Abstract
Background
Studies have shown that microRNA (miRNA) regulates gene expression of osteoporosis (OS). It is known that miR-197-3p is abnormally expressed in osteoporosis. This study is to investigate the mechanism of miR-197-3p in regulating osteoblast differentiation.
Methods
Rats were ovariectomized to establish an animal model of postmenopausal osteoporosis. The expression of miR-197-3p and KLF10 was detected in ovariectomized rat models. Primary osteoblasts and MC3T-E1 cells were divided into the control group, miR-197-3p inhibitor group, NC inhibitor group and miR-197-3p inhibitor + si-KLF10 group. The expression of miR-197-3p and Kruppel-like factor 10 (KLF10) was detected by qRT-PCR and Western blot. The relationship between miR-197-3p and KLF10 was analyzed by bioinformatics and luciferase reporter assay. Cell viability was evaluated by MTT assay. The ALP activity measurement and mineralization analysis were performed.
Results
The expression of miR-197-3p was significantly raised in ovariectomized osteoporosis rats. During the differentiation of osteoblasts, the expression of miR-197-3p was significantly decreased, while the expression of KLF10 was significantly raised in primary osteoblasts and MC3E3T1 cells. The expression of RUNX2, ALP, OCN and OSX in miR-197-3p inhibitor group and MC3T3-E1 group was significantly raised, and the cell survival rate and mineralized nodule were raised as well. KLF10 may be the downstream target gene of miR-197-3p. After co-transfection of miR-197-3p inhibitor and si-klf10, ALP, Runx2, OCN and OSX mRNA, cell survival rate and mineralized nodule were significantly decreased in primary osteoblasts and MC3T3-E1 cells.
Conclusion
MiR-197-3p Inhibition promoted osteoblast differentiation and reduced OS by up-regulating KLF10.
Keywords: osteoporosis, miR-197-3p, KLF10, osteogenic differentiation, MC3T3-E1 cells
Background
Osteoporosis is a public health problem with about 200 million patients worldwide.1 Among women over 60 in China, the prevalence of osteoporosis is as high as 40% −50%, of which about 30% −50% experience osteoporosis-related fractures.2 It seriously affects the physical health and life quality, and even lead to shortened life span.3 And osteoporosis and its related fractures seriously increase the financial and human burden of the country and family.4 Although there are many kinds of medicines for treating osteoporosis, but most of the medicines for osteoporosis still have many shortcomings such as poor safety, large side effects, and failure to effectively reduce the risk of fractures.5 Exploring its exact pathogenesis has important clinical value for the prevention and treatment of osteoporosis.
Although there are many factors that affect osteoporosis, most of them are caused by the effects of osteoblasts and osteoclasts.6 The regulation of differentiation of osteoblasts and the balance between osteoblasts and osteoclasts are the keys of normal bone metabolism.7 Out of control of these processes will lead to bone deformity and bone-related diseases. Osteoblasts are mainly produced in the body from mesenchymal stem cells (BMSCs) in the bone marrow.8 However, the molecular mechanism of weakened osteogenesis of BMSCs in patients with osteoporosis is not completely clear. Finding effective methods to restore the osteogenic ability of BMSCs may be the key to the treatment. After ovariectomy, the estrogen level in rats gradually decreases, accompanied by changes such as gradual loss of bone mass and destruction of bone tissue structure, which leads to postmenopausal osteoporosis. Therefore, we established an animal model of postmenopausal osteoporosis by ovariectomy.9
MicroRNAs (miRNAs) have regulatory functions on the expression of protein molecules. MiRNAs are widely present in the cells of animal and plant eukaryotes and are involved in regulating biological growth.10 Scholars believe that miRNAs play a critical role in the metabolic activities of bone tissue cells in organisms.11 MiRNAs maintain the metabolic balance of bone by regulating the phenotypic differentiation of mesenchymal stem cells, and ultimately affect the metabolic homeostasis and bone formation.12 MiRNAs are expected to provide new directions for the treatment of metabolic bone disease and bone defect disease. In addition, miRNAs play a critical role in the osteogenic differentiation of mesenchymal precursor cells. However, there is a wide range of miRNAs which need further exploration and research.9 As an important member of miRNAs family, miR-197-3p is overexpressed in many kinds of tumors, including lung cancer, bladder cancer, liver cancer and pancreatic cancer.13,14 Its overexpression is thought to play a role in the occurrence and development of tumors, at the same time, miR-197-3p was down-regulated in the process of osteogenic differentiation and up-regulated in the process of osteoporosis. KLF10 could promote osteogenic differentiation, while miR-197-3p interacted with KLF10 but the role of miR-197-3p in osteogenic differentiation of osteoporosis has not been reported.
Most studies found that miRNAs negatively regulate target genes at the post-transcriptional level, thereby regulating cell proliferation, differentiation and apoptosis.15 KLF10 is a protein encoded by the KLF10 gene.16 KLF10 is expressed in cells which participate in cell proliferation, differentiation, and apoptosis.17 Multiple studies have shown that KLF10 is the key of osteoblast differentiation, bone formation and mineralization.18 Based on the above research, it was proposed that miR-197-3p may influence osteoporosis pathogenesis through regulating KLF10 expression. The aim of the study is to explore the role and mechanism of miR-197-3p in osteogenic differentiation, and to verify the role of KLF10 in osteogenic differentiation regulated by miR-197-3p.
Methods
Ethics Statement
The study was approved by the Jiangxi Provincial People’s hospital’s Experimental Animal Ethics Committee. All the animals in this study received the standard care of National Institutes of Health’s Guide to the Care and Use of Laboratory Animals (NIH Publication No. 85).
Establishment of Ovariectomized Rat Model of Osteoporosis
Eighteen female Sprague-Dawley rats (weight 280–300 g) were obtained from Shanghai Slark Experimental Animal Co., Ltd. They were randomly divided into the OVX group and the sham group (Sham), and 9 rats in each group. The osteoporosis model of ovariectomized rats was made according to the methods in the literature except the sham operation group. The animals were anesthetized of sodium pentobarbital (30mg/kg), and the abdominal cavity was opened. The bilateral ovaries were removed and sutured layer by layer. 20,000 U of penicillin was injected intramuscularly for 3 consecutive days. 5 days after ovariectomy, the rats were examined by vaginal smear, and the rats with incomplete ovariectomy were removed. The abdominal cavity was opened and sutured in rats of the sham operation group. Each group was given corresponding drug intervention, in which the sham operation group and the control group were given equal volumes of distilled water once a day for 90 d. After one week, rats were sacrificed after intraperitoneal anesthesia with ketamine and xylazine.
BMD Measurement and Bone Histomorphometric Analysis
The separated femurs were measured for BMD using the American LUNARDPX-L 6843 dual-energy X-ray bone densitometer, each was measured twice, and the average value was taken. The BMD results were expressed in g/cm.2 The femur was scanned by Skyscan (Brooke micro CT, Kentucky, Belgium) and analyzed by Skyscan software. The measurement was performed with 40 kV and 250 μA.
Dual-Luciferase Reporter Gene Assay
The wild-type (WT) luciferase reporter plasmid KLF10-WT and the mutant KLF10-MT plasmid containing the KLF10 3ʹUTR region were constructed. The miR-197-3p mimic or miRNA mimic was then transfected into the cells by Lipofectamine 2000 (Invitrogen). The luciferase activity was measured by the dual luciferase assay system (Promega).
Culture, Differentiation and Transfection of Osteoblasts
The primary skull osteoblasts were isolated. Firstly, primary osteoblasts/pre-osteoblasts MC3T3-E1 cells (obtained by ATCC) were cultured in α-MEM medium with 10% FBS. The cells were cultured in an incubator for 24 h. And the transfected cells were cultured in osteoblast medium (OM) containing 10% FBS, 50 μ g/mL ascorbic acid and 5 mm β - glycerophosphate for 7 days. After the induction of osteogenesis, the cells were divided into the control group, miR-197-3p inhibitor group, NC inhibitor group and miR-197-3p inhibitor + si-KLF10 group. MiR-197-3p mimics, inhibitors and control oligonucleotides were all obtained from RiboBio (China), and siRNA was synthesized by Jima Biotech (Shanghai, China). Lipofectamine TM 2000 transfection reagent (Invitrogen, USA) was used to transfect oligonucleotides and plasmids for 48 h.
qRT-PCR
Total RNA samples from bone marrows and cultured MRC-5 cells were extracted using the Trizol reagent (#15596026; Thermo Fishier Scientific) according to the manufacturer’s instructions. The cDNA library synthesis and relative expression levels were performed using the TransScript® Green Two-Step qRT-PCR SuperMix (#AQ201-01; Transgen, Beijing, China) and the TransScript Green miRNA Two-Step qRT-PCR SuperMix (AQ202-01; Transgen, Beijing, China) following the manufacturer’s instructions. PCR assays were performed in duplicate on the 7500 real‑time PCR machine (Applied Biosystems; Thermo Fisher Scientific, Inc.). The cycling conditions were as follows: Incubation for 2 min at 50°C followed by another incubation step at 95°C for 10 min, 15 sec at 95°C and 1 min at 60°C for 40 cycles. Reaction specificity was evaluated by melting curve analysis, which was performed by heating the plate from 55°C to 95°C and measuring SYBR Green I (Takara Biotechnology Co., Ltd.) dissociation from the amplicons. The expression of miR-197-3p and genes was calculated using the2−ΔΔCT method. The primers used for qRT-PCR analysis are listed in Table 1.
Table 1.
Sequences of Primers Used in qRT-PCR
| Gene | Forward Primer (5ʹ-3ʹ) | Reversed Primer (5ʹ-3ʹ) |
|---|---|---|
| miR-197-3p | GCCGGGTTGTAAACGATCCTACGG | GTGATTCCGTGTCGTTAGTGG |
| U6 | GCTTCGGCAGCACATATACTAAAAT | CGCTTCAGAATTTGCGTGTCAT |
| KLF10 | ATTGCACCGGCGTTCGGACAC | CTGGAAGTGCGTAGCCCAGATCGT |
| ALP | TCAGGGCAATGAGGTCACAT | CCTCTGGTGGCATCTCGTTA |
| RUNX2 | GACTGTGGTTACCGTCATGGC | ACTTGGTTTTTCATAACAGCGGA |
| OCN | CCCTGAGTCTGACAAAGCCT | GCGGTCTTCAAGCCATACTG |
| OSX | CCTCTGCGGGACTCAACAAC | TGCCTGGACCTGGTGAGATG |
| GAPDH | AACGGATTTGGTCGTATTGGG | TCGCTCCTGGAAGATGGTGAT |
MTT Assay
The anti-proliferative and cytotoxic effects of the target compound on osteoblasts were examined by MTT assay. 2×105 osteoblast cells were seeded in a 96-well plate. The cells were then treated with ECH for 24 h. 10 μL MTT solution was added and incubated for 4 h. After that, 150 μL of DMSO was added to the well. The absorbance was measured at 570 nm by an ELISA reader (bio Tek El 800, USA).
ALP Activity and Mineralization Analysis
After transfection 7 days of the osteoblasts with the plasmid, the ALP activity was detected by the ALP test kit (Applygen, Beijing, China). And the cells were lysed with cold lysis buffer. Then, the supernatant was incubated at 4°C. The absorbance value was measured at 570nm by ELISA microplate reader (LAB-EYE 1800L, Chuangmeng, Shanghai). The mineralization ability of osteoblasts was detected by Alizarin red staining (ARS) (Nanjing Jiancheng Biotechnology Research Institute). Osteoblasts were added 4% paraformaldehyde. Then, osteoblasts were left for 10 min and washed twice with PBS. 200 μL Alizarin Red dye was added for 30 minutes. Finally, the cells were observed and pictures were taken under a microscope.
Western Blot
The supernatant was discarded after centrifugation, lysate was added. After 30 min, it was freezed at −20°C overnight. The supernatant was centrifuged at 4°C. After protein quantification, PAGE-SDS gel was used for electrophoresis separation of protein samples for 1.5 h. After transferred to PVDF blotting membrane for 1.5 h, it was sealed in TPST containing 5% skimmed milk for 4 h. Then, it was incubated with anti-KLF10 (1:1000, Youliante, Shanghai, China) or anti-GAPDH antibody (1:1000, Youliante, Shanghai, China) overnight. After that, anti-rabbit secondary antibody (1:1000, Youliante, Shanghai, China) was added and incubated for 1 h. Relative protein expression was expressed as the ratio of the gray value of the target band/GAPDH band.
Statistical Methods
The data were analyzed by SPSS19.0 statistical software. The results of data analysis were shown as mean ± standard deviation (mean ±SD). Multigroup data analysis was completed by one-way ANOVA. LSD test was used for subsequent analysis. P < 0.05 indicated the difference was significant.
Result
Expression of miR-197-3p and KLF10 in Ovariectomized Rat Model of Osteoporosis
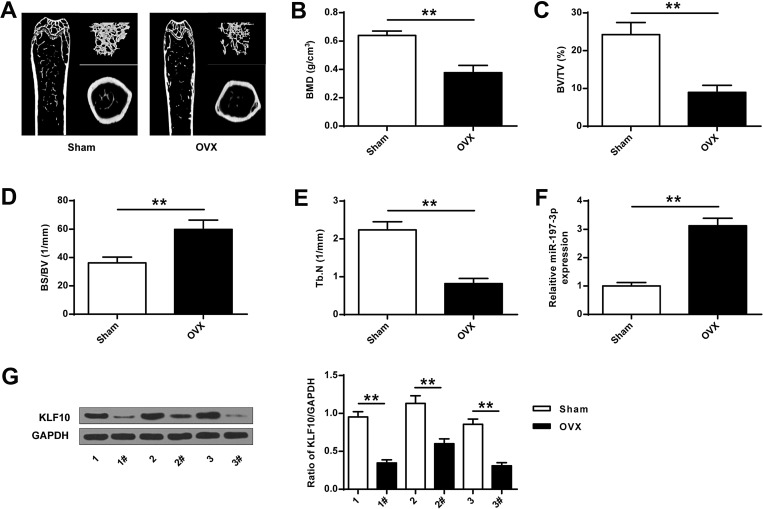
As shown in Figure 1A, compared with the Sham group, representative micro-CT images of the right femur, bone mineral density was significantly decreased in the OVA group. Compared with the Sham group, the expression of BMD, BV/TV and Tb.N in the OVA group was significantly reduced, while the expression of BS/BV was significantly increased (P <0.01), indicating that a rat model of osteoporosis with ovariectomy was successfully established (Figure 1B-E). Then, the expression of miR-197-3p in the OVA group was found to be significantly increased (P <0.01), and the protein expression level of KLF10 was significantly decreased (P <0.01) (Figure 1F and G).
Figure 1.
MiR-197-3p and KLF10 expression in ovariectomized osteoporosis rat models. (A) representative micro-CT images of the right femur. (B) Bone mineral density (BMD) of femoral tissue. (C) Micro-CT analysis of TV/TV in OVX and Sham groups. (D) Micro-CT analysis of BS/BV in OVX and Sham rats. (E) Micro-CT analysis of Tb.N in OVX and Sham groups. (F). MiR-197-3p expression in femoral tissue of OVX and Sham groups. (G) Protein expression of KLF10 in femoral tissue of OVX and Sham groups. N = 3. ** P <0.01.
Expression of miR-197-3p and KLF10 in Osteogenic Differentiation
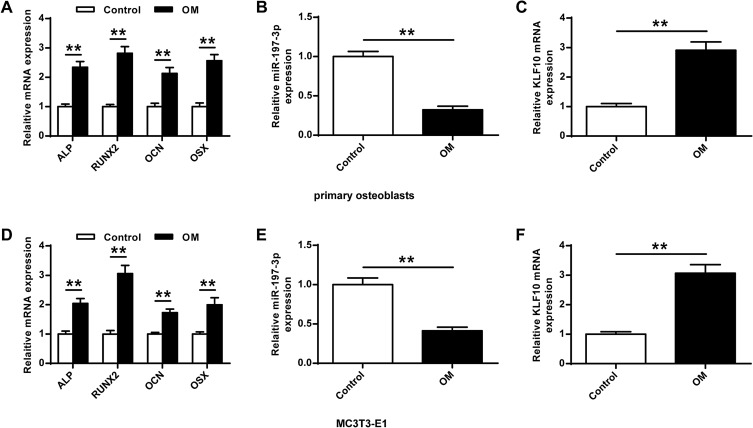
Next, the role of miR-197-3p in osteogenic differentiation was analyzed. As shown in Figure 2A and D, the expression of genes (OCN, RUNX2, ALP, and OSX) was significantly increased (P <0.01) in the primary osteoblasts and MC3E3T1 cells of OM group compared with the Control group. In addition, miR-197-3p expression was significantly reduced in primary osteoblasts and MC3E3T1 cells during osteoblast differentiation, indicating that miR-197-3p might be involved in osteogenesis induction (P <0.01) (Figure 2B and E). The expression of KLF10 in primary osteoblasts and MC3E3T1 cells was significantly increased (P<0.01), indicating that KLF10 might be involved in osteogenesis induction (P <0.01, Figure 2C and F).
Figure 2.
MiR-197-3p and KLF10 expression levels in osteogenic differentiation. (A) Levels of osteogenesis-related genes in primary osteoblasts including ALP, RUNX2, OCN, and OSX after 7 d of OM treatment. (B) Expression of miR-197-3p in primary osteoblasts 7 d after OM treatment. (C) Expression of KLF10 in primary osteoblasts 7 d after OM treatment. (D) Levels of osteogenesis-related genes in MC3T3-E1 cells include levels of OCN, RUNX2, ALP and OSX 7 d after OM treatment. (E) MiR-197-3p expression in MC3T3-E1 cells 7 d after OM treatment. (F) On the 7th day after OM treatment, the expression of KLF10 in MC3T3-E1 cells. N = 3. ** P <0.01.
KLF10 Was the Target Gene of miR-197-3p
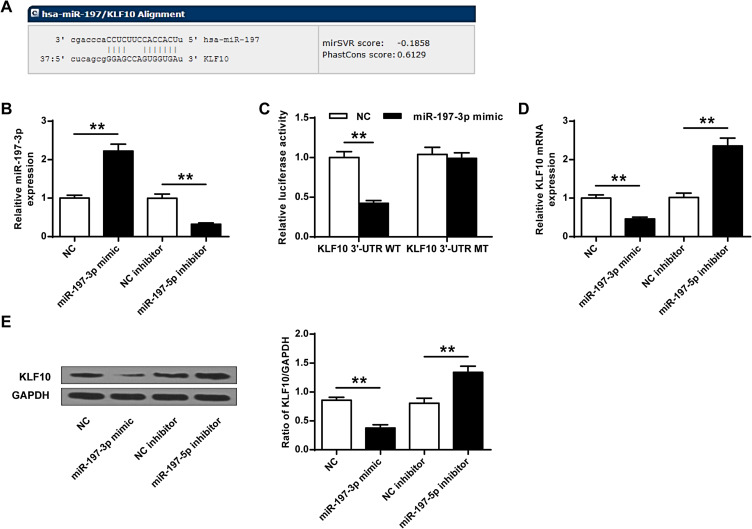
We predicted through online prediction tool microrna.org (http://www.microrna.org/microrna/home.do) and found KLF10 was identified as a potential target for miR-197-3p (Figure 3A). Compared with NC group, the expression of miR-197-3p in the miR-197-3p mimic group was significantly increased (P<0.01), while the expression of miR-197-3p in the miR-197-3p inhibitor group was significantly reduced, indicating that transfection was successful (P<0.01) (Figure 3B). And miR-197-3p mimic and KLF10-WT co-transfected cells had significantly reduced luciferase activity (P<0.05), but not KLF10-MUT (Figure 3C). And the mRNA and protein expression of KLF10 in miR-503-3p mimic group was significantly reduced (P <0.01), while the mRNA and protein expression of KLF10 in the miR-503-3p inhibitor group was significantly increased (P <0.05, Figure 3D and E).
Figure 3.
KLF10 was the target gene of miR-197-3p. (A) KLF10 and miR-197-3p were predicted on the target gene prediction website. (B) The expression of miR-197-3p. (C) The targeting relationship between KLF10 and miR-197-3p was detected by dual luciferase reporter gene analysis. (D) The effect of miR-197-3p on KLF10 mRNA expression. (E) The effect of miR-197-3p on KLF10 protein expression. N = 3. ** P <0.01.
Expression of Osteogenesis-Related Genes in Each Group
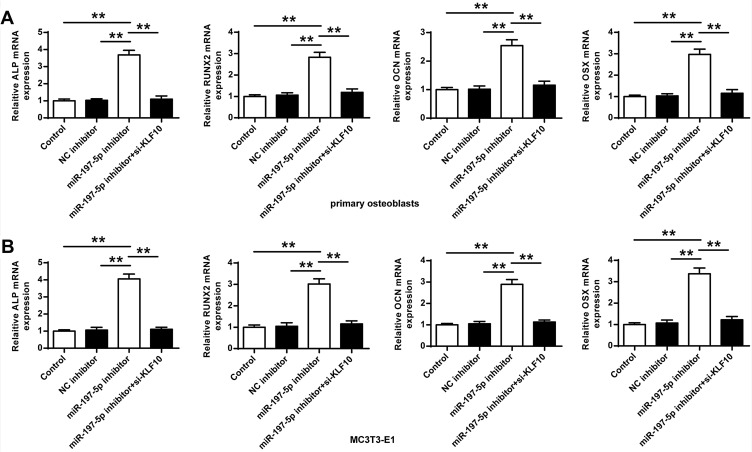
Next, whether miR-197-3p played a role in osteogenic differentiation through KLF10 was analyzed, the mRNA expression of OCN, RUNX2, ALP and OSX in primary osteoblasts and MC3T3-E1 cells of miR-197-3p inhibitor group was significantly increased (P < 0.01) compared with the control group and NC inhibitor group. In addition, compared with miR-197-3p inhibitor group, the expression of OCN, RUNX2, ALP and OSX in primary osteoblasts and MC3T3-E1 cells after co-transfection of miR-197-3p inhibitor with si-KLF10 were significantly reduced (P < 0.01) (Figure 4).
Figure 4.
mRNA expression of OCN, RUNX2, ALP and OSX (A) in primary osteoblasts (B) in MC3T3-E1 cells. N = 3. ** P <0.01.
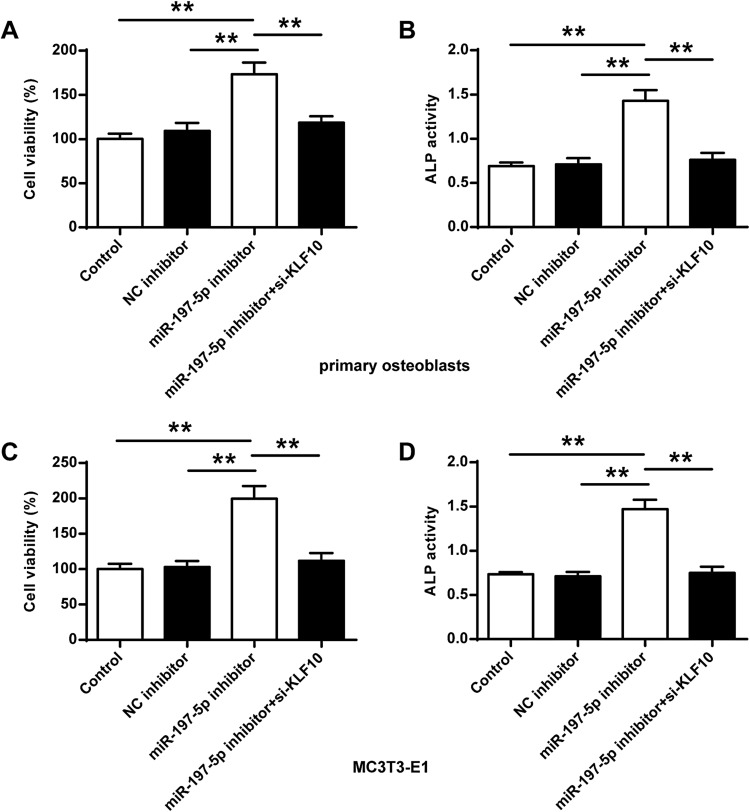
Comparison of Cell Viability and ALP Activity of Osteoblasts
As shown in Figure 5, the cell survival rate and ALP activity in the primary osteoblasts and MC3T3-E1 cells in the miR-197-3p inhibitor group were significantly improved compared with the control group and the NC inhibitor (P <0.01). Compared with the miR-197-3p inhibitor group, the cell viability and ALP activity in primary osteoblasts and MC3T3-E1 cells were significantly reduced after co-transfection of miR-197-3p inhibitor with si-KLF10 (P <0.01).
Figure 5.
Comparison of osteoblast viability and ALP activity in different groups. (A, B) Cell viability and ALP activity in primary osteoblasts. (C, D) Cell viability in MC3T3-E1 cells. N = 3. ** P <0.01.
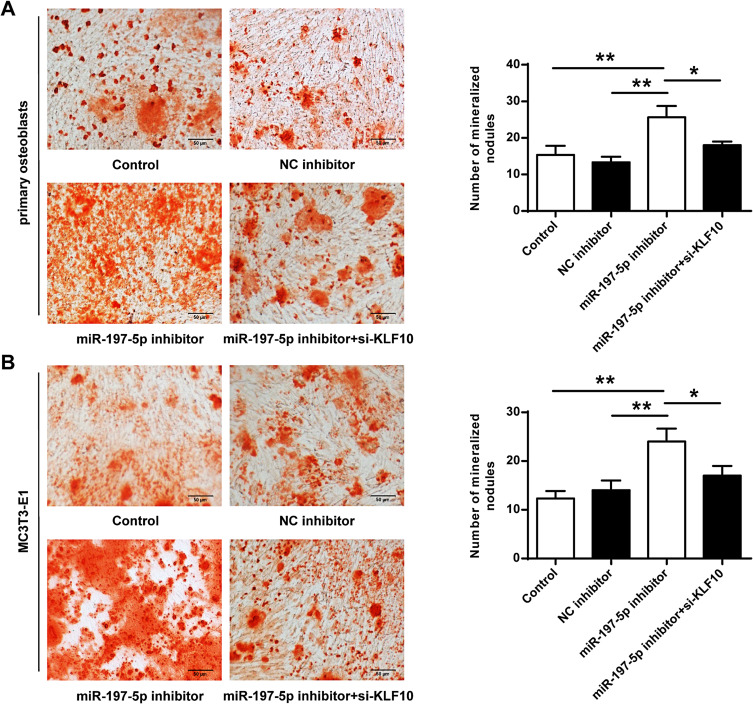
Comparison of Mineralized Nodules of Osteoblasts
As shown in Figure 6, the mineralized nodules in primary osteoblasts and MC3T3-E1 cells were significantly increased in the miR-197-3p inhibitor group compared with the control group and NC inhibitor group (P <0.01). In addition, compared with the miR-197-3p inhibitor group, the mineralized nodules were significantly reduced in primary osteoblasts and MC3T3-E1 cells co-transfected with miR-197-3p inhibitor and si-KLF10 (P <0.01). As shown in Supplementary Figure 1, compared with the control group and NC inhibitor group, there were calcified nodules and extensive deposition of red particles in the miR-197-3p inhibitor group. Compared with the miR-197-3p inhibitor group, calcified nodules were significantly decreased in primary osteoblasts and MC3T3-E1 cells co-transfected with miR-197-3p inhibitor and si-KLF10 (P <0.01).
Figure 6.
Mineralized nodules of primary osteoblasts and MC3T3-E1 cells. (A) Mineralized nodules of primary osteoblasts. (B) The mineralized nodules of MC3T3-E1 cells. N = 3. * P <0.05, ** P <0.01.
Discussion
Osteoporosis is a systemic skeletal disease characterized and risk of fractures.19 As the population ages, the global prevalence of osteoporotic fracture rate will increase.20 Osteoporosis is a chronic disease that consumes a lot of medical and economic resources of the country.21 Therefore, it is important to define how to effectively prevent and reverse the pathological process of osteoporosis. At present, the clinical treatment methods for osteoporosis.22,23 However, clinical bone defects, fracture nonunion and nonunion have always been difficult to treat, so effective methods for promoting bone formation are still under study. Osteoblasts are derived from BMSC.24 Inhibition of osteogenic differentiation can cause post-traumatic bone defects.25 Therefore, it is of significance to study the mechanism of osteogenic differentiation in the treatment of osteoporosis.
The main diagnostic and evaluation criteria for osteoporosis include the measurement of BMD and the detection of biochemical indicators of bone metabolism.26,27 Current research has found that BS/TV, BV/TV, Tb.N and Tb.Sp may be sensitive variables of bone trabecular structure changes, which predict the development of osteoporosis.28,29 In this study, BMD, BV/TV and TB. N levels were decreased in OVX group, while BS/BV level was increased significantly, indicating that ovariectomized osteoporosis rat model had been successfully established.
Recently, miRNAs play critical roles in the directional differentiation of mesenchymal stem cells into osteoblasts and development by inhibiting the translation and expression of target mRNA or degradation of target mRNA.30 miRNA has a regulatory effect on osteogenic differentiation of different cells.31 However, osteogenic differentiation of stem cells may involve multiple factors and different signaling pathways to coordinate with each other to jointly regulate osteogenic differentiation of stem cells.32 The osteogenic differentiation of stem cells may involve multiple factors.33 Therefore, it is of significance to study the role of key miRNAs on osteogenic differentiation of BMSCs for the OS. Studies have found that miR-17, miR-27a, miR-140-5p and miR-654-5p can directly inhibit the expression of BMP2 to regulate osteogenic differentiation.34 MiR-197-3p, as a member of miRNA family, can regulate the proliferation of tumor cells.35 In this study, it was found that the expression of miR-197-3p in OVX group was increased. During osteoblast differentiation, the miR-655-3p level in primary osteoblasts and MC3E3T1 cells of the OM group was significantly reduced. Moreover, ALP activity and osteogenesis-related genes (RUNX2, OSX, and OCN) expression were significantly reduced. In the miR-197-3p inhibitor group, ALP, RUNX2, OCN and OSX mRNA, cell survival rate and mineralized nodules in primary osteoblasts and MC3E3T1 cells were significantly increased. These results suggest that inhibition of miR-197-3p may promote osteoblast differentiation and relieve osteoporosis.
Krüppel-like factors (KLFs) are a class of transcription factors with three C2H2 zinc finger structures at the C-terminus, which can bind a variety of specific proteins to mediate transcriptional regulation.36 It is found that KLFs play a critical role in the differentiation, phenotype maintenance and physiological function regulation of various types of human cells.37 KLF10 can inhibit skeletal muscle formation, and KLF10 gene expression is down-regulated during skeletal muscle formation.38 However, during the differentiation of mouse skeletal muscle cells, the expression of KLF10 is increased. KLF10 gene expression can be detected in myoblasts and myotubes, suggesting that KLF10 in skeletal muscle tissue may be more complex.39 This study found that the expression of KLF10 in OVX group was increased. During osteoblast differentiation, the expression of KLF10 in the OM group was increased in primary osteogenesis cells and MC3E3T1 cells. Luciferase reporter assay showed that there was indeed a targeting relationship between KLF10 and miR-197-3p. MiR-197-3p negatively regulated the expression of KLF10. ALP, RUNX2, OCN, OSX. Cell survival rate and mineralized nodules were significantly down-regulated in primary osteoblasts and MC3T3-E1 cells co-transfected with miR-197-3p inhibitor and si-KLF10. These results suggest miR-197-3p inhibition advances osteoblast differentiation and reduces osteoporosis by KLF10.
Conclusion
MiR-197-3p promoted osteoblast differentiation and reduced osteoporosis by KLF10, indicating that miR-197-3p can be an important medium for osteoblast differentiation.
Acknowledgments
I would like to thank all the authors for their contribution to this article.
Funding Statement
There is no funding to report.
Ethics
The study was approved by the Jiangxi Provincial People’s hospital’s Experimental Animal Ethics Committee. NO.125765. All the animals in this study received the standard care of National Institutes of Health’s Guide to the Care and Use of Laboratory Animals (NIH Publication No. 85).
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
The authors declare that they have no competing interests.
References
- 1.Papaleontiou M, Banerjee M, Reyes〨astelum D, Hawley ST. Risk of osteoporosis and fractures in patients with thyroid cancer: a case control study in U.S. veterans. Oncologist. 2019;24(9):theoncologist.2019–0234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Li X-D, Liu -T-T, Wang W-Z, Zhang J-G. Efficacy of weight adjusted bone mineral content in osteoporosis diagnosis in Chinese female population. Chinese Med J. 2019;132(7):1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Geusens P, Bergh J. Management of Male Osteoporosis. 2019. [Google Scholar]
- 4.Maluta T, Toso G, Negri S, Samaila EM. Correlation between hip osteoarthritis and proximal femoral fracture site: could it be protective for intracapsular neck fractures? A retrospective study on 320 cases. Osteoporosis Int. 2019;30(3):1–6. [DOI] [PubMed] [Google Scholar]
- 5.Ya-Ping X, Jie Z, Lin-na J, Medica XX. Review for Treatment Effect and Signaling Pathway Regulation of Kidney-Tonifying Traditional Chinese Medicine on Osteoporosis;2018. [DOI] [PubMed] [Google Scholar]
- 6.Wang J, Chen L, Zhang Y, Li C-G. Association between serum vitamin B 6 concentration and risk of osteoporosis in the middle-aged and older people in China: a cross-sectional study. Open BSJB. 2019;9(7):e028129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ye CF, Pan YM, Zhou HJ. Regulation of vitamin D receptor and Genistein on bone metabolism in mouse osteoblasts and the molecular mechanism of osteoporosis. J Biol Regulators Homeostatic agents. 2018;32(3):497–505. [PubMed] [Google Scholar]
- 8.Yu X, Shen G, Ren H, Zhang Z. TGFβ﹊nduced factor homeobox 2 blocks osteoblastic differentiation through targeting pSmad3/HDAC4/H4ac/Runx2 axis. Physiology. 2019;234(1). [DOI] [PubMed] [Google Scholar]
- 9.Jung Koo H, Sohn E-H, Kim Y-J, Jang S-A, Namkoong S, Chan Kang S. Effect of the combinatory mixture of Rubus coreanus Miquel and Astragalus membranaceus Bunge extracts on ovariectomy-induced osteoporosis in mice and anti-RANK signaling effect. J Ethnopharmacol. 2014;151(2):951–959. doi: 10.1016/j.jep.2013.12.008 [DOI] [PubMed] [Google Scholar]
- 10.Carrasco‐Rozas A, Fernández‐Simón E, Lleixà MC, et al. Identification of serum microRNAs as potential biomarkers in Pompe disease. Ann Clin Transl Neurol. 2019:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Arriaga MA, Ding M, Gutierrez AS. The application of microRNAs in biomaterial scaffold-based therapies for bone tissue engineering. J SACJB. 2019. [DOI] [PubMed] [Google Scholar]
- 12.Li Z, Hassan MQ, Jafferji M, Aqeilan RI. Correction: biological functions of miR-29b contribute to positive regulation of osteoblast differentiation. Chemistry. 2019;294(25):10018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen Y, Yang C. miR‑197‑3p‑induced downregulation of lysine 63 deubiquitinase promotes cell proliferation and inhibits cell apoptosis in lung adenocarcinoma cell lines. Mol Med Rep. 2018;17(3):3921–3927. doi: 10.3892/mmr.2017.8333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang -Y-Y, Wu Z-Y, Wang G-C, Liu K, Niu X-B. LINC00312 inhibits the migration and invasion of bladder cancer cells by targeting miR-197-3p. Tumour Biol. 2016;37(11):14553–14563. doi: 10.1007/s13277-016-5303-8 [DOI] [PubMed] [Google Scholar]
- 15.Jane B, Uros K, Punit S, et al. Copy number and expression alterations of miRNAs in the ovarian cancer cell line OVCAR-3: impact on Kallikrein 6 protein expression. Clin Chem. 2020(1):1. [DOI] [PubMed] [Google Scholar]
- 16.Mishra VK, Subramaniam M, Kari V, et al. Krüppel-like transcription factor KLF10 Suppresses TGF{beta}-induced epithelial-to-mesenchymal transition via a negative feedback mechanism. Cancer Res. 2017;77(9):2387. doi: 10.1158/0008-5472.CAN-16-2589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Elalfy MS, Sherif NHKE, Kamal TM, Aly NH. Klf10 gene, a secondary modifier and a pharmacogenomic biomarker of hydroxyurea treatment among patients with hemoglobinopathies. J Ped Hematol Oncol. 2017;39(3). [DOI] [PubMed] [Google Scholar]
- 18.Ko FC, Martins JS, Reddy P, et al. Acute Phosphate Restriction Impairs Bone Formation and Increases Marrow Adipose Tissue in Growing Mice. 2016:31. [DOI] [PubMed] [Google Scholar]
- 19.Binkley N, Blank RD, Leslie WD, et al. Osteoporosis in crisis: it’s time to focus on fracture. 2017;32. [DOI] [PubMed]
- 20.Curtis EM, Moon RJ, Harvey NC, Cooper CJ. The impact of fragility fracture and approaches to osteoporosis risk assessment worldwide. Int J Orthopaedic Trauma Nurs. 2017;26(3):289–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Forciea MA, Mclean RM, Qaseem AJ. Treatment of low bone density or osteoporosis to prevent fractures in men and women. Ann Internal Med. 2017;167(12):904. doi: 10.7326/L17-0490 [DOI] [PubMed] [Google Scholar]
- 22.Nelson RE, Ma J, Miller K, Lawrence P. The impact of a musculoskeletal training program on residents’ recognition and treatment of osteoporosis. BMC Med Educ. 2019;19:1. doi: 10.1186/s12909-019-1653-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Matzkin EG, DeMaio M, Charles JF, Surgeons CC. Diagnosis and treatment of osteoporosis: what orthopaedic surgeons need to know. 2019;1. [DOI] [PubMed]
- 24.Jing H, Liao L, Su X, Shuai Y, Jin YJ. Declining histone acetyltransferase GCN5 represses BMSC-mediated angiogenesis during osteoporosis. FASEB J. 2017;31:10. doi: 10.1096/fj.201700118R [DOI] [PubMed] [Google Scholar]
- 25.Deng L, Hu G, Jin L, Wang C. Involvement of microRNA-23b in TNF-α-reduced BMSC osteogenic differentiation via targeting runx2. Bone Metabolism. 2017;36(6):1–13. [DOI] [PubMed] [Google Scholar]
- 26.Ott SM. Review: osteoporosis drugs may improve BMD and reduce fractures in some patients with CKD. 2017;167(4):JC19. [DOI] [PubMed] [Google Scholar]
- 27.Woitge HW, Eva H, Keck AV, Beatrice A, Seibel MJ, Martin PJCC. Biochemical markers of bone formation in patients with plasma cell dyscrasias and benign osteoporosis. Clin Chem. 2020;4:4. [PubMed] [Google Scholar]
- 28.Kim JY, Lee S, Choi YY, Bae SC. Atypical bone change of spine caused by epidural venous thrombosis in systemic lupus erythematosus with antiphospholipid syndrome. Korean J Internal Med. 2017;32(3):573–574. doi: 10.3904/kjim.2016.075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sornay‐Rendu E, Boutroy S, Duboeuf F, Chapurlat RD. Bone MRtOJotASf, Research M. Bone microarchitecture assessed by HR‐pQCT as predictor of fracture risk in postmenopausal women: the OFELY Study. 2017:32. [DOI] [PubMed] [Google Scholar]
- 30.Wang H, Hu Z, Shi F, Dong J, Zhang GJCD. Osteoblast-targeted delivery of miR-33-5p attenuates osteopenia development induced by mechanical unloading in mice. Disease. 2018;9:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yin N, Zhu L, Ding L, et al. MiR-135-5p promotes osteoblast differentiation by targeting HIF1AN in MC3T3-E1 cells. Cell ; Mol Biol Letters. 2019;24(1). doi: 10.1186/s11658-019-0177-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Melguizo‐Rodríguez L, Manzano‐Moreno FJ, Luna‐Bertos ED, et al. Effect of olive oil phenolic compounds on osteoblast differentiation. Eur J Clin Invest. 2018;48:4. doi: 10.1111/eci.12904 [DOI] [PubMed] [Google Scholar]
- 33.Dong Z, Xu M, Liu L, Jiang C, Wu CJ. Silicate-based bioceramics regulating osteoblast differentiation through BMP2 signalling pathway. J Mater Chem B. 2017;5:35. [DOI] [PubMed] [Google Scholar]
- 34.Son HE, Kim KM, Kim EJ, Jang WG. Kisspeptin-10 (KP-10) stimulates osteoblast differentiation through GPR54-mediated regulation of BMP2 expression and activation. Sci Rep. 2018;8(1):2134. doi: 10.1038/s41598-018-20571-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mcmanus DD, Rong J, Huan T, Lacey S, Levy DJBG. Messenger RNA and MicroRNA transcriptomic signatures of cardiometabolic risk factors. BMC Genomics. 2017;18(1):139. doi: 10.1186/s12864-017-3533-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pollak NM. Krüppel-like factors: crippling and uncrippling metabolic pathways. JACC. Basic Transl Sci. 2018;3(1):132–156. doi: 10.1016/j.jacbts.2017.09.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhuang ZH, Zhong Y, Chen YH, Hereditas ZW. Research progress on the roles of Krüppel-like factors in muscle tissues. Yi Chuan = Hereditas. 2018;40(9):733–748. doi: 10.16288/j.yczz.18-095 [DOI] [PubMed] [Google Scholar]
- 38.DiMario, DiMario JX. KLF10 gene expression modulates fibrosis in dystrophic skeletal muscle. Am J Pathol. 2018;188(5):1263–1275. doi: 10.1016/j.ajpath.2018.01.014 [DOI] [PubMed] [Google Scholar]
- 39.Malayannan S, Muzaffer C, Pitel KS, et al. TIEG1 modulates β-catenin sub-cellular localization and enhances Wnt signaling in bone. 2017;9:9. [DOI] [PMC free article] [PubMed] [Google Scholar]