Abstract
Diagnosis of COVID-19 in end-stage kidney disease (ESKD) patients on hemodialysis is challenging, as the symptoms are often atypical. Herein, we reported a confimed case of COVID-19 in a patient on maintenance hemodialysis. A 38-year-old man with ESKD on regular hemodialysis initially presented with progressive shortness of breath and dry cough, without fever. He had lymphopenia, and chest X-ray suggested pulmonary edema with cardiomegaly and suspected bilateral bronchopneumonia. The patient clinically improved after 7 days of hospitalization, and was subsequently discharged from hospital. Ten days after being discharged, the patient was re-admitted with progressive shortness of breath and dry cough, without fever. SARS-CoV-2 infection was later confirmed by a qualitative RT-PCR test and the diagnosis COVID-19 pneumonia was established. We presented a case of atypical presentation of COVID-19 in an ESKD patient on maintenance hemodialysis with a brief review of the current literature.
Keywords: COVID-19, Hemodialysis, End-Stage Kidney Disease, Atypical presentation
Introduction
In December 2019, an infectious pneumonia caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) emerged in Wuhan, China. The disease then rapidly spread worldwide, and the World Health Organization (WHO) declared coronavirus disease 2019 (COVID-19) as a global public health emergency [1]. The spectrum of the disease ranges from asymptomatic to fatal multiorgan failure with features of hyperinflammation and hypercoagulable state [2–5]. Cardiac arrhythmias in COVID-19 are gaining attention as potential complications of SARS-CoV-2 because of its association with increased risk of poor outcome in COVID-19 [6, 7]. Clinical characteristics of patients associated with a high risk of severe disease and death include older age, underlying cardiac disease, chronic kidney disease, diabetes, and hypertension [8–13]. End-stage kidney disease (ESKD) patients on regular hemodialysis are considered to be at high risk for SARS-CoV-2 infection. This special population is vulnerable because they usually have several comorbidities, suppressed immunity, and inevitable frequent visits to the dialysis unit. Information about symptoms, laboratory and radiologic abnormalities, and the use of rapid diagnostic tests for COVID-19 in patients on maintenance hemodialysis is limited. Herein, we report our first experience with a confirmed COVID-19 case in a patient with ESKD on regular hemodialysis.
Case report
A 38-year-old male ESKD patient on regular hemodialysis presented with shortness of breath and dry cough for 3 days. He had a history of fever 3 days prior admission. He denied any history of contact with a confirmed COVID-19 patient or travel to epidemic areas. He had been on hemodialysis three times weekly through an AV Fistula since 2016. He had a history of hypertension, but no diabetes or cardiovascular, cerebrovascular, or chronic obstructive pulmonary disease (COPD), nor chronic liver disease. His regular medications included Irbesartan, Amlodipine, calcium carbonate, and folic acid.
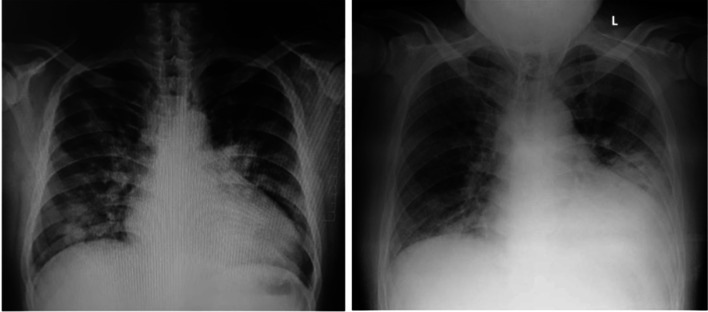
On the first admission, his physical examination revealed tachypnea, peripheral oxygen saturation of 90% within room air, no fever, with normal jugular venous pressure (5 + 2 cm H2O). Chest examination revealed bilateral crackles. He did not have peripheral edema. Laboratory tests showed anemia (hemoglobin level 8.8 g/dL), a white blood cell count of 5160 cells/µL, with a low absolute lymphocyte count of 570 cells/µL, and a thrombocyte count of 153,000 cells/µL (Table 1). Chest X-ray showed bilateral peripheral opacities, suggesting pulmonary edema with cardiomegaly, and suspected bilateral bronchopneumonia (Fig. 1). After one hemodialysis session, the symptoms slightly improved but still with an increased work of breathing. The patient was diagnosed with community-acquired pneumonia and was started on empirical antibiotics. COVID-19 was not suspected at first, as he denied any epidemiological exposure.
Table 1.
Laboratory results according to the day of illness
| Measure | Baseline | Day 3 (1st admission) | Day 7 | Day 21 (2nd admission) | Day 23 | Day 28 |
|---|---|---|---|---|---|---|
| Hemoglobin (gr/dL) | 9.3 | 8.6 | 9.1 | 9.2 | 6.1 | 10.9 |
| White blood cell count (103/uL) | 7.20 | 5.16 | 6.15 | 19.56 | 16.81 | 9.15 |
| Neutrophil count (/uL) | 6.500 | 4.130 | 5.290 | 15.450 | 14.620 | 6.490 |
| Lymphocyte count (/uL) | 1.296 | 570 | 490 | 920 | 660 | 1.360 |
| Platelet count (103/uL) | 162 | 153 | 138 | 189 | 263 | 240 |
| Ureum (mg/dL) | 108 | 167 | 116.4 | 164 | 113 | 146 |
| Creatinine (mg/dL) | 7.24 | 12.6 | 9.26 | 9.34 | 9.23 | 8.46 |
| Procalcitonin (ng/mL) | 4.29 | |||||
| SARS-CoV-2 rapid IgG/IgM combine antibody test | Negative | Positive | ||||
| PCR SARS-CoV-2 | Positive | Negativea |
PCR polymerase chain reaction, SARS-CoV-2 severe acute respiratory syndrome Coronavirus 2
aThe test was negative for two times over a period of 24 h
Fig. 1.
Chest X-ray: day 3 of illness/first admission (left); day 21 of illness second admission (right)
On the fifth day of hospitalization, even though the patient’s respiratory condition began to improve, his blood tests still showed a very low absolute lymphocyte count (490 cells/µL) relative to his baseline values (Table 1), with a total white blood cell count of 6150 cells/µL. Suspicion of viral pneumonia was raised, and a SARS-CoV-2 antibody test was performed. The result was negative. Due to the early surge of COVID-19 pandemic in our hospital, the patient was “semi-isolated” in a single-patient room to minimize contact with other patients. The patient was discharged on the seventh day of hospitalization and advised to re-do the SARS-CoV-2 antibody test after 10–14 days.
Ten days after the patient was discharged from hospital, he presented with a progressive shortness of breath and dry cough, without fever. His physical examination revealed tachypnea, peripheral oxygen saturation was 88% in room air, and he was afebrile. Laboratory tests showed anemia (hemoglobin level 9.2 g/dL), a white blood cell count of 15,452 cells/µL, with a low absolute lymphocyte count of 920 cells/µL, and a platelet count of 153,000 cells/µL. His repeat chest X-rays demonstrated evolution of the bilateral multifocal opacities, consistent with the atypical pneumonia (Fig. 1). The patient was tested with the Rapid IgM/IgG Combined Antibody test, with a positive result. SARS-CoV-2 infection was later confirmed by the qualitative RT-PCR test of nasopharyngeal and oropharyngeal specimens, and the diagnosis of COVID-19 pneumonia was established. Due to the high level of serum procalcitonin (Table 1), co-infection with bacterial pneumonia was suspected. He was treated with oxygen supplementation, supportive packed red cells transfusion, empirical intravenous antibiotics, prophylactic-dose of heparin, regular hemodialysis, and received a COVID-19 regimen according to the local standard of care for in early pandemics, consisting of Hydroxychloroquine, Azithromycin, and Oseltamivir for 5 days. The patient did not receive any erythropoietin stimulating agent during hospitalization due to concerns of increasing the risk of thromboembolic event. His clinical condition improved gradually, and after repeated negative qualitative RT-PCR tests, the patient was discharged from the hospital (Table 2).
Table 2.
Timeline
| Day of illness | Events |
|---|---|
| Day 1 | The onset of shortness of breath, cough, and fever |
| Day 3 | Persistent shortness of breath and cough. Patient was admitted to hospital |
| Day 7 | SARS-CoV-2 Rapid IgG/IgM combine antibody test: negative |
| Day 9 | Clinically improved. He was discharged from hospital, and advised to re-check the antibody in the next 10–14 days |
| Day 21 (23 May 2020) |
Shortness of breath, cough after dialysis session. He was admitted again to the hospital SARS-CoV-2 Rapid IgG/IgM combine antibody test: positive Later confirmed with PCR: Positive |
| Day 28 | Clinically improved. PCR: Negative |
PCR polymerase chain reaction, SARS-CoV-2 severe acute respiratory syndrome coronavirus 2
Discussion
During the COVID-19 pandemic, ESKD patients on hemodialysis are considered to be at high risk for SARS-CoV-2 infection. They are likely to be immunocompromised with a high prevalence of multiple comorbidities [14, 15]. Regular compulsory visits to the dialysis facility, a place where peoples are crowding for certain amount of time, also increase the risk of being exposed to this communicable disease. The prevalence of COVID-19 and its severity among hemodialysis patients varies worldwide [16]. Xiong et al. reported 154 cases of COVID-19 among 7153 hemodialysis patients in China [17], while Goicoechea et al. reported 36 confirmed COVID-19 cases among 283 hemodialysis patients in Spain [18]. Moreover, Alberici et al. reported 94 patients positive for SARS-CoV-2 infection within an overall population of 643 hemodialysis patients in Italy [19].
Our center is the top referral hospital for COVID-19 in the West Java Region, and the case presented here was our very first confirmed case of COVID-19 in a hemodialysis patient at the beginning of pandemic (our patient was diagnosed as COVID-19 on 23 May 2020). Initially, we were quite startled with surprisingly rare case of COVID-19 among relatively high prevalence of ESKD patients on chronic dialysis in West Java which accounts for a total of 33,828 patients in this specific region [20]. It seemed that researchers around the globe were also wondering this same question, “Why COVID-19 may be less severe in dialysis patients?” [21]. These controversial issues regarding the low prevalence and severity of COVID-19 among ESKD on dialysis aroused when an initial study reported a lesser severity of COVID-19 among those individuals [22]. The surge of new COVID-19 cases among hemodialysis patients in our region after this first case report, along with the result of our previous meta-analysis resolved the issues and revealed the high overall prevalence of COVID-19 among dialysis patients, and high rates of severe COVID-19 and mortality among hospitalized ESKD patients on dialysis (18.4%, 45.3%, and 26.8%, respectively) [16].
The clinical manifestation of COVID-19 in hemodialysis patients can be atypical from the general population. The main symptoms of COVID-19 pneumonia in the general population include fever, cough, and dyspnea [9]. In our case, the patient presented with dyspnea and cough. Wu et al. found that fever is reported in 47% of COVID-19 patients on hemodialysis, versus 90% in the control group (with normal kidney function) [23]. Reports showed that typical symptoms of COVID-19 pneumonia such as fever, cough, and dyspnea are less likely to be found in the hemodialysis population [17, 19, 24]. The wide spectrum of symptoms in the hemodialysis population makes it difficult to screen patients with SARS-CoV-2 infection. Symptoms can mimic uremia conditions or fluid overload from inadequate dialysis. We have summarized evidence from the current literature regarding the clinical manifestation of COVID-19 in ESKD patients on hemodialysis in Table 3.
Table 3.
Clinical characteristic of COVID-19 in hemodialysis patients
| Author | Place | Case (n) | Male (%) | Age (mean/median) | Comorbidities | Clinical manifestation | Laboratory findings | Radiologic findings |
|---|---|---|---|---|---|---|---|---|
| Xiong [17] | Wuhan, China | 131 | 57.3 | 63.3 |
Cardiovascular disease 68.7% Diabetes 22.9% Hepatitis B 8.4% Hepatitis C 2.3% COPD 3.8% Cancer 1.5% |
Fever 51.9% Cough 37.4% Dyspnea 26% Fatigue 45% Sputum production 29% Nausea/vomiting 18% Diarrhea 13% Sore throat 7.7% |
Hb 10.5 (9.1–11.8) WBC 5.0 (3.8–7.3) Lymphocyte 0.7 (0.5–1.1) Neutrophils 3.9 (3.0–6.1) PLT 144.2 (107–186) |
CT scan image: Ground-glass 82.1% Cord Shadow 7.7% Consolidation 4.3% Lesion region: Bilateral 86.7% Left lung 6.2% Right lung 7.1% |
| Alberici [19] | Brescia, Italy | 94 | 65.9 | 72 |
Hypertension 93% Diabetes 43% Vascular Disease 23% Cardiac Failure 18% Ischemic Cardiac disease 17% Cancer 12% COPD 11% |
Fever 68% Cough 23% Dyspnea 25% Gastrointestinal 6% Pharyngitis 2% Myalgia 17% |
WBC 5.0 (3.9–6.4) Lymphocyte 0.7 (0.5–1.0) Neutrophils 3.5 (2.6–4.7) PLT 162 (126–229) |
Chest X-ray: No infiltrates 30% Unilateral infiltrates 25% Bilateral infiltrates 45% |
| Valeri [24] | New York, US | 59* | 56 | 63 |
Hypertension 98% Diabetes 69% Coronary artery disease 46% Cardiac failure 18% Pulmonary disease 17% |
Fever 49% Cough 39% Dyspnea 36% Fatigue 22% Gastrointestinal 15% Myalgia 8% Altered mentation 5% |
Hb 10.6 (9.7–11.8) WBC 6.0 (4.5–7.8) Lymphocyte 0.8 (0.5–1.2) |
Chest X-ray: Bilateral opacities 59% Unilateral opacity 10% Pleural effusion 7% Pulmonary Edema 5% No acute findings 19% |
| Wu [23] | Wuhan, China | 49 | 63 | 62 |
Hypertension 92% Diabetes 20% Cardiovascular disease 20%; Cerebrovascular disease 2% Cancer 8% COPD 2% Chronic liver disease 6% |
Fever 47% Cough 49% Dyspnea 45% Sputum Production 33% Fatigue 59% Anorexia 57% Diarrhea 12% Dizziness 14% Nausea 8% Vomiting 4% Sore throat 7.7% |
Hb 10.4 (8.2–12.2) WBC 5.6 (4.7–7.6) Lymphocyte 0.8 (0.5–1.0) Neutrophils 4.0 (3.1–5.6) PLT 169 (120–234) |
CT scan: Bilateral opacities 82% Unilateral opacity 10% No abnormality 8% |
| Goicoechea [18] | Madrid, Spain | 36 | 64 | 71 |
Hypertension 97% Diabetes 64% Coronary heart disease 22% Dyslipidemia 67% COPD 19% |
Fever 67% Cough 44% Fatigue 25% Gastrointestinal 17% |
Hb 10.6 (9.2–12.0) Lymphocyte 0.79 (0.32–1.26) PLT 164 (98–230) |
Chest X-ray: Bilateral peripheral ground-glass opacity 61% Unilateral opacity 19% Normal X-ray 19% |
Hb hemoglobin (g/L), WBC white blood cells (109/L); Lymphocyte (109/L); Neutrophils (109/L); PLT platelet (109/L)
*2 patients on PD, 57 patients on HD
The patient presented here had lymphopenia, a prominent laboratory finding of SARS-CoV-2 infection in the general population [25]. Low lymphocyte counts are common in chronic dialysis patients. Yoon et al. found an increased susceptibility of apoptosis of naive T cell lymphocytes and memory T cells in patients with ESKD [26]. Ma et al. showed that T cell, Th cell, killer T cell, and NK cell counts are reduced in dialysis patients, irrespective of SARS-CoV-2 infection [27]. Therefore, lymphopenia with an absolute cut-off value may not be helpful to identify individuals with SARS-CoV-2 infection in hemodialysis patients. Nevertheless, lymphopenia that is lower than the previous baseline values might still provide an insight of underlying COVID-19, as we consistently found that lymphopenia is observed in hemodialysis patients with COVID-19 from many reports [17–19, 24, 28].
SARS-CoV-2 qualitative RT-PCR should be performed when there is a suspicion of viral pneumonia, but due to logistic limitations we used the SARS-CoV-2 Rapid IgG/IgM Combined Antibody Test as the initial screening. Initially, a couple of weeks after the WHO announced the pandemic status, there were still limitations in COVID-19 testing. Nasopharyngeal and oropharyngeal specimens had to be sent to the central laboratory in Jakarta (115 km away from Bandung), and the results were available after 5–7 days. In our case, the SARS-CoV-2 Antibody Test was first negative (on the seventh day after onset of symptoms) and later positive (on the 21st day). We considered the possibility that the patient might have been infected by SARS-CoV-2 already on the first admission, but the antibody response was delayed.
In the general population, the IgM SARS-CoV-2 antibody can be detected 3–6 days after infection, while IgG can be detected after 8 days. Sun et al. found out that in up to 75% patients, IgM/IgG could be possibly detected in the first week [29]. Ying et al. showed that sensitivity, specificity, and accuracy of the Rapid IgG/IgM Combined Antibody Test for SARS-CoV-2 were different between three groups according to the interval between symptom onset and sample collection: “0–7 days,” “8–15 days,” and “> 16 days.” Sensitivity was 18.7%, 100%, and 100%; specificity was 77.8%, 50%, and 64.3%; and accuracy was 40%, 87.5%, and 93.9%, respectively [30]. A majority of patients develop an antibody response in the second week after onset of symptoms. However, analysis of the dynamics of SARS-CoV-2 antibody is still ongoing in the general population, and even less is known about these dynamics in hemodialysis patients.
The kinetics of the antibody response to SARS-CoV-2 infection in hemodialysis patients remain to be determined. De Vriese et al. reported the first evaluation of potential antibody responses in six hemodialysis patients. They found that the IgG response against SARS-CoV-2 nucleocapsid (N) protein occurred in the second week after symptom onset [31]. It remains to be confirmed whether those results are applicable to the entire hemodialysis population [32]. With the limited data available, the use of antibody-detecting rapid diagnostic tests is not recommended. It could be used in limited resource facilities with precaution at 10–14 days after onset of symptoms. However, further investigation to characterize the antibody response to SARS-CoV-2 infection in hemodialysis patients is encouraged to improve disease surveillance and epidemiologic research.
In summary, despite our several limitations in regard to the absence of several radiological and laboratory investigations (e.g., Pulmonary CT Scan, echocardiography, C-reative protein, and d-dimer), we have reported our very first case of ESKD on hemodialysis patient with COVID-19 in our region. Atypical presentations of COVID-19 on this specific population are relatively frequent according to the current literature. More vigilant suspicion, early screening, and additional preventive precautions cannot be over-emphasized in dealing with COVID-19 among ESKD patients on hemodialysis.
Funding
The authors received no financial support for the authorship and/or publication of this article.
Data availability statement
All the relevant data are available and included in the manuscript.
Compliance with ethical standards
Conflict of interest
The authors declare that there is no conflict of interest.
Patient consent confirmation statement
Patient consent was obtained to publish the findings of this case report.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Rizky Andhika, Email: rizkyandhikaipd@gmail.com.
Afiatin Makmun, Email: afiatinmakmun@gmail.com.
Yovita Hartantri, Email: yhartantri@gmail.com.
Indra Wijaya, Email: indrawijayaipd@gmail.com.
Ian Huang, Email: ianhuang2108@gmail.com.
References
- 1.Zhu N, Zhang D, Wang W, Li X, Yang B, Song J, Zhao X, Huang B, Shi W, Lu R, Niu P, Zhan F, Ma X, Wang D, Xu W, Wu G, Gao GF, Tan W. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 2020;382(8):727–733. doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lim MA, Pranata R, Huang I, Yonas E, Soeroto AY, Supriyadi R. Multiorgan failure with emphasis on acute kidney injury and severity of COVID-19: systematic review and meta-analysis. Can J Kidney Heal Dis. 2020;7:1–12. doi: 10.1177/2054358120938573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wijaya I, Andhika R, Huang I. The use of therapeutic-dose anticoagulation and its effect on mortality in patients with COVID-19: a systematic review. Clin Appl Thromb. 2020;26:1076029620960797. doi: 10.1177/1076029620960797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Huang I, Pranata R, Lim MA, Oehadian A, Alisjahbana B. C-reactive protein, procalcitonin, D-dimer, and ferritin in severe coronavirus disease-2019: a meta-analysis. Ther Adv Respir Dis. 2020;14:1–14. doi: 10.1177/1753466620937175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wijaya I, Andhika R, Huang I. Hypercoagulable state in COVID-19 with diabetes mellitus and obesity: is therapeutic-dose or higher-dose anticoagulant thromboprophylaxis necessary? Diabetes Metab Syndr Clin Res Rev [Internet]. 2020;14(5):1241–2. Available from: https://linkinghub.elsevier.com/retrieve/pii/S1871402120302630. [DOI] [PMC free article] [PubMed]
- 6.Pranata R, Huang I, Raharjo SB. Incidence and impact of cardiac arrhythmias in coronavirus disease 2019 (COVID-19): a systematic review and meta-analysis. Indian Pacing Electrophysiol J [Internet]. 2020;20(5):193–8. Available from: https://linkinghub.elsevier.com/retrieve/pii/S0972629220300875. [DOI] [PMC free article] [PubMed]
- 7.Yasmin Kusumawardhani N, Huang I, Martanto E, Sihite TA, Nugraha ES, Prodjosoewojo S, Hamijoyo L, Hartantri Y. Lethal arrhythmia (Torsade de Pointes ) in COVID-19: an event synergistically induced by viral associated cardiac injury, hyperinflammatory response, and treatment drug? Clin Med Insights Case Reports [Internet]. 2020;13:117954762097239. Available from: http://journals.sagepub.com/doi/10.1177/1179547620972397. [DOI] [PMC free article] [PubMed]
- 8.Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, Zhang L, Fan G, Xu J, Gu X, Cheng Z, Yu T, Xia J, Wei Y, Wu W, Xie X, Yin W, Li H, Liu M, Xiao Y, Gao H, Guo L, Xie J, Wang G, Jiang R, Gao Z, Jin Q, Wang J, Cao B. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang D, Hu B, Hu C, Zhu F, Liu X, Zhang J, Wang B, Xiang H, Cheng Z, Xiong Y, Zhao Y, Li Y, Wang X, Peng Z. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA J Am Med Assoc. 2020;323(11):1061–1069. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Huang I, Lim MA, Pranata R. Diabetes mellitus is associated with increased mortality and severity of disease in COVID-19 pneumonia—a systematic review, meta-analysis, and meta-regression: Diabetes and COVID-19. Diabetes Metab Syndr Clin Res Rev. 2020;14(4):395–403. doi: 10.1016/j.dsx.2020.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pranata R, Lim MA, Huang I, Raharjo SB, Lukito AA. Hypertension is associated with increased mortality and severity of disease in COVID-19 pneumonia: a systematic review, meta-analysis and meta-regression. J Renin-Angiotensin-Aldosterone Syst. 2020;21(2):147032032092689. doi: 10.1177/1470320320926899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yonas E, Alwi I, Pranata R, Huang I, Lim MA, Gutierrez EJ, Yamin M, Siswanto BB, Virani SS. Effect of heart failure on the outcome of COVID-19—a meta analysis and systematic review. Am J Emerg Med. 2020;S0735–6757(20):30602–1. doi: 10.1016/j.ajem.2020.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pranata R, Supriyadi R, Huang I, Permana H, Lim MA, Yonas E, Soetedjo NNM, Lukito AA. The association between chronic kidney disease and new onset renal replacement therapy on the outcome of COVID-19 patients: a meta-analysis. Clin Med Insights Circ Respir Pulm Med. 2020;14:1179548420959165. doi: 10.1177/1179548420959165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pranata R, Huang I, Lim MA, Wahjoepramono PEJ, July J. Impact of cerebrovascular and cardiovascular diseases on mortality and severity of COVID-19—systematic review, meta-analysis, and meta-regression. J Stroke Cerebrovasc Dis. 2020 doi: 10.1016/j.jstrokecerebrovasdis.2020.104949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pranata R, Soeroto AY, Ian H, Lim MA, Santoso P, Permana H, Lukito AA. Effect of chronic obstructive pulmonary disease and smoking on the outcome of COVID-19. Int J Tuberc Lung Dis. 2020;24(April):1–10. doi: 10.5588/ijtld.20.0278. [DOI] [PubMed] [Google Scholar]
- 16.Andhika R, Huang I, Wijaya I. The severity of COVID‐19 in end‐stage kidney disease patients on chronic dialysis. Ther Apher Dial. 2020;50(6):1744–9987.13597. Available from: https://onlinelibrary.wiley.com/doi/10.1111/1744-9987.13597. [DOI] [PMC free article] [PubMed]
- 17.Xiong F, Tang H, Liu L, Tu C, Tian JB, Lei CT, Liu J, Dong JW, Chen WL, Wang XH, Luo D, Shi M, Miao XP, Zhang C. Clinical characteristics of and medical interventions for COVID-19 in hemodialysis patients in Wuhan. China. J Am Soc Nephrol. 2020;31(7):1387–1397. doi: 10.1681/ASN.2020030354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Goicoechea M, Sánchez Cámara LA, Macías N, Muñoz de Morales A, Rojas ÁG, Bascuñana A, Arroyo D, Vega A, Abad S, Verde E, García Prieto AM, Verdalles Ú, Barbieri D, Delgado AF, Carbayo J, Mijaylova A, Acosta A, Melero R, Tejedor A, Benitez PR, Pérez de José A, Rodriguez Ferrero ML, Anaya F, Rengel M, Barraca D, Luño J, Aragoncillo I. COVID-19: clinical course and outcomes of 36 hemodialysis patients in Spain. Kidney Int. 2020;98(1):27–34. [DOI] [PMC free article] [PubMed]
- 19.Alberici F, Delbarba E, Manenti C, Econimo L, Valerio F, Pola A, Maffei C, Possenti S, Lucca B, Cortinovis R, Terlizzi V, Zappa M, Saccà C, Pezzini E, Calcaterra E, Piarulli P, Guerini A, Boni F, Gallico A, Mucchetti A, Affatato S, Bove S, Bracchi M, Costantino EM, Zubani R, Camerini C, Gaggia P, Movilli E, Bossini N, Gaggiotti M, Scolari F. A report from the Brescia Renal COVID Task Force on the clinical characteristics and short-term outcome of hemodialysis patients with SARS-CoV-2 infection. Kidney International. 2020;98(1):20–26. doi: 10.1016/j.kint.2020.04.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Indonesian Society of Nephrology. 11th Report of Indonesian Renal Registry 2018 [Internet]. Indonesian Renal Registry. 2018. Available from: https://www.indonesianrenalregistry.org/data/IRR2018.pdf.
- 21.Pisani A, Rizzo M, Angelucci V, Riccio E. Covid-19 experience in hemodialysis patients: a cue for therapeutic heparin-based strategies? Nephron. 2020;144(8):383–385. doi: 10.1159/000508638. [DOI] [PubMed] [Google Scholar]
- 22.Ma Y, Ph D, Diao B, Ph D, Lv X, Liang W, Ph D, Zhu J, Ph D, Liu L, Ph D, Zhang S, Shen B, Ph D, Wang H, Ph D. COVID-19 in hemodialysis (HD) patients: report from one HD center in Wuhan, China. medRxiv. 2020 doi: 10.1101/2020.02.24.20027201. [DOI] [Google Scholar]
- 23.Wu J, Li J, Zhu G, Zhang Y, Bi Z, Yu Y, Huang B, Fu S, Tan Y, Sun J, Li X. Clinical features of maintenance hemodialysis patients with 2019 novel coronavirus-infected pneumonia in Wuhan. China. Clin J Am Soc Nephrol. 2020;15(8):1139–1145. doi: 10.2215/CJN.04160320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Valeri AM, Robbins-Juarez SY, Stevens JS, Ahn W, Rao MK, Radhakrishnan J, Gharavi AG, Mohan S, Ali HS. Presentation and outcomes of patients with ESKD and COVID-19. J Am Soc Nephrol. 2020;31(7):1409–1415. doi: 10.1681/ASN.2020040470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Huang I, Pranata R. Lymphopenia in severe coronavirus disease-2019 (COVID-19): systematic review and meta-analysis. J Intensive Care. 2020;8(1):36. Available from: https://www.jintensivecare.biomedcentral.com/articles/10.1186/s40560-020-00453-4. [DOI] [PMC free article] [PubMed]
- 26.Yoon JW, Gollapudi S, Pahl MV, Vaziri ND. Naïve and central memory T cell lymphopenia in end-stage renal disease. Kidney Int. 2006;70(2):371–376. doi: 10.1038/sj.ki.5001550. [DOI] [PubMed] [Google Scholar]
- 27.Ma Y, Diao B, Lv X, Zhu J, Liang W, Liu L, Bu W, Cheng H, Zhang S, Yang L, Shi M, Ding G, Shen B, Wang H. novel coronavirus disease in hemodialysis (HD) patients: Report from one HD center in Wuhan China. medRxiv. 2020 doi: 10.1101/2020.02.24.20027201. [DOI] [Google Scholar]
- 28.Kuroki Y, Hiyama K, Minami J, Takeuchi M, Shojima M, Matsueda S, Nagae H, Nakano T. The first case of COVID-19 pneumonia in a hemodialysis patient in Japan. CEN Case Rep. 2020;9(4):404–408. doi: 10.1007/s13730-020-00495-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sun B, Feng Y, Mo X, Zheng P, Wang Q, Li P, Peng P, Liu X, Chen Z, Huang H, Zhang F, Luo W, Niu X, Hu P, Wang L, Peng H, Huang Z, Feng L, Li F, Zhang F, Li F, Zhong N, Chen L. Kinetics of SARS-CoV-2 specific IgM and IgG responses in COVID-19 patients. Emerg Microbes Infect. 2020;9(1):940–948. doi: 10.1080/22221751.2020.1762515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liu Y, Liu Y, Diao B, Ren F, Wang Y, Ding J, Huang Q. Diagnostic indexes of a rapid IgG/IgM combined antibody test for SARS-CoV-2. medRxiv. 2020 doi: 10.1101/2020.03.26.20044883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.De Vriese AS, Reynders M. IgG antibody response to SARS-CoV-2 infection and viral RNA persistence in patients on maintenance hemodialysis. Am J Kidney Dis. 2020;76(3):440–441. doi: 10.1053/j.ajkd.2020.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dudreuilh C, Moutzouris D-A. Is SARS-CoV-2 serology relevant for hemodialysis patients with COVID-19? Am J Kidney Dis. 2020;S0272-6386(20)30784-8. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All the relevant data are available and included in the manuscript.