Abstract
This study was conducted to assess the clinical spectrum, management, and outcome of SARS-CoV-2-related multisystem inflammatory syndrome in children (MIS-C). We reviewed medical records of children with MIS-C diagnosis seen at the Children’s Hospital of Michigan in Detroit between April and June 2020. Thirty-three children were identified including 22 who required critical care (group 1) and 11 with less intense inflammation (group 2). Children in group 1 were older (median 7.0 years) than those in group 2 (median 2.0 years). Abdominal pain was present in 68% of patients in group 1. Hypotension or shock was present in 17/22 patients in group 1. Thirteen (39.4%) had Kawasaki disease (KD)–like manifestations. Five developed coronary artery dilatation; All resolved on follow-up. Intravenous immunoglobulin (IVIG) was given to all patients in group 1 and 7/11 in group 2. Second-line therapy was needed in 13/22 (group 1) for persisting inflammation or myocardial dysfunction; 12 received infliximab. All patients recovered.
Conclusion: MIS-C clinical manifestations may overlap with KD; however, MIS-C is likely a distinct inflammatory process characterized by reversible myocardial dysfunction and rarely coronary artery dilatation. Supportive care, IVIG, and second-line therapy with infliximab were associated with a favorable outcome.
|
What is Known: • Multisystem inflammatory syndrome in children (MIS-C) manifestations include fever, gastrointestinal symptoms, shock, and occasional features of Kawasaki disease (KD). • Treatment includes immunomodulatory agents, most commonly IVIG and corticosteroids. | |
|
What is New: • Spectrum of MIS-C varies from mild to severe inflammation and coronary artery dilatation occurred in 5/22 (23%) critically ill patients. • IVIG and infliximab therapy were associated with a favorable outcome including resolution of coronary dilatation; only 2/33 received corticosteroids. |
Keywords: COVID-19, MIS-C, Kawasaki disease, IVIG, Infliximab
Introduction
The pandemic of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has rapidly spread worldwide since it was first identified in China in December 2019 [1]. Infections in adults are characterized by severe interstitial pneumonia, acute respiratory distress syndrome, multiorgan failure and even death. Immune responses including a cytokine storm during coronavirus disease (COVID-19) have been shown to play an important role in the pathogenesis of tissue injury and multisystem involvement [1, 2]. Most COVID-19 infections in children are mild or asymptomatic [3–5]. However, there are recent reports of clusters of children who developed severe systemic inflammatory response related to SARS-CoV-2 infection [6–8].
The first report of pediatric systemic inflammatory syndrome related to SARS-CoV-2 occurred on April 26, 2020, from the UK that described an increasing number of otherwise healthy children who were hospitalized with a severe inflammatory syndrome characterized by hypotension, multisystem involvement, and elevated inflammatory markers [9]. Affected children tested positive for infection with SARS-CoV-2 or had epidemiological link to an infected contact. In early May 2020, the New York State Department of Health issued a health advisory of children with multisystem inflammatory syndrome with similar clinical presentation who tested positive for SARS-CoV-2 by RT-PCR or serology [10]. On May 14, 2020, the US Centers for Disease Control (CDC) published the case definition of this syndrome which was called the multisystem inflammatory syndrome in children (MIS-C) associated with COVID-19 [11]. Additional reports of this inflammatory syndrome from other countries including Italy, France, Switzerland, and the UK have followed [6–8]. Children with MIS-C can develop clinical features that mimic other inflammatory disorders such as Kawasaki disease, toxic shock syndrome (streptococcal or staphylococcal), and macrophage activation syndrome [6, 12].
The first cases of COVID-19 in the state of Michigan were reported on March 10, 2020, followed by a rapid increase in COVID-19 cases and deaths. On March 27, 2020, the US Surgeon General declared Metro Detroit which had most of the cases in Michigan a “hot spot” in the USA. At our hospital, which serves a large proportion of children in Metro Detroit, we have noted an increasing number of children admitted with “Kawasaki disease (KD)” diagnosis during April 2020. By the end of April and in May 2020, we noted an unexpectedly large number of children who were admitted to our pediatric intensive care unit (PICU) for hypotension and shock in the setting of multisystem inflammation. These patients tested positive for SARS-CoV-2 either by RT-PCR or by serology or had history of exposure to adult contacts with COVID-19.
The aim of our study was to review the clinical spectrum of illness in children with MIS-C at our hospital. We describe the clinical findings in children who required intensive care due to severe inflammation and those who had less severe inflammation or self-limited illness. We discuss the differences between the two groups and the similarities and differences with KD. We also present our experience in the use of anti-inflammatory medications including intravenous immunoglobulin (IVIG) and the role of the tumor necrosis factor (TNF)-α antagonist infliximab in severe and IVIG-resistant cases. The echocardiographic findings of these patients at baseline and outpatient follow-up are also presented.
Patients and methods
Children admitted to the Children’s Hospital of Michigan (CHM) with the diagnosis of MIS-C during the period from April 1, 2020, to June 5, 2020, were included in this study. CHM is a tertiary care teaching pediatric institution with 228-bed capacity including a 48-bed pediatric intensive care unit. For this study, we reviewed the patients’ demographic data, COVID-19 exposure, laboratory findings including microbiologic and radiographic data, electrocardiogram (ECG) and echocardiogram findings, clinical course, response to treatment, and outcome. Patients were diagnosed with multisystem inflammatory syndrome in children (MIS-C) associated with COVID-19 using US Center for diseases Control (CDC) criteria published on May 14, 2020 [11]. To better delineate the spectrum of MIS-C, we included patients with COVID-19 infection or exposure who presented with fever and elevated inflammatory markers but had no features of organ failure or myocardial dysfunction and did not require intensive care. KD features in patients with KD-like clinical presentations were categorized as classic or incomplete according to the 2017 criteria of the American Heart Association (AHA) [13].
Cardiac evaluation included baseline echocardiogram (ECG) as well as blood high-sensitivity troponin I and brain natriuretic peptide (BNP) levels. Troponin I levels were repeated daily if the patient remained febrile. All patients who were admitted to the intensive care unit were monitored with continuous telemetry. Repeat ECG and echocardiogram were obtained in case of worsening clinical condition as well as at 2- and 6-week outpatient follow-up. Coronary artery Z score was calculated based on the formula by McCrindle et al. [14].
Laboratory studies included baseline complete blood count (CBC); inflammatory markers including C-reactive protein (CRP), erythrocyte sedimentation rate (ESR), ferritin, d dimer, and lactate dehydrogenase (LDH); and coagulation studies such as fibrinogen, prothrombin time (PT), and partial thromboplastin time (PTT). Baseline assessment of liver function (alanine aminotransferase (ALT) and aspartate aminotransferase (AST), kidney function (blood urea nitrogen (BUN), and creatinine) and creatine kinase (CK) were obtained as well as evaluation of any other end-organ involvement as clinically indicated. CBC, CRP, ferritin, d dimer, CK, and LDH were trended daily if the patient remained febrile. Patients with evidence of myocardial dysfunction and multiorgan dysfunction were managed in the PICU. Testing for SARS-CoV-2 included RT-PCR from nasopharyngeal swabs using Cepheid Xpert Xpress SARS-CoV-2 Assay (Cepheid Inc., Sunnyvale, CA, USA) and serum immunoglobulin (Ig) G immunoassay (Abbott Laboratories, Lake Bluff, IL, USA) that detects antibodies against the viral nucleocapsid protein. The study was approved by the Institutional Review Board at Wayne State University. A waiver of consent was granted.
Statistical analysis
Data on different clinical variables and frequencies were analyzed using SPSS version 20. A non-parametric Fisher’s exact test was employed to examine potential differences in categorical variables between study groups. Continuous data were described as median and interquartile (IQR) values. A p value of ≤ 0.05 was considered statistically significant.
Results
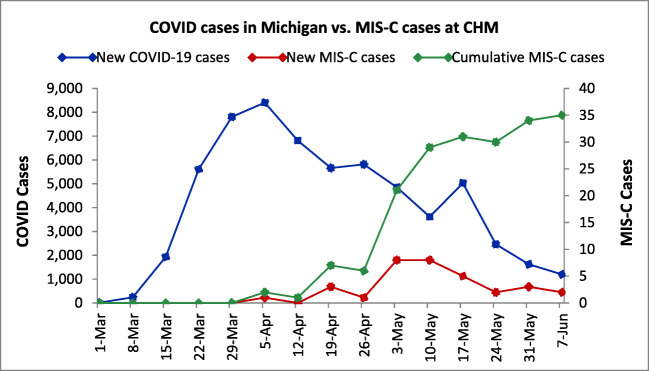
Between April 1 and June 5, 2020, 33 children with MIS-C associated with COVID-19 were identified. Of these included 22 children who required critical care due to hypotension, myocardial dysfunction, or multisystem involvement (group 1) and 11 children whose systemic inflammation findings were not as severe and did not warrant critical care (group 2). The age range of all study patients was 3 months to 17 years (median 6.0, IQR range 3.0–9.0 years). Patients who required critical care were older (median 7.0, IQR range 6.0–10.0 years) than those who did not (median 2.0, IQR 1.3–5.0 years). Of the 33 patients, 18 were females including 14/22 (64%) in group 1 patients, and 25 were African American including 18/22 (82%) in group 1 (Table 1). None of our study patients had underlying heart disease or immunocompromising conditions. Clinical characteristics of study patients are listed in Table 1. Among 33 children with MIS-C 13 (39.4%) had overlapping features with classic or incomplete KD. The peak incidence of cases of MIS-C occurred 4 weeks after the peak incidence of new cases in the state of Michigan (Fig. 1).
Table 1.
Clinical characteristic and cardiac findings: comparison between 22 children who required critical care (group 1) versus 11 with less severe inflammation (group 2)
| Demographic characteristics | |||||
|---|---|---|---|---|---|
| Total | Total (%) | Group 1 (critical) (%) | Group 2 (noncritical) (%) | p values# | |
| 33 | 22 | 11 | |||
| Age (year) | |||||
| Median (IQR range) | 6.0 (3.0–9.0) | 7.0 (6.0–10.0) | 2.0 (1.3–5.0) | < 0.01 | |
| < 1 year | 4 (12.1) | 1 (4.5) | 3 (27.3) | 0.10 | |
| 1–5 | 10 (30.3) | 4 (18.2) | 6 (54.5) | 0.04 | |
| 6–10 | 14 (42.4) | 12 (54.5) | 2 (18.2) | 0.05 | |
| 11–18 | 5 (15.2) | 5 (22.7) | 0 (0) | 0.16 | |
| Sex | |||||
| M | 15 (45.5) | 8 (36.4) | 7 (63.6) | 0.13 | |
| F | 18 (54.5) | 14 (63.6) | 4 (36.4) | ||
| Ethnicity | |||||
| AA | 25 (75.8) | 18 (81.8) | 7 (54.5) | 0.23 | |
| Caucasian | 3 (9.1) | 0 (0) | 3 (27.3) | 0.03 | |
| ME | 2 (6.1) | 2 (9.1) | 0 (0) | 0.44 | |
| Hispanic | 2 (6.1) | 1 (4.5) | 1 (9.1) | 0.56 | |
| Other | 1 (3.0) | 1 (4.5) | 0 (0) | 0.67 | |
| Comorbidities | |||||
| Asthma | 7 (21.2) | 7 (31.8) | 0 (0) | 0.04 | |
| Obesity | 6 (18.2) | 5 (22.7) | 1 (9.1) | 0.33 | |
| Type 2 DM | 1 (3.0) | 1 (4.5) | 0 (0) | 0.67 | |
| Obesity + asthma | 3 (9.1) | 3 (13.6) | 0 (0) | 0.28 | |
| Clinical presentation | |||||
| Fever | 33 (100) | 22 (100) | 11 (100) | 1.00 | |
| GI symptoms | |||||
| Vomiting | 15 (45.5) | 10 (45.5) | 5 (45.5) | 0.45 | |
| Diarrhea | 17 (51.5) | 12 (54.5) | 5 (45.5) | 0.45 | |
| Abdominal pain | 17 (51.5) | 15 (68.2) | 2 (18.2) | 0.01 | |
| Respiratory distress | 4 (12.1) | 4 (18.2) | 0 (0) | 0.18 | |
| Skin rash | 19 (57.6) | 14 (63.6) | 5 (45.5) | 0.27 | |
| Neck tenderness | 6 (18.2) | 6 (27.3) | 0 (0) | 0.07 | |
| Lymphadenopathy | 13 (39.4) | 8 (36.4) | 5 (45.5) | 0.45 | |
| KD diagnosis | |||||
| Classic | 7 (21.2) | 6 (27.3) | 1 (9.1) | 0.23 | |
| Incomplete | 6 (18.2) | 4 (18.2) | 2 (18.2) | 0.67 | |
| Chest pain | 4 (12.1) | 4 (18.2) | 0 (0) | 0.18 | |
| Hypotension | 17 (51.5) | 17 (77.3) | 0 (0) | < 0.01 | |
| Cardiac involvement | |||||
| Coronaries | |||||
| Dilated | 5 (18.2) | 5 (27.3) | 0 (0) | ||
| Aneurysms | 0 (0) | ||||
| Ejection fraction (%)* | |||||
| > 55 | 17 (53.1) | 6 (31.8) | 11 (100) | ||
| 30–54 | 13 (40.6) | 13 (59.5) | 0 (0) | ||
| < 30 | 2 (6) | 2 (9.1) | 0 (0) | ||
PICU pediatric intensive care unit, AA African American, ME Middle Eastern, DM diabetes mellitus, GI gastrointestinal
#Italics indicate significant p values (≤ 0.05)
*Lowest EF during admission, available for 32 patients
Fig. 1.
Weekly new cases of COVID-19 in the state of Michigan with a peak incidence on the week of April 5, 2020 (left). Weekly new cases of MIS-C at CHM (right) with a peak 4–5 weeks later
All study patients had documented infection or exposure to SARS-CoV-2. Infection was confirmed in 18/22 (82%) patients who required intensive care including 5 who tested positive by both nasopharyngeal RT-PCR and serum IgG. Exposure to a family member who tested positive for COVID-19 was documented in 16/33 (48.5%) patients.
All study patients had fever (temperature > 38.5C) and decreased activity. Gastrointestinal symptoms such as abdominal pain, vomiting, or diarrhea were common. Abdominal pain was reported in 17/33 (52%) of patients including 15/22 (68%) in group 1 (Table 1). Surgical evaluation was requested for 6 patients who had abdominal tenderness concerning for peritonitis; none had surgical intervention. Abdominal ultrasound was done for 13 patients in groups 1 and 10 showed abnormal findings including 5 with hepatomegaly/splenomegaly, 3 with prominent or enlarged abdominal lymph nodes mainly noted in right lower quadrant suggestive of mesenteric adenitis, and one with gallbladder wall thickening and pericholecystic fluid. Echogenic kidneys indicating renal kidney disease were also seen in 2 patients. Computed tomography (CT) scans of the abdomen were done for 3 patients in group 1 and revealed acute pancreatitis, duodenitis, jejunitis, and ascites in one patient; enlarged lymph nodes in the right lower quadrant in one patient; and acalculous cholecystitis in another. Abdominal imaging was abnormal in two children in group 2: one had mild splenomegaly and dilated common bile duct by ultrasound, and one had multiple enlarged lymph nodes in the right lower quadrant by CT scan.
At time of PICU admission, 17 of 22 (77%) were hypotensive or had cardiogenic shock and 16 of 17 required intravenous inotropic agents for 1 to 6 days (median 3 days) including epinephrine: 16, dopamine: 5, and milrinone: 4 patients. Six of 22 (27%) required intubation and mechanical ventilation for 4 to 8 days (median 6 days). A 6-year-old female had cardiac arrest and required cardiopulmonary resuscitation. Two patients required veno-arterial extracorporeal membrane oxygenation (V-A ECMO) for severe myocardial dysfunction including the child who had cardiac arrest and a 17-year-old male; both were successfully decannulated after 6 and 4 days respectively. Unfractionated or low molecular weight heparin was used for prophylaxis of thromboembolism in all 6 mechanically ventilated patients. Two patients required packed red cell transfusion and 2 received transfusion with fresh frozen plasma.
All patients had elevation of inflammatory markers. Other laboratory findings included lymphopenia, hyponatremia, hypoalbuminemia, evidence of acute kidney injury (AKI), and mild liver transaminase elevation. The laboratory parameters of the two study groups are listed in Table 2. Cytokine levels were not routinely tested. Interleukin (IL)-6 levels were elevated in all 4 patients in group 1 who were tested.
Table 2.
Laboratory data in study patients: comparison between 22 children who required critical care (group 1) versus 11 with less severe inflammation (group 2)
| Laboratory data (medians) | |||||
|---|---|---|---|---|---|
| Total number | Total (IQR) | Group 1 (critical) (IQR) | Group 2 (noncritical) (IQR) | Reference value | p values# |
| 33 | 22 | 11 | |||
| C-reactive protein (mg/l) | 171.4 (126.0–291.8) | 267.2 (170.6–316.1) | 126 (38.6–154.5) | < 5 | < 0.01 |
| ESR (mm/h) | 62 (36.5–103.0) | 62 (42.0–112.0) | 70.5 (25.0–95.5) | < 13 | 0.99 |
| Sodium (mMol/l) | 130 (128–133) | 128.5 (127–131) | 133 (132–135) | 136–145 | 0.02 |
| Creatinine (mg/dl) | 0.52 (0.37–0.8) | 0.71 (0.48–1.06) | 0.35 (0.29–0.43) | < 0.6 | 0.04 |
| Albumin (g/dl) | 2.6 (2.3–3.1) | 2.5 (2.2–2.7) | 3.2 (3.1–3.8) | < 4.7 | < 0.01 |
| ALT (U/l) | 32 (20–56) | 40 (20–78) | 23 (18–33) | < 52 | 0.10 |
| AST (U/l) | 40 (33–90) | 44.5 (33–99) | 40 (29–64) | < 39 | 0.34 |
| LDH (U/l) | 397 (329–475) | 390 (330–447) | 432.5 (290–477) | < 271 | 0.77 |
| CK (U/l) | 133 (57–307) | 199 (64–554) | 90 (52–133) | < 233 | 0.07 |
| WBC (K/CUMM) | 13.3 (8.8–18.7) | 13.8 (9.6–18.6) | 9.9 (8.5–19.5) | 4.1–11.3 | 0.61 |
| ALC (K/CUMM) | 900 (600–1900) | 800 (600–1100) | 2100 (1100–3400) | 800–7900 | < 0.01 |
| Fibrinogen (mg/dl) | 488 (429–686) | 607 (433–687) | 480 (428–516) | < 466 | 0.55 |
| PT (s) | 12 (11.4–12.7) | 12.5 (11.4–13) | 11.5 (11.3–12) | < 11.7 | 0.10 |
| PTT (s) | 31.6 (30.1–33.5) | 31.8 (30.6–34.8) | 30.3 (29.6–31.1) | < 33.1 | 0.06 |
| d dimer (mg/l FEU) | 4.3 (2.2–7.9) | 5.1 (4.1–8.2) | 2.1 (1.5–3.2) | < 0.5 | 0.06 |
| Ferritin (ng/ml) | 382 (183.8–828.9) | 681.1 (380.3–869.1) | 121.6 (75.1–272.7) | < 336 | < 0.01 |
| Troponin (ng/l) | 96.5 (27–161) | 131.5 (89–437) | 26 (11–44) | < 17 | < 0.01 |
| BNP (pg/ml) | 966.5 (498–2028) | 1524.5 (555–2301) | 281.5 (25–566) | < 101 | 0.03 |
| IL-6 (pg/ml)* | 22 (15.5–91) | 22 (15.5–91) | N/A | < 6 | |
IQR interquartile, ESR erythrocyte sedimentation rate, ALT alanine aminotransferase, AST aspartate aminotransferase, LDH lactate dehydrogenase, CK creatine kinase, WBC white blood cells, ALC absolute lymphocyte count, PT prothrombin time, PTT partial thromboplastin time, BNP brain natriuretic peptide, IL-6 interleukin 6
#Italics indicate significant p values (≤ 0.05)
*Only available for 4 patients
ECG and telemetry monitoring showed frequent isolated premature ventricular contractions in one patient and accelerated junctional rhythm with atrioventricular dissociation in 4 patients. The arrhythmia resolved during inpatient stay and no arrhythmia was detected on follow-up outpatient ECG of all patients except for the patient who had premature ventricular contractions. The patients did not report any palpitations or syncope on outpatient follow-up. A 24-h Holter monitor was obtained during outpatient follow-up on the patient with persistent premature ventricular contractions. It showed isolated premature ventricular contraction burden of 11%.
Echocardiography was performed in all patients and showed depressed left ventricular function (ejection fraction < 55%) in 15/22 (68%) patients in group 1. Small or trace pericardial effusions were reported in 6 patients including 4 in group 1 and 2 in group 2. Dilatation of the coronary arteries (Z scores > 2.0) was detected in 5/22 (23%) patients in group 1. Three (3 months, 3 years, and 7 years) had clinical features that overlapped with KD, and the other 2 were teenagers who had concomitant severe left ventricular dysfunction (Table 3; patients #1, 3, 4, 10, and 13). The left main coronary artery (LMCA) was dilated in 4 patients, left anterior descending artery (LAD) in 3 patients, and right coronary artery in 1 patient. Coronary dilatation markedly improved or resolved in all patients on follow-up. No patient had coronary artery Z score > 2.5 on outpatient follow-up echocardiograms (Table 3).
Table 3.
Summary of 13 children with MIS-C who required intensive care and second-line therapy (infliximab/a second IVIG dose) following first-line therapy with IVIG
| Age/sex/ethnicity | KD-like features | COVID-19 tests | ECG findings | ECHO findings * | Anti-inflammatory treatment | Supportive care | Outcome/follow-up |
|---|---|---|---|---|---|---|---|
|
#1 17 year Male AA |
No | COVID-19 PCR-positive; COVID-19 IgG-positive | ECG: sinus rhythm with 1st-degree AV block, RBBB | SF 12.6%, EF 26.9% Z scores: LMCA 4.2, LAD 2.77, RCA 1.87 | IVIG on D#1 Infliximab on D#4 D#8 Pulse methylprednisolone 1 g IV daily × 3 | Mechanical ventilation (6 days) ECMO (4 days) Vasopressors (6 days) | Improved (LOS 22 days, PICU 18 days) ECHO D#77: NL anatomy and function. Z scores: LMCA 2.1, LAD 0.08, RCA 0.04 |
|
#2 14 years Female AA |
No | COVID-19 PCR-negative COVID-19 IgG-positive | ECG: accelerated junctional rhythm, prolonged QTc | SF 23.3%, EF 46.4% Z scores: LMCA 0.58, LAD − 0.41, RCA − 0.35 | IVIG on D#1 Infliximab on D#2 | Mechanical ventilation (4 days) Vasopressors (4 days) | Improved (LOS 14 days, PICU 7 days) ECHO D#26: NL |
| #3 3 months Female AA | Yes (classic) | COVID-19 PCR-positive COVID-19 IgG-NA | SF 36.1%, EF 68.3% Z scores: LMCA 2.2, LAD 3.72, RCA 0.39 | IVIG on D#1 Infliximab on D#3 | Improved (LOS 7 days, PICU 3 days) ECHO D#59: NL LV function. NL coronary arteries | ||
|
#4 3 years Male AA |
Yes (classic) | COVID-19 PCR-negative COVID-19 IgG-positive | SF 21%, EF 43.2% Z scores: LMCA 5.02, LAD 0.97, RCA − 0.88 | IVIG on D#1 IVIG D#4 Infliximab on D#6 | Vasopressors (2 days) | Improved (LOS 8 days, PICU 5 days) D#80 ECHO: NL function. NL coronary arteries | |
|
#5 7 years Male South Asian |
Yes (Incomplete) | COVID-19 PCR-negative COVID-19 IgG-negative COVID-19-exposed | ECG: PVCs and trigeminy | SF 36.4%, EF 67.2% Z scores: LMCA 0.32, LAD 0.66, RCA − 0.17 | IVIG on D#1 Infliximab on D#3 | Improved (LOS 7 days, PICU 3 days) ECHO D#65: NL | |
|
#6 8 years Female ME |
Yes (classic) | COVID-19 PCR-positive COVID-19 IG-positive | SF 30%, EF 58.2% Z scores: LMCA − 0.47, LAD − 0.43, RCA 0.59 | IVIG on D#1 Infliximab on D#3 | Vasopressors (4 days) Oxygen supplement (4 days) | Improved (LOS 10 days, PICU 4 days) ECHO D#73: NL | |
|
#7 6 years Male AA |
No | COVID-19 PCR-negative COVID-19 IgG-positive | ECG: accelerated junctional rhythm, borderline prolonged QTc | SF 26%, EF 51.7% Z scores: LMCA − 0.27, LAD 0.69, RCA − 0.22 | IVIG on D#1 Infliximab on D#2 | Vasopressors (2 days) Oxygen supplement (2 days) | Improved (LOS 5 days, PICU 2 days) ECHO D#24: NL EKG D#24: NL |
|
#8 9 years Female AA |
No | COVID-19 PCR-negative COVID-19 IgG-positive | SF 17.2%, EF 36.5% Z scores: LMCA − 0.47, LAD − 0.43, RCA 0.59 | IVIG on D#1 Infliximab on D#2 | Vasopressors (3 days) Oxygen supplement (1 days) | Improved (LOS 5 days, PICU 3 days) ECH0 D#73: NL | |
|
#9 11 years Male AA |
No | COVID-19 PCR-negative COVID-19 IgG-positive | SF 25.7%, EF 50.8% Z scores: LMCA 0.87, LAD 1.48, RCA 1.69 | IVIG on D#1 Infliximab on D#3 | Mechanical ventilation (5 days) Vasopressors (4 days) | Improved (LOS 9 days, PICU 7 days) ECHO D#67: NL | |
|
#10 15 years Male AA |
Yes (incomplete) | COVID-19 PCR-positive COVID-19 IgG-positive | ECG: accelerated junctional rhythm | SF 8.1%, EF 17.5% Z scores: LMCA 0.32, LAD 1.17, RCA 3.18 | IVIG on D#1 Infliximab on D#2 | Mechanical ventilation (5 days) Vasopressors (6 days) | Improved (LOS 8 days, PICU 8 days) ECHO D#164: EF 58.3%. Z score: LMCA 1.21, RCA 1.51 |
|
#11 10 years Female AA |
No | COVID-19 PCR-neagtive COVID-19 IgG-positive | SF 24.1%, EF 48.2% Z scores: LMCA 0.32, LAD 0.63, RCA 0.51 | IVIG on D#1 Infliximab on D#2 | Vasopressors (6 days) Oxygen supplement (6 days) | Improved (LOS 10 days, PICU 7 days) ECHO D#50: NL | |
|
#12 7 years Female AA |
No | COVID-19 PCR-negative COVID-19 IgG-positive | SF 38.8%, EF 69.9% Z scores: LMCA − 0.46, LAD − 0.13, RCA − 0.14 | IVIG on D#1 Infliximab on D#3 | Improved (LOS 7 days, PICU 3 days) | ||
|
#13 7 years Male AA |
Yes (classic) | COVID-19 PCR-negative COVID-19 IgG-positive | SF 25.9%, EF 51.1% Z scores: LMCA 2.37, LAD 0.65, RCA 0.79 | IVIG on D#1 IVIG on D#3 | Mechanical ventilation (5 days) Vasopressors (4 days) | Improved (LOS 13 days, PICU 8 days) D#45 ECHO: EF 61.6%. Z scores: LMCA 1.4. LAD − 1.7. RCA − 2.7. |
MIS-C patients with dilated coronary arteries are numbered in bold IVIG intravenous immunoglobulins, D# day of PICU admission/IVIG administration, ECHO echocardiogram, SF shortening fraction, EF ejection fraction, LMCA left main coronary artery, LAD left anterior descending artery, RCA right coronary artery, ECMO extracorporeal membrane oxygenation, NL normal, LV left ventricle, RBBB right bundle branch block, PE pericardial effusion, NA not available, ECG electrocardiogram, PVC premature ventricular contraction, LOS length of hospital stay, PICU pediatric intensive care unit, AA African American, ME Middle Eastern *Significant echocardiographic findings during hospitalization; no coronary aneurysms were detected
First-line anti-inflammatory treatment was intravenous immunoglobulins (IVIG) at a dose of 2 g/kg which was given to all patients in group 1 and to 7/11 (64%) patients in group 2. For obese patients, the dose of IVIG was adjusted for the weight that correlates with the height percentile in standard CDC growth charts. Second-dose IVIG therapy was given to 2 patients in group 2 due to persistent fever 48 h after first infusion, and one received infliximab after second IVIG infusion. Thirteen (59%) patients in group 1 received second-line therapy due to persistent fever and severe inflammatory state as evidenced by rising inflammatory markers or due to severely depressed myocardial systolic function on presentation. All 5 patients who had coronary artery dilatation required second-line therapy. High-dose infliximab (10 mg/kg) was given to 12/13 (92%) of patients who required second-line therapy, and 2/13 (15%) received repeat IVIG infusion. One patient who required V-A ECMO also received pulse methylprednisolone therapy (1 g daily × 3 days) due to persistent fever and persistent myocardial dysfunction following IVIG and infliximab therapy (Table 3, patient #1). The other patient who required V-A ECMO was initially treated with IVIG concomitantly with a 5-day course of IV methylprednisolone at 2 mg/kg/day for 5 days. Details of clinical findings, timeline of second-line therapy, and echocardiographic findings in relation to therapy are included in Table 3. All patients who received anti-inflammatory therapy with IVIG or infliximab were also given moderate-dose aspirin (30–50 mg/kg) until afebrile for 48 to 72 h. This was followed by low-dose aspirin (3–5 mg/kg) once daily.
Antiviral therapy with remdesivir was given to 2 patients who tested positive for SARS-CoV-2 RT-PCR and who also required V-A ECMO. Both were diagnosed with severe MIS-C and were not suffering from adult-type COVID-19. Both had radiological findings that were more likely due to vascular congestion than primary pulmonary involvement and both tested positive for SARS-CoV-2 IgG. Concomitant infections were rare. Rapid group A Streptococcus antigen test was positive in 2 patients in group 1. Blood cultures were obtained from all patients; all but one was negative (patient #2, Table 3). Antibiotics were given to 27/33 (82%) patients including all but one patient in group 1. The most common antibiotics used were ceftriaxone and clindamycin. The BioFire FilmArray Respiratory Panel (Biofire Diagnostics, Salt Lake City, UT, USA) was tested in 18/33 (55%) study patients and all were negative.
All study patients had favorable outcome, and all were discharged home in stable condition. At time of discharge, all had normal ventricular function except one who had mild decrease in ejection fraction which normalized after 2 weeks. Complications were noted in one patient (#1, Table 3) who developed a hematoma and thrombosis of the internal jugular vein at the site V-A ECMO cannulation, hypertension, and stress-related neuropsychiatric problems related to his PICU stay. Hematoma formation in this patient was likely due to concomitant use of enoxaparin and aspirin after decannulation in the setting of systemic hypertension. No other thrombotic or embolic events were diagnosed in other patients. Two patients were noted to have increased left ventricular mass on repeat echocardiography (Table 3; patients #1 and #4) likely due to myocardial edema, but this has resolved with subsequent follow-up. No adverse drug reactions or infectious complications were attributed to infliximab use. For group 1 patients, the median PICU stay was 4.5 days (IQR 2–7 days), and the median hospital stay was 7.5 days (IQR 5–9.25 days). For group 2, the median hospital stay was 4 days (IQR 2–7 days).
Discussion
Our study patients with MIS-C had a wide spectrum of clinical presentations varying from mild, self-limited disease to severe systemic inflammation. However, myocardial dysfunction was a consistent finding in patients with severe inflammation. Children with MIS-C who required critical care were older than those who had less severe disease. Gastrointestinal manifestations were common. Five of 22 (23%) MIS-C patients who required critical care also developed coronary dilatation. Infliximab was used successfully as a second-line anti-inflammatory therapy in MIC-S patients who required critical care. All patients in our study had a favorable outcome including resolution of coronary artery dilatation. Of note, only two patients in our study received steroid therapy. Recent reports from the USA and other countries around the world indicate a rise in the number of cases of MIS-C. The syndrome has overlapping features with toxic shock syndrome (TSS), classic and incomplete KD, and macrophage activation syndrome (MAS) [6–8, 15–17]. The severity of illness in our critically ill patients was higher than other reports [17, 18]. This was demonstrated by 27% receiving mechanical ventilation, 73% requiring inotropic support, and 9% receiving ECMO.
The majority (76%) of children with MIS-C in our study were African American including 18/22 (82%) who required intensive care management. Other studies have also reported increased prevalence of MIS-C in ethnic minorities especially among African ethnicities [15]. It is however unclear whether the increased prevalence MIS-C in African Americans is due to genetic predisposition versus a reflection of widespread SARS-CoV-2 infection in their communities. In contrast, despite widespread infection with SARS-CoV-2 in China, South Korea, and Japan in the early months of the pandemic, there have been no reports of increased KD or MIS-C cases in Asian populations that are known to have higher incidence of KD than other ethnic groups [12, 19].
The clinical manifestation showed predominance of abdominal symptoms rather than chest pain or primary respiratory complaints. Abdominal pain was common in our patients and was present in 68% of patients who were admitted to PICU. GI complications were the presenting symptoms in 84% of cases in one study and often accompanied by fever and skin rash in 100% and 71% of patients respectively [20]. Findings on abdominal imaging in our patients included enlarged lymph nodes, hepatosplenomegaly, mesenteric adenitis, acalculous cholecystitis, pancreatitis, ascites, and bowel wall thickening and inflammation. These findings are not typically seen in KD. An 8-year-old boy in group 2 presented with prolonged fever, loss of taste, and large abdominal lymph nodes concerning for malignancy without other clinical features or cardiovascular manifestations. MIS-C should be considered in patients who present with significant GI symptoms with recent SARS-CoV-2 infection or exposure. The prodromal GI symptoms, clinical signs of peritoneal inflammation, and abdominal lymph node enlargement raise concerns of viral replication occurring in the GI tract. The pathophysiological mechanism of GI involvement is postulated to be related to the affinity of the SARS-CoV-2 to angiotensin-converting enzyme-2 receptor (ACE2) which is located in multiple human cells including enterocytes in the ilium and colon [21]. ACE2 receptor, which in part mediates inflammation, may be upregulated during SARS-CoV-2 infection [21]. GI involvement is present in most patients with MIS-C and any segment of the GI tract may be affected. However, the inflammation predominates in the terminal ilium and colon [22]. Sahn et al. reported GI involvement in 34/35 patients with MIS-C with more than 50% of those who had abdominal CT imaging showing terminal ileitis and bowel wall thickening, features that are similar to inflammatory bowel disease [22]. Bowel obstruction was reported in 2/19 (10.5%) patients with MIS-C who warranted imaging including one who required ileocolic resection due to worsening intestinal obstruction [22]. Histopathology revealed transmural lymphocytic inflammation and the ileocolic mass revealed necrotizing lymphadenitis [22].
Severe myocarditis may occur in the late phase of adult COVID-19 which is characterized by cytokine storm, hyperinflammation, and multiorgan damage including myocarditis and laboratory findings suggestive of MAS or TSS [23]. In contrast, the rapid resolution of systolic myocardial dysfunction in our MIS-C patients suggests that the mechanism of heart failure is likely secondary to systemic inflammatory response causing myocardial edema and stunning, rather than primary myocardial injury as seen in adults with COVID-19 [7]. In addition, the frequent detection of IgG antibody to SARS-CoV-2 with rare detection of the virus by PCR in nasopharyngeal samples suggest that MIS-C is a post-infectious inflammatory process in contrast to the progressively worsening acute infection in adult patients [7, 24].
Children with MIS-C who develop coronary artery dilatation may represent a subset of patients with a more severe phenotype who need close monitoring and long-term cardiac follow-up of coronary artery abnormalities as well as subtle structural abnormalities and function. In a recent report from the UK, Whittaker et al. have not found any difference in clinical or laboratory markers among patients with MIS-C who developed coronary artery dilatation when compared to those who did not develop coronary abnormalities [8]. Because the long-term prognosis of patients across the spectrum of MIS-C presentations is unclear, sequential echocardiography may be advisable for all patients as recommended for KD patients [8].
The levels of CRP and other markers of inflammation in MIS-C patients are higher than those seen in KD patients [6, 7]. In our study, patients who required critical care were found to have significantly higher levels of CRP, ferritin, and d dimers as well as troponin I and BNP levels than those with milder or self-limited inflammation. In addition, hyponatremia, AKI, lymphopenia, and hypoalbuminemia were more common among PICU patients than those with less severe disease. These laboratory findings may serve as markers to predict the progression of disease.
All of our 22 PICU study patients and 7/11 of those with less severe inflammation were treated with IVIG (2 g/kg). Like other reports, some of our patients were treated with supportive care alone [8]. Some received IVIG treatment due to the diagnosis of complete or incomplete KD. The use of second-line therapy was common among PICU patients likely due to the intense inflammation that was not adequately controlled by IVIG. Second-line therapy with high-dose infliximab (10 mg/kg) was used in 12/22 PICU patients and was successful in controlling the inflammatory process as evidenced by resolution of fever, improvement of cardiac function, and improvement of coronary artery dilatation (Table 3). Patients in different MIS-C reports were treated with different immunomodulatory mediations including IVIG, corticosteroids, anakinra, tocilizumab, and infliximab [7, 8, 15–17, 25]. However, details were not reported regarding indications, time, and relation to echocardiographic changes. In addition, the clinical response of patients to each regimen has not been reported. Treatment regimen was not controlled or randomized in our case series, but we followed a standardized protocol where patients who require systemic anti-inflammatory therapy will receive IVIG. Second-line therapy with high-dose infliximab was given to patients with persistent fever and inflammation, including those with myocardial dysfunction that failed to improve after IVIG. If further therapy was needed, pulse steroid therapy with methylprednisolone 30 mg/kg daily (max dose 1 g) for 1–3 days was given [26]. All patients who require anti-inflammatory therapy were given aspirin as directed by AHA recommendation for treatment of KD [13].
Timely administration of anti-inflammatory therapy may be essential to prevent complications and need for escalation of supportive care. Of note, the 2 patients with the most severe presentation and who required ECMO were the earliest patients who were admitted to our PICU. One received IVIG and the other (Table 3, #1) received infliximab on day 4. Subsequently, we had 3 more patients with severe myocardial dysfunction who were considered for ECMO therapy. All 3 received high-dose infliximab immediately after IVIG infusion which may have resulted in faster resolution of fever and inflammation and subsequently averted the need for ECMO. Our findings suggest that adjunctive anti-inflammatory therapy besides IVIG should be considered early in the management of patients with intense inflammation and severe myocardial dysfunction.
The lack of adequate response to IVIG in cases with intense inflammation in our case series warrants further evaluation of the inflammatory pathways involved in MIS-C pathogenesis. Although MIS-C seems to be a distinct entity from KD, the similarities in clinical presentations including cardiac involvement and response to treatment provide information on the possible underlying mechanisms of both disorders [8, 12]. Our patients who were refractory to IVIG therapy responded to infliximab, a TNF-α blocker that has been used successfully to treat IVIG refractory KD [27]. TNF-α, a proinflammatory cytokine, has been demonstrated to be associated with severe KD complications [28]. The levels of many proinflammatory cytokines, chemokines, and soluble adhesion molecules can be elevated in the acute stage KD [29–31]. The IL-6 level was elevated in patients MIS-C as well as other cytokines such as IL-8 and TNF-α [7, 25]. The role of different cytokines in the pathogenesis of MIS-C remains to be determined. However, two recent studies have demonstrated TNF-α to be particularly elevated in MIS-C and was an immunological marker that discriminated between MIS-C from severe COVID-19 patients [32, 33].
In conclusion, our large single-center experience with MIS-C suggests that the clinical manifestations may overlap with KD. However, the inflammatory process is likely unique to SARS-CoV-2 infection and is characterized by reversible myocardial dysfunction and/or coronary artery dilatation in some children. Certain laboratory markers such as elevated serum CRP, ferritin, or d dimer; low albumin, sodium, or ALC may be predictors of more severe disease manifestations. Supportive care, IVIG, and second-line therapy with infliximab were associated with excellent outcome in our patients including those with high disease severity. Treatment with a single dose of infliximab may provide an effective alternative to a prolonged and tapering course of glucocorticoids.
Abbreviations
- AHA
American Heart Association
- ALC
Absolute lymphocyte count
- ALT
Alanine aminotransferase
- AST
Aspartate aminotransferase
- BNP
Brain natriuretic peptide
- BUN
Blood urea nitrogen
- CBC
Complete blood count
- CDC
Centers for Disease Control and Prevention
- CK
Creatine kinase
- COVID-19
Coronavirus disease 2019
- CRP
C-reactive protein
- CT
Computed tomography
- ECG
Electrocardiogram
- ECMO
Extracorporeal membrane oxygenation
- ESR
Erythrocyte sedimentation rate
- GI
Gastrointestinal
- IL
Interleukin
- IQR
Interquartile
- IVIG
Intravenous immunoglobulin
- KD
Kawasaki disease
- KDSS
Kawasaki disease shock syndrome
- LDH
Lactate dehydrogenase
- LMCA
Left main coronary artery
- MAS
Macrophage activation syndrome
- PICU
Pediatric intensive care unit
- PT
Prothrombin time
- PTT
Partial thromboplastin time
- MIS-C
Multisystem inflammatory syndrome in children
- RT-PCR
Reverse transcription-polymerase chain reaction
- SARS-CoV-2
Severe acute respiratory syndrome coronavirus 2
- TNF
Tumor necrosis factor
- TSS
Toxic shock syndrome
Authors’ contributions
NAH: Conception or design of the work, data collection, data analysis and interpretation, drafting, and finalizing the article. BIA: Conception or design of the work, critical revision of the first draft of the manuscript, and contribution with important intellectual content. MPDL: Data collection, data analysis, and interpretation. EJM, HSA, CK, BT, ACE, and US: Critical revision of the first draft of the manuscript and contribution with important intellectual content. JYA: Conception or design of the work, critical revision of the article, and contribution with important intellectual content. All the authors approved the final version of the manuscript to be published and agree to be accountable for all aspects of the work.
Data availability
N/A
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
The study was approved by the Wayne State University Institutional Review Board (IRB-20-04-2115). A waiver of consent was granted.
Consent to participate
N/A
Consent for publication
N/A
Code availability
N/A
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Nahed Abdel-Haq, Email: nabdel@dmc.org.
Basim I. Asmar, Email: basmar@wayne.edu
Maria P. Deza Leon, Email: mdezaleo@dmc.org
Eric J. McGrath, Email: emcgrath@med.wayne.edu
Harbir S. Arora, Email: harora@dmc.org
Katherine Cashen, Email: kcashen@dmc.org.
Bradley Tilford, Email: btilford@dmc.org.
Ahmad Charaf Eddine, Email: acharafe@dmc.org.
Usha Sethuraman, Email: usethura@dmc.org.
Jocelyn Y. Ang, Email: jang@dmc.org
References
- 1.Zhu N, Zhang D, Wang W, Li X, Yang B, Song J, Zhao X, Huang B, Shi W, Lu R, Niu P, Zhan F, Ma X, Wang D, Xu W, Wu G, Gao GF, Tan W. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 2020;382:727–733. doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shi S, Qin M, Shen B, Cai Y, Liu T, Yang F, Gong W, Liu X, Liang J, Zhao Q, Huang H, Yang B, Huang C. Association of cardiac injury with mortality in hospitalized patients with COVID-19 in Wuhan, China. JAMA Cardiol. 2020;5:802. doi: 10.1001/jamacardio.2020.0950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Liu W, Zhang Q, Chen J, Xiang R, Song H, Shu S, Chen L, Liang L, Zhou J, You L, Wu P, Zhang B, Lu Y, Xia L, Huang L, Yang Y, Liu F, Semple MG, Cowling BJ, Lan K, Sun Z, Yu H, Liu Y. Detection of Covid-19 in children in early January 2020 in Wuhan, China. N Engl J Med. 2020;382:1370–1371. doi: 10.1056/NEJMc2003717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.CDC Coronavirus Disease 2019 in children - United States, February 12-April 2, 2020. MMWR Morb Mortal Wkly Rep. 2020;69:422–426. doi: 10.15585/mmwr.mm6914e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Xu Y, Li X, Zhu B, Liang H, Fang C, Gong Y, Guo Q, Sun X, Zhao D, Shen J, Zhang H, Liu H, Xia H, Tang J, Zhang K, Gong S. Characteristics of pediatric SARS-CoV-2 infection and potential evidence for persistent fecal viral shedding. Nat Med. 2020;26:502–505. doi: 10.1038/s41591-020-0817-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Verdoni L, Mazza A, Gervasoni A, Martelli L, Ruggeri M, Ciuffreda M, Bonanomi E, D’Antiga L. An outbreak of severe Kawasaki-like disease at the Italian epicentre of the SARS-CoV-2 epidemic: an observational cohort study. Lancet. 2020;395:1771–1778. doi: 10.1016/S0140-6736(20)31103-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Belhadjer Z, Méot M, Bajolle F, Khraiche D, Legendre A, Abakka S, Auriau J, et al. Circulation. 2020. Acute heart failure in multisystem inflammatory syndrome in children (MIS-C) in the context of global SARS-CoV-2 pandemic. [DOI] [PubMed] [Google Scholar]
- 8.Whittaker E, Bamford A, Kenny J, Kaforou M, Jones CE, Shah P, Ramnarayan P, Fraisse A, Miller O, Davies P, Kucera F, Brierley J, McDougall M, Carter M, Tremoulet A, Shimizu C, Herberg J, Burns JC, Lyall H, Levin M (2020) Clinical characteristics of 58 children with a pediatric inflammatory multisystem syndrome temporally associated with SARS-CoV-2. JAMA 324(3):259–69 [DOI] [PMC free article] [PubMed]
- 9.Royal College of Paediatrics and Child Health-UK (2020) Guidance - paediatric multisystem inflammatory syndrome temporally associated with COVID-19 (PIMS). https://www.rcpch.ac.uk/resources/guidance-paediatric-multisystem-inflammatory-syndrome-temporally-associatedcovid-19-pims. Accessed 26 June 2020
- 10.New York State Department of Health (NYS DOH) Bureau of Communicable Disease Control (2020) Health advisory: pediatric multisystem inflammatory syndrome potentially associated with Coronavirus Disease (COVID-19) in children. https://www.health.ny.gov/press/releases/2020/docs/2020-05-06_covid19_pediatric_inflammatory_syndrome.pdfAccessed 26 June 2020
- 11.CDC (2020) Information for healthcare providers about multisystem inflammatory syndrome in children (MIS-C). https://www.cdc.gov/mis-c/hcp/. Accessed 28 June 2020
- 12.Loke YH, Berul CI, Harahsheh AS. Multisystem inflammatory syndrome in children: is there a linkage to Kawasaki disease? Trends Cardiovasc Med. 2020;30:389–396. doi: 10.1016/j.tcm.2020.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McCrindle BW, Rowley AH, Newburger JW, Burns JC, Bolger AF, Gewitz M, Baker AL, Jackson MA, Takahashi M, Shah PB, Kobayashi T, Wu MH, Saji TT, Pahl E. Diagnosis, treatment, and long-term management of Kawasaki disease: a scientific statement for health professionals from the American Heart Association. Circulation. 2017;135:e927–e999. doi: 10.1161/CIR.0000000000000484. [DOI] [PubMed] [Google Scholar]
- 14.McCrindle BW, Li JS, Minich LL, Colan SD, Atz AM, Takahashi M, Vetter VL, Gersony WM, Mitchell PD, Newburger JW. Coronary artery involvement in children with Kawasaki disease: risk factors from analysis of serial normalized measurements. Circulation. 2007;116:174–179. doi: 10.1161/CIRCULATIONAHA.107.690875. [DOI] [PubMed] [Google Scholar]
- 15.Riphagen S, Gomez X, Gonzalez-Martinez C, Wilkinson N, Theocharis P. Hyperinflammatory shock in children during COVID-19 pandemic. Lancet. 2020;395:1607–1608. doi: 10.1016/S0140-6736(20)31094-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chiotos K, Bassiri H, Behrens EM, Blatz AM, Chang J, Diorio C, Fitzgerald JC, Topjian A, John ARO (2020) Multisystem inflammatory syndrome in children during the COVID-19 pandemic: a case series. J Pediatr Infect Dis Soc 9(3):393–8 [DOI] [PMC free article] [PubMed]
- 17.Feldstein LR, Rose EB, Horwitz SM, Collins JP, Newhams MM, Son MBF, Newburger JW, Kleinman LC, Heidemann SM, Martin AA, Singh AR, Li S, Tarquinio KM, Jaggi P, Oster ME, Zackai SP, Gillen J, Ratner AJ, Walsh RF, Fitzgerald JC, Keenaghan MA, Alharash H, Doymaz S, Clouser KN, Giuliano JS Jr, Gupta A, Parker RM, Maddux AB, Havalad V, Ramsingh S, Bukulmez H, Bradford TT, Smith LS, Tenforde MW, Carroll CL, Riggs BJ, Gertz SJ, Daube A, Lansell A, Coronado Munoz A, Hobbs CV, Marohn KL, Halasa NB, Patel MM, Randolph AG, Overcoming COVID-19 Investigators. CDC COVID-19 Response Team Multisystem inflammatory syndrome in U.S. children and adolescents. N Engl J Med. 2020;383:334–346. doi: 10.1056/NEJMoa2021680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cheung EW, Zachariah P, Gorelik M, Boneparth A, Kernie SG, Orange JS, Milner JD (2020) Multisystem inflammatory syndrome related to COVID-19 in previously healthy children and adolescents in New York City. JAMA 324(3):294–296 [DOI] [PMC free article] [PubMed]
- 19.Shulman ST (2020) Pediatric COVID-associated multi-system inflammatory syndrome (PMIS). J Pediatr Infect Dis Soc 9(3):285–286 [DOI] [PMC free article] [PubMed]
- 20.Miller J, Cantor A, Zachariah P, Ahn D, Martinez M, Margolis K. Gastrointestinal symptoms as a major presentation component of a novel multisystem inflammatory syndrome in children (MIS-C) that is related to COVID-19: a single center experience of 44 cases. Gastroenterology. 2020;159:1571–1574.e2. doi: 10.1053/j.gastro.2020.05.079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Agarwal A, Chen A, Ravindran N, To C. Thuluvath PJ. Gastrointestinal and liver manifestations of COVID-19. J Clin Exp Hepatol. 2020;10:263–265. doi: 10.1016/j.jceh.2020.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sahn B, Eze OP, Edelman MC, Chougar CE, Thomas RM, Schleien CL, Weinstein T (2020) Features of intestinal disease associated with COVID-related multisystem inflammatory syndrome in children. J Pediatr Gastroenterol Nutr Publish Ahead of Print [DOI] [PMC free article] [PubMed]
- 23.Akhmerov A, Marbán E. COVID-19 and the heart. Circ Res. 2020;126:1443–1455. doi: 10.1161/CIRCRESAHA.120.317055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hendren NS, Drazner MH, Bozkurt B, Cooper LT., Jr Description and proposed management of the acute COVID-19 cardiovascular syndrome. Circulation. 2020;141:1903–1914. doi: 10.1161/CIRCULATIONAHA.120.047349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Waltuch T, Gill P, Zinns LE, Whitney R, Tokarski J, Tsung JW, Sanders JE. Features of COVID-19 post-infectious cytokine release syndrome in children presenting to the emergency department. Am J Emerg Med. 2020;38:2246.e3–2246.e6. doi: 10.1016/j.ajem.2020.05.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dove ML, Jaggi P, Kelleman M, Abuali M, Ang JY, Ballan W, Basu SK, Campbell MJ, Chikkabyrappa SM, Choueiter NF, Clouser KN, Corwin D, Edwards A, Gertz SJ, Ghassemzadeh R, Jarrah RJ, Katz SE, Knutson SM, Kuebler JD, Lighter J, Mikesell C, Mongkolrattanothai K, Morton T, Nakra NA, Olivero R, Osborne CM, Panesar LE, Parsons S, Patel RM, Schuette J, Thacker D, Tremoulet AH, Vidwan NK, Oster ME (2020) Multisystem inflammatory syndrome in children: survey of protocols for early hospital evaluation and management. J Pediatr S0022–3476(20):31293–2
- 27.Mori M, Hara T, Kikuchi M, Shimizu H, Miyamoto T, Iwashima S, Oonishi T, Hashimoto K, Kobayashi N, Waki K, Suzuki Y, Otsubo Y, Yamada H, Ishikawa C, Kato T, Fuse S. Infliximab versus intravenous immunoglobulin for refractory Kawasaki disease: a phase 3, randomized, open-label, active-controlled, parallel-group, multicenter trial. Sci Rep. 2018;8:1994. doi: 10.1038/s41598-017-18387-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Maury CP, Salo E, Pelkonen P. Elevated circulating tumor necrosis factor-alpha in patients with Kawasaki disease. J Lab Clin Med. 1989;113:651–654. [PubMed] [Google Scholar]
- 29.Furukawa S, Matsubara T, Jujoh K, Yone K, Sugawara T, Sasai K, Kato H, Yabuta K. Peripheral blood monocyte/macrophages and serum tumor necrosis factor in Kawasaki disease. Clin Immunol Immunopathol. 1988;48:247–251. doi: 10.1016/0090-1229(88)90088-8. [DOI] [PubMed] [Google Scholar]
- 30.Lin CY, Lin CC, Hwang B, Chiang B. Serial changes of serum interleukin-6, interleukin-8, and tumor necrosis factor alpha among patients with Kawasaki disease. J Pediatr. 1992;121:924–926. doi: 10.1016/S0022-3476(05)80343-9. [DOI] [PubMed] [Google Scholar]
- 31.Schiller B, Elinder G. Inflammatory parameters and soluble cell adhesion molecules in Swedish children with Kawasaki disease: relationship to cardiac lesions and intravenous immunoglobulin treatment. Acta Paediatr (Oslo Norway) 1999;1992(88):844–848. doi: 10.1080/08035259950168766. [DOI] [PubMed] [Google Scholar]
- 32.Diorio C, Henrickson SE, Vella LA, McNerney KO, Chase JM, Burudpakdee C, Lee JH, et al. Multisystem inflammatory syndrome in children and COVID-19 are distinct presentations of SARS-CoV-2. J Clin Invest. 2020;130:5967–5975. doi: 10.1172/JCI140970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pouletty M, Borocco C, Ouldali N, Caseris M, Basmaci R, Lachaume N, Bensaid P, Pichard S, Kouider H, Morelle G, Craiu I, Pondarre C, Deho A, Maroni A, Oualha M, Amoura Z, Haroche J, Chommeloux J, Bajolle F, Beyler C, Bonacorsi S, Carcelain G, Koné-Paut I, Bader-Meunier B, Faye A, Meinzer U, Galeotti C, Melki I. Paediatric multisystem inflammatory syndrome temporally associated with SARS-CoV-2 mimicking Kawasaki disease (Kawa-COVID-19): a multicentre cohort. Ann Rheum Dis. 2020;79:999–1006. doi: 10.1136/annrheumdis-2020-217960. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
N/A