Abstract
Coronavirus disease (COVID-19) caused by novel severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is an ongoing pandemic and presents a public health emergency. It has affected millions of people and continues to affect more, despite the tremendous social preventive measures. The therapeutic strategy relies on suppressing infectivity and inflammation, along with immune modulation. The identification of candidate drugs effective for COVID-19 is crucial, thus many natural products including phytochemicals are also being proposed for repurposing and evaluated for their potential in COVID-19. Among numerous phytochemicals, limonene (LMN), a dietary terpene of natural origin has been recently showed to target viral proteins in the in-silico studies. LMN is one of the main compounds identified in many citrus plants, available and accessible in diets and well-studied for its therapeutic benefits. Due to dietary nature, relative safety and efficacy along with favorable physicochemical properties, LMN has been suggested to be a fascinating candidate for further investigation in COVID-19. LMN showed to modulate numerous signaling pathways and inhibits inflammatory mediators, including cytokines, chemokines, adhesion molecules, prostanoids, and eicosanoids. We hypothesized that given the pathogenesis of COVID-19 involving infection, inflammation, and immunity, LMN may have potential to limit the severity and progression of the disease owing to its immunomodulatory, anti-inflammatory, and antiviral properties. The present article discusses the possibilities of LMN in SARS-CoV-2 infections based on its immunomodulatory, anti-inflammatory, and antiviral properties. Though, the suggestion on the possible use of LMN in COVID-19 remains inconclusive until the in-silico effects confirmed in the experimental studies and further proof of the concept studies. The candidature of LMN in COVID-19 treatment somewhat appear speculative but cannot be overlooked provided favorable physiochemical and druggable properties. The safety and efficacy of LMN are necessary to be established in preclinical and clinical studies before making suggestions for use in humans.
Keywords: COVID-19, SARS-CoV-2, Limonene, Terpenes, Essential oils, Immunomodulators, Nutrition, Pharmaceutical science, Physiology, Pharmacology, Immunology, Toxicology, Alternative medicine, Evidence-based medicine
COVID-19; SARS-CoV-2; Limonene; Terpenes; Essential Oils; Immunomodulators; Nutrition; Pharmaceutical Science; Physiology; Pharmacology; Immunology; Toxicology; Alternative Medicine; Evidence-Based Medicine.
1. Introduction
The severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection, termed coronavirus disease 2019 (COVID-19), is an ongoing pandemic and represents a public health emergency; it has affected millions and continues to affect more despite tremendous social preventive measures. So far, there is no cure for the disease, and no specific medicine or vaccine is available for its treatment except supportive management [1, 2]. The current pace of research for finding therapeutic agents for SARS-CoV-2 with regard to the repurposing of drugs has been a remarkable approach in drug discovery. Identifying candidate drugs effective for COVID-19 is crucial until the development of a vaccine against it. Currently, numerous efforts are ongoing to accelerate the discovery and development of preventive and effective therapeutic molecules against SARS-CoV-2 infections [3]. In the past few months since the emergence of COVID-19, several compounds have emerged as promising alternatives such as remdesivir, lopinavir, ritonavir, interferon-β, ribavirin, chloroquine/hydroxychloroquine, azithromycin, tocilizumab, and ivermectin [4]. The repurposing of drugs mainly concentrated on antivirals, antibiotics, anti-inflammatory and immunomodulatory agents [5]. The use of the above-mentioned drugs in COVID-19 patients is mostly empirical because of the lack of randomized controlled trials demonstrating their efficacy and safety. Considering the emergence of COVID-19-related mortality, effective medications are required to improve the prognosis of patients and curb the spread of the virus [5]. From a pharmacological perspective, all these drugs have the potential to block the virus from entering host cells, prevent viral replication, and/or attenuate the exacerbation of the host's immune response [5].
The clinical manifestations of COVID-19 range from mild to severe disease with the extensive involvement of the lungs, from pneumonia to acute respiratory distress syndrome (ARDS), acute liver injury, acute cardiac injury, and neurological manifestations that may lead to multi-organ failure with poor prognosis [6, 7]. Severe lung disease with extensive alveolar damage and progressive respiratory failure leads to deadly outcomes [8]. The fatalities are higher in elderly people with cardiometabolic diseases, cancer, those that are immunocompromised, or with co-morbidities. A recently published report showed a case with significant interstitial lymphocyte infiltrates in the lung tissue, lymphopenia, and hyperactivation of T-cells in peripheral blood [6, 7]. Inflammatory and immune responses are important to eliminate infection, but may have a significant impact on SARS-CoV-2 pathogenesis, and may play a role in the expression of the clinical spectrum of COVID-19 disease [9]. Following SARS-CoV-2 infections, abnormal release of cytokines and pro-inflammatory molecules are closely related to lung injury [6, 7]. Such uncontrolled release of cytokines, namely interleukin (IL)-1β, IL-6, and monocyte chemoattractant protein (MCP)-1, coupled with a decreased number of natural killer cells may result in the so-called “cytokine storm” [10]. Immune dysregulation, rather than viremia levels per se, has been related to the massive pro-inflammatory cytokine secretion by alveolar macrophages and subsequent CD4+ and CD8+ T cell dysfunction observed in SARS-CoV-2 infection [11]. Hence, until specific vaccines become available, the use of antiviral agents alone may not be sufficient to stop the cytokine storm and respiratory distress in severely ill patients. In an attempt to reduce overall mortality, it is therefore essential to identify new therapeutics capable of preventing or mitigating the cytokine storm and sequelae. Immunomodulators of natural origin could be valuable for use as preventive as well as therapeutic adjuvant in mitigating the cytokine storm and associated sequelae. COVID-19-driven complications and their pathogenesis mainly involve the immune-inflammatory cascade; therefore, the available approaches emphasize this cascade to reduce inflammation and immune modulation [9, 12]. Several research projects are ongoing across pharmaceutical, biotechnological, and academic sectors for the discovery of novel drugs, as well as vaccines, for COVID-19.
Among the numerous therapeutic avenues to be explored, natural products have received much attention owing to the increased application of their anti-inflammatory, immunomodulatory, and antioxidant properties; moreover, they are regarded as important sources of new antimicrobial, anti-inflammatory, and immunomodulatory compounds [13, 14]. Essential oils (EOs) are known for their potent antiviral, anti-inflammatory, and immunomodulatory activities [15, 16]. Given the interplay between viral infections and the immune-inflammatory axis, EOs appear to be promising therapeutic agents in the interference with the redox immune-inflammatory cascade as they could play a significant role in the regulation of immune-inflammatory processes in the airways [15, 16]. Since ancient times, EOs have been used in traditional medicine and food and have proven safety and efficacy. They have shown beneficial effects in numerous diseases and exert systemic effects; therefore, they represent potential candidates for the management and treatment of COVID-19 [17, 137]. EOs are complex mixtures of lipophilic and volatile terpene compounds predominantly found in aromatic plants. These are commonly consumed in diets and are known to exert potent antimicrobial, anti-inflammatory, immunomodulatory, and antioxidant properties [18]. Several experimental and a few clinical studies have demonstrated that EOs could be valuable agents for the treatment of immune system-related diseases [15, 16]. EOs alone or in combination are also very well indicated for the treatment of respiratory infections caused by bacteria or viruses. These are believed to be safe and synergistic with potent pharmacological effects, including antihistamine and antioxidant effects [19]. Among the many constituents of EOs, one of the most common compounds that have received attention is limonene (LMN), due to its action specifically on SARS-CoV-2. A recent study showed that LMN obtained from the EOs of geranium and lemon is effective in reducing the epithelial expression of angiotensin-converting enzyme 2 (ACE2), an integral membrane glycoprotein, the receptor that facilitates the binding of the SARS-CoV-2 spike via its receptor-binding domain and further releases its RNA, which gets translated into viral proteins [20]. Furthermore, these proteins form a replication complex to generate additional RNA that assembles with the viral proteins so new viruses can be released. Thus, blocking ACE2 in epithelial cells may prevent virus entry into the host cells and eventually inhibit viral infection. In addition, LMN was found to reduce the mRNA levels of transmembrane protease serine 2 (TMPRSS2), which is expressed in human lung cells [20]. TMPRSS2, as a host cell factor, is critical for S-protein priming during the entry and spread of SARS-CoV-2 and is dispensable for development and homeostasis; thus, inhibiting TMPRSS2 may block viral entry and represent an attractive drug target [21]. ACE2 and TMPRSS2 receptors are crucial viral gateways in oral, lung, and intestinal epithelial cells and the ocular surface constituting important routes of SARS-CoV-2 invasion; therefore, downregulation of these receptors can be important in preventing the entry and spread of the virus [22, 23]. The interruption of entry at any stage of fusion/replication of SARS-CoV-2 may become a potential strategy for the development of antiviral agents [20, 24]. In another recent study, LMN derived from the EO of Ammoides verticillata was studied in silico for activity against SARS-CoV-2, which showed favorable properties for drug-likeness [25].
LMN is a cyclic monoterpene that constitutes about 98% of the EOs in the peel, leaf, and flower of many citrus fruits, including oranges, mandarins, lemons, pummelos, grapefruits, and limes [26]. The concentration of LMN varies between 32% and 98% depending on the variety, for example, in bergamot (32–45%), lemon (45–76%) and sweet orange (68–98%) [27]. In addition to citrus fruits, it is also abundantly found in edible plants such as neroli, thyme, rosemary, lavender, ginger, perilla, sage, cranberries, mint, cherries, lemongrass, lavenders, hemp, licorice, hops, mushrooms, celery, cardamom, and caraway [26, 27]. LMN is widely used in foods, pharmaceuticals, beverages, and industrial solvents, owing to its flavor and aroma [26]. It also possesses numerous therapeutic effects, including anti-inflammatory [28], antioxidant [29], antiviral [30], immunomodulatory [31, 32, 33], anti-nociceptive [34], anticancer [35], antidiabetic [36], analgesic [37], cardioprotective [38, 39], neuroprotective [40], hepatoprotective [29] and gastroprotective [41]. A comprehensive review is available demonstrating the health benefits of LMN in health and diseases elsewhere and is out of scope of the present review [136]. In the present review, we present a perspective of probable use of LMN on infection, immunity and inflammation within the scope of COVID-19 pandemic.
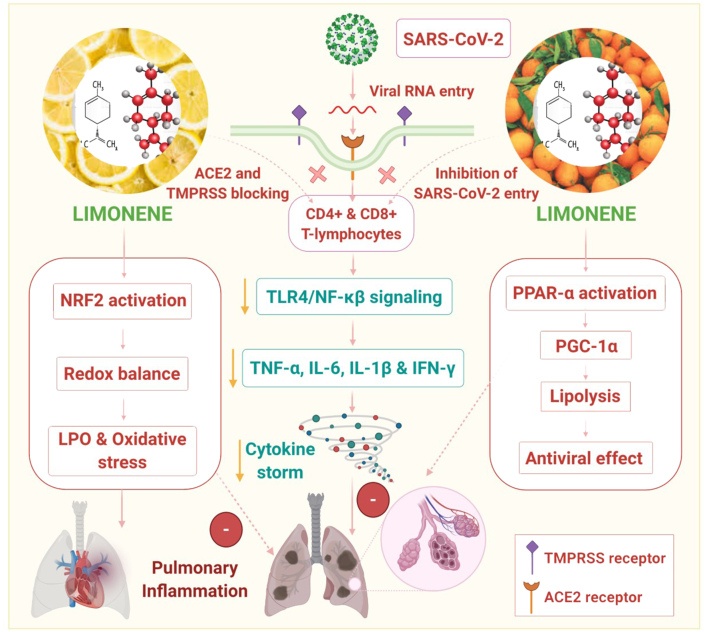
In this article, we hypothesized that LMN could be a candidate compound for potential use in COVID-19 due to its notable immunomodulatory, anti-inflammatory, and antiviral properties which may limit the severity and progression of the disease. Herein, we discuss the possible mechanisms of LMN in SARS-CoV-2 infections based on previous and recent findings on its immunomodulatory, anti-inflammatory, and antiviral properties. A scheme for the effect of LMN on infection, inflammation, and immunity in the context of SARS-CoV-2 is also proposed and depicted in Figure 1.
Figure 1.
Possible propsed effect of limonene (LMN) on infection, inflammation, and immunity in the context of SARS-CoV-2.
1.1. LMN as a potential immunomodulator
The immune system is the central player dealing with all types of infections, including SARS-CoV-2. Immunomodulation is aimed at modifying the immune response either via augmentation to prevent infections in a state of immunodeficiency or by suppressing the immune system in allergies or autoimmune diseases, where the goal is to weaken the immune system. In the early stages of SARS-CoV-2 infection, macrophages exhibit a pro-inflammatory phenotype (M1), producing nitric oxide, IL-6, IL-8, IL-1β, ROS, MCP-1, CXCL-10, and TNF-α, which mediate host defense against the virus, but also promote lung injury. TNF-α is a principal initiator of inflammation, a mediator of cell death, differentiation, and immune modulation. Meanwhile, anti-inflammatory macrophages (M2) become activated and regulate healing once the pathogenic agent is eliminated, restoring the lung tissue. Additionally, natural killer (NK) cells play an important role in the innate immune system by eliminating virally infected cells. LMN administered orally enhanced IFN-γ production in KHYG-1 cells, a human NK cell line, in addition to reducing the stress response [31].
Aside from these, LMN also showed immunomodulatory potential by exhibiting anti-inflammatory activity mainly on Th2 cytokines, including IL-4, IL-5, and IL-10, in mouse primary splenocytes [42]. LMN isolated from Citrus latifolia had potential anti-inflammatory effects in peritonitis, likely by inhibiting pro-inflammatory mediators present in inflammatory exudate and leukocyte chemotaxis [43]. LMN and its metabolites, limonene-1-2-diol and perillic acid, were shown to inhibit the production of IFN-γ, IL-2, TNF-α, IL-4, and IL-13 by CD3+ CD4+ T cells, and the production of IFN-γ, IL-2, and TNF-α by CD3+ CD8+ T cells [33]. Additionally, LMN showed favorable modulation of upregulated CD25, CD69, and CD40L by activated T lymphocytes. LMN also showed immunoregulatory activity in an LPS-induced pleurisy model by inhibiting NO and the cytokines IFN-γ and IL-4 [44]. LMN was observed to inhibit LPS-stimulated inflammation by blocking the NF-κB, JNK, and ERK pathways in macrophages [45].
COVID-19 has a significant impact on mental health and may adversely impact immune function in the health workers as well as in post-recovery survivors [46]. Psychosocial issues such as stress, anxiety, and depression are believed to increase susceptibility to viral upper respiratory infections [47]. Psychological distress is linked with immune-inflammatory responses and suggests that psychoneuroimmunity can be important in COVID-19 infections. Many of the EOs are known for relieving stress, treating depression, and aiding with insomnia. Subsequently, LMN and its metabolites have been shown useful in alleviating depression, anxiety, stress mediating anti-inflammatory, immunomodulatory, and antioxidant properties [48]). Mechanistically, antidepressant activities were shown to mediate Gamma aminobutyric acid (GABAergic), monoaminergic, and neurotrophic mechanisms, as evidenced by the inhibition of hypothalamic-pituitary-adrenal axis hyperactivity and the reduction of monoamine neurotransmitter levels [49, 50]).
LMN also showed to control hyperlocomotion by regulating dopamine and serotonin levels [51], and exert potent anxiolytic effects in experimental models mediating antioxidant action [52, 53]. LMN-containing plants such as Aloysia polystachya, traditionally used in anxiety, has been evaluated in adults following oral intake for eight weeks in a randomized, double-blind, placebo-controlled, phase 2 clinical trial and was found to be superior to placebo for the treatment of anxiety [54]. Thus, considering its anxiolytic, antidepressant, and sedative properties, LMN could be an important agent to favorably modulate psychoneuroimmunity in COVID-19 patients.
To repurpose or identify drug candidates for COVID-19, it is beneficial if the drug has a favorable safety and efficacy profile, coupled with a multitargeted mechanism of action capable of synergistically mitigating the cytokine storm and acting as an immunomodulatory agent rather than an immunosuppressant drug. Regarding the possible concerns about immunosuppression during acute infections, it is important to emphasize that LMN improves survival in animals and does not increase mortality during infections. In this context, LMN could be a promising candidate based on encouraging preclinical reports and its relative safety in few studies in humans. However, further evidences are still needed to confirm its beneficial activities in preclinical studies and translate these benefits to therapeutic effects in humans, either as a plausible agent or an adjuvant in the treatment of COVID-19.
1.2. LMN as a potential anti-inflammatory agent in inflammation
The innate immune response is related to the inflammatory process since the release of inflammatory mediators (cytokines, ROS, etc.) by the various cells of the immune system (neutrophils, macrophages, lymphocytes) activates adaptive immunity but enhances inflammation. T helper cells, in turn, activate other types of cells (monocytes, B cells, etc.) via the release of cytokines, such as TNF-α, IL-1β, and IL-2, which progress the inflammatory cascade [55]. Several studies have demonstrated the anti-inflammatory activity of LMN, its metabolites and derivatives, or plants containing high amounts of LMN in LPS-induced macrophages, monocytes, or eosinophils. It was found that LMN can inhibit pro-inflammatory cytokines, inflammatory mediators such as iNOS and COX-2, and the production of NO and prostaglandin E2 [28], [56], and reduce CD18 frequency in human lymphocytes [57].
Furthermore, LMN activates peroxisome proliferator-activated receptor (PPAR)-α signaling and inhibits liver X receptor (LXR)-β signaling [58, 59]. PPAR-α agonists showed favorable modulation of lipid metabolism by increasing the ability of hormone nuclear receptors, such as PPAR-α and estrogen-related receptor alpha (ERR-α), to drive the transcription of fatty acid oxidation enzymes by increasing the levels of peroxisome proliferator-activated receptor-gamma coactivator 1α (PGC-1α). The role of activating PPAR-α and lipolysis was shown to reduce hepatitis C virus genotype-associated lipid metabolic disorders in liver diseases [60]. PPAR-α activation also beneficially influences inflammation in alveolar epithelial cells and suggests the potential usefulness of PPAR-α in ARDS [61]. LMN, being natural and dietary could be a safer alternative or add on with synthetic PPAR-α. Taken together, the role of LMN as a PPAR-α agonist seems promising to regulate lipid metabolism, along with additional regulatory roles on cell proliferation and differentiation, vascular homeostasis and atherosclerosis, the immune system, and inflammation. Thus, LMN may be a candidate to modulate the orchestrated immune-inflammatory events in COVID-19.
Patients with COVID-19 usually present with acute respiratory distress, causing acute lung injuries characterized, in particular, by elevated levels of IL-6, which correlate with the severity of the disease pathology, prognosis, and mortality [10, 62]. Acute lung injury refers to a characteristic form of parenchymal lung disease, characterized by bilateral pulmonary infiltrates, alveolar-capillary vasculitis with neutrophil infiltration, and pro-inflammatory cytokine release in COVID-19. Elevated IL-6 levels have also been demonstrated to contribute to acute lung injury in murine models [63], similar to those observed in patients with severe acute respiratory syndrome and those with COVID-19, where inhibition of IL-6 appears to mitigate acute lung injury [63, 64]. Many therapeutic strategies have been developed against IL-6 in the context of SARS-CoV-2-induced cytokine storm, including one effective agent, tocilizumab. However, it causes numerous adverse effects such as liver damage, thrombocytopenia, leukopenia, serious infections, gastrointestinal perforations, hypertension, skin reactions, and anaphylaxis [65]. Furthermore, in healthy elderly humans, dietary supplementation with LMN was found to reduce IL-6 [66]. LMN acts as a ligand and an agonist for adenosine A(2A) receptors. The adenosine A(2A) receptor has been shown to exert anti-inflammatory effects in a murine model of acute lung injury [67]. This indicates that LMN may have a protective effect on pulmonary function, mediating the amelioration of inflammatory mediators and the reduction in lung resistance and elastance. Taken together, LMN could be important to target acute lung injury in COVID-19.
In extra-pulmonary manifestations of SARS-CoV-2 infections, cardiac injury also occurs in patients with critical illness. Patients with cardiovascular diseases, such as ischemic heart disease and hypertension, have an increased risk of severe disease and death. Systemic infection and inflammation may cause acute thrombosis by activating platelets, vasoconstriction of the coronary artery, hypoxemia, enhanced sympathetic tone, altered heart rate, coagulation pattern, and impaired endothelium [68]. LMN has been shown to inhibit atherogenesis by inhibiting endothelial adhesion molecules, inducing an antiarrhythmic effect [69], and amelioration of acute myocardial injury by restoring hemodynamics, cardiac function, suppressing oxidative stress, cardiomyocyte cell death, and inflammation by inhibiting the mitogen-activated protein kinase (MAPK)/NF-κB pathway [39, 69]. Infants exposed to LMN odors for 2 min had a significantly decreased heart rate and an increased parasympathetic nervous activity [70]. Cardiovascular complications in patients with COVID-19 arise from altered sympathetic activity, coagulation, hemodynamics following infections, and inflammation. Given the protective effect of LMN on all these factors, it may have a therapeutic role in cardiovascular risk factors and squeal in COVID-19.
In patients with COVID-19, one of the common issues is liver injury/dysfunction. This may be due to the virus itself or other concurrent conditions, such as hepatotoxicity caused by the drugs (such as antipyretics or immunomodulatory agents) used in COVID-19 management, along with the co-existence of systemic inflammation, acute respiratory distress syndrome-induced hypoxia, multiple organ failure, or the presence and progression of chronic liver diseases [71]. Patients with COVID-19 suffering from severe liver dysfunction are more vulnerable and have a poor prognosis. The hepatoprotective effects of LMN have also been demonstrated against acetaminophen-induced hepatotoxicity; it acts by inhibiting mitochondrial oxidative stress, lipid peroxidation, and improving glutathione levels [72, 73]. LMN attenuated pro-inflammatory cytokines, increased levels of antioxidants including glutathione, corrected altered lipid profiles, and restored liver function parameters in drug-induced liver toxicity in diabetic animals [36] and it was reported that LMN also reduced chronic stress-induced hepatotoxicity [29]. Intestinal inflammation may also occur in patients with COVID-19 due to the SARS-CoV-2-mediated reduction of mucosal ACE2 following viral entry, resulting in elevated angiotensin levels as well as elevated TNF-α and tryptophan deficiency [74]. LMN showed protective effects on the epithelial barrier and ameliorated intestinal inflammation by inhibiting TNF-α-induced NF-κB translocation [66] as well as the NOS, COX-2, LOX, PGE2, TGF-β, and ERK1/2 signaling pathways [75]. It also increased the antioxidant and mucosal defense; moreover, it attenuated pro-inflammatory cytokines, such as NF-κB, and reduced neutrophil infiltration [41].
In COVID-19, there is an increased risk of secondary bacterial infections in critically ill patients, causing complications and even death [76]. LMN is an important constituent in the EOs of many plants, such as Monarda punctate [77], Schinus molle [78], and Juniperus spp [79]. LMN in these plants have been attributed to exert potent antibacterial effects against frequently encountered infection-causing pathogens, including Streptococcus pyogenes, methicillin-resistant Staphylococcus aureus, Streptococcus pneumoniae, Haemophilus influenzae, Escherichia coli, Klebsiella pneumoniae, Mycoplasma pneumoniae, and Brochothrix thermosphacta. Additionally, LMN also acts as an analgesic as it exhibits antihyperalgesic effects against mechanical hyperalgesia mainly by inhibiting the synthesis of pro-inflammatory cytokines and their attenuating their consequences [37, 80]. The other mechanism demonstrated for the analgesic effect of LMN is the inhibition of nociception induced by oxidative stress-induced TRPA1 activation in TRPA1-gene-deficient mice [81]. Many derivatives have been developed for LMN, and one of the epoxide derivatives of LMN inhibited inflammatory mediators, vascular permeability, migration of neutrophils, and systemic and peripheral analgesic-dependent effects of the opioid system [82].
Furthermore, LMN also showed reactive oxygen species or free radical scavenging and Fe2+ chelating activity against free radicals in numerous in vitro assays such as ABTS, DPPH, FRAP, and ORAC [83, 84], promoting mitochondrial biogenesis [59] and improving endogenous antioxidants in vivo in many tissues [78, 84]. Prolonged oxidative stress causes DNA damage, alters the transcription of various genes, impairs macromolecules, and causes plasma membrane damage following lipid peroxidation. Oxidative stress also initiates and contributes to numerous signaling pathways, including inflammasomes, Nrf2/Keap1, TLR4/HMGB1, MAPK, and SIRT/PGC1-α, leading to the release of inflammatory mediators and cytokines that sustain inflammation involving metabolic reprogramming of innate immune cells. LMN has been shown to modulate a majority of the signaling pathways that contribute to the redox immune-inflammatory signaling that results in organoprotective effects. In addition to lungs, COVID-19 affects almost all organ systems, including the heart, brain, liver, kidney, intestine, and the coagulation system. Thus, the organoprotective effects demonstrated in experimental models in vivo are encouraging to speculate the therapeutic benefits of LMN.
1.3. LMN potential as an antiviral agent
The antiviral properties of LMN as well as plants containing LMN are represented in Table 1. The antiviral activity of LMN is mediated mainly by inhibiting viral replication through direct action on the virus. LMN was reported to exhibit antiviral activity against HSV-1 strain KOS using RC-37 cells by diminishing its infectivity by up to 100% via inhibition of replication in early phases of infection, in a dose-dependent manner [30]. Its antiviral activity was found to be comparable to that of acyclovir. LMN present in the EO of Citrus reshni is attributed to moderate inhibition of the avian influenza A virus (H5N1) [85]. The EO of Lippia alba containing LMN showed inhibition of yellow fever virus in Vero cells as evidenced from the MTT assay [86]. The EO of Jungia polita and Buddleja cordobensis showed inhibitory activity against dengue virus type 2 (DENV-2) [87]. The antiviral activities of LMN against HSV-1, influenza, yellow fever, and dengue virus is appealing and suggest that it needs to be tested for its antiviral activity in further in vitro or in vivo studies.
Table 1.
The antiviral activities of LMN or LMN containing plants.
| Sources | Inhibitory concentration (IC 50) | Virus | Mechanisms | Ref. |
|---|---|---|---|---|
| LMN | 5.9 μg/ml | Simplex virus type 1 using RC-37 cells | Plaque reduction | [30] |
| Cleopatra mandarin | 2.45 | Avian influenza A | Plaque reduction assay | [85] |
| Heterotheca latifolia | >150, 90 and >250 | HSV-1, JUNV and DENV-2 | Plaque reduction assay (Vero cells) | [123] |
| Lippia junelliana | >150, 20 and >250 | HSV-1, JUNV and DENV-2 | Plaque reduction assay (Vero cells) | [123] |
| Lippia turbinate | >150, 14 and >250 | HSV-1, JUNV and DENV-2 | Plaque reduction assay (Vero cells) | [123] |
| Aloysia triphylla | >250 | DENV-2, JUNV and HSV-1 | Plaque reduction assay (Vero cells) | [87] |
| Gaillardia megapotamica | 140.6, 49.8, and 99.1 | DENV-2, JUNV and HSV-1 | Plaque reduction assay | [87] |
| Pectis odorata | 39.6, 36.6, and 71.5 | DENV-2, JUNV and HSV-1 | Plaque reduction assay | [87] |
| Lippia alba | 100 | Yellow fever virus | Plaque reduction assay | [124] |
| Citrus bergamia | 100% inhibition at 0.3% | Influenza virus type A H1N1 | - | [125] |
| Citrus limonum | - | Vero cells, HSV-1 | Plaque reduction assay | [126] |
| Juniperus communis | >10000 | HSV-1 | Plaque reduction assay | [126] |
| Eucalyptus cinereal | 13 μg/ml | Coxsakie virus B3 | [127] | |
| Lepechinia salviifolia | 68.8, 81.9 | HSV-1 and HSV-2 | Plaque reduction assay | [128] |
| Lepechinia vulcanicola | 112, 68.9 | HSV-1 and HSV-2 | Plaque reduction assay | [128] |
| Lippia alba | 10.1, 0.4, 32.6, 21.1, 4.9 | DENV-1, 2, 3, 4, YFV 17 DD | Plaque reduction assay (Vero cells) | [129] |
| Lippia citriodora Kunth | 19.4 μg/ml | Yellow fever virus | Plaque reduction assay (Vero cells) | [129] |
| Lippia alba | 4.3 and 15.2 | Yellow fever virus | Plaque reduction | [86] |
| Mentha suaveolens | 0.87 μg/ml | Cytopathogenic murine norovirus | Plaque reduction assay | [130] |
| Trachyspermum ammi | 80% reduction at 500 μg/ml | Japanese encephalitis virus | Plaque reduction assay | [131] |
1.4. LMN's selectivity for respiratory systems
In a systematic review, LMN was found to be effective in both preventing and controlling respiratory system injuries through its anti-inflammatory properties [88]. LMN attenuated acute lung injury by inhibiting inflammatory cells, pro-inflammatory cytokines, blocking phosphorylation of IκBα , NF-κB-p65, p38-MAPK, c-Jun NH2-terminal kinase (JNK), and extracellular signal-regulated kinase induced by lipopolysaccharide (LPS) [89]. LMN and the ozone-LMN reaction mixture were found to reduce inflammation in the lungs due to their anti-inflammatory and antioxidant properties [32].
LMN also showed suppression of airway inflammation by reducing airway hyper-responsiveness and eosinophilic infiltration and increasing T helper (Th) 2 cytokine production. Furthermore, it reduced the levels of IL-5, IL-13, eotaxin (a potent eosinophil-specific chemoattractant), MCP-1, and TGF-β in the eosinophils and lungs, while preserving lung histology [90]. LMN also reduced goblet cell metaplasia, smooth muscle thickness, and fibrosis of the airways [91]. LMN ameliorated ovalbumin-induced inflammatory and oxidative stress [92]. LMN also enhanced mucociliary clearance and ciliary beat frequency and was found to be comparable to salbutamol and superior to that of N-acetylcysteine [93]. One of the formulations containing LMN, known as Myrtol®, has been demonstrated to possess antioxidant, anti-inflammatory, and antimicrobial properties, along with secretolytic and bronchospasmolytic effects. This formulation has been suggested for use in several acute and chronic infections of the upper and lower airway system such as acute and chronic rhinosinusitis, acute and chronic bronchitis, and chronic obstructive pulmonary disease [94].
1.5. Pros and cons on safety and efficacy of LMN
A recent review comprehensively presented regulatory toxicology data, pharmacokinetic aspects and drug delivery strategies for LMN [95]. For a long time, LMN has been consumed in human diets and is used for medicinal purposes to promote health and well-being. It is also an important constituent of many herbal formulations. The lemon balm which is one of the source of LMN has been given the status of “generally recognized as safe” (GRAS) that is indicative of relative dietary safety within certain limits. The tolerable daily intake for LMN has been proposed 0.1 mg/kg/day that equates to 6 mg/day in humans [Filipsson et al. 1998] and in a questionnaire based dietary assessment survey the estimated consumption of LMN through consumption of citrus foods (fruit, juice and peel) was found that the mean intake of LMN was about 13 mg/day [96]. Numerous studies have demonstrated the safety profile of LMN following oral intake [97], and also showed safe in humans [54, 66]. LMN is considered a low toxic food additive that is devoid of genotoxicity [98], immunotoxicity [99], mutagenicity [98], and carcinogenicity. It has been shown to possess satisfactory bioavailability in the liver, kidney, brain, and blood following oral intake. It has been conveniently given as an inhalation, since it appears bioavailable and effective in the respiratory tract [95]. LMN has also been shown to improve the stability, drug delivery, and bioavailability of many agents such as lycopene, curcumin, and glimepiride, and exert a synergistic anti-inflammatory activity [100, 101, 102, 103]. It has also been observed that LMN behaves as a prodrug because its metabolites exhibit better therapeutic potency and efficacy [104]. Perillic acid is an important bioactive metabolite and a biomarker of LMN metabolism in human plasma. Despite the demonstrated potential organoprotective effects, in some studies showed to manifest skin sensitization, neurotoxicity and hepatotoxicity following the use of this agent [95].
In a recent systematic review, LMN has been revealed effective in preventing and controlling respiratory system injuries mediating anti-inflammatory actions [88]. Additionally, another recent review presents that LMN upon exposure to humans and animals do not support sensitization of the airways at indoor levels by inhalation that include other selected fragrances [105]. The literature reveals that LMN and/or its reaction mixtures may exhibit anti-inflammatory properties in murine inhalational models. The authors opined that reported lung function effects are likely based on perception rather than toxic effects of the fragrances in humans [105, 106]. There is limited information on long term effects of ozonolysis products and available information is inadequate to confirm whether ozone-initiated reactions with LMN at usual indoor levels elicit airway effects in humans [105].
LMN is not a very stable compound and it forms oxidation products viz. 4-acetyl-1-methylcyclohexene, 4-oxopentanal, and 3-isopropenyl-6-oxoheptanal and secondary organic aerosols upon ozonolysis which elicit adverse effects on airways [107, 108]. Effects of LMN oxidation products of LMN such as 4-acetyl-1-methylcyclohexene, 4-oxopentanal, and 3-isopropenyl-6-oxoheptanal were showed to induce inflammatory cytokines in human bronchial epithelial (16HBE14o-) and alveolar epithelial (A549) cell lines with no effect on cellular viability [107]. The pharmacokinetics of LMN has been studied in mice following oral and inhalation routes of delivery [109]. The tissue distribution showed a patterned decrease in the liver > kidney > heart > lung > spleen tissues. The toxicokinetics of LMN were studied in human volunteers exposed by inhalation. LMN metabolized readily and showed high pulmonary uptake and long half-life following slow elimination phase with accumulation in adipose tissues and eliminated unchanged in the expired air and in urine. No irritative symptoms or effects on respiratory and nervous system was observed [110].
Despite numerous data demonstrating organoprotective effects including chemopreventive effects, adverse effects such as hepatotoxicity characterized by parenchymal and matrix injury in the liver [111], nephrotoxicity [112], and skin sensitivity [97] are reported with LMN. However, recent studies demonstrated hepatoprotective [29] and antifibrotic effects of LMN in liver [113]. LMN metabolite 1,2-epoxide reported to induce nephrotoxicity by causing hyaline droplet after binding with α2-globulin and mice lacks it are resistant to hyaline droplet nephropathy [112]. Some of the important activities and effects of LMN relevant to infection, immunity and inflammation and organoprotective properties in preclinical studies are presented in Table 2.
Table 2.
Some important activities and effects of LMN relevant to infection, immunity and inflammation and organoprotective properties in experimental models.
| Diseased condition | Experimental model | Effects and mechanisms observed | Ref. |
|---|---|---|---|
| Pulmonary hypertension | Monocrotaline-induced pulmonary hypertension, lung injury, & ventricular hypertrophy in rats | - reduced pulmonary arterial media thickness, interstitial fibrosis - reduced macrophages and lymphocytes around the pulmonary veins |
[132] |
| Acute lung injury | Atomized LPS-induced acute lung injury in BALB/c mice | - inhibited neutrophils and inflammatory cytokines - attenuated MPO activity and lung injury - prevented NF-κB activation in lungs |
[133] |
| Acute lung injury | Intratracheal instillation of LPS (0.5 mg/kg)-induced acute lung injury | - reduced morpho- and histo-logical changes - inhibited inflammatory cytokines, myeloperoxidase - blocked phosphorylation of IκBα, NF-κB p65, p38 MAPK, JNK and ERK |
[89] |
| Cardiac arrhythmias | Cardiac arrhythmia in Langendorff model | - improved hemodynamic and electrocardiographic changes in rats | [69] |
| Myocardial infarction | Isoproterenol-induced murine MI model | - attenuated ST elevation, infarct area, histopathology - restored antioxidants, suppressed apoptosis |
[38] |
| Myocardial infarction | Isoproterenol (85 mg/kg, s.c.)-induced myocardial infarction in rats | - mitigated injury by MAPK/NF-κB pathway - improved hemodynamics and cardiac enzymes - inhibits inflammatory markers & infract area - inhibits apoptosis altering Bcl-2 and Bax |
[39] |
| Dyslipidemia | high-fat diet-fed obesity in C57BL/6 mice | - reduced lipid accumulation and adipocytes - corrected lipid profile & fasting blood glucose - activated PPAR-α, and inhibited LXR-β signaling |
[58] |
| Liver fibrosis | CCl4-induced liver fibrosis in Wistar rats | - prevented serum aminotransferases, total cholesterol - restored antioxidants & decreased MDA - decreased TNF-α, TGF-β, α-SMA and hydroxyproline |
[113] |
| Acetaminophen-hepatotoxicity | Cytochrome P450 isoform-specific substrates in the liver microsomes of mouse | - prevented bioactivation of procarcinogens and enhanced conjugation of proximal carcinogens - inhibited p-nitrophenol hydroxylase in microsomes - inhibited 7-ethoxyresorufin O-deethylase in microsomes from PB- and BNF-treated - no protection with acute doses, but LMN (1.0% diet x 10 d) improved liver GSH |
[72] |
| Hepatic dysfunction | Chronic immobilization-induced liver dysfunction in rats | - corrected liver enzymes and reduced infiltrated cells in the liver parenchyma and improved glutathione - reduced MDA, TNF-α, IL-1β, -6 and NF-κB |
[29] |
| Cerebral ischemia | Ischemia-induced cerebral injury in hypertensive SHRsp rats | - decreased infarct size & improved neurobehavor - decreased MDA, IL-1β, MCP-1 and COX-2 - increased antioxidants, & increased DHE-staining |
[134] |
| Allergic inflammation | LMN or LMN-ozone to ovalbumin- sensitized mice | - LMN not exacerbated airway allergy - reduced allergic inflammation & oxidative stress |
[32] |
| Lung inflammation and airway responsiveness | Ovalbumin-induced inflammation in A2AKO mice using whole-body plethysmography | - attenuated induced airway responsiveness - reduced levels of eosinophilsand neutrophils - reduced airway inflammation and reactivity via activation of A2AAR with support of A2B receptors |
[135] |
| Immunomodulatory property | absorptive pathway and the immune responses of the lung, the phagocytic function of alveolar macrophages (Mphi) after p.o. and Con A-stimulated proliferation of splenocytes | - LMN taken up from thoracic duct lymph moves to the lung and reach maximum levels in lungs - increased number and phagocytic activity of alveolar Mphi & Con A-stimulated proliferation of splenocytes - directly activates immune response of alveolar Mphi - indirectly activates via activated lymphocytes |
[99] |
2. Limitations
Majority of the experimental research directed on therapeutic benefits of phytochemicals are based on ethnopharmacological usage of the particular plant rich in these phytochemicals. Many of them have been also evaluated for their antiviral properties in addition to their anti-inflammatory and immunoregulatory roles in numerous immune related disease models. Since the emergence of COVID-19, the repurposing of drugs begins first with target identification and continues to be used in screening of druggable agents against viral infections [114]. In the past few months, a significant number of natural products including plant extracts and phytochemicals have been proposed for their possible use as a preventive agent or as an adjuvant in COVID-19 [115]. Majority of them proposed their use on the basis of their potent immunomodulatory, anti-inflammatory, and antimicrobial properties. Few of the phytochemicals have been screened in molecular docking studies for their potential activity against viral targets using the in-silico tools [116, 117, 118].
Among numerous phytochemicals, LMN has been shown to target host specific ACE2 receptors , binding domains for viral proteins in the in-silico studies [20]. LMN is one of the main compounds identified in many citrus plants, found dietary available and accessible and well-studied for its therapeutic benefits. However, the safety and efficacy of LMN in preclinical and clinical trials are yet to be necessary to establish for its evidence-based use and application in humans. The dietary nature and pleiotropic effects make LMN a fascinating candidate for further investigation. The candidature of LMN in COVID-19 treatment somewhat appears speculative but cannot be overlooked provided favorable physiochemical and druggable properties along with dietary use. However, the suggestion on the possible use of LMN in COVID-19 remains inconclusive until the in-silico effects are confirmed in the experimentaland further proof of the concept studies.
It is noteworthy to emphasize here that the antiviral potential of natural products was showed effective in the in vitro studies until recent years [119, 120, 121], whereas the SARS-CoV emerged in 2003. But, none of them have been evaluated meticulously to translate their effects in humans despite their potential efficacy in preclinical studies due to many reasons including lack of integrated approach. A recent report suggested that if an integrated and rigorous approach could have been followed since the epidemic of SARS-CoV, we may have the possibility that some progressed to the clinical studies and developed some useful agents in the process of drug discovery and development, which involves testing of druggable compounds from laboratory to clinics [122]. This encourages that phytochemicals should be investigated and validated in the preclinical models of COVID-19 and if appears efficacious, can be evaluated further in humans. In present manuscript, the possible candidature of LMN as a preventive agent or adjuvant in COVID-19 has been proposed based on the previously reported potent pharmacological activity against infection, inflammation and immunity in experimental models of human diseases involving dysregulated immune-inflammatory and redox homeostasis. There are reports of long-term complications in some patients even after recovery from COVID-19. The progression and complications of COVID-19 involves cytokine storm; therefore, compounds potentially inhibit proinflammatory cytokines and chemokines, in addition to their organ-protective effects. Thus, given the tissue protective effects and effect on numerous tissue remodeling effects, LMN could be a candidate to be investigated for possible use in combating the long-term complications in COVID-19.
There is no data available from preclinical or clinical studies to suggest whether LMN can protect against COVID-19 or be useful in treatment of COVID-19. There is paucity of preclinical and clinical data on infection, inflammation and immunity in context to COVID-19. The recent availability of animal models could be important in evaluating its preclinical efficacy. There is lack of clinical data and rigorous pharmacokinetics in humans. Therefore, the preclinical evaluation including duration of use and dose to be explored, the safety and interaction with concomitant drugs should be considered before the possible indication of LMN as a preventive agent or therapeutic adjuvant. Taking in to consideration the dietary safety of LMN in humans, and efficacy of LMN in various disease models in experimental studies, LMN may be a valuable agent to be investigated further in COVID-19. However, until now there is no direct evidence available on the antiviral activity of LMN on SARS-CoV-2. Further studies are encouraged before making any recommendation on the clinical usage and pharmaceutical development of LMN.
3. Conclusions and future remarks
In summary, based on evidence, it can be concluded that LMN and its metabolites possess essential pharmacological bioactivities for infection, immunity, and inflammation, as far as COVID-19 is concerned. Its potent anti-inflammatory activity mediating multiple pathways and mediators of inflammation, including inhibition of pro-inflammatory cytokines, chemokines, and adhesion molecules, along with the suppression of macrophage infiltration and neutrophil-endothelial cell interaction, might constitute a promising approach to inhibit cytokine storm, which is a major cause of mortality in patients with COVID-19. The potential of LMN as an immunomodulatory, as a potent antioxidant in improving host cellular immunity against infection, and its ability to interfere with ACE2 receptors, along with its antibacterial activity may further help in controlling symptoms and worsening of the diseases, secondary infections, complications, progression, and eventually death.
Collectively, the immunomodulatory, anti-inflammatory, and antiviral properties of LMN, along with its various pharmacological and molecular mechanisms, make it a promising candidate for SARS-CoV-2 infection. Recently, the animal models of COVID-19 become available that may facilitate preclinical evaluations to distinguish whether these candidate compounds are likely to become effective drugs. Furthermore, its safety status, drug-likeliness properties, pharmacological actions, and molecular mechanisms provide a rationale for the use of LMN as a nutritional and/or adjuvant strategy against SARS-CoV-2. However, it is important to highlight that no human studies have yet demonstrated the effect of LMN on SARS-CoV-2. Therefore, the suggestion on the use remains inconclusive until the preclinical and clinical studies provide evidence on safety and efficacy in COVID-19.
Declarations
Author contribution statement
All authors listed have significantly contributed to the development and the writing of this article.
Funding statement
The works in laboratory of Dr. Shreesh Ojha is supported by the United Arab Emirates University.
Data availability statement
Data included in article/supplementary material/referenced in article.
Competing interest statement
The authors declare no conflict of interest. The opinion of the authors is solely based on available literature on infection, immunity and inflammation and speculated its candidature for possible use COVID-19 based on the reported data from limited experimental studies. The authors opinions are hypothesized solely based on previous published observations and does not promote the use of LMN in any forms for COVID-19 until the evidence become available from proof of the concept studies.
Additional information
No additional information is available for this paper.
References
- 1.Lai C.C., Shih T.P., Ko W.C., Tang H.J., Hsueh P.R. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and coronavirus disease-2019 (COVID-19): the epidemic and the challenges. Int. J. Antimicrob. Agents. 2020:105924. doi: 10.1016/j.ijantimicag.2020.105924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Li H., Liu S.M., Yu X.H., Tang S.L., Tang C.K. Coronavirus disease 2019 (COVID-19): current status and future perspectives. Int. J. Antimicrob. Agents. 2020:105951. doi: 10.1016/j.ijantimicag.2020.105951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Altay O., Mohammadi E., Lam S., Turkez H., Boren J., Nielsen J. Current status of COVID-19 therapies and drug repositioning applications. iScience. 2020:101303. doi: 10.1016/j.isci.2020.101303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wu R., Wang L., Kuo H.-C.D., Shannar A., Peter R., Chou P.J. An update on current therapeutic drugs treating COVID-19. Current Pharmacology Reports. 2020;6:56–70. doi: 10.1007/s40495-020-00216-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.S F, D X, Y W, L L, X Z, W Z Research progress on repositioning drugs and specific therapeutic drugs for SARS-CoV-2. Future Med. Chem. 2020 doi: 10.4155/fmc-2020-0158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.D W, B H, C H, F Z, X L, J Z Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in wuhan, China. J. Am. Med. Assoc. 2020:323. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Z X, L S, Y W, J Z, L H, C Z Pathological findings of COVID-19 associated with acute respiratory distress syndrome. The Lancet Respiratory medicine. 2020;8 doi: 10.1016/S2213-2600(20)30076-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.X Y, Y Y, J X, H S, J X, H L Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. The Lancet Respiratory medicine. 2020;8 doi: 10.1016/S2213-2600(20)30079-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jw S., C Z, X F, Fp M, Z X, P X Immunological and inflammatory profiles in mild and severe cases of COVID-19. Nat. Commun. 2020:11. doi: 10.1038/s41467-020-17240-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.H H, Q M, C L, R L, L Z, W W Profiling serum cytokines in COVID-19 patients reveals IL-6 and IL-10 are disease severity predictors. Emerg. Microb. Infect. 2020;9 doi: 10.1080/22221751.2020.1770129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.R C, Ar F, R V, M M, J Z, Dk M. Dysregulated type I interferon and inflammatory monocyte-macrophage responses cause lethal pneumonia in SARS-CoV-infected mice. Cell Host Microbe. 2016;19 doi: 10.1016/j.chom.2016.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.A A, Dg M., A T, C M, S G Immunopathology of SARS-CoV-2 infection: immune cells and mediators, prognostic factors, and immune-therapeutic implications. Int. J. Mol. Sci. 2020:21. doi: 10.3390/ijms21134782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mj K., M L, P P, R C NF-κβ signaling and chronic inflammatory diseases: exploring the potential of natural products to drive new therapeutic opportunities. Drug Discov. Today. 2014;19 doi: 10.1016/j.drudis.2013.11.002. [DOI] [PubMed] [Google Scholar]
- 14.Nj S., Gl J. From petri dish to patient: bioavailability estimation and mechanism of action for antimicrobial and immunomodulatory natural products. Front. Microbiol. 2019;10 doi: 10.3389/fmicb.2019.02470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gr G., Abs V., Gh H., VKDS C., G J, JSS Q. Essential oils and its bioactive compounds modulating cytokines: a systematic review on anti-asthmatic and immunomodulatory properties. Phytomedicine : international journal of phytotherapy and phytopharmacology. 2020:73. doi: 10.1016/j.phymed.2019.152854. [DOI] [PubMed] [Google Scholar]
- 16.ÉM dL., AWC F., dAT R.B., AEBP L., Rg dOJ., GES M. Essential oils and their major compounds in the treatment of chronic inflammation: a review of antioxidant potential in preclinical studies and molecular mechanisms. Oxidative medicine and cellular longevity. 2018;2018 doi: 10.1155/2018/6468593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.A-K S., E K, Dw M. Essential oils and functional herbs for healthy aging. Neural regeneration research. 2019:14. doi: 10.4103/1673-5374.245467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.ZAA A., A A, SHM S., A K, MM A, D L Essential oils: extraction techniques, pharmaceutical and therapeutic potential - a review. Curr. Drug Metabol. 2018;19 doi: 10.2174/1389200219666180723144850. [DOI] [PubMed] [Google Scholar]
- 19.L-dR S., vV S.F. Odoriferous therapy: a review identifying essential oils against pathogens of the respiratory tract. Chem. Biodivers. 2020:17. doi: 10.1002/cbdv.202000062. [DOI] [PubMed] [Google Scholar]
- 20.Kj S.K., GV M., CS W., CC C., YC C., Lp L. Vol. 9. 2020. Geranium and lemon essential oils and their active compounds downregulate angiotensin-converting enzyme 2 (ACE2), a SARS-CoV-2 spike receptor-binding domain. (Epithelial Cells. Plants (Basel, Switzerland)). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.M H, H K-W, S S, N K, T H, S E SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020:181. doi: 10.1016/j.cell.2020.02.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.S L, Rl C., T T, NC K., MA S., T M SARS-CoV-2 receptor ACE2 and TMPRSS2 are primarily expressed in bronchial transient secretory cells. EMBO J. 2020;39 doi: 10.15252/embj.20105114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.R Z, MF G.C., BT M., Q Z, Pw R., NM S. TMPRSS2 and TMPRSS4 promote SARS-CoV-2 infection of human small intestinal enterocytes. Science immunology. 2020:5. doi: 10.1126/sciimmunol.abc3582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.N R, Z B, M Y, G C, L R, H K . Vol. 25. Molecules; Basel, Switzerland: 2020. (Virtual Screening of Natural Products against Type II Transmembrane Serine Protease (TMPRSS2), the Priming Agent of Coronavirus 2 (SARS-CoV-2)). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.I A, F H, BB S., S G In silico study the inhibition of angiotensin converting enzyme 2 receptor of COVID-19 by Ammoides verticillata components harvested from Western Algeria. J. Biomol. Struct. Dynam. 2020 doi: 10.1080/07391102.2020.1763199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bora H., Kamle M., Mahato D.K., Tiwari P., Kumar P. 2020. Citrus Essential Oils (CEOs) and Their Applications in Food: an Overview. Plants (Basel) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.S M, B M Biochemical characterization of blood orange, sweet orange, lemon, bergamot and bitter orange. Phytochemistry. 2003;62 doi: 10.1016/s0031-9422(02)00631-3. [DOI] [PubMed] [Google Scholar]
- 28.C S, Aj L., BM N., F J, C C, AF M. Standardised comparison of limonene-derived monoterpenes identifies structural determinants of anti-inflammatory activity. Sci. Rep. 2020:10. doi: 10.1038/s41598-020-64032-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.R A, M A-R, S A . Naunyn-Schmiedeberg's archives of pharmacology; 2020. Hepatoprotective effect of limonene against chronic immobilization induced liver damage in rats. [DOI] [PubMed] [Google Scholar]
- 30.A A, P S Antiviral activity of monoterpenes beta-pinene and limonene against herpes simplex virus in vitro. Iran. J. Microbiol. 2014;6 [PMC free article] [PubMed] [Google Scholar]
- 31.R T, A M, K S, T W, K N, K N Immunostimulatory effect of kumquat (Fortunella crassifolia) and its constituents, β-cryptoxanthin and R-limonene. Food & function. 2019;10 doi: 10.1039/c8fo01971a. [DOI] [PubMed] [Google Scholar]
- 32.JS H., Aw N., Ik K., JB S., MD P., Sw H. Limonene and its ozone-initiated reaction products attenuate allergic lung inflammation in mice. J. Immunot. 2016;13 doi: 10.1080/1547691X.2016.1195462. [DOI] [PubMed] [Google Scholar]
- 33.CM L., NT L. D-Limonene modulates T lymphocyte activity and viability. Cell. Immunol. 2012:279. doi: 10.1016/j.cellimm.2012.09.002. [DOI] [PubMed] [Google Scholar]
- 34.Hg A.-F., EWM P., MM R., Pp M., AAS A., RSS B. D-limonene exhibits superior antihyperalgesic effects in a β-cyclodextrin-complexed form in chronic musculoskeletal pain reducing Fos protein expression on spinal cord in mice. Neuroscience. 2017;358 doi: 10.1016/j.neuroscience.2017.06.037. [DOI] [PubMed] [Google Scholar]
- 35.YM M., M A-F, X X, J Y Biochemical significance of limonene and its metabolites: future prospects for designing and developing highly potent anticancer drugs. Biosci. Rep. 2018;38 doi: 10.1042/BSR20181253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.M B, Hg A., S A, T Ç, N A, Ü Ü.B. d-limonene ameliorates diabetes and its complications in streptozotocin-induced diabetic rats. Food Chem. Toxicol. : Int. J. Publ. Brit. Indust. Biolog. Res. Ass. 2017:110. doi: 10.1016/j.fct.2017.09.020. [DOI] [PubMed] [Google Scholar]
- 37.AC P., PN M., DSB M., J C, J S, X K Limonene reduces hyperalgesia induced by gp120 and cytokines by modulation of IL-1 β and protein expression in spinal cord of mice. Life Sci. 2017:174. doi: 10.1016/j.lfs.2016.11.017. [DOI] [PubMed] [Google Scholar]
- 38.AO D., dS D.S., L H, R M-D-S, Tk R., T OB D-limonene ameliorates myocardial infarction injury by reducing reactive oxygen species and cell apoptosis in a murine model. J. Nat. Prod. 2019;82 doi: 10.1021/acs.jnatprod.9b00523. [DOI] [PubMed] [Google Scholar]
- 39.NS Y. D-Limonene mitigate myocardial injury in rats through MAPK/ERK/NF-κB pathway inhibition. Korean J. Physiol. Pharmacol.: Off. J. Korean Physiol. Soc. Korean Soc. Pharmacol. 2020;24 doi: 10.4196/kjpp.2020.24.3.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.M S, QF L., B C, C S, B L, C Y Neuroprotective effects of limonene (+) against aβ42-induced neurotoxicity in a Drosophila model of alzheimer's disease. Biol. Pharm. Bull. 2020;43 doi: 10.1248/bpb.b19-00495. [DOI] [PubMed] [Google Scholar]
- 41.MC dS., Aj V., Fp B., Ch P., Rh N., AL R. Gastroprotective effect of limonene in rats: influence on oxidative stress, inflammation and gene expression. Phytomedicine : international journal of phytotherapy and phytopharmacology. 2019;53 doi: 10.1016/j.phymed.2018.09.027. [DOI] [PubMed] [Google Scholar]
- 42.CM K., JY L. Anti-inflammatory effects of 27 selected terpenoid compounds tested through modulating Th1/Th2 cytokine secretion profiles using murine primary splenocytes. Food Chem. 2013:141. doi: 10.1016/j.foodchem.2013.04.044. [DOI] [PubMed] [Google Scholar]
- 43.R K, FC F.-Q., CF E.-S., R G, EL S., CA B.-A. 2013. Evaluation of Anti-inflammatory Activity of Citrus Latifolia Tanaka Essential Oil and Limonene in Experimental Mouse Models. Evidence-Based Complementary and Alternative Medicine : eCAM. 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.MC S., AC S., MF R., Oj M-d-L, Mg H. Evaluation of anti-inflammatory activity of essential oils from two Asteraceae species. Pharmazie. 2003;58 [PubMed] [Google Scholar]
- 45.KN K., Yj K., HM Y., YM H., Sw R., Yj J. Anti-inflammatory effect of essential oil and its constituents from fingered citron (Citrus medica L. var. sarcodactylis) through blocking JNK, ERK and NF-κB signaling pathways in LPS-activated RAW 264.7 cells. Food Chem. Toxicol. : an international journal published for the British Industrial Biological Research Association. 2013;57 doi: 10.1016/j.fct.2013.03.017. [DOI] [PubMed] [Google Scholar]
- 46.Rp R. COVID-19 and mental health: a review of the existing literature. Asian journal of psychiatry. 2020:52. doi: 10.1016/j.ajp.2020.102066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.A P, R Z, Dh B. Influence of psychological stress on upper respiratory infection-a meta-analysis of prospective studies. Psychosom. Med. 2010:72. doi: 10.1097/PSY.0b013e3181f1d003. [DOI] [PubMed] [Google Scholar]
- 48.PA dA., JF B., MC B. Anti-stress effects of d-limonene and its metabolite perillyl alcohol. Rejuvenation Res. 2014;17 doi: 10.1089/rej.2013.1515. [DOI] [PubMed] [Google Scholar]
- 49.W Z, M Y, H Y Sub-chronic effects of s-limonene on brain neurotransmitter levels and behavior of rats. J. Nutr. Sci. Vitaminol. 2009;55 doi: 10.3177/jnsv.55.367. [DOI] [PubMed] [Google Scholar]
- 50.Ll Z., ZY Y., G F, JN R., Kj Y., SY P. Antidepressant-like effect of citrus sinensis (L.) osbeck essential oil and its main component limonene on mice. J. Agric. Food Chem. 2019;67 doi: 10.1021/acs.jafc.9b00650. [DOI] [PubMed] [Google Scholar]
- 51.J Y Limonene inhibits methamphetamine-induced locomotor activity via regulation of 5-HT neuronal function and dopamine release. Phytomedicine : international journal of phytotherapy and phytopharmacology. 2014:21. doi: 10.1016/j.phymed.2013.12.004. [DOI] [PubMed] [Google Scholar]
- 52.AN W., A Z, G B, Mk D.S., BT B., dCM K. Citrus essential oils inhalation by mice: behavioral testing, GCMS plasma analysis, corticosterone, and melatonin levels evaluation. Phytother Res. : PT. 2018;32 doi: 10.1002/ptr.5964. [DOI] [PubMed] [Google Scholar]
- 53.AA dA., RB dC., OA S., Dp dS., RM dF. Potential antioxidant and anxiolytic effects of (+)-limonene epoxide in mice after marble-burying test. Pharmacol. Biochem. Behav. 2014:118. doi: 10.1016/j.pbb.2014.01.006. [DOI] [PubMed] [Google Scholar]
- 54.F C, FS C., PA B., DC A., MA A., EZ M. Aloysia polystachya (Griseb.) Moldenke (Verbenaceae) powdered leaves are effective in treating anxiety symptoms: a phase-2, randomized, placebo-controlled clinical trial. J. Ethnopharmacol. 2019:242. doi: 10.1016/j.jep.2019.112060. [DOI] [PubMed] [Google Scholar]
- 55.JR B. TNF-mediated inflammatory disease. J. Pathol. 2008:214. doi: 10.1002/path.2287. [DOI] [PubMed] [Google Scholar]
- 56.Wj Y., Nh L., Cg H. Limonene suppresses lipopolysaccharide-induced production of nitric oxide, prostaglandin E2, and pro-inflammatory cytokines in RAW 264.7 macrophages. J. Oleo Sci. 2010;59 doi: 10.5650/jos.59.415. [DOI] [PubMed] [Google Scholar]
- 57.BE S., MHF O., PGM dA., AB M., JVW S., Vg A. Effect of essential oil from Ageratum fastigiatum on beta-integrin (CD18) expression on human lymphocytes stimulated with phorbol myristate acetate in vitro. Nat. Prod. Res. 2019 doi: 10.1080/14786419.2019.1569653. [DOI] [PubMed] [Google Scholar]
- 58.L J, Y Z, S F, M G, Y G, X L Preventive and ameliorating effects of citrus D-limonene on dyslipidemia and hyperglycemia in mice with high-fat diet-induced obesity. Eur. J. Pharmacol. 2013:715. doi: 10.1016/j.ejphar.2013.06.022. [DOI] [PubMed] [Google Scholar]
- 59.J L, Jw Y. Monoterpene limonene induces brown fat-like phenotype in 3T3-L1 white adipocytes. Life Sci. 2016;153 doi: 10.1016/j.lfs.2016.04.010. [DOI] [PubMed] [Google Scholar]
- 60.T P, R S, K M, RB R., R R Transforming growth factor β acts as a regulatory molecule for lipogenic pathways among hepatitis C virus genotype-specific infections. J. Virol. 2019;93 doi: 10.1128/JVI.00811-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.M H, A B, RE M., N S, I V, S H PPAR-α activation reduced LPS-induced inflammation in alveolar epithelial cells. Exp. Lung Res. 2015:41. doi: 10.3109/01902148.2015.1046200. [DOI] [PubMed] [Google Scholar]
- 62.Ej G.-B., Mg N., N R, K A, A A, N A Complex immune dysregulation in COVID-19 patients with severe respiratory failure. Cell Host Microbe. 2020;27 doi: 10.1016/j.chom.2020.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Jl G., S S, C K, L S, E L, T W Pleiotropic effects of interleukin-6 in a "two-hit" murine model of acute respiratory distress syndrome. Pulm. Circ. 2014;4 doi: 10.1086/675991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.C P, C T, A V, DS G., G P Lung under attack by COVID-19-induced cytokine storm: pathogenic mechanisms and therapeutic implications. Ther. Adv. Respir. Dis. 2020:14. doi: 10.1177/1753466620933508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.S Z, L L, A S, Y C, Z Q Rational use of tocilizumab in the treatment of novel coronavirus pneumonia. Clin. Drug Invest. 2020:40. doi: 10.1007/s40261-020-00917-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.PA dA R.O., JF B., JD S., MV U., MC B. Oral administration of d-limonene controls inflammation in rat colitis and displays anti-inflammatory properties as diet supplementation in humans. Life Sci. 2013;92 doi: 10.1016/j.lfs.2013.04.013. [DOI] [PubMed] [Google Scholar]
- 67.A R, V F-d-P, Ml P., LB V., Dp M-S, WM Q.-F. Cannabidiol, a non-psychotropic plant-derived cannabinoid, decreases inflammation in a murine model of acute lung injury: role for the adenosine A(2A) receptor. Eur. J. Pharmacol. 2012:678. doi: 10.1016/j.ejphar.2011.12.043. [DOI] [PubMed] [Google Scholar]
- 68.V C, V C, R E, ME C., F I, M P . 2020. Epidemiology, Prognosis and Clinical Manifestation of Cardiovascular Disease in COVID-19. Expert Review of Cardiovascular Therapy. [DOI] [PubMed] [Google Scholar]
- 69.GAD N., DS S., BS L., CML V., AAS A., AO D. Bradycardic and antiarrhythmic effects of the D-limonene in rats. Arq. Bras. Cardiol. 2019:113. doi: 10.5935/abc.20190173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Y T, K I Heart rate and heart rate variability in infants during olfactory stimulation. Ann. Hum. Biol. 2019;46 doi: 10.1080/03014460.2019.1622775. [DOI] [PubMed] [Google Scholar]
- 71.G F, KI Z., QQ Y., RS R., G T, CD B. COVID-19 and liver dysfunction: current insights and emergent therapeutic strategies. Journal of clinical and translational hepatology. 2020;8 doi: 10.14218/JCTH.2020.00018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.MM R., D C Effects of D-limonene on hepatic microsomal monooxygenase activity and paracetamol-induced glutathione depletion in mouse. Xenobiotica; the fate of foreign compounds in biological systems. 1993;23 doi: 10.3109/00498259309166786. [DOI] [PubMed] [Google Scholar]
- 73.Kp D., M S, K B, T D Protective effect of Premna tomentosa extract (L. verbanacae) on acetaminophen-induced mitochondrial dysfunction in rats. Mol. Cell. Biochem. 2005:272. doi: 10.1007/s11010-005-7142-6. [DOI] [PubMed] [Google Scholar]
- 74.M G, Sg R., JS L. Letter: intestinal inflammation, COVID-19 and gastrointestinal ACE2-exploring RAS inhibitors. Aliment. Pharmacol. Ther. 2020;52 doi: 10.1111/apt.15814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.L Y, J Y, Z S D-limonene exhibits anti-inflammatory and antioxidant properties in an ulcerative colitis rat model via regulation of iNOS, COX-2, PGE2 and ERK signaling pathways. Mol. Med. Rep. 2017;15 doi: 10.3892/mmr.2017.6241. [DOI] [PubMed] [Google Scholar]
- 76.Y F, Q Y, M X, H K, H C, Y F Secondary bacterial infections in critical ill patients with coronavirus disease 2019. Open forum infectious diseases. 2020:7. doi: 10.1093/ofid/ofaa220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.H L, T Y, FY L., Y Y, ZM S. Antibacterial activity and mechanism of action of Monarda punctata essential oil and its main components against common bacterial pathogens in respiratory tract. Int. J. Clin. Exp. Pathol. 2014;7 [PMC free article] [PubMed] [Google Scholar]
- 78.MM R., S A, F C, MT T., C-M J. Antioxidant, antimicrobial and toxicological properties of Schinus molle L. essential oils. J. Ethnopharmacol. 2014:151. doi: 10.1016/j.jep.2013.10.063. [DOI] [PubMed] [Google Scholar]
- 79.RS D., HM H., DA G., ASA A., BEN E.M., FM H. Efficacy-directed discrimination of the essential oils of three Juniperus species based on their in-vitro antimicrobial and anti-inflammatory activities. J. Ethnopharmacol. 2020:259. doi: 10.1016/j.jep.2020.112971. [DOI] [PubMed] [Google Scholar]
- 80.AC P., JA S., EC K., SA O., AS F., J C Antihyperalgesic and antidepressive actions of (R)-(+)-limonene, α-phellandrene, and essential oil from Schinus terebinthifolius fruits in a neuropathic pain model. Nutr. Neurosci. 2015;18 doi: 10.1179/1476830514Y.0000000119. [DOI] [PubMed] [Google Scholar]
- 81.T K, Y H, K T, T I, M T, T O . Vol. 20. 2016. Involvement of Transient Receptor Potential A1 Channel in Algesic and Analgesic Actions of the Organic Compound Limonene. European Journal of Pain (London, England) [DOI] [PubMed] [Google Scholar]
- 82.dA A.A., RO S., LA N., TV dB., dS D.P., AL B. Physio-pharmacological investigations about the anti-inflammatory and antinociceptive efficacy of (+)-Limonene epoxide. Inflammation. 2017;40 doi: 10.1007/s10753-016-0496-y. [DOI] [PubMed] [Google Scholar]
- 83.P P, A A, J M, R W, B G, A F In vitro anti-inflammatory and radical scavenging properties of chinotto (citrus myrtifolia raf.) essential oils. Nutrients. 2018;10 doi: 10.3390/nu10060783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Bh A., M G, W D, BS R., N S, W M Potential anti-inflammatory and antioxidant effects of Citrus aurantium essential oil against carbon tetrachloride-mediated hepatotoxicity: a biochemical, molecular and histopathological changes in adult rats. Environ. Toxicol. 2019;34 doi: 10.1002/tox.22693. [DOI] [PubMed] [Google Scholar]
- 85.Nagy M.M., Al-Mahdy D.A., Aziz O.M.A.E., Kandil A.M., Tantawy M.A., Alfy T.S.M.E. Chemical Composition and Antiviral Activity of Essential Oils from Citrus Reshni Hort. 2018. Ex Tanaka (Cleopatra Mandarin) Cultivated in Egypt. [Google Scholar]
- 86.LA G E.S., RE O. Comparative study on in vitro activities of citral, limonene and essential oils from Lippia citriodora and L. alba on yellow fever virus. Natural product communications. 2013;8 [PubMed] [Google Scholar]
- 87.CB D., Ml P., LB T., CC G., F M, NV A. Evaluation of chemical and antiviral properties of essential oils from South American plants. Antiviral Chem. Chemother. 2005;16 doi: 10.1177/095632020501600404. [DOI] [PubMed] [Google Scholar]
- 88.HSR S., FO dC., Er S., NGL S., S S, DN S. Anti-inflammatory activity of limonene in the prevention and control of injuries in the respiratory system: a systematic review. Curr. Pharmaceut. Des. 2020;26 doi: 10.2174/1381612826666200320130443. [DOI] [PubMed] [Google Scholar]
- 89.G C, M W, X X, Lw S., F L, S Z Suppression of MAPK and NF-κB pathways by limonene contributes to attenuation of lipopolysaccharide-induced inflammatory responses in acute lung injury. Inflammation. 2013;36 doi: 10.1007/s10753-012-9571-1. [DOI] [PubMed] [Google Scholar]
- 90.R H, NN R., H N, HS S., M S, N S Anti-inflammatory effects of limonene from yuzu (Citrus junos Tanaka) essential oil on eosinophils. J. Food Sci. 2010;75 doi: 10.1111/j.1750-3841.2010.01541.x. [DOI] [PubMed] [Google Scholar]
- 91.R H, H N, SA B., Nr N., BA M., N D Limonene inhalation reduces allergic airway inflammation in Dermatophagoides farinae-treated mice. Inhal. Toxicol. 2012;24 doi: 10.3109/08958378.2012.675528. [DOI] [PubMed] [Google Scholar]
- 92.H B, O R, D W, E K Prophylactic treatment of asthma by an ozone scavenger in a mouse model. Bioorg. Med. Chem. Lett. 2015;25 doi: 10.1016/j.bmcl.2014.11.035. [DOI] [PubMed] [Google Scholar]
- 93.F B, C B, M E, T W, Ej V. Effect of myrtol standardized and other substances on the respiratory tract: ciliary beat frequency and mucociliary clearance as parameters. Adv. Ther. 2012;29 doi: 10.1007/s12325-012-0014-z. [DOI] [PubMed] [Google Scholar]
- 94.M P, A G Is Myrtol® standardized a new alternative toward antibiotics? Phcog. Rev. 2016;10 [Google Scholar]
- 95.C R, PC B, P G, A U Review of toxicological assessment of d-limonene, a food and cosmetics additive. Food Chem. Toxicol. : Int. J. Publ. Brit. Indust. Biolog. Res. Ass. 2018:120. doi: 10.1016/j.fct.2018.07.052. [DOI] [PubMed] [Google Scholar]
- 96.IA H., V H, E G, R W, D A Development of a questionnaire and a database for assessing dietary d-limonene intake. Publ. Health Nutr. 2002;5 doi: 10.1079/PHN2002371. [DOI] [PubMed] [Google Scholar]
- 97.Yw K., Mj K., BY C., Bd Y., Sk L., SM C. Safety evaluation and risk assessment of d-Limonene. J. Toxicol. Environ. Health B Crit. Rev. 2013;16 doi: 10.1080/10937404.2013.769418. [DOI] [PubMed] [Google Scholar]
- 98.SD T., H T, W P, P S, J A The male rat carcinogens limonene and sodium saccharin are not mutagenic to male Big Blue rats. Mutagenesis. 2001;16 doi: 10.1093/mutage/16.4.329. [DOI] [PubMed] [Google Scholar]
- 99.M H, K U, J M, S Y, Y K Distribution and immune responses resulting from oral administration of D-limonene in rats. J. Nutr. Sci. Vitaminol. 2002;48 doi: 10.3177/jnsv.48.155. [DOI] [PubMed] [Google Scholar]
- 100.Xl H., Xj L., QF Q., YS L., Wk Z., HB T. Anti-inflammatory and antinociceptive effects of active ingredients in the essential oils from Gynura procumbens, a traditional medicine and a new and popular food material. J. Ethnopharmacol. 2019;239 doi: 10.1016/j.jep.2019.111916. [DOI] [PubMed] [Google Scholar]
- 101.AA Y., RC G., M N, MA M., D A, A P Development and evaluation of nanoemulsion and microsuspension formulations of curcuminoids for lung delivery with a novel approach to understanding the aerosol performance of nanoparticles. Int. J. Pharm. 2019;557 doi: 10.1016/j.ijpharm.2018.12.042. [DOI] [PubMed] [Google Scholar]
- 102.Y G, X M, J Z, P S, H W, Y Z Oral delivery of lycopene-loaded microemulsion for brain-targeting: preparation, characterization, pharmacokinetic evaluation and tissue distribution. Drug Deliv. 2019;26 doi: 10.1080/10717544.2019.1689312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Mr A., M A, A A, RM S, A M Formulation design and development of matrix diffusion controlled transdermal drug delivery of glimepiride. Drug Des. Dev. Ther. 2018;12 doi: 10.2147/DDDT.S147082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Ir H., Mg R., AM B., RM G., Mk M., Bp N. Inhibition of protein prenylation by metabolites of limonene. Biochem. Pharmacol. 1999;57 doi: 10.1016/s0006-2952(98)00349-9. [DOI] [PubMed] [Google Scholar]
- 105.P W, GD N Effects by inhalation of abundant fragrances in indoor air - an overview. Environ. Int. 2017:101. doi: 10.1016/j.envint.2017.01.013. [DOI] [PubMed] [Google Scholar]
- 106.P W Indoor air pollutants in office environments: assessment of comfort, health, and performance. Int. J. Hyg Environ. Health. 2013:216. doi: 10.1016/j.ijheh.2012.08.001. [DOI] [PubMed] [Google Scholar]
- 107.D L, P L, J B-M, M C Inflammatory effects induced by selected limonene oxidation products: 4-OPA, IPOH, 4-AMCH in human bronchial (16HBE14o-) and alveolar (A549) epithelial cell lines. Toxicol. Lett. 2016:262. doi: 10.1016/j.toxlet.2016.08.023. [DOI] [PubMed] [Google Scholar]
- 108.X N, SSH H., KF H., Y H, J C, Z S Indoor secondary organic aerosols formation from ozonolysis of monoterpene: an example of d-limonene with ammonia and potential impacts on pulmonary inflammations. Sci. Total Environ. 2017:579. doi: 10.1016/j.scitotenv.2016.11.018. [DOI] [PubMed] [Google Scholar]
- 109.C C, Y S, Y H, J S, W L, H F Determination of d-limonene in mice plasma and tissues by a new GC-MS/MS method: comparison of the pharmacokinetics and tissue distribution by oral and inhalation administration in mice. Biomed. Chromatogr. : BMC (Biomed. Chromatogr.) 2019;33 doi: 10.1002/bmc.4530. [DOI] [PubMed] [Google Scholar]
- 110.F-F A., A L, M H, Ew H., Z W d-limonene exposure to humans by inhalation: uptake, distribution, elimination, and effects on the pulmonary function. J. Toxicol. Environ. Health. 1993;38 doi: 10.1080/15287399309531702. [DOI] [PubMed] [Google Scholar]
- 111.CAF R., RCDS S., MF A., RB B., Dp dS., MFFM D. Histopathological and biochemical assessment of d-limonene-induced liver injury in rats. Toxicology reports. 2015;2 doi: 10.1016/j.toxrep.2015.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.LD L.-M., D C d-Limonene induced hyaline droplet nephropathy in alpha 2u-globulin transgenic mice. Fund. Appl. Toxicol. : official journal of the Society of Toxicology. 1994;23 doi: 10.1006/faat.1994.1141. [DOI] [PubMed] [Google Scholar]
- 113.SB A., MU R., B F, B A, I H, Sp A. Antifibrotic effects of D-limonene (5(1-methyl-4-[1-methylethenyl]) cyclohexane) in CCl 4 induced liver toxicity in Wistar rats. Environ. Toxicol. 2018;33 doi: 10.1002/tox.22523. [DOI] [PubMed] [Google Scholar]
- 114.S B, KE L., VD D., Sg K. Computational insights into tetracyclines as inhibitors against SARS-CoV-2 M pro via combinatorial molecular simulation calculations. Life Sci. 2020:257. doi: 10.1016/j.lfs.2020.118080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.YS W., P B, RE V., MA K., Nk P. Current pharmaceutical design; 2020. Natural Products: A Rich Source of Antiviral Drug Lead Candidates for the Management of COVID-19. [DOI] [PubMed] [Google Scholar]
- 116.VN B., A K, Pk S, S M, A D, G B Determination of potential inhibitors based on isatin derivatives against SARS-CoV-2 main protease (m pro): a molecular docking, molecular dynamics and structure-activity relationship studies. J. Biomol. Struct. Dynam. 2020 doi: 10.1080/07391102.2020.1845800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.A B., A S., U M. Molecular docking study of potential phytochemicals and their effects on the complex of SARS-CoV2 spike protein and human ACE2. Sci. Rep. 2020:10. doi: 10.1038/s41598-020-74715-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.LS B., EN G., GB A., Lk K., MA A., CN M. Alkaloids from cryptolepis sanguinolenta as potential inhibitors of SARS-CoV-2 viral proteins: an in silico study. BioMed Res. Int. 2020:2020. doi: 10.1155/2020/5324560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Dw K., Kh S., Mj C.-L., KY O., Jw O., Jk C. Phenolic phytochemical displaying SARS-CoV papain-like protease inhibition from the seeds of Psoralea corylifolia. J. Enzym. Inhib. Med. Chem. 2014;29 doi: 10.3109/14756366.2012.753591. [DOI] [PubMed] [Google Scholar]
- 120.S S, D S, K W, R Z, B S, A K Kaempferol derivatives as antiviral drugs against the 3a channel protein of coronavirus. Planta Med. 2014:80. doi: 10.1055/s-0033-1360277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.JY P., Hj Y., Hw R., Sh L., KS K., Kh P. Evaluation of polyphenols from Broussonetia papyrifera as coronavirus protease inhibitors. J. Enzym. Inhib. Med. Chem. 2017;32 doi: 10.1080/14756366.2016.1265519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.A P, Mk K., M H, S G Natural plant products: a less focused aspect for the COVID-19 viral outbreak. Front. Plant Sci. 2020:11. doi: 10.3389/fpls.2020.568890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.CC G, L T, N A, S C, C D, EB D. Virucidal activity of essential oils from aromatic plants of San Luis, Argentina. Phytother Res. : PT. 2003;17 doi: 10.1002/ptr.1305. [DOI] [PubMed] [Google Scholar]
- 124.R M, RE O M., JR., EE S Inhibitory effect of essential oils obtained from plants grown in Colombia on yellow fever virus replication in vitro. Ann. Clin. Microbiol. Antimicrob. 2009;8 doi: 10.1186/1476-0711-8-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Setzer W.N. Essential oils as complementary and alternative medicines for the treatment of influenza. American Journal of Essential Oils and Natural Products. 2016;4:16–22. [Google Scholar]
- 126.M M, M K, T N, T Y, H K, J I The inhibitory effect of essential oils on herpes simplex virus type-1 replication in vitro. Microbiol. Immunol. 2003:47. doi: 10.1111/j.1348-0421.2003.tb03431.x. [DOI] [PubMed] [Google Scholar]
- 127.Elaissi A., Rouis Z., Salem N.A.B., Mabrouk S., Salem Yb, Salah K.B.H. Chemical composition of 8 eucalyptus species' essential oils and the evaluation of their antibacterial, antifungal and antiviral activities. BMC Compl. Alternative Med. 2012;12:1–15. doi: 10.1186/1472-6882-12-81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Brand Y.M., Roa-Linares V.C., Betancur-Galvis L.A., Durán-García D.C., Stashenko E. 2015. Antiviral Activity of Colombian Labiatae and Verbenaceae Family Essential Oils and Monoterpenes on Human Herpes Viruses. [Google Scholar]
- 129.RE O, R M, FA T E.S. Memorias do Instituto Oswaldo Cruz; 2010. Virucidal activity of Colombian Lippia essential oils on dengue virus replication in vitro; p. 105. [DOI] [PubMed] [Google Scholar]
- 130.Moussaoui N.E., Laboratory of Biology and Health DoB, Faculty of Sciences, University Abdelmalek Essaâdi M’hannech II, BP 2121, Tetouan 93002, Morocco, Sanchez G, Institute of Agrochemistry and Food Technology (IATA). Spanish Council for Scientific Research (CSIC) S, Khay EO, Laboratory of Biology and Health DoB, Faculty of Sciences, University Abdelmalek Essaâdi, M’hannech II, BP 2121, Tetouan 93002, Morocco, et al. Antibacterial and Antiviral Activities of Essential Oils of Northern Moroccan Plants. Biotechnology Journal International. 2013:318–331. [Google Scholar]
- 131.Roy Roy, S Cp, Chowdhary A. 2015. Evaluation of Antiviral Activity of Essential Oil of. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.C T, B V, C C, M-H J.M., P B Effect of limonene and sobrerol on monocrotaline-induced lung alterations and pulmonary hypertension. Int. Arch. Allergy Immunol. 1995;107 doi: 10.1159/000237000. [DOI] [PubMed] [Google Scholar]
- 133.Pj Y., LM W., Sh W., WY C., H X, DM M. Standardized myrtol attenuates lipopolysaccharide induced acute lung injury in mice. Pharmaceut. Biol. 2016;54 doi: 10.1080/13880209.2016.1216132. [DOI] [PubMed] [Google Scholar]
- 134.X W, G L, W S Protective effects of D-Limonene against transient cerebral ischemia in stroke-prone spontaneously hypertensive rats. Experimental and therapeutic medicine. 2018;15 doi: 10.3892/etm.2017.5509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.M P, D N, M K, KM F., S R, A S Limonene-induced activation of A 2A adenosine receptors reduces airway inflammation and reactivity in a mouse model of asthma. Purinergic Signal. 2020;16 doi: 10.1007/s11302-020-09697-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Vieira A.J., Beserra F.P., Souza M.C., Totti B.M., Rozza A.L. Limonene: Aroma of innovation in health and disease. Chem. Biol. Interact. 2018;283:97–106. doi: 10.1016/j.cbi.2018.02.007. PMID: 29427589. [DOI] [PubMed] [Google Scholar]
- 137.Asif M., Saleem M., Saadullah M., Yaseen H.S., Al Zarzour R. COVID-19 and therapy with essential oils having antiviral, anti-inflammatory, and immunomodulatory properties. Inflammopharmacology. 2020;28(5):1116–1153. doi: 10.1007/s10787-020-00744-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data included in article/supplementary material/referenced in article.