Abstract
Methods
Colitis was induced in mice using 2,4,6-trinitrobenzene-sulfonic acid (TNBS), and mice were subsequently treated with either a PD-1 inhibitor or 5-amino-salicylic acid (ASA) as a positive control. Body weight, disease activity index (DAI), colon length, and tissue damage were evaluated, and the enteric microbiota was profiled using high-throughput 16S rRNA sequencing of fecal samples from the experimental mice.
Results
TNBS caused mice to experience IBD-like symptoms, which were attenuated by the PD-1 inhibitor, as indicated by a decrease in DAI scores (p = 0.0002). Furthermore, in this mouse model of IBD, PD-1 inhibition improved the alpha diversity as well as restored the beta diversity of the enteric microbiome. It also significantly enriched the abundance of short-chain fatty acid- (SCFA-) producing bacteria of the Firmicutes (p < 0.05) and Bacteroidetes (p < 0.05) phyla but depopulated Proteobacteria (p < 0.05).
Conclusion
PD-1 inhibition can partly mitigate TNBS-induced colitis and restore the enteric microbiota by enriching the abundance of SCFA-producing bacteria.
1. Introduction
Inflammatory bowel disease (IBD) includes two chronic and nonspecific disease entities, ulcerative colitis and Crohn's disease [1], and clinically presents as abdominal pain, diarrhea, and bloody stool. Etiological studies have proposed genetic factors, a disproportionate immune response, an impaired mucoepithelial barrier, and dysbacteriosis as the putative pathogenic mechanisms for the development of IBD [2, 3].
Recent studies have suggested that aberrant immune activation against the enteric microbiota forms the pathogenic foundation of IBD [4, 5]. Experiments on enteric microbiota from IBD patients have revealed that a decreased abundance of the Firmicutes and Bacteroidetes phyla with an increased abundance of Proteobacteria is significantly correlated to the severity of IBD, and genera of the Firmicutes phylum, such as the Clostridium, Ruminococcus, Lachnospira, and Roseburia genera, as well as Faecalibacterium prausnitzii are reduced in IBD patients [6].
Immunocheckpoint blockade (ICB) has recently been popularized as a treatment for malignancies and other diseases. Clinically, not only does ICB possess the adverse effect of IBD-like enteritis itself, but during or after the course of ICB therapy, a significantly higher proportion of patients with IBD developed either common gastrointestinal adverse events (diarrhea, abdominal pain, etc.) compared to patients without IBD, or had a flare-up of their IBD symptoms [7–10], thus indicating the role of ICB in the progression of IBD.
However, there have not been many studies that have investigated the role of the PD-1/programmed death ligand 1 (PD-L1) axis in intestinal inflammation. Kanai et al. [11] found that PD-1 was highly expressed on T cells in the inflamed colon, while blockade of PD-L1 suppressed experimental colitis. In contrast, Scandiuzzi et al. [12] showed that PD-L1 expressed by the intestinal epithelium regulated intestinal inflammation by inhibiting innate immune cells. Moreover, Park et al. [13] suggested that the absence of PD-1 was protective against experimental colitis through the gut microbiota. Therefore, we aimed to investigate the pathogenic connection between the PD-1/PD-L1 pathway, the progression of IBD, and the enteric microbiota using a mouse model of 2,4,6-trinitrobenzene-sulfonic acid- (TNBS-) induced colitis.
2. Materials and Methods
2.1. Drugs and Reagents
The PD-1 inhibitor was purchased from TopAlliance Biosciences Inc. (Shanghai, China). TNBS and 5-amino-salicylic acid (ASA) were purchased from Sigma (St. Louis, MO, USA).
2.2. Experimental Animals, Induction of Colitis, and Treatment Regimen
Male BALB/c mice (23.1-28.4 g) were purchased from Guangdong Medical Laboratory Animal Center (GDMLAC; Certificate number SYXK 2013-0002, Foshan, China), and the experimental procedures were performed in accordance with the guidelines approved by the Animal Ethics Committee of GDMLAC. The protocols were approved by the Committee on the Ethics of Animal Experiments of GDMLAC. Mice were housed under a 12 h light/dark cycle with controlled temperature (24°C) and humidity (50-70%) and had access to food and water ad libitum.
All experimental mice in this study received a diet of SPF maintaining mice feed-1003 (provided by GDMLAC, production license No. (2019) 05073, executive standard: GB14924.3-2010). The ingredients included corn, soybean meal, flour, wheat flour, Peru fish meal, calcium hydrogen phosphate, stone powder, sodium chloride, vegetable oil, vitamins, amino acids, and minerals, and the food was sterilized via cobalt-60 irradiation.
The mice were acclimatized for seven days and randomly assigned to one of four groups: control (n = 12), TNBS (n = 10), TNBS+5-ASA (n = 12), and TNBS+PD-1 inhibitor (n = 12) groups (Supplementary Figure 1). Colitis was induced by rectal administration of TNBS as described by Morris et al. [14]. Briefly, the mice were fasted for 24 h, anesthetized with 0.4% pentobarbital sodium (0.1 mL/10 g) via intraperitoneal injection, and administered 25 mg TNBS per kg body weight in 50% ethanol. The mice in the control group were given an enema of only 50% ethanol. The TNBS-treated (0.1 mL/10 g) mice in the ASA or PD-1 treatment groups were, respectively, administered with either 5-ASA (150 mg/kg) through transgastric feeding or the PD-1 inhibitor (3 mg/kg) through intraperitoneal injections to avoid the acidic degradation of the PD-1 inhibitor in the stomach. The mice were monitored daily for weight loss, stool consistency, and the presence of blood on the anus or in the stool. Following the treatment regimen, stool samples were collected, and colonic tissues were resected after the mice were euthanized. The colon length was recorded, and the tissues were fixed overnight in 10% neutral buffered formalin.
2.3. Disease Activity Index
The disease activity index (DAI) was scored according to a modified version of a previously described method [15] and included the following parameters: (A) weight loss percentage (0: none, 1: 1-5%, 2: 5-10%, 3: 10-15%, and 4: >15%), (B) stool consistency (0: normal, 1: pasty and not sticking to the anus, 2: pasty and slightly sticking to the anus, 3: pasty and stuck to the anus, and 4: watery), and (C) rectal bleeding (0: hemoccult (-), 1: hemoccult (±), 2: hemoccult (+), 3: hemoccult (++), and 4: obvious blood in stool).
2.4. Histopathological Evaluation
The mice were euthanized under anesthesia, and the entire colon from cecum to anus was removed. The colon was opened longitudinally along the mesenteric border, and its contents were flushed with cold saline. The cleaned tissue samples were fixed in buffered formalin, embedded in paraffin, cut into 5 μm sections, and stained with hematoxylin and eosin (H&E). The slides were histopathologically evaluated by two investigators blinded to the conditions and graded with the following parameters: (A) inflammation (0: no infiltration of inflammatory cells, 1: infiltration in the lamina propria, 2: infiltration into the submucosa, and 3: transmural infiltration), (B) ulceration (0: no ulceration, 1: one or two ulcers, 2: three or four ulcers, and 3: more than four ulcers), (C) mucosal hyperplasia (0: normal, 1: slightly thickened mucosa with minimal fibrosis, 2: mucosal thickening with fibrous hyperplasia, and 3: extensive mucosal thickening and fibrous hyperplasia or granulation), and (D) edema (0: none, 1: 0-30%, 2: 30-70%, and 3: >70%).
2.5. Fecal Sample Collection and Extraction of Genomic DNA
Approximately two or three pellets of fresh feces per mouse were collected in sterile plastic tubes and stored at -80°C. Total genomic DNA was extracted using the QIAamp DNA Stool Mini Kit (Qiagen, Dusseldorf, Germany) and quantified at 260 nm with the NanoDrop 2000 BioAnalyzer (Thermo Fisher Scientific, Inc., Waltham, MA, USA).
2.6. PCR Amplification and Illumina Sequencing
Bar-coded V4-515 forward 5′-GTGCCAGCMGCCGCGGTAA-3′ and V4-806 reverse 5′-GGACTACHVGGGTWTCTAAT-3′ primers were used to amplify the bacterial 16S rRNA V4 fragments. The Phusion High-Fidelity PCR Master Mix (New England Biolabs, Beverly, MA, USA) was used, and amplified products 400-450 bp in length were purified. Sequencing libraries were generated using the TruSeq DNA PCR-Free Sample Preparation Kit (Illumina, San Diego, CA, USA) according to the manufacturer's recommendations, and index codes were added. The library quality was assessed on the Qubit@ 2.0 Fluorometer (Thermo Scientific, Carlsbad, CA, USA) and Agilent Bioanalyzer 2100 system and sequenced using the Illumina HiSeq 2500 platform (Tianjin Novogene Bioinformatics Technology Co., Ltd.).
2.7. Data Analysis
SPSS statistical software was used to perform all analyses, and the data were expressed as the mean ± standard deviation (SD). t-tests and Wilcoxon signed-rank tests were used to compare two groups when appropriate, and one-way analysis of variance (ANOVA) with Tukey's tests was used for comparisons of multiple groups. A p value less than 0.05 was considered statistically significant.
3. Results
3.1. Intestinal Inflammation in TNBS-Induced Colitis Is Partially Alleviated by Blocking PD-1/PD-L1
For generating the experimental models, 10 mice were treated by rectal administration of TNBS, all of which showed significant weight loss and higher DAI scores associated with diarrhea, rectal bleeding, and shorter colon lengths compared to the control mice, indicating the successful establishment of colitis as a mouse model of IBD, with a success rate of 100% (1 of 10 died on the seventh day due to severe diarrhea and rectal bleeding).
Administration of either the PD-1 inhibitor or 5-ASA (as positive control) prevented the mice with IBD-like colitis from weight loss (Figure 1(a)) and improved their DAI scores (p = 0.0002) compared to the untreated mice with colitis (Figure 1(b)). In addition, treatment with the PD-1 inhibitor mitigated inflammation along the intestinal mucosa in mice with TNBS-induced colitis as indicated by a decrease in cell infiltration and reorganization of the intestinal villi structure (Figures 2(b) and 2(c)).
Figure 1.
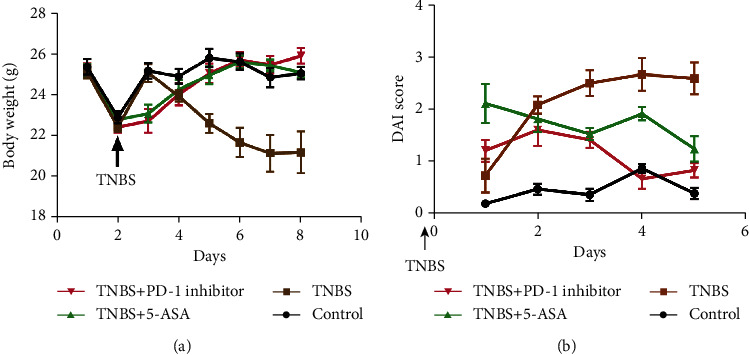
Inhibition of programmed cell death protein 1 (PD-1) improved the physiological indices in mice with TNBS colitis. (a) Body weight and (b) disease activity index (DAI) in the various treatment groups. Data is expressed as the mean ± standard deviation (SD), ∗p < 0.05.
Figure 2.
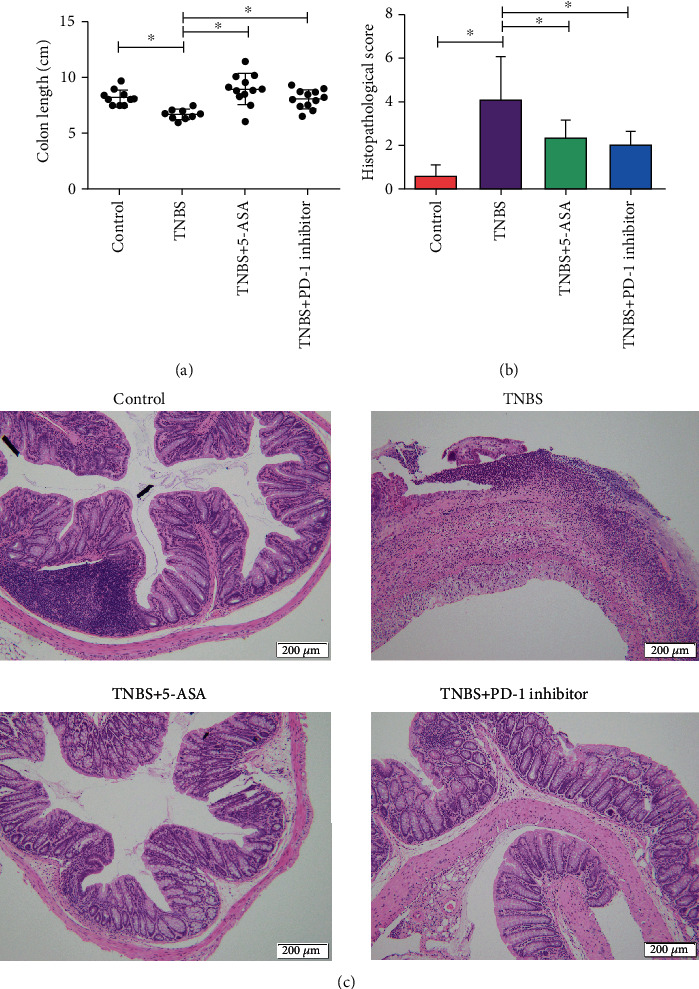
Inhibition of programmed cell death protein 1 (PD-1) restored macroscopic and histopathological damage caused by TNBS-induced colitis. Representative pictures showing the (a) colon length and (b, c) histopathological appearance of colonic tissue (scale bar = 200 μm). Data are expressed as the mean ± standard deviation (SD), ∗p < 0.05.
3.2. PD-1 Inhibition Increases Enteric Microbiota Diversity in Mice with Colitis
The Shannon index was used to estimate the alpha diversity, a measure of taxa richness, and evenness within a sample. Both the PD-1 inhibitor and 5-ASA increased the low alpha diversity after TNBS-induced colitis (Figure 3(a)). In addition, principal component analysis (PCA) was used to evaluate the beta diversity by comparing the similarity in microbial communities between samples. The PD-1 inhibitor reversed the changes in the enteric microbiota caused by TNBS (Figure 3(b)).
Figure 3.
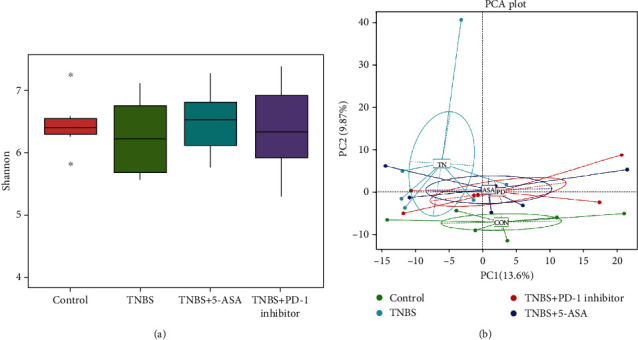
Inhibition of PD-1 increased the alpha diversity and restored the beta diversity of the gut microbiota in mice with TBNS colitis. (a) Shannon index and (b) principal component analysis (PCA). Each dot represents one fecal sample.
3.3. PD-1 Inhibitor Restores the Gut Microbiota and Increases the Abundance of Short-Chain Fatty Acid-Producing Bacteria
The relationship between gut microbial composition and IBD has been widely investigated, and IBD patients show a significant decline in the diversity of their intestinal microbiome along with a decrease in the abundance of Firmicutes and an increase in the abundance of Proteobacteria, which are designated as signatures of IBD [4].
The heat maps for the predictive operational taxonomic units (OTUs) were plotted according to the species annotations and abundance at the phylum and genus levels, and a cluster analysis was performed (Figure 4). These results suggested that the inhibition of PD-1 increased the abundance of Butyricicoccus, Ruminiclostridium, Ruminiclostridium 9, Oscillibacter, Ruminiclostridb-5, Rikenella, Roseburia, Anaerotruncus, Lachnospiraceae bacterium NK4A136, and an unidentified Lachnospiraceae but decreased that of Acinetobacter, Bacteroides, Parabacteroides, and Alloprevotella. Metastats was used to analyze the relative abundance of bacteria in each group at different levels. Inhibition of PD-1 increased the abundance of Deferribacteres, Clostridium_sensu_stricto_1, Empedobacter, and Mucispirillum and decreased that of Rhizobiales, Candidatus_Arthromitus, and Turicibacter (Figure 5). As shown in the network map in Figure 6, the mice in the control groups possessed Firmicutes, Bacteroidetes, and Proteobacteria as the core genera, while TNBS-induced colitis resulted in the enrichment of Actinobacteria and Cyanobacteria. After treatment with the PD-1 inhibitor, the relative abundance of Firmicutes and Bacteroidetes was restored in the mice with TNBS-induced colitis to levels comparable to the control groups. Taken together, inhibiting PD-1/PD-L1 signaling restored the enteric microbiota dysbiosis in mice with colitis by enriching the abundance of SCFA-producing bacteria as well as mucosal immune-related bacteria.
Figure 4.
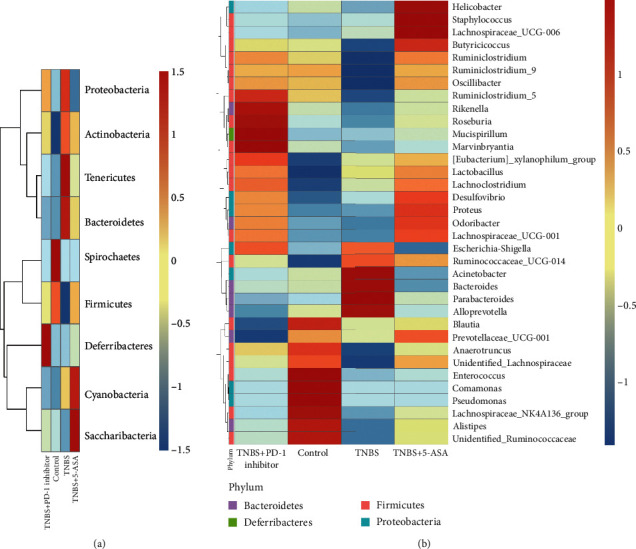
Cluster heat map analysis of the (a) top nine predictive operational taxonomic units (OTUs) at the phylum level and (b) top 35 predictive OTUs at the genus level. Blue indicates a negative correlation, and red indicates a positive correlation. The X-axis indicates the sample information, and the Y-axis contains the annotation information for the species. The tree on the left indicates species clustering.
Figure 5.
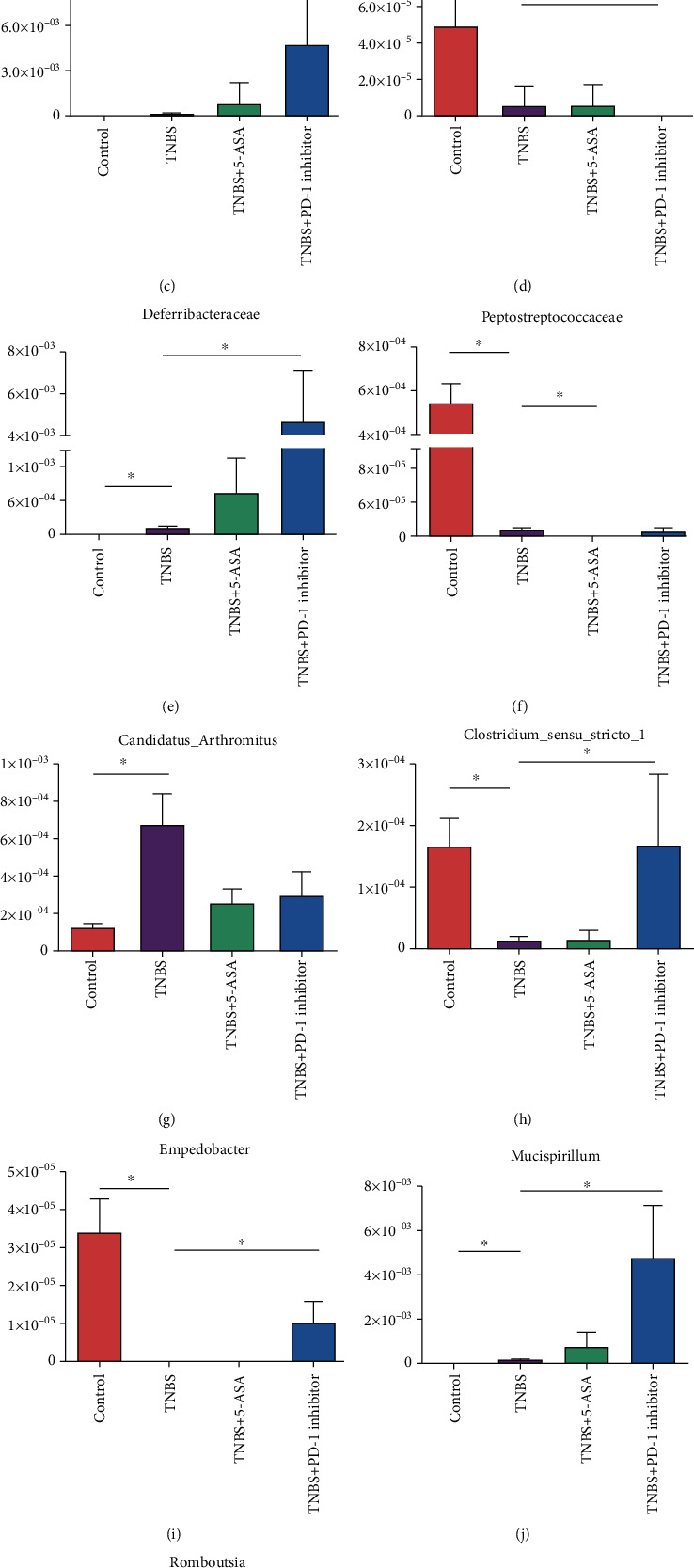
Differential bacterial communities identified by Metastats at the level of (a) phylum, (b) class, (c, d) order, (e, f) family, and (g–l) genus; ∗p < 0.05.
Figure 6.
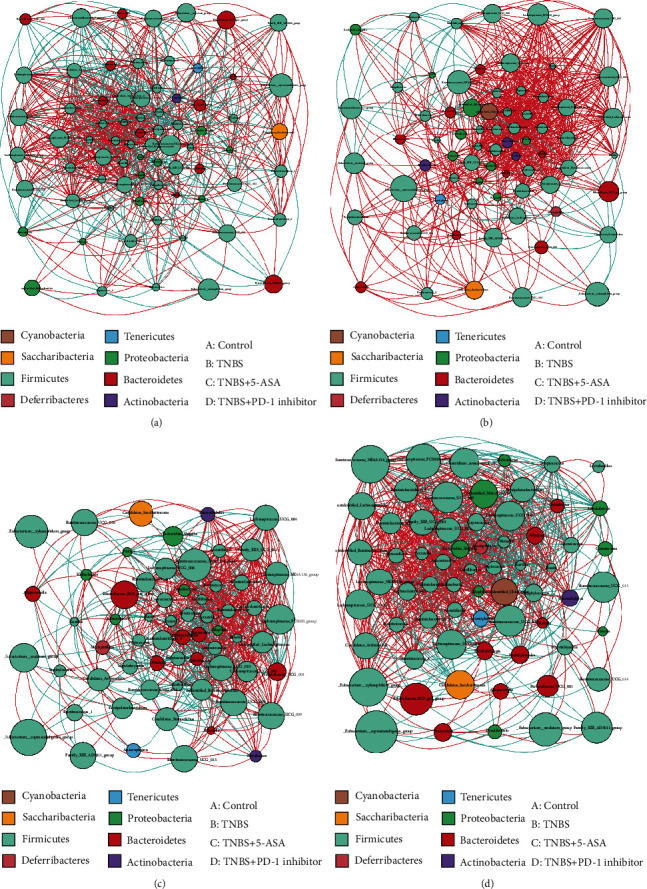
Network maps showing the interactions of the operational taxonomic units (OTUs) among all the samples at the genus level. Each point represents one genus: (a) control group; (b) TNBS group; (c) TNBS+5-ASA group; (d) TNBS+PD-1 inhibitor group.
4. Discussion
The pathogenesis of IBD in genetically susceptible hosts has been proposed to begin with a breakdown of the intestinal epithelial barrier, followed by a disproportionate immune response to the enteric microbiota, which results in a loss of intestinal homeostasis [16]. Such dysbiosis in the intestinal symbionts of IBD patients can be indicated by the low-level alpha diversity [17] and manifests as a decrease and an increase in the abundance of Firmicutes and Proteobacteria, respectively [18]. A study of the enteric microbiota in gnotobiotic mice under an inflammatory microenvironment [19] revealed that the enteric microbiota potentiates the formation and maturation of CD4+ T cells, and the severely damaged intestinal mucoepithelial barrier in IBD patients exposes the gut microbial antigens to the immune cells, which elicit an excessive immune response. Furthermore, various metabolites of the intestinal bacteria, such as SCFAs, can increase the number of immunosuppressive Treg cells in the laminae propria mucosae [20, 21] and promote mucin secretion as well as strengthen the epithelial barrier. Studies show that maintaining a minimal concentration of SCFAs in the intestine is beneficial to the repair and regeneration of the mucosal barrier, whereas chronic lack of SCFAs can thin the intestinal mucosa and weaken the integrity of the intestinal mucosal barrier, resulting in the translocation of pathogenic bacteria and increased exposure to antigenic substances [22–26].
Since the PD-1/PD-L1 pathway regulates T cell activation and immune tolerance and has been a potent therapeutic target in autoimmune diseases [27], recent studies began to focus on the role of the PD-1/PD-L1 signaling axis in the pathogenesis of IBD and possible novel therapeutic strategies. However, very few studies made significant progress. We used a common murine model of chronic IBD that involved rectal administration of TNBS [14, 28, 29], and we administered a PD-1 inhibitor, or 5-ASA as a positive control, to determine the pathogenic association of the PD-1/PD-L1 pathway with IBD. Our findings revealed that inhibition of PD-1 can significantly improve the physiological status of mice with colitis based on the evaluation of body weight along with DAI scores, and it also reduced both macroscopic and microscopic colonic lesions.
Past studies have focused more on the immunological regulation of the PD-1/PD-L1 pathway. However, Kawamoto et al. [30] investigated the effect of the PD-1/PD-L1 pathway on the enteric microbiota and reported a significant decrease in the relative abundance of P. aeruginosa and Bifidobacteria in the intestinal microbiota of PD-1−/− mice, whereas Vetizou et al. [31] were followed to show that the introduction of Bacteroides fragilis alleviated colitis caused by cytotoxic T-lymphocyte-associated protein 4 (CTLA-4) inhibition. Based on the knowledge from these previous studies and with our experimental model, we then analyzed the diversity and abundance of the enteric microbiota in the different experimental groups and upheld that PD-1 inhibition increased microbial diversity and altered the abundance of various phyla and genera, especially bacteria that produced SCFAs.
Lachnospiraceae, Rikenellaceae, Ruminococcaceae, Butyricicoccus, and Roseburia are important SCFA-producing intestinal symbiotic bacteria, which ferment dietary fiber into acetic acid, propionic acid, and butyric acid [32]. More specifically, Eeckhaut et al. [33] revealed a lower abundance of Butyricicoccus in IBD patients, while the introduction of Butyricicoccus in mice with TNBS-induced colitis reduced levels of proinflammatory factors including myeloperoxidase (MPO), tumor necrosis factor alpha (TNF-α), and interleukin- (IL-) 12 and improved intestinal mucosal barrier function. Several other studies [34–37] have also reported that IBD patients possessed a lower abundance of Lachnospiraceae, a bacteria that can protect the intestinal tract through the production of butyrate and decolonize C. difficile during pseudomembranous colitis. More importantly, cancer patients with a higher abundance of Rikenellaceae undergoing anti-CTL4 therapy were less likely to suffer from CTLA-4-associated enteritis [31]. The abundance of the butyrate-producing actinomycete Roseburia [38] is decreased in ulcerative colitis patients, and another butyrate-producing bacteria, P. sphaeroides, is associated with disease activity in patients with Crohn's disease [39, 40]. Ruminococcaceae and Lachnospiraceae, the two most abundant bacteria of the Clostridium spp, decompose various fibrous polysaccharides and are associated with the PD-1/PD-L1 pathway [41]. The SCFA-producing bacteria also promote protein synthesis that can affect the intestinal immune response [42]. Schaffler et al. [43] found that an increased abundance of Anaerotruncus enhanced the therapeutic effect against Crohn's disease. Taken together, SCFA-producing enteric bacteria not only manufacture SCFAs as the main source of energy for the intestinal microbes but also help to maintain intestinal microbial homeostasis by inhibiting the growth of pathogenic bacteria, promoting colonic mucus secretion, and enhancing mucosal barrier function [44]. Butyrate enemas have been used to manage mucosal inflammation in IBD, highlighting the therapeutic potential of SCFAs for the treatment of IBD [45, 46].
An alteration in the abundance of certain enteric microbes has been associated with intestinal mucosal immune regulation. For example, Bacteroides acidifaciens induces the formation of germinal centers and regulates immunoglobulin A (IgA) and IgB-producing plasma cells [47], while Clostridium leptum induces immunotolerant dendritic cells and Treg cells [48–50]. IgA is the major antibody on the surface of the intestinal mucosa which can penetrate the intestinal lumen via the multi-immunoglobulin receptor (pIgR) present on intestinal epithelial cells to mediate intestinal mucosal immunity [51, 52]. Other enteric microbes such as Mucispirillum and Candidatus_Arthromitus also possess a strong correlation with the production and secretion of T cell-dependent IgA [53, 54]. Importantly, the abundance of Mucispirillum increased significantly after inhibition of PD-1, and therefore, it may serve as a potential marker for the inhibition of this pathway [55, 56].
Although the cross-talk between the PD-1/PD-L1 pathway, gut microbiota, and intestinal mucosal immunity has been investigated in detail [57, 58], the exact involvement of the PD-1/PD-L1 axis in the pathogenesis of IBD remains elusive. PD-1 inhibitors significantly improved the outcome of TNBS-induced colitis by restoring the intestinal microbiome. However, the dosage, withdrawal criteria, and other immunological effects of PD-1 inhibition require additional studies.
5. Conclusion
PD-1 inhibition can partly alleviate TNBS-induced colitis and restore the gut microbiota by increasing the abundance of SCFA-producing bacteria, but the exact effects and mechanisms require additional study.
Acknowledgments
The study was funded by the National Natural Science Foundation of China (Nos. 81700487 and 81871905), Natural Science Foundation of Guangdong Province (No. 2018A030310672), China Postdoctoral Science Foundation (2019M652978), Guangdong Medical Science and Technology Research Fund (A2019243), and Innovative Clinical Technique of Guangzhou (2019GX05).
Contributor Information
Yong-jian Zhou, Email: eyzhouyongjian@scut.edu.cn.
Yu-qiang Nie, Email: eynieyuqiang@scut.edu.cn.
Data Availability
The dataset used and analyzed during the current study is available from the corresponding authors on reasonable request.
Ethical Approval
The study was approved by Guangdong Medical Laboratory Animal Center (GDMLAC; Certificate number SYXK 2013-0002). All subsequent studies were performed in accordance with the guidelines approved by the Animal Ethics Committee of GDMLAC. The protocols were approved by the Committee on the Ethics of Animal Experiments of GDMLAC.
Disclosure
An earlier version of this research was presented as a conference abstract in “AGA Abstracts” (https://www.gastrojournal.org/article/S0016-5085(19)39773-2/).
Conflicts of Interest
The authors declare that they have no competing interests.
Authors' Contributions
Xu HM and Zhou YL designed the study and drafted the article; Xu J performed statistical analysis and interpretation of the data; Li YF and Zhao C participated in animal experiments and recorded the general status of experimental animals; Huang HL performed sample collection and DNA extraction; Du YL and He J interpreted the data and revised the article; Zhou YJ and Nie YQ designed and organized the study, interpreted the data, and revised the article. H.M.X., Y.L.Z., and J.X. contributed equally to this article.
Supplementary Materials
Supplementary Figure 1: process of TNBS-induced colitis model and solvent control. Preparation of TNBS ethanol solution: in 10 mL of 50 mg/mL TNBS solution, add 10 mL absolute ethanol and mix well. Preparation of 50% ethanol solution: in 2 mL of absolute ethanol, add 2 mL sterile water for injection and mix well. Step 1 (fasting): all mice were fasted for 24 hours. Step 2 (anesthesia): mice were anesthetized by intraperitoneal injection of pentobarbital sodium at the dose of 40 mg/kg and 0.1 mL/10 g body weight. Step 3 (enema): 25 mg/L TNBS ethanol solution (model group)/50% ethanol solution (control group) was aspirated by syringe. The soft rubber tube connected with the syringe was gently inserted approximately 3 cm into the mouse through the anus, and the contents of the syringe were slowly injected into the intestinal cavity of mice. Step 4 (inversion): after administration, the plastic tube was slowly removed and the tail of the mouse was lifted. The mouse was kept inverted for 1 min.
References
- 1.Lozupone C. A., Stombaugh J. I., Gordon J. I., Jansson J. K., Knight R. Diversity, stability and resilience of the human gut microbiota. Nature. 2012;489(7415):220–230. doi: 10.1038/nature11550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sheehan D., Moran C., Shanahan F. The microbiota in inflammatory bowel disease. Journal of Gastroenterology. 2015;50(5):495–507. doi: 10.1007/s00535-015-1064-1. [DOI] [PubMed] [Google Scholar]
- 3.Larsen O., Claassen E. The mechanistic link between health and gut microbiota diversity. Scientific reports. 2018;8(1, article 2183) doi: 10.1038/s41598-018-20141-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Matsuoka K., Kanai T. The gut microbiota and inflammatory bowel disease. Seminars in Immunopathology. 2015;37(1):47–55. doi: 10.1007/s00281-014-0454-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Maloy K. J., Powrie F. Intestinal homeostasis and its breakdown in inflammatory bowel disease. Nature. 2011;474(7351):298–306. doi: 10.1038/nature10208. [DOI] [PubMed] [Google Scholar]
- 6.Nagao-Kitamoto H., Kamada N. Host-microbial cross-talk in inflammatory bowel disease. Immune Network. 2017;17(1):1–12. doi: 10.4110/in.2017.17.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gopalakrishnan V., Spencer C. N., Nezi L., et al. Gut microbiome modulates response to anti-PD-1 immunotherapy in melanoma patients. Science. 2018;359(6371):97–103. doi: 10.1126/science.aan4236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Routy B., Le Chatelier E., Derosa L., et al. Gut microbiome influences efficacy of PD-1-based immunotherapy against epithelial tumors. Science. 2018;359(6371):91–97. doi: 10.1126/science.aan3706. [DOI] [PubMed] [Google Scholar]
- 9.Sivan A., Corrales L., Hubert N., et al. Commensal Bifidobacterium promotes antitumor immunity and facilitates anti-PD-L1 efficacy. Science. 2015;350(6264):1084–1089. doi: 10.1126/science.aac4255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Matson V., Fessler J., Bao R., et al. The commensal microbiome is associated with anti–PD-1 efficacy in metastatic melanoma patients. Science. 2018;359(6371):p. 104. doi: 10.1126/science.aao3290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kanai T., Totsuka T., Uraushihara K., et al. Blockade of B7-H1 suppresses the development of chronic intestinal inflammation. Journal of Immunology. 2003;171(8):4156–4163. doi: 10.4049/jimmunol.171.8.4156. [DOI] [PubMed] [Google Scholar]
- 12.Scandiuzzi L., Ghosh K., Hofmeyer K. A., et al. Tissue-expressed B7-H1 critically controls intestinal inflammation. Cell Reports. 2014;6(4):625–632. doi: 10.1016/j.celrep.2014.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Park S. J., Kim J., Song M., Sung Y. C., Lee S., Park Y. PD-1 deficiency protects experimental colitis via alteration of gut microbiota. BMB Reports. 2017;50(11):578–583. doi: 10.5483/BMBRep.2017.50.11.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Morris G. P., Beck P. L., Herridge M. S., Depew W. T., Szewczuk M. R., Wallace J. L. Hapten-induced model of chronic inflammation and ulceration in the rat colon. Gastroenterology. 1989;96(3):795–803. doi: 10.1016/0016-5085(89)90904-9. [DOI] [PubMed] [Google Scholar]
- 15.Murthy S. N., Cooper H. S., Shim H., Shah R. S., Ibrahim S. A., Sedergran D. J. Treatment of dextran sulfate sodium-induced murine colitis by intracolonic cyclosporin. Digestive Diseases and Sciences. 1993;38(9):1722–1734. doi: 10.1007/BF01303184. [DOI] [PubMed] [Google Scholar]
- 16.Peloquin J. M., Nguyen D. D. The microbiota and inflammatory bowel disease: insights from animal models. Anaerobe. 2013;24:102–106. doi: 10.1016/j.anaerobe.2013.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Manichanh C., Rigottier-Gois L., Bonnaud E., et al. Reduced diversity of faecal microbiota in Crohn's disease revealed by a metagenomic approach. Gut. 2006;55(2):205–211. doi: 10.1136/gut.2005.073817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Frank D. N., Robertson C. E., Hamm C. M., et al. Disease phenotype and genotype are associated with shifts in intestinal-associated microbiota in inflammatory bowel diseases. Inflammatory Bowel Diseases. 2011;17(1):179–184. doi: 10.1002/ibd.21339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tlaskalová-Hogenová H., Štěpánková R., Kozáková H., et al. The role of gut microbiota (commensal bacteria) and the mucosal barrier in the pathogenesis of inflammatory and autoimmune diseases and cancer: contribution of germ-free and gnotobiotic animal models of human diseases. Cellular & Molecular Immunology. 2011;8(2):110–120. doi: 10.1038/cmi.2010.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ince M. N., Blazar B. R., Edmond M. B., Tricot G., Wannemuehler M. J. Understanding luminal microorganisms and their potential effectiveness in treating intestinal inflammation. Inflammatory Bowel Diseases. 2016;22(1):194–201. doi: 10.1097/MIB.0000000000000599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lathrop S. K., Bloom S. M., Rao S. M., et al. Peripheral education of the immune system by colonic commensal microbiota. Nature. 2011;478(7368):250–254. doi: 10.1038/nature10434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Han X., Song H., Wang Y., Sheng Y., Chen J. Sodium butyrate protects the intestinal barrier function in peritonitic mice. International Journal of Clinical and Experimental Medicine. 2015;8(3):4000–4007. [PMC free article] [PubMed] [Google Scholar]
- 23.Kelly C. J., Zheng L., Campbell E. L., et al. Crosstalk between microbiota-derived short-chain fatty acids and intestinal epithelial HIF augments tissue barrier function. Cell Host & Microbe. 2015;17(5):662–671. doi: 10.1016/j.chom.2015.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Macia L., Tan J., Vieira A. T., et al. Metabolite-sensing receptors GPR43 and GPR109A facilitate dietary fibre-induced gut homeostasis through regulation of the inflammasome. Nature Communications. 2015;6(1):p. 6734. doi: 10.1038/ncomms7734. [DOI] [PubMed] [Google Scholar]
- 25.Velasquez-Manoff M. Gut microbiome: the peacekeepers. Nature. 2015;518(7540):S3–S5. doi: 10.1038/518S3a. [DOI] [PubMed] [Google Scholar]
- 26.Macia L., Thorburn A. N., Binge L. C., et al. Microbial influences on epithelial integrity and immune function as a basis for inflammatory diseases. Immunological Reviews. 2012;245(1):164–176. doi: 10.1111/j.1600-065X.2011.01080.x. [DOI] [PubMed] [Google Scholar]
- 27.Francisco L. M., Sage P. T., Sharpe A. H. The PD-1 pathway in tolerance and autoimmunity. Immunological Reviews. 2010;236(1):219–242. doi: 10.1111/j.1600-065X.2010.00923.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wirtz S., Neufert C., Weigmann B., Neurath M. F. Chemically induced mouse models of intestinal inflammation. Nature Protocols. 2007;2(3):541–546. doi: 10.1038/nprot.2007.41. [DOI] [PubMed] [Google Scholar]
- 29.Fichtner-Feigl S., Young C. A., Kitani A., Geissler E. K., Schlitt H. J., Strober W. IL-13 signaling via IL-13R alpha2 induces major downstream fibrogenic factors mediating fibrosis in chronic TNBS colitis. Gastroenterology. 2008;135(6):2003–2013. doi: 10.1053/j.gastro.2008.08.055. [DOI] [PubMed] [Google Scholar]
- 30.Kawamoto S., Tran T. H., Maruya M., et al. The inhibitory receptor PD-1 regulates IgA selection and bacterial composition in the gut. Science. 2012;336(6080):485–489. doi: 10.1126/science.1217718. [DOI] [PubMed] [Google Scholar]
- 31.Vetizou M., Pitt J. M., Daillere R., et al. Anticancer immunotherapy by CTLA-4 blockade relies on the gut microbiota. Science. 2015;350(6264):1079–1084. doi: 10.1126/science.aad1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tao J. H., Duan J. A., Jiang S., Guo J. M., Qian Y. Y., Qian D. W. Simultaneous determination of six short-chain fatty acids in colonic contents of colitis mice after oral administration of polysaccharides from Chrysanthemum morifolium Ramat by gas chromatography with flame ionization detector. Journal of Chromatography. B, Analytical Technologies in the Biomedical and Life Sciences. 2016;1029-1030:88–94. doi: 10.1016/j.jchromb.2016.07.002. [DOI] [PubMed] [Google Scholar]
- 33.Eeckhaut V., Machiels K., Perrier C., et al. Butyricicoccus pullicaecorum in inflammatory bowel disease. Gut. 2013;62(12):1745–1752. doi: 10.1136/gutjnl-2012-303611. [DOI] [PubMed] [Google Scholar]
- 34.Meehan C. J., Beiko R. G. A phylogenomic view of ecological specialization in the Lachnospiraceae, a family of digestive tract-associated bacteria. Genome Biology and Evolution. 2014;6(3):703–713. doi: 10.1093/gbe/evu050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Reeves A. E., Koenigsknecht M. J., Bergin I. L., Young V. B. Suppression of Clostridium difficile in the gastrointestinal tracts of germfree mice inoculated with a murine isolate from the family Lachnospiraceae. Infection and Immunity. 2012;80(11):3786–3794. doi: 10.1128/IAI.00647-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Louis P., Flint H. J. Diversity, metabolism and microbial ecology of butyrate-producing bacteria from the human large intestine. FEMS Microbiology Letters. 2009;294(1):1–8. doi: 10.1111/j.1574-6968.2009.01514.x. [DOI] [PubMed] [Google Scholar]
- 37.Yilmaz B., Juillerat P., Øyås O., et al. Microbial network disturbances in relapsing refractory Crohn’s disease. Nature Medicine. 2019;25(2):323–336. doi: 10.1038/s41591-018-0308-z. [DOI] [PubMed] [Google Scholar]
- 38.Machiels K., Joossens M., Sabino J., et al. A decrease of the butyrate-producing species Roseburia hominis and Faecalibacterium prausnitzii defines dysbiosis in patients with ulcerative colitis. Gut. 2014;63(8):1275–1283. doi: 10.1136/gutjnl-2013-304833. [DOI] [PubMed] [Google Scholar]
- 39.Shen Z., Zhu C., Quan Y., et al. Update on intestinal microbiota in Crohn's disease 2017: mechanisms, clinical application, adverse reactions, and outlook. Journal of Gastroenterology and Hepatology. 2017;32(11):1804–1812. doi: 10.1111/jgh.13861. [DOI] [PubMed] [Google Scholar]
- 40.Pryde S. E., Duncan S. H., Hold G. L., Stewart C. S., Flint H. J. The microbiology of butyrate formation in the human colon. FEMS Microbiology Letters. 2002;217(2):133–139. doi: 10.1111/j.1574-6968.2002.tb11467.x. [DOI] [PubMed] [Google Scholar]
- 41.Vetizou M., Trinchieri G. Anti-PD1 in the wonder-gut-land. Cell Research. 2018;28(3):263–264. doi: 10.1038/cr.2018.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sagheddu V., Patrone V., Miragoli F., Puglisi E., Morelli L. Infant early gut colonization by Lachnospiraceae: high frequency of Ruminococcus gnavus. Frontiers in Pediatrics. 2016;4:p. 57. doi: 10.3389/fped.2016.00057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Schäffler H., Herlemann D. P., Klinitzke P., et al. Vitamin D administration leads to a shift of the intestinal bacterial composition in Crohn's disease patients, but not in healthy controls. Journal of digestive diseases. 2018;19(4):225–234. doi: 10.1111/1751-2980.12591. [DOI] [PubMed] [Google Scholar]
- 44.Barcelo A., Claustre J., Moro F., Chayvialle J. A., Cuber J. C., Plaisancie P. Mucin secretion is modulated by luminal factors in the isolated vascularly perfused rat colon. Gut. 2000;46(2):218–224. doi: 10.1136/gut.46.2.218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Geirnaert A., Calatayud M., Grootaert C., et al. Butyrate-producing bacteria supplemented in vitro to Crohn's disease patient microbiota increased butyrate production and enhanced intestinal epithelial barrier integrity. Scientific reports. 2017;7(1, article 11450) doi: 10.1038/s41598-017-11734-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Martin-Gallausiaux C., Béguet-Crespel F., Marinelli L., et al. Butyrate produced by gut commensal bacteria activates TGF-beta1 expression through the transcription factor SP1 in human intestinal epithelial cells. Scientific reports. 2018;8(1, article 9742) doi: 10.1038/s41598-018-28048-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yanagibashi T., Hosono A., Oyama A., et al. IgA production in the large intestine is modulated by a different mechanism than in the small intestine: Bacteroides acidifaciens promotes IgA production in the large intestine by inducing germinal center formation and increasing the number of IgA+ B cells. Immunobiology. 2013;218(4):645–651. doi: 10.1016/j.imbio.2012.07.033. [DOI] [PubMed] [Google Scholar]
- 48.Rehman A., Rausch P., Wang J., et al. Geographical patterns of the standing and active human gut microbiome in health and IBD. Gut. 2016;65(2):238–248. doi: 10.1136/gutjnl-2014-308341. [DOI] [PubMed] [Google Scholar]
- 49.Li Y. N., Huang F., Cheng H. J., Li S. Y., Liu L., Wang L. Y. Intestine-derived Clostridium leptum induces murine tolerogenic dendritic cells and regulatory T cells in vitro. Human Immunology. 2014;75(12):1232–1238. doi: 10.1016/j.humimm.2014.09.017. [DOI] [PubMed] [Google Scholar]
- 50.Li Y. N., Huang F., Liu L., Qiao H. M., Li Y., Cheng H. J. Effect of oral feeding with Clostridium leptum on regulatory T-cell responses and allergic airway inflammation in mice. Annals of Allergy, Asthma & Immunology. 2012;109(3):201–207. doi: 10.1016/j.anai.2012.06.017. [DOI] [PubMed] [Google Scholar]
- 51.McCoy K. D., Ronchi F., Geuking M. B. Host-microbiota interactions and adaptive immunity. Immunological Reviews. 2017;279(1):63–69. doi: 10.1111/imr.12575. [DOI] [PubMed] [Google Scholar]
- 52.Clavel T., Gomes-Neto J. C., Lagkouvardos I., Ramer-Tait A. E. Deciphering interactions between the gut microbiota and the immune system via microbial cultivation and minimal microbiomes. Immunological Reviews. 2017;279(1):8–22. doi: 10.1111/imr.12578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bunker J. J., Flynn T. M., Koval J. C., et al. Innate and adaptive humoral responses coat distinct commensal bacteria with immunoglobulin a. Immunity. 2015;43(3):541–553. doi: 10.1016/j.immuni.2015.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Atarashi K., Tanoue T., Ando M., et al. Th17 cell induction by adhesion of microbes to intestinal epithelial cells. Cell. 2015;163(2):367–380. doi: 10.1016/j.cell.2015.08.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yin X., Heeney D., Srisengfa Y., Golomb B., Griffey S., Marco M. Bacteriocin biosynthesis contributes to the anti-inflammatory capacities of probiotic Lactobacillus plantarum. Beneficial Microbes. 2018;9(2):333–344. doi: 10.3920/BM2017.0096. [DOI] [PubMed] [Google Scholar]
- 56.Robertson B. R., O'Rourke J. L., Neilan B. A., et al. Mucispirillum schaedleri gen. nov., sp. nov., a spiral-shaped bacterium colonizing the mucus layer of the gastrointestinal tract of laboratory rodents. International Journal of Systematic and Evolutionary Microbiology. 2005;55(3):1199–1204. doi: 10.1099/ijs.0.63472-0. [DOI] [PubMed] [Google Scholar]
- 57.Botticelli A., Zizzari I., Mazzuca F., et al. Cross-talk between microbiota and immune fitness to steer and control response to anti PD-1/PDL-1 treatment. Oncotarget. 2017;8(5):8890–8899. doi: 10.18632/oncotarget.12985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Schroeder B. O., Backhed F. Signals from the gut microbiota to distant organs in physiology and disease. Nature Medicine. 2016;22(10):1079–1089. doi: 10.1038/nm.4185. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1: process of TNBS-induced colitis model and solvent control. Preparation of TNBS ethanol solution: in 10 mL of 50 mg/mL TNBS solution, add 10 mL absolute ethanol and mix well. Preparation of 50% ethanol solution: in 2 mL of absolute ethanol, add 2 mL sterile water for injection and mix well. Step 1 (fasting): all mice were fasted for 24 hours. Step 2 (anesthesia): mice were anesthetized by intraperitoneal injection of pentobarbital sodium at the dose of 40 mg/kg and 0.1 mL/10 g body weight. Step 3 (enema): 25 mg/L TNBS ethanol solution (model group)/50% ethanol solution (control group) was aspirated by syringe. The soft rubber tube connected with the syringe was gently inserted approximately 3 cm into the mouse through the anus, and the contents of the syringe were slowly injected into the intestinal cavity of mice. Step 4 (inversion): after administration, the plastic tube was slowly removed and the tail of the mouse was lifted. The mouse was kept inverted for 1 min.
Data Availability Statement
The dataset used and analyzed during the current study is available from the corresponding authors on reasonable request.


