old/26 women and 1 man | − | Cream alone and cream + nystatin | For every 5 ml of cream, 1 ml was replaced by 1 ml of M. alternifolia cream and homogenized for 20 seconds | − | Culture and CFU count of samples from the palate mucosa in all sessions | + | [40] |
|
| ||||||||||||
| Melaleuca alternifólia | Oral solution | Phase II, single-center open study | 13 patients | USA | 18 and 65 years old/men and women | 1 (never returned) | − | Rinse using 15 ml of the solution for 30–60 s four times a day for 14 days | 2 and 4 weeks | Assessment of signs and symptoms of oropharyngeal candidiasis, mycological assessments included a KOH test, yeast quantification, and in vitro susceptibility studies | + | [41] |
Note: statistically significant reduction (+) or not (−) in the CFU count and in the signs and symptoms of oral candidiasis in relation to the positive control or placebo. †Good result.
Figure 2.
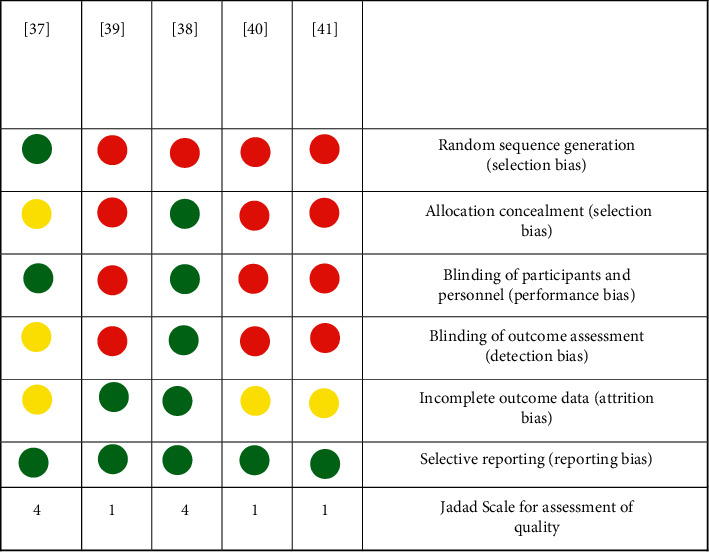
Risk-of-bias summary of the clinical trials included in this literature review. Red (−) stands for high risk of bias, green (+) stands for low risk of bias, and yellow (?) stands for unclear risk of bias. Overall, the studies are compliant with the CONSORT guidelines for clinical trials of herbal interventions, showing a low risk of bias.
4. Discussion
The main antimicrobial mechanisms of EO and their constituents are associated with their ability to increase cell membrane permeability due to lipophilicity of their molecules, resulting in extravasation of ions and cellular contents and cell lysis [39–41]. In this review, the selected data suggest that some EO and phytochemicals are promising for the treatment of oral candidiasis and warrant further nonclinical, clinical, and toxicological investigation for pharmaceutical purposes [42–44].
Next, a brief summary of the most active EO and isolated compounds will be presented based on in vitro and clinical studies. Information on ethnopharmacological knowledge, biological properties, and chemical composition is further discussed.
4.1. Essential Oils and Phytochemicals with Promising Antifungal Activity against Candida spp
The Allium genus, which belongs to the Amaryllidaceae family, contains approximately 700 species of plants, such as Allium cepa (onion), Allium sativum (garlic), Allium schoenoprasum (chives), and Allium tuberosum (garlic chives). All are important due to their commercial character and nutritional value [45]. Allium tuberosum is a perennial plant that grows in many countries in Asia and whose aerial parts are edible green vegetables common to the Chinese. A. tuberosum has an odor similar to the smell of garlic and other Allium plants due to the presence of sulfur-containing compounds [46].
Several pharmacological activities are attributed to this species, including antidiabetic and hepatoprotective [47], antiparasitic [48], antibacterial [49], and antifungal activities against fungi of the Aspergillus genus [50]. This species has been reported to have strong antifungal activity against Candida parapsilosis isolates and inhibitory effects on biofilm formation [19].
Cinnamomum cassia, popularly known as China cinnamon, is an herb belonging to the Lauraceae family, occurring in several countries such as India, China, Uganda, Vietnam, Bangladesh, and Pakistan. It is intensely aromatic, with a sweet taste and bitter touch. Its peels have been used in different ways, either as a flavoring in various Asian cuisines or in traditional medicine for the treatment of diabetes mellitus and peptic ulcer [51]. The major compound of C. cassia is cinnamaldehyde (75–90%). Other phytoconstituents, present in trace amounts, include eugenol, benzoic acid, cinnamic acid, salicylic acid, cinnamyl alcohol, and their corresponding esters and aldehydes [52].
C. cassia has been shown to have anti-inflammatory, antioxidant, anticancer, antipyretic, antiangiogenic, larvicidal, and antifungal properties [53]. C. cassia was reportedly active against four Candida spp. strains, namely, C. albicans and C. tropicalis, C. glabrata, and C. krusei, as well as against Aspergillus, Fusarium, and three dermatophyte isolates (Microsporum gypseum, Trichophyton rubrum, and T. mentagraphytes). C. Cassia EO was effective in reducing the number of pseudohyphae in C. albicans cultures, which is considered an important virulence factor [54]. Mouse models and in vitro assays have also proved the antiproliferative activity of C. cassia EO against oral candidiasis (C. albicans infection). Cinnamaldehyde was reported as the main compound responsible for the antifungal effects observed in C. cassia EO [55].
Cinnamomum zeylanicum, popularly known as cinnamon, is a very common spice that has been used by different cultures around the world for several centuries. It is obtained from the bark and leaves of trees of the genus Cinnamomum, a perennial tropical plant that has two main varieties, namely, Cinnamomum zeylanicum and Cinnamomum cassia. In addition to its culinary uses, in native Ayurvedic medicine, cinnamon is used as an alternative to treat respiratory, digestive, and gynecological diseases [56]. Four of the main components of the EO obtained from C. zeylanicum bark are trans-cinnamaldehyde, cinnamaldehyde, eugenol, and linalool, which represent 82.5% of the total EO composition [57]. In vitro and in vivo studies in animals and humans have shown important biological activities attributed to C. zeylanicum EO, such as anti-inflammatory, antimicrobial, reduction of cardiovascular diseases, and increase of cognitive function [58]. Some studies reported that C. zeylanicum EO has antifungal activity against Candida spp. most likely by disrupting yeast cell wall [14, 24, 25], which suggests that this EO may be a promising candidate for the treatment of oral candidiasis.
Coriandrum sativum L. is a small plant belonging to the Apiaceae family, popularly known as coriander. Coriander leaves and seeds are widely used in folk medicine as a cholesterol-lowering agent, digestive stimulant, and antihypertensive [11], in addition to its use as a spice in food preparation. The main components present in C. sativum EO are linalool (55.09%), α-pinene (7.49%), 2,6-octadien-1-ol, 3,7-dimethyl-acetate, geraniol (4.83%), 3-cyclohexene-1-methanol, α, α, 4-trimethyl- (4.72%), hexadecanoic acid (2.65%), acid tetradecanoic (2.49%), 2-α-pinene (2.39%), citronellyl acetate (1.77%), and undecanal (1.29%) [59]. Pharmaceutical formulations containing C. sativum also revealed antibacterial [60], antioxidant [61], hepatoprotective, and anticonvulsant properties. C. sativum EO also showed strong antifungal effects against Candida spp. strains [16].
Citral (3,7-dimethyl-2-6-octadienal) is a racemic mixture composed of geranial (trans-citral, citral A) and neral (cis-citral, citral B) isomers, which are acyclic and monounsaturated aldehydes naturally occurring in many citric fruits, as well as in other herbs or spices [62]. Citral has become a raw material of great importance due to its characteristic lemon aroma and has been used as a flavoring ingredient in the food, perfumery and cosmetic industries [63]. Citral showed fungicidal activity against Candida spp. strains isolated from denture wearers after 2 hours of exposure and caused major morphological changes [34]. Leite et al. [64] demonstrated a strong antifungal activity of citral against C. albicans strains via mechanisms other than cell wall biosynthesis or ergosterol complexation. Thus, citral can be considered a promising candidate for the development of novel antifungal leads.
Thymol is a monoterpene found in essential oils extracted from plants belonging to the Lamiaceae family such as the genera Thymus, Ocimum, Origanum, Satureja, Thymbra, and Monarda [65–67]. This molecule is a phytoconstituent with several biological activities described, including anti-inflammatory and antinociceptive [68], local anesthetic [69], and antifungal and antibacterial [70] activities. Thymol has been reported to have strong antifungal activity against strains of the Candida genus, acting on the fungal cell membrane and producing a synergistic effect when used with nystatin to inhibit the growth of these strains [36].
4.2. Clinical Studies of Essential Oils for the Treatment of Oral Candidiasis
While numerous studies are carried out to determine the antifungal activity of EO in vitro, only a few formulations reach the clinical stage and even less become a commercial product. As seen in this review, few clinical trials have been carried out to test experimental formulations containing EO and/or isolated constituents against oral candidiasis. Currently, the most common formulations for the treatment of oral candidiasis are for external use, such as oral solutions, gels, and creams, which are normally safe [71].
Sabzghabaee et al. [37] evaluated the clinical efficacy of a gel containing Pelargonium graveolens EO for the treatment of prosthetic stomatitis. This study presented a low risk of bias for aspects related to randomization and blinding and showed high methodological quality according to Jadad's scale [18]. Another clinical study, conducted by Amanlou et al. [38], showed that Zataria multiflora EO is also effective to treat prosthetic stomatitis. Denture wearers applied the gel containing 0.1% of Z. multiflora EO four times a day for two weeks. The presence of erythema on the palate surface of participants was significantly reduced as well as CFU counts of yeast strains. Although limitations related to randomization were observed in the study by Amanlou et al. [38], it showed a low risk of bias, which suggests that Z. multiflora EO may be a favorable therapeutic alternative for the treatment of prosthetic stomatitis.
Despite the favorable outcomes of EO on oral candidiasis and prosthetic stomatitis reported by the authors of the studies selected in this review, only the studies with P. graveolens (popular names: fragrant-leaf geranium-Port., rose geranium-Engl., and geranium-Span.) and Z. multiflora (popular name: thyme of shiraz-Engl.) met high methodological quality standards. Further research should consider the chemical standardization of these EO and the adoption of appropriate methodological strategies for further clinical testing.
This literature review shows that the most promising EOs were obtained from Allium tubeorosum, Cinnamomum cassia, Cinnamomum zeylanicum, and Coriandrum sativum L. Among the phytochemicals, the citral and the thymol were the most active. The clinical trials selected in this review provided evidence that the EO from Pelargonium graveolens and Zataria multiflora are potentially effective to treat oral candidiasis. Further nonclinical and clinical studies with these EO are warranted to determine their potential use and safety for the treatment of oral candidiasis.
Acknowledgments
This study was financed in part by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior–Brasil (CAPES)–Finance Code 001.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- 1.Jabra-Rizk M. A., Kong E. F., Tsui C., et al. Candida albicans pathogenesis: fitting within the host-microbe damage response framework. Infection and Immunity. 2016;84(10):2724–2739. doi: 10.1128/iai.00469-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Garcia-Cuesta C., Sarrion-Pérez M. G., Bagán J. V. Current treatment of oral candidiasis: a literature review. Journal of Clinical Experimental Dentistry. 2014;6(5):576–582. doi: 10.4317/jced.51798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ribeiro D. I., Alves M. S., Faria M. G. I., et al. Determinação da atividade antifúngica dos óleos essenciais de Curcuma longa L. (Zingiberaceae) e de Achillea millefolium (Asteraceae) cultivadas no noroeste do Paraná. Arquivo de Ciências da Saúde. 2010;14(2):103–109. [Google Scholar]
- 4.Wisplinghoff H., Ebbers J., Geurtz L., et al. Nosocomial bloodstream infections due to Candida spp. in the USA: species distribution, clinical features and antifungal susceptibilities. International Journal of Antimicrobial Agents. 2014;43(1):78–81. doi: 10.1016/j.ijantimicag.2013.09.005. [DOI] [PubMed] [Google Scholar]
- 5.Zida A., Bamba S., Yacouba A., Ouedraogo-traore R., Guiguemdé R. T. Anti Candida albicans natural products, sources of new antifungal drugs: a review. Journal de Mycologie Medicale. 2016;27(1):1–19. doi: 10.1016/j.mycmed.2016.10.002. [DOI] [PubMed] [Google Scholar]
- 6.Bakkali F., Averbeck S., Averbeck D., Idaomar M. Biological effects of essential oils-a review. Food and Chemical Toxicology. 2008;46(2):446–475. doi: 10.1016/j.fct.2007.09.106. [DOI] [PubMed] [Google Scholar]
- 7.Gyawali R., Ibrahim S. A. Natural products as antimicrobial agents. Food Control. 2014;46(2):412–429. doi: 10.1016/j.foodcont.2014.05.047. [DOI] [Google Scholar]
- 8.Morais-Braga M. F. B., Sales D. L., Carneiro J. N. P., et al. Psidium guajava L. and Psidium brownianum Mart ex DC.: chemical composition and anti-Candida effect in association with fluconazole. Microbial Pathogenesis. 2016;95:200–207. doi: 10.1016/j.micpath.2016.04.013. [DOI] [PubMed] [Google Scholar]
- 9.Santos K. K. A., Matias E. F. F., Souza C. E. S., et al. Anti-candida activity of Mentha arvensis and Turnera ulmifolia. Journal of Medicinal Food. 2012;15(3):322–324. doi: 10.1089/jmf.2011.0128. [DOI] [PubMed] [Google Scholar]
- 10.Cardoso N. N. R., Alviano C. S., Blank A. F., et al. Synergism effect of the essential oil from Ocimum basilicum var. Maria Bonita and its major components with fluconazole and its influence on ergosterol biosynthesis. Evidence-Based Complementary and Alternative Medicine. 2016;2016:12. doi: 10.1155/2016/5647182.5647182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shreaz S., Wani W. A., Behbehani J. M., et al. Cinnamaldehyde and its derivatives, a novel class of antifungal agents. Fitoterapia. 2016;112:116–131. doi: 10.1016/j.fitote.2016.05.016. [DOI] [PubMed] [Google Scholar]
- 12.Rajkowska K., Nowak A., Kunicka-Styczyńska A., Siadura A. Biological effects of various chemically characterized essential oils: investigation of the mode of action against Candida albicans and hela cells. RSC Advances. 2016;6(99):97199–97207. doi: 10.1039/c6ra21108a. [DOI] [Google Scholar]
- 13.Singh S., Fatima Z., Hameed S. Citronellal-induced disruption of membrane homeostasis in Candida albicans and attenuation of its virulence attributes. Revista da Sociedade Brasileira de Medicina Tropical. 2016;49(4):465–472. doi: 10.1590/0037-8682-0190-2016. [DOI] [PubMed] [Google Scholar]
- 14.Castro R. D. D., Lima E. O. Anti-candida activity and chemical composition of Cinnamomum zeylanicum Blume essential oil. Brazilian Archives of Biology and Technology. 2013;56(5):749–755. doi: 10.1590/s1516-89132013000500005. [DOI] [Google Scholar]
- 15.Liberati A., Altman D. G., Tetzlaff J., et al. The prisma statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. Plos Medicine. 2009;6:1–28. doi: 10.1371/journal.pmed.1000100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Freires I., Denny C., Benso B., de Alencar S., Rosalen P. I. Antibacterial activity of essential oils and their isolated constituents against cariogenic bacteria: a systematic review. Molecules. 2015;20(4):7329–7358. doi: 10.3390/molecules20047329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gagnier J. J., Boon H., Rochon P., et al. Recommendations for reporting randomized controlled trials of herbal interventions: explanation and elaboration. Journal of Clinical Epidemiology. 2006;59(11):1134–1149. doi: 10.1016/j.jclinepi.2005.12.020. [DOI] [PubMed] [Google Scholar]
- 18.Jadad A. R., Moore R. A., Carroll D., et al. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Controlled Clinical Trials. 1996;17(1):1–12. doi: 10.1016/0197-2456(95)00134-4. [DOI] [PubMed] [Google Scholar]
- 19.Furletti V. F., Teixeira I. P., Obando-Pereda G., et al. Action of Coriandrum sativum L. essential oil upon oral Candida albicans biofilm formation. Evidence-Based Complementary and Alternative Medicine. 2011;2011:9. doi: 10.1155/2011/985832.985832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vieira J. N., Gonçalves C. L., Villarreal J. P. V., et al. Chemical composition of essential oils from the Apiaceae family, cytotoxicity, and their antifungal activity in vitro against Candida species from oral cavity. Brazilian Journal of Biology. 2019;79(3):432–437. doi: 10.1590/1519-6984.182206. [DOI] [PubMed] [Google Scholar]
- 21.Ivera-yañez C. R., Terrazas L. I., Jimenez-Estrada M., et al. Anti-Candida activity of Bursera morelensis Ramirez essential oil and two compounds, α-Pinene and γ-Terpinene an in vitro study. Molecules. 2017;5(12):1–13. doi: 10.3390/molecules22122095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Veilleux M. P., Grenier D. Determination of the effects of Cinnamon bark fractions on Candida albicans and oral epithelial cells. BMC Complementary Medicine and Therapies. 2019;19(303):1–12. doi: 10.1186/s12906-019-2730-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Almeida L., Cavalcanti Y. C., Castro R. D., Lima E. O. Atividade antifúngica e alterações morfológicas induzidas pelo óleo essencial de cinnamomum cassia frente cepas de Candida albicans isoladas de pacientes HIV positivos. Pesquisa Brasileira em Odontopediatria e Clínica Integrada. 2012;12(3):393–398. doi: 10.4034/pboci.2012.123.15. [DOI] [Google Scholar]
- 24.Castro R. D., Lima E. O. Screening da atividade antifúngica de óleos essenciais sobre cepas de Candida. Pesquisa Brasileira em Odontopediatria e Clínica Integrada. 2011;11(3):341–345. [Google Scholar]
- 25.Oliveira F., Andrade L., de Sousa É., Sousa D. P. Anti-ulcer activity of essential oil constituents. Molecules. 2014;19(5):5717–5747. doi: 10.3390/molecules19055717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rangel M. d. L., Aquino S. G. d., Lima J. M. d., Castellano L. R., Castro R. D. d. In vitro effect of Cinnamomum zeylanicum blume essential oil on Candida spp. involved in oral infections. Evidence-Based Complementary and Alternative Medicine. 2018;2018:13. doi: 10.1155/2018/4045013.4045013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Freires I. A., Murata R. M., Furletti V. F., et al. Coriandrum sativum L. (Coriander) essential oil: antifungal activity and mode of action on Candida spp., and molecular targets affected in human whole-genome expression. PLoS One. 2014;9(6):1–13. doi: 10.1371/journal.pone.0099086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Peixoto L. R., Rosalen P. L., Ferreira G. L. S., et al. Antifungal activity, mode of action and anti-biofilm effects of Laurus nobilis Linnaeus essential oil against Candida spp. Archives of Oral Biology. 2017;73:179–185. doi: 10.1016/j.archoralbio.2016.10.013. [DOI] [PubMed] [Google Scholar]
- 29.Cavalcanti Y. W., Almeida L. F. D., Padilha W. W. N. Atividade antifúngica de três óleos essenciais sobre cepas de Candida. Revista Odontológica Do Brasil Central. 2011;20(52):197–201. [Google Scholar]
- 30.Rasteiro V. M. C., Costa A. C. B. P., Araújo C. F., et al. Essential oil of Melaleuca alternifolia for the treatment of oral candidiasis induced in immunosuppressed mouse model. BMC Complementary Alternative Medicine. 2014;14(489):1–10. doi: 10.1186/1472-6882-14-489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ninomiya K., Maruyama N., Inoue S., et al. The essential oil of Melaleuca alternifolia (tea tree oil) and its main component, terpinen-4-ol protect mice from experimental oral candidiasis. Biological and Pharmaceutical Bulletin. 2012;35(489):861–865. doi: 10.1248/bpb.35.861. [DOI] [PubMed] [Google Scholar]
- 32.Sharifzadeh A., Khosravi A. R., Ahmadian S. Chemical composition and antifungal activity of Satureja hortensis L. essentiall oil against planktonic and biofilm growth of Candida albicans isolates from buccal lesions of HIV+ individuals. Microbial Pathogenesis. 2016;96:1–9. doi: 10.1016/j.micpath.2016.04.014. [DOI] [PubMed] [Google Scholar]
- 33.Souza C. M. C., Pereira Junior S. A., Moraes T. d. S., et al. Antifungal activity of plant-derived essential oils on Candida tropicalis planktonic and biofilms cells. Medical Mycology. 2016;54(5):515–523. doi: 10.1093/mmy/myw003. [DOI] [PubMed] [Google Scholar]
- 34.Freire J. C. P., Júnior J. K. d. O., Silva D. d. F., Sousa J. P. d., Guerra F. Q. S., de Oliveira Lima E. Antifungal activity of essential oils against Candida albicans strains isolated from users of dental prostheses. Evidence-Based Complementary and Alternative Medicine. 2017;2017:9. doi: 10.1155/2017/7158756.7158756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dias I. J., Trajano E. R. I. S., Castro R. D., et al. Antifungal activity of linalool in cases of Candida spp. isolated from individuals with oral candidiasis. Brazilian Journal of Biology. 2018;78(2):368–374. doi: 10.1590/1519-6984.171054. [DOI] [PubMed] [Google Scholar]
- 36.Castro R. D., Souza T. M. P. A., Bezerra L. M. D., et al. Antifungal activity and mode of action of thymol and its synergism with nystatin against Candida species involved with infections in the oral cavity: an in vitro study. BMC Complementary Alternative Medicine. 2015;15(417):1–7. doi: 10.1186/s12906-015-0947-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sabzghabaee A. M., Shirdare Z., Ebadian B., Aslani A., Ghannadi A. Clinical evaluation of the essential oil of Pelargonium graveolens for the treatment of denture stomatitis. Dental Reseach Journal. 2011;8(1):105–108. doi: 10.4103/1735-3327.95922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Amanlou M., Beitollahi J. M., Abdollahzadeh S., Tohidast-Ekrad Z. Miconazole gel compared with Zataria multiflora Boiss. gel in the treatment of denture stomatitis. Phytotherapy Research. 2006;20(11):966–969. doi: 10.1002/ptr.1986. [DOI] [PubMed] [Google Scholar]
- 39.Vazquez J. A., Zawawi A. A. Efficacy of alcohol-based and alcohol-free melaleuca oral solution for the treatment of fluconazole refractory oropharyngeal candidiasis in patients with AIDS. HIV Clinical Trials. 2002;3(5):379–385. doi: 10.1310/99dy-8q52-306a-v0aj. [DOI] [PubMed] [Google Scholar]
- 40.Catalán A., Pacheco J. G., Martínez A., Mondaca M. A. In vitro and in vivo activity of Melaleuca alternifolia mixed with tissue conditioner on Candida albicans. Oral Surgery, Oral Medicine, Oral Pathology, Oral Radiology, and Endodontology. 2008;105(3):327–332. doi: 10.1016/j.tripleo.2007.08.025. [DOI] [PubMed] [Google Scholar]
- 41.Jandourek A., Vaishampayan J. K., Vazquez J. A. Efficacy of melaleuca oral solution for the treatment of fluconazole refractory oral candidiasis in AIDS patients. Aids. 1998;12(9):1033–1037. doi: 10.1097/00002030-199809000-00011. [DOI] [PubMed] [Google Scholar]
- 42.Anibal P. C., Sardi J. d. C. O., Peixoto I. T. A., Moraes J. J. d. C., Höfling J. F. Conventional and alternative antifungal therapies to oral candidiasis. Brazilian Journal of Microbiology. 2010;41(4):824–831. doi: 10.1590/s1517-83822010000400001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Vergis J., Gokulakrishnan P., Agarwal R. K., Kumar A. Essential oils as natural food antimicrobial agents: a review. Critical Reviews in Food Science and Nutrition. 2015;55(10):1320–1323. doi: 10.1080/10408398.2012.692127. [DOI] [PubMed] [Google Scholar]
- 44.Dagli N., Dagli R., Mahmoud R., Baroudi K. Essential oils, their therapeutic properties, and implication in dentistry: a review. Journal of International Society of Preventive and Community Dentistry. 2015;5(5):335–340. doi: 10.4103/2231-0762.165933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Alizadeh B., Royandazagh S. D., Khawar K. M., Ozcan S. Micropropagation of garlic chives (Allium tuberosum rottl. Ex spreng) using mesocotyl axis. Journal of Animal and Plant Sciences. 2013;23(2):543–549. [Google Scholar]
- 46.Lombardino J. G., Lowe J. A. The role of the medicinal chemist in drug discovery-then and now. Nature Reviews Drug Discovery. 2004;3(10):853–862. doi: 10.1038/nrd1523. [DOI] [PubMed] [Google Scholar]
- 47.Ika R. S., Erfan E. Antioxidant and hepatoprotective activity of garlic chives (Allium tuberosum) ethanolic extract on doxorubicin-induced liver injured rats. International Journal Pharma Medicine and Biological Science. 2017;6(1):20–23. doi: 10.18178/ijpmbs.6.1.20-23. [DOI] [Google Scholar]
- 48.Angayarkanni T., Dhivya R., Santny A. S. Phytochemical screening, antioxidant activity and antimicrobial activity of Allium tuberosum. World Journal Pharmaceutical Reseach. 2017;6(16):991–1004. doi: 10.20959/wjpr201716-10256. [DOI] [Google Scholar]
- 49.Kocevski D., Du M., Kan J., Jing C., Lacanin I., Pavlovic H. Antifungal effect of Allium tuberosum, Cinnamomum cassia, and Pogostemon cablin essential oils and their components against sporulation of Aspergillus species. Journal of Food Science. 2013;78(5):731–737. doi: 10.1111/1750-3841.12118. [DOI] [PubMed] [Google Scholar]
- 50.Yong-hong H., Zhen-chuan M., Bing-yan X. Chinese leek (Allium tuberosum Rottler ex Sprengel) reduced disease symptom caused by root-knot nematode. Journal of Integrative Agriculture. 2016;15(2):364–372. doi: 10.1016/S2095-3119(15)61032-2. [DOI] [Google Scholar]
- 51.Block E. Fifty years of smelling sulfur. Journal of Sulfur Chemistry. 2013;34(1-2):158–207. doi: 10.1080/17415993.2012.717294. [DOI] [Google Scholar]
- 52.Lopes D., Godoy R. L. O., Gonçalves S. L., Koketsu M., Oliveira A. M. Sulphur constituents of the essential oil of nira (Allium tuberosum Rottl.) cultivated in Brazil. Flavour and Fragrance Journal. 1997;12(4):237–239. doi: 10.1002/(sici)1099-1026(199707)12:4<237::aid-ffj644>3.0.co;2-9. [DOI] [Google Scholar]
- 53.Hu G., Sheng C., Mao R., Ma Z., Lu Y., Wei D. Essential oil composition of Allium tuberosum seed from China. Chemistry of Natural Compounds. 2013;48(6):1091–1093. doi: 10.1007/s10600-013-0476-5. [DOI] [Google Scholar]
- 54.Kumar S., Kumari R., Mishra S. Pharmacological properties and their medicinal uses of Cinnamomum: a review. Journal of Pharmacy and Pharmacology. 2019;71(12):1735–1761. doi: 10.1111/jphp.13173. [DOI] [PubMed] [Google Scholar]
- 55.Ng L.-T., Wu S.-J. Antiproliferative activity of Cinnamomum cassia constituents and effects of pifithrin-alpha on their apoptotic signaling pathways in Hep G2 cells. Evidence-Based Complementary and Alternative Medicine. 2011;2011:6. doi: 10.1093/ecam/nep220.492148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ooi L. S. M., Li Y., Kam S.-L., Wang H., Wong E. Y. L., Ooi V. E. C. Antimicrobial activities of cinnamon oil and cinnamaldehyde from the chinese medicinal herb Cinnamomum cassia Blume. The American Journal of Chinese Medicine. 2006;34(3):511–522. doi: 10.1142/s0192415x06004041. [DOI] [PubMed] [Google Scholar]
- 57.Taguchi Y., Takizawa T., Ishibashi H., et al. Therapeutic effects on murine oral candidiasis by oral administration of cassia (Cinnamomum cassia) preparation. Nippon Ishinkin Gakkai Zasshi. 2010;51(1):13–21. doi: 10.3314/jjmm.51.13. [DOI] [PubMed] [Google Scholar]
- 58.Ranasinghe P., Pigera S., Premakumara G. A. S., et al. Medicinal properties of “true” cinnamon (Cinnamomum zeylanicum): a systematic review. Evidence-Based Complementary and Alternative Medicine. 2013;13(275):1–10. doi: 10.1186/1472-6882-13-275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Chericoni S., Prieto J. M., Iacopini P., Cioni P., Morelli I. In vitro activity of the essential oil of Cinnamomum zeylanicum and eugenol in peroxynitrite-induced oxidative processes. Journal of Agricultural and Food Chemistry. 2005;53(12):4762–4765. doi: 10.1021/jf050183e. [DOI] [PubMed] [Google Scholar]
- 60.Jayaprakasha G. K., Rao L. J. M. Chemistry, biogenesis, and biological activities of Cinnamomum zeylanicum. Critical Reviews in Food Science and Nutrition. 2011;51(6):547–562. doi: 10.1080/10408391003699550. [DOI] [PubMed] [Google Scholar]
- 61.Chung I.-M., Ahmad A., Kim E.-H., et al. Immunotoxicity activity from the essential oils of coriander (Coriandrum sativum) seeds. Immunopharmacology and Immunotoxicology. 2012;34(3):499–503. doi: 10.3109/08923973.2011.637500. [DOI] [PubMed] [Google Scholar]
- 62.Galvão L. C. d. C., Furletti V. F., Bersan S. M. F., et al. Antimicrobial activity of essential oils against Streptococcus mutans and their antiproliferative effects. Evidence-Based Complementary and Alternative Medicine. 2012;2012:12. doi: 10.1155/2012/751435.751435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Harsha S. N., Anilakumar K. R. In vitro free radical scavenging and DNA damage protective property of Coriandrum sativum L. leaves extract. Journal of Food Science and Technology. 2012;51(8):1533–1539. doi: 10.1007/s13197-012-0648-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Leite M. C. A., Bezerra A. P. d. B., Sousa J. P. d., Guerra F. Q. S., Lima E. d. O. Evaluation of antifungal activity and mechanism of action of citral against Candida albicans. Evidence-Based Complementary and Alternative Medicine. 2014;2014:9. doi: 10.1155/2014/378280.378280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Licata M., Tuttolomondo T., Dugo G., et al. Study of quantitative and qualitative variations in essential oils of Sicilian oregano biotypes. Journal of Essential Oil Research. 2015;27(4):293–306. doi: 10.1080/10412905.2015.1045088. [DOI] [PubMed] [Google Scholar]
- 66.Mancini E., Senatore F., Del Monte D., et al. Studies on chemical composition, antimicrobial and antioxidant activities of five Thymus vulgaris L. essential oils. Molecules. 2015;20(7):12016–12028. doi: 10.3390/molecules200712016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Sarwar A., Latif Z. GC-MS characterisation and antibacterial activity evaluation of Nigella sativa oil against diverse strains of Salmonella. Natural Product Research. 2015;29(5):447–451. doi: 10.1080/14786419.2014.947493. [DOI] [PubMed] [Google Scholar]
- 68.Mendes S. S., Bomfim R. R., Jesus H. C. R., et al. Evaluation of the analgesic and anti-inflammatory effects of the essential oil of Lippia gracilis leaves. Journal of Ethnopharmacology. 2010;129(3):391–397. doi: 10.1016/j.jep.2010.04.005. [DOI] [PubMed] [Google Scholar]
- 69.Haeseler G., Maue D., Grosskreutz J., et al. Voltage-dependent block of neuronal and skeletal muscle sodium channels by thymol and menthol. European Journal of Anaesthesiology. 2002;19(8):571–579. doi: 10.1097/00003643-200208000-00005. [DOI] [PubMed] [Google Scholar]
- 70.Marchese A., Orhan I. E., Daglia M., et al. Antibacterial and antifungal activities of thymol: a brief review of the literature. Food Chemistry. 2016;210(1):402–414. doi: 10.1016/j.foodchem.2016.04.111. [DOI] [PubMed] [Google Scholar]
- 71.Marques A., Lima C., Alviano D., Alviano C., Esteves R., Kaplan M. Traditional use, chemical composition and antimicrobial activity of Pectis brevipedunculata essential oil: a correlated lemongrass species in Brazil. Emirates Journal of Food and Agriculture. 2013;25(10):798–808. doi: 10.9755/ejfa.v25i10.16408. [DOI] [Google Scholar]


