Abstract
As an important phenomenon to monitor disease development, cell signaling usually takes place at the interface between organisms/cells or between organisms/cells and abiotic materials. Therefore, finding a strategy to build the specific biomedical interfaces will help regulate information transmission and produce better therapeutic results to benefit patients. In the past decades, plasmas containing energetic and active species have been employed to construct various interfaces to meet biomedical demands such as bacteria inactivation, tissue regeneration, cancer therapy, and so on. Based on the potent functions of plasma modified surfaces, this mini-review is aimed to summarize the state-of-art plasma-activated interfaces and provide guidance to researchers to select the proper plasma and processing conditions to design and prepare interfaces with the optimal biological and related functions. After a brief introduction, plasma-activated interfaces are described and categorized according to different criteria including direct plasma-cells interfaces and indirect plasma-material-cells interfaces and recent research activities on the application of plasma-activated interfaces are described. The authors hope that this mini-review will spur interdisciplinary research efforts in this important area and expedite associated clinical applications.
Keywords: Plasma-assisted processes, Bioactive interfaces, Biomedical engineering
Graphical abstract
Highlights
-
•
The Interfaces between organisms/cells and abiotic materials are crucial for cell signaling.
-
•
Plasmas containing energetic and active species are potent tool to construct biomedical interfaces.
-
•
The objective here is to summarize recent plasma-activated interfaces to spur interdisciplinary efforts for clinical applications.
1. Introduction
Cells and organisms have a complicated system to carry out specific missions that follow a sequence of steps bridged by signal transduction. Signal communication which usually takes place at the interfaces between the external substances and internal biological environment regulates the biological behavior especially that associated with disease development [1]. For instance, an imbalance of blood glucose on the two sides of cell membranes triggers ion channels for calcium exchange [2,3]. In fact, most physiological processes related to metabolism, apoptosis, and other self-repairing activities rely on signal transduction at the interfaces [4,5] and the proper interfaces are crucial to biological functions and biomaterials research.
Many efficient bio-interfaces have been designed to address different biomedical demands, for example, improving therapeutics to relieve patients from discomfort and plasma technology has emerged to be very useful in biomedical engineering and biomaterials research. The active species in plasmas interact with pathogenic microorganisms directly killing them but they are friendly to normal cells. Moreover, proper plasma treatment can foster tissue regeneration and inhibit tumor progression [[6], [7], [8], [9]]. The recent development of interdisciplinary research has linked plasma science with nanotechnology and nanomaterials. For instance, the active species in plasmas can be introduced to the interfaces of biomaterials in biosystems associated with orthopedics, dentistry, and vascular implants [[10], [11], [12]]. There have also been in-depth investigations of plasma physics and plasma medicine to elucidate the mechanisms on how interfaces regulate various biochemical responses. This mini-review aims to summarize recent advances in plasma-activated interfaces and describes the working principles of plasmas in creating such interfaces. Following a brief introduction of plasmas, direct and indirect plasma-based interfaces and corresponding applications are described. The general guidelines on the selection of various parameters and processes to cater to specific applications such as plasma medicine are also discussed.
2. Plasmas
As the fourth fundamental state of matters in addition to solid, liquid, and gas, a plasma is an electrically neutral system comprising electrons, ions, excited particles, and neutral particles [13]. Besides being the main state in extraterrestrial matters, plasmas exist in the form of lightning and aurora on earth [[14], [15], [16]]. Plasmas can be classified as nonthermal and thermal ones depending on the temperature and also as metal and non-metal plasmas according to the ingredients. As the metastable active species in plasmas are quite energetic, processes incorporating man-made plasmas have high efficiency and productivity and are widely used commercially, especially in the microelectronics and coatings industry [[17], [18], [19]].
2.1. Nonthermal and thermal plasmas
Temperature is a critical plasma factor and must be considered in biomedical engineering because biological matters and tissues can only tolerate a narrow temperature window. Plasmas are classified as thermal and non-thermal ones according to the average temperature of the neutral and charged particles. Both nonthermal and thermal plasmas are usually initiated by high-energy electrons excited by external sources such as direct current (DC) discharge, pulsed DC discharge, radio frequency (RF), and electron cyclotron resonance (ECR) [17]. Electron excitation is followed by energy transfer via collisions between electrons and particles until the temperature equilibrates. The temperature difference (Tdiff) between electrons and particles is related to the ratio of the electric field (E) to pressure (p) with the following relationship: Equation (1): Tdiff ∝ (E/p)2. Hence, a small E/p accelerates the local thermodynamic equilibrium and enables the formation of thermal plasma in which the temperature of electrons (Te) is equal to that of ions and gas (Ti and Tg). However, in many cases, plasmas rarely reach thermodynamic equilibrium with Te » Ti » Tg, so that the average temperature is lower and this kind of plasma is categorized as a nonthermal one [20]. A nonthermal plasma also has a small fraction of ionization, whereas a thermal plasma is nearly fully ionized [21]. The high temperature and ionization rate in thermal plasmas facilitate applications such as welding, cutting, and plasma spraying albeit limited to heat-resistant materials [[22], [23], [24], [25], [26]]. On the other hand, cold ions and gases in nonthermal plasmas create a milder environment which is more suitable for biomaterials and biosystems that are prone to thermal damage. In this way, a plasma-activated interface can be constructed to cater to different applications such as bacteria sterilization, wound healing, and cancer therapy while keeping normal tissues undamaged [[27], [28], [29], [30]].
2.2. Plasma sources
Both thermal and nonthermal plasmas are sustained by two electrodes connected to an external power supply. The space between the two electrodes is filled with one or more gases at atmospheric or low pressure and the plasma is created when a voltage is applied between the electrodes. Generally, a pressure as low as a few Torr and small current can sustain the discharge in a nonthermal plasma [31,32]. Low-pressure discharges can be further classified as DC, RF, and ECR ones and are effective for etching and deposition [33]. As vacuum can cause irreversible damage such as excessive swelling to tissue/cells, low-pressure plasma is suitable for materials modification rather than direct interactions with biological tissues/cells [34]. Pulsed power excitation can be extended to atmospheric pressure to produce corona discharges that are widely used in activating polymeric materials such as fabrics to improve adhesion and dyeing effectiveness [35]. Atmospheric-pressure nonthermal plasmas are normally more suitable for direct interactions with tissues that actually constitute one of the electrodes. The floating-electrode dielectric barrier discharge (FE-DBD) can be generated to sterilize surfaces and interfaces without damaging living tissues [36]. When the pressure is high and external resistance is small, a current as large as 1 A can be obtained, and the hot arcs are usually coupled with the gas glow discharge to form a high-pressure thermal plasma source which has many applications in welding and cutting [25,37]. These power sources can ionize metals or nonmetals to modify the various surface properties of materials and more details will be provided in the following sections.
3. Plasma-activated interfaces
The milder working conditions of nonthermal atmospheric-pressure plasmas allow direct interactions with biological tissues and biomaterials but on the other hand, low-pressure plasmas are more suitable for introducing external species into the surface of the material before they come into contact with body fluids and tissues, for example, plasma-treated orthopedic and dental implants. Such plasma-activated interfaces can be divided into two categories: direct interfaces and indirect interfaces.
3.1. Direct interfaces activated by plasmas
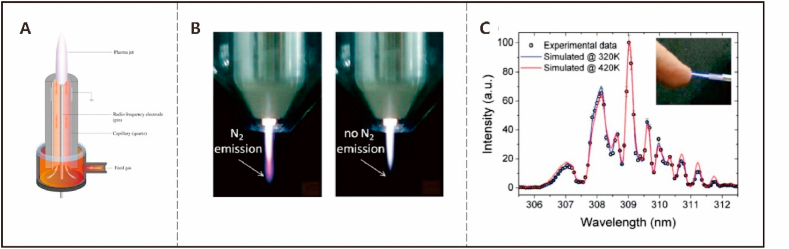
The operating conditions of atmospheric-pressure nonthermal plasmas are mild enough so that the cells, microorganisms, and tissues can serve as one of the electrodes. In this way, electrons, ions, or particles in the plasma can carry out the designated functions including sterilization, wound healing, blood coagulation, and treatment of skin disease and cancer [[38], [39], [40], [41]]. Taking the antibacterial process by the low-pressure radio frequency or microwave discharge as an example [42], the active species in the nonthermal plasma can kill microorganisms such as bacteria, viruses, and fungi but do not cause thermal damage in normal tissue [43,44]. Generally, a noble gas is ionized in the plasma to react with components at the interface subsequently triggering a burst of reactive oxygen species (ROS) such as ·OH, , and 1O2, which interfere with the body metabolism [45]. The large electron and ion densities also contribute to the death of microorganisms via physical collision and interruption of the charge balance at the interface. Compared to indirect interfaces on pretreated biomaterials, direct interfaces work more swiftly and precisely and have many potential applications. For instance, the fledgling field of plasma medicine is derived as an interdisciplinary subject from plasma technology (Fig. 1) [[46], [47], [48]].
Fig. 1.
Examples of atmospheric pressure plasma jet: (a) Schematic set-up of the atmospheric pressure plasma jet [47]; (b) Photograph of the modified plasma jet with two different shielding gas conditions [48]; (c) Measured and simulated optical emission spectra around the OH line at 309 nm for the sub-microsecond pulsed atmospheric argon plasma jet [46]; Reprinted with permission from Refs. [[46], [47], [48]].
3.2. Indirect interfaces activated by plasmas
The interfaces at which tissues/cells interact with biomaterials pretreated by plasmas are indirect ones with many applications in biomaterials and biomedical engineering. Indirect interfaces can be constructed on a myriad of biomaterials such as polydopamine [49], titanium [50], and silicone [51] to produce bone implants, artificial organs, medical devices for diagnosis, and sensors. The main methods to fabricate indirect interfaces include etching, sputtering, deposition, and ion implantation.
Sputtering and etching are processes in which undesired components and species are removed from the surface and the desired surface morphology can be formed. Inert gases such as argon are normally employed in physical sputtering in which energy is transferred from the plasma species to atoms in the near-surface of the substrate via collisional cascades. The clean interface resulting from sputtering can be the precursor for further deposition or grafting and the exposed sublayer can also be successively etched by the plasma. Physical sputtering using an inert plasma is usually isotropic but shows a dependence on the crystal orientation of the substrate. On the other hand, the use of reactive gases in reactive ion etching can produce anisotropic features and so the surface topography can be adjusted controllably by varying the plasma species and conditions [52,53].
Plasma deposition which is an efficient method in biomaterials design and engineering is usually carried out with a low-pressure plasma in the pulsed alternating current (AC) or DC mode [54]. After sputter cleaning described above, the surface is exposed to the plasma to conduct deposition. Plasma deposition is a mature and common technique to produce thick and thin coatings and modify the surface chemistry and structure of a multitude of industrial materials. The chamber can be filled with a high-density plasma to produce a high deposition rate and various microstructures on the substrates [55,56]. By using the proper plasma constituents and instrumental parameters, surface structures with metallic or non-metallic species or a combination can be formed to improve specific properties such as hardness, adhesion strength, friction, and other chemical and biological properties.
As an advanced plasma technique, plasma immersion ion implantation and deposition (PIII&D) is a versatile means to modify and functionalize materials surface. As a non-line-of-sight process, it boasts high efficiency and versatility and is particularly useful for samples and industrial components with a complex shape [57,58]. In PIII&D, ion implantation and deposition can be carried out in the same vacuum chamber by varying the sample voltages on the fly, that is, high negative pulsed voltages for ion implantation and low or zero sample biases for deposition. A gas plasma (air, oxygen, nitrogen, argon, etc.) is usually sustained by an external power source such as DC, RF, and ECR, whereas metallic plasmas are usually generated by vacuum arc plasma sources [59,60]. By mixing ion implantation and deposition and using different plasma sources and parameters, surfaces with improved biological, physical, and/or chemical properties can be produced selectively to cater to different biomedical applications to be described in the following section.
4. Applications
Plasma-activated interfaces can address diverse issues in biomedical engineering. Generally, direct plasma treatment such as plasma medicine is gentle enough so that living tissues can be treated directly but the effects may be limited due to possible side effects and hardware restriction. In contrast, many artificial materials can withstand the harsher vacuum and plasma conditions, and indirect interfaces can be prepared to boost the various mechanical and biological functions. A brief summarization for the plasma-activated interfaces has been listed in Table 1.
Table 1.
Plasma-activated interfaces for biomedical engineering.
| Type of surfaces | application | Plasma sources | Other remarks/mechanisms |
|---|---|---|---|
| Direct interfaces activated by nonthermal plasma | Bacterial killing | CAP jet with Ar [61], DBD with mixture of He and O2 [62], air [[63], [64], [65]], mixture of O2, CO2 and N2 [66] | Oxidation, membrane damage, cell leakage in S. aureus, E. coli, fungi in plant |
| Anticancer | FE-DBD with air [67], DBD powered by AC [68] with N2 [67], CAP with He [69,70], DBD assisted Au nanoparticle [71], CAP integrated with microneedles [72] | MMP decrease, mitochondrial enzymatic dysfunction, and mitochondrial morphological alteration in lung [68,69], apoptosis in cervical [73], targeting the cell cycle in Keratinocytes cells [70] and glioblastoma [71] | |
| Dermatological diseases | DBD [74], kinpen09 with Ar [75], DBD with air [76,78], He [77], CAP powered by AC with Ar [[79], [80], [81]] | T cells proliferation [75], ulcustreatment [76], promoting inflammation, re-epithelialization and wound contraction in cutaneous wound [79], inhibiting the proliferation of the hyperproliferative keratinocytes [80], stimulating angiogenesis [81], clearance of early inflammation [77,78] | |
| Indirect interfaces activated by plasma | Bacterial killing | Ag PIII [[82], [83], [84], [85]], Ag deposited DLC [86], codoping of Mg and Ag [87], PIII of Zn on Ti [88], immersion in polyurethane [89] and PEEK [90], N PIII on Ti–6Al–4V [91], combination of O and N PIII [92], O and H2O immersion in Ti [93] | Killing via Ag+ leaching [82,83], electron transfer at the interface [84,85], Zn2+ leaching [88], increased hydrophilicity [89], electrostatic repelling [91], formation of polar groups [92] |
| Enhanced biocompatibility | Ceramic-like structure by Si–Ag PIII [94], codoping of Mg and Ag [87], Zn PIII on Ti [88] and TiO2 [95,96], O PIII on Ti [97,98], O plasma modified PEEK [99], combination of Ag and N PIII [100] | Regulating the iNOS and nNOS signal pathways [94], galvanic effects matters [87], stimulating proliferation of osteoblastic MC3T3-E1 cells as well as rat mesenchymal stem cells [88,95,96], higher TiO2 component [97], enhanced blood response [97], the surface topography enhanced osseointegration [99] | |
| Corrosion resistance/wear resistance | Ag deposited DLC [86], O PIII on Ti [97], N PIII on Ti–6Al–4V [91], Ti–O/Ti–N coating on Ti [101] | Enhancement of the passive region of Ti specimens [97], formation of TiN [91] | |
| Surface etching | Silicon etching [102], O2 plasma etching [103] | Superhydrophobic and superoleophobic surfaces [103] |
4.1. Direct interfaces
4.1.1. Direct interfaces attacking microorganisms
Atmospheric-pressure nonthermal plasmas are usually generated between electrodes under a gas flow. Previous studies have validated the anti-microbial effects of nonthermal plasmas against Gram-positive and Gram-negative bacteria, fungi, and viruses in infection prevention. Compared to traditional antimicrobial strategies, nonthermal plasmas have several advantages. Firstly, nonthermal plasmas can interact directly with materials, medical devices, tumors, and the superficial part of the body and construct a barrier against microbial species [61,67,74,104]. Secondly, nonthermal plasmas with better safety are applicable to heat-sensitive surfaces to complement autoclave strategies and chemical sterilization using ethylene oxide [62,105]. Thirdly, the mild conditions enable simpler engineering design and commercialization of nonthermal plasma devices with advantages such as simplicity, low cost, and portability [106,107]. In the process, gases such as air, O2, N2, He, and Ar are used to generate plasmas to supply electrons, ROS, reactive nitrogen species (RNS), ions, particles, and ultraviolet (UV) radiation, among which UV radiation and ROS play key roles in killing microbial species [63]. UV irradiation can destroy the genetic materials and etch the bacterial membrane by photo-desorption. Furthermore, the ROS can interact with micro-organisms to trigger chain reactions to disrupt metabolism [64,108] and as a result, physiological changes such as membrane damage, leakage of intracellular macromolecules, and DNA fragmentation occur in the treated microorganisms [65,109]. The mechanisms corroborated by experimental results indicate that the plasma species and power sources should be chosen carefully to produce sufficient UV and ROS to kill bacteria.
4.1.2. Direct interfaces attacking cancer cells
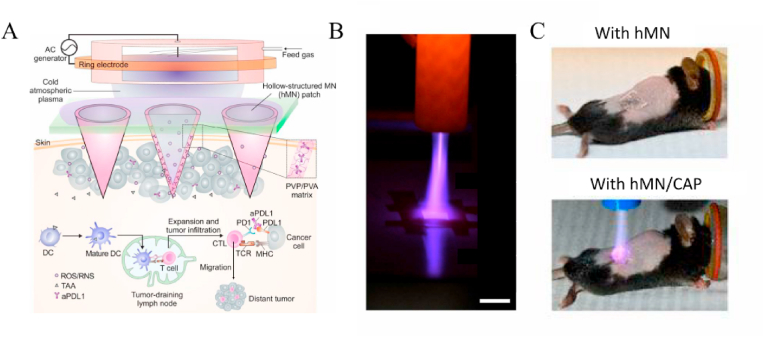
The reactive species in plasma can not only react with the surface of the tissue but also penetrate relative depth with a prolonged treatment duration, which opens up the application fields in oncology. Plasma needles and torches powered by DBD discharges generate a plasma that can be transported to the tumor site via the gas flow to create a microenvironment containing active species [68,69,73]. Mechanistic studies reveal that the ROS and RNS are able to interfere with the cell cycle of cancer cells and trigger apoptosis of cells in the S-phase leading to death [69]. Moreover, the expression of γH2A.X (pSer139), an oxidative stress reporter indicating S-phase damage, is boosted after the cold atmospheric plasma (CAP) treatment, thereby verifying the influence of the CAP treatment on the cells [110]. ROS accumulation can also be amplified with the assistance of gold nanoparticles to produce synergistic anticancer effects [71,111]. Consequently, specific materials can be selected to work in conjunction with CAP-based therapy for tumor treatment. Chen et al. have designed a plasma setup with hollow microneedles for transdermal treatment of cancer by promoting tumor immunology (Fig. 2) [72]. The hollow-structured microneedles (hMN) patch served as microchannels to facilitate transportation of CAP through the skin. However, conventional plasma devices cannot be miniaturized easily and they are usually used in superficial tumor treatment. In the future, interdisciplinary technological advancement is expected to expand anticancer interfaces enabling the treatment of different types of cancer. Nevertheless, it should be kept in mind that different cancer cells may respond to the plasma treatment differently due to the different mitochondria susceptibility to the reactive species [68] and more research is needed in this important area.
Fig. 2.
Design, processing, and animals experiments of hMN enhanced CAP mediated therapy: (A) Illustration of the transdermal CAP mediated immune checkpoint blockade therapy; (B) Penetration test of the CAP through hMN patch (Scale bar, 1 cm); (C) Schematic of B16F10 melanoma-bearing mice with different treatments. Reprinted with permission from Ref. [72].
4.1.3. Direct interfaces for tissue engineering
Despite the longstanding trend that reactive species are primarily toxic to tissues, recent studies in terms of molecular biology revealed that reactive species at low concentration are second messenger regulating cellular physiology and signaling [112,113], which creates the therapeutic window for plasma-generated reactive species in tissue regeneration. Mechanistic investigations on the molecular level disclose that sufficient nitric oxide at the interface plays a key role in the proliferation of mammalian cells [75,114]. Boasting a mild temperature, flexibility, and portability, atmospheric-pressure nonthermal plasmas allow efficient, painless, and contactless treatment without harming healthy tissues in the vicinity [76,115]. Plasmas can activate the interfaces to accelerate wound healing by inhibition of bacteria as well as regulation of the differentiation of dermal and epidermal cells [79,116]. Moreover, they are effective in activating the immune system in the treatment of atopic dermatitis and relieving patients of pruritus [80,117]. Tissue regeneration has been observed from the treatment of facial skin to smooth wrinkles and blood vessels to speed up coagulation [77,78,81]. It is also important that little side effects have been observed [44,118] thereby boding well for clinical applications [119,120].
Above all, the actual state of knowledge is that the cocktail effects of the multiple species in plasma endow it the property of “Jack of all trades”. Hence, plasma dosage must be precisely manipulated on the way to clinical routine. Apart from the basic understanding of the biomedical efficacy, the underlying mechanism still deserves tireless efforts but has yet been clearly explained due to the less maturity of the current biological and cyber technologies. It is foreseeable that the development of advanced technology including virtual simulation and molecular biology should offer multidimensional cognitions on the reaction process guiding the precise manipulation of plasma medicine.
4.2. Indirect interfaces
Besides the aforementioned direct plasma treatment in which UV, electrons, and free radicals are produced in situ and participate in the direct interactions with biological tissues, plasmas can be used to produce interfaces on artificial materials to interact with biosystems indirectly. The development of indirect surfaces relies on the design of advanced materials that can be adopted in vivo. Implant materials with acceptable biocompatibility usually do not possess sufficient inherent antibacterial ability, although biocompatibility and bacterial resistance are highly desirable in many clinical applications. For example, titanium alloys and polyetheretherketone (PEEK) possess poor intrinsic antibacterial ability and biocompatibility, but the properties can be improved by modifying the physicochemical characteristics on the surface [[121], [122], [123], [124], [125]]. Meanwhile, the mechanical strength of implants should also be taken into consideration. For example, hip replacement implants should possess bone-like mechanical strength to avoid bone loss caused by stress shielding and should also be biodegradable to avoid a second surgery. In many cases, plasmas are employed to endow interfaces with the aforementioned multi-functionalities while preserving the favorable bulk attributes of the substrate, which may be challenging for traditional chemical strategies such as physical vapor deposition, chemical vapor deposition, annealing, sol-gel deposition [126], acid etching [127], anodic oxidation [128] and chemical etching [129,130]. Inert gases such as argon are mostly used to generate reactive species in activating direct surfaces and to etch the top surface in activating indirect surfaces. Metallic and non-metallic species can be introduced to biomaterials to modifying selective functionalities. Metallic elements are usually used to improve the bacterial resistance and osteogenesis, whereas nonmetallic elements are usually deposited or implanted to introduce polar functional groups and modify the physicochemical properties without changing the surface topography. Nonmetallic elements from plasmas can also be utilized to etch surfaces with the aid of colloidal lithography [102,103]. The following section will describe some of the important applications.
4.2.1. Metallic element: Silver (Ag)
Ag can be introduced to biomaterials to improve the antibacterial ability [131] and as a broad-spectrum antibacterial agent, Ag rarely leads to drug resistance [132]. Ag is commonly dispersed in the solution acting as a bactericide but the dosage is usually beyond the toxic level. By using plasma technology, Ag ions in the plasma are injected into the biomaterials to form nanoparticles [82]. The size, distribution, and depth distribution of the Ag nanoparticles can be tuned by adjusting the plasma parameters and Ag nanoparticles with a size of 5 nm have been found to produce the highest antibacterial rate. Besides, interfaces modified with the proper amount of Ag nanoparticles by plasmas are generally biocompatible and normal tissues can adhere and proliferate well [83,133]. For example, Ag-doped diamond-like carbon (DLC) films formed by plasma treatment possess good antibacterial ability, low friction, atomically smooth topography, as well as corrosion resistance [86]. Ag can be combined with other elements at the interfaces to achieve multiple functions [94,134]. For instance, Liu et al. have refined polyamine 66 by Ag and Si doping. The surface hardness and elastic modulus enhanced by Si results in better bone regeneration and good antibacterial efficiency is rendered by Ag. Compared to Ag doping alone, fewer Ag ions are released during the first 3 weeks after implantation and an upregulated expression of bone-generation genes (iNOS and nNOS) is detected as well [94].
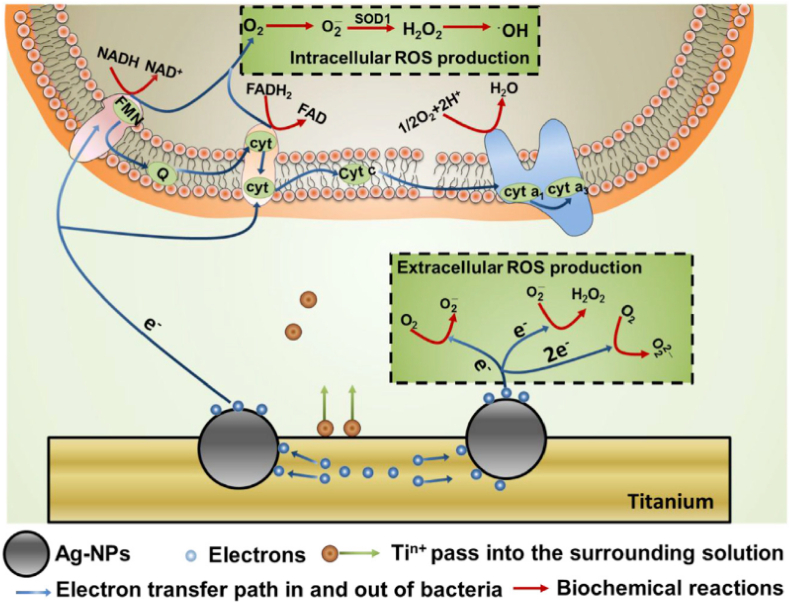
The antibacterial mechanism of Ag has been investigated. It is shown that Ag nanoparticles dispersed in a solution or embedded in the substrate react with bacteria by different mechanisms. Ag nanoparticles dispersed in a solution usually attack bacteria directly in the form of Ag ion release. When embedded in the substrate, Ag nanoparticles react with the substrate and the bacteria simultaneously. Ti ions are leached into the solution and sufficient electrons accumulate on the Ag nanoparticles and are transferred to the attached bacteria influencing the respiratory chain leading to ROS burst, membrane rupture, and final death as illustrated in Fig. 3 [84]. This electron-transfer mediated antibacterial process is size-dependent that Ag nanoparticles in the range of 5–25 nm fare the best in accumulating electrons but on the other hand, larger particles are toxic to normal tissues and can induce serious cytosolic content leakage and lysis [85]. The galvanic effect can be extended to the interfaces doped with Mg and Ag. This hybrid system facilitates the release of Mg but reduces the leaching of Ag ions. In this way, the antibacterial efficacy and osteogenic properties are improved while the toxicity is reduced [134]. In conclusion, Ag nanoparticles introduced by plasma technology create antibacterial surfaces with multiple functionalities and the antibacterial concept demonstrated on Ag embedded Ti suggests a new strategy for efficient bacterial killing.
Fig. 3.
Generation of oxidative stress in the solution and in the bacteria. Reprinted with permission from Ref. [84].
4.2.2. Metallic element: Zinc (Zn)
As an important trace element to support physiological metabolism, Zn can stimulate osteointegration by improving cell adhesion, immune functions, alkaline phosphatase activity, and skeletal development [[135], [136], [137]]. Moreover, the oxidative stress exerted by Zn ions produces antibacterial effects [138] and Zn is usually coated on dental or orthopedic implants to enhance the retention strength, osseointegration, and antibacterial properties [88,95]. For instance, Fang et al. have produced Zn-incorporated Ti by PIII and the materials provide a habitable environment for osteoblasts to adhere and proliferate. The Zn-decorated interface also inhibits bacteria growth [139] but Zn films are usually not durable due to dissolution in the osseointegration process [140]. Zhao et al. have combined plasma spraying with plasma ion implantation to produce crystalline TiO2 from which Zn ions are released at a small rate to ensure prolonged support during osteogenesis while providing bacterial resistance against S. aureus [141].
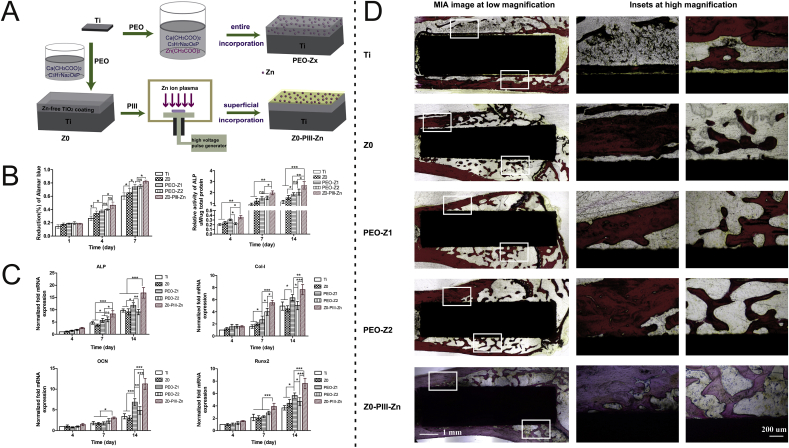
Although release of Zn ions can be slowed down, uncontrolled release can disrupt the original homeostasis resulting in lack of other trace elements such as iron and calcium [142]. Therefore, it is essential to synthesize Zn-incorporated implants in a controllable manner to satisfy clinical demand. PIII&D is a controllable technique to incorporate the desired elements into the interfaces. Jin et al. have reported controlled Zn incorporation by altering the implantation time and the sample with the optimal amount exhibits excellent osteogenic activity and antibacterial effects without undesirable side effects [88]. Qiao et al. have found that Zn ions at the interface act as the primary factor triggering the osteogenic loop [96] and “bulk-doped” and “surface-doped” coatings have been prepared by single plasma electrolyte oxidation (PEO) and PEO combined Zn PIII&D (Z0-PIII-Zn), respectively, to study the mechanism (Fig. 4). PEO produces a uniform 3D distribution of Zn forming a “bulk-doped” surface which can be tuned by adjusting the amount or treatment duration. Compared with single PEO treatment, delayed release of Zn from the “surface-doped” Z0-PIII-Zn sample can be maintained at an acceptable level for a long time resulting in superior osseointegration and the osteogenic genes OCN, Col-1 and Runx2 are upregulated to promote bone remodeling. The histological analysis confirms that all the PEO coatings (Z0, PEO-Zx, and Z0-PIII-Zn) show the formation of new bone filling the gaps between the implants and bones. The bone-to-implant contact (BIC) index of Z0-PIII-Zn is significantly higher than the other groups, verifying that Z0-PIII-Zn induces better osteogenisis than the other samples. These results provide evidence that PIII&D is a controllable technique to deliver the proper amount of elements to satisfy clinical needs.
Fig. 4.
Design, processing, cellular and animals experiments of Zinc incorporated implants for stimulation of bone growth: (A) Schematic presentation of the two zinc incorporation strategies: bulk incorporation and surface incorporation; (B) Cell proliferation and quantitative ALP activity of rBMSCs on different Zn-incorporated coating; (C) Expression of osteogenic-related genes in MCSs on different groups measured by quantitative real-time RT-PCR; (D) Histological analysis of section of each implant after 12 weeks in the rat models. Reprinted with permission from Ref. [96].
4.2.3. Nonmetallic element: Oxygen
An oxygen plasma can be used to produce an oxide layer on biomaterials to obtain the desirable properties. For example, after oxygen PIII, Ti is covered by a dense oxide layer with improved corrosion resistance, cell adhesion, and proliferation, as well as differentiation of human bone marrow mesenchymal stem cells [97,143,144]. The interactions between O-PIII Ti and blood have been investigated and a higher voltage produces an interface dominated by the rutile phase of TiO2 which can activate platelets to accelerate blood coagulation and inhibit bacteria adhesion [98]. The favorable properties produced by oxygen PIII are beneficial to Ti bone implants.
Oxygen plasma etching can be used to create the desired surface topography. For example, the surface energy of O-PIII modified PEEK increases significantly giving rise to enhanced hydrophilicity and subsequently better osseointegration and stability [99]. Another advantage of oxygen plasma etching is to generate an isotropic surface. Argon and oxygen plasma treatment has been performed on polystyrene (PS) monolayers and the anisotropic process of the argon plasma etches the monolayer vertically while keeps the monolayer unchanged in horizontal direction. During oxygen plasma etching, the size of the PS spheres decreases contributing to isotropic etching. Consequently, different morphologies can be produced by plasma etching as exemplified by pillar-like arrays prepared by a combination of argon and oxygen plasma as well as cone-like arrays produced by the oxygen plasma solely [49].
4.2.4. Nonmetallic element: Nitrogen
Nitrogen-bearing functional groups can be formed readily by plasma technology. For chemically and biologically inert implants, nitrogen plasma treatment can create N-containing groups such as –NH2 to provide a bridge between the implant surface and proteins to enhance cell adhesion. N-PIII also activates the bio-interface between microorganisms and implants by altering the micro-macro structure on the surface. For example, N-PIII increases the surface roughness of polyurethane (PU) which shows increased fractal dimensions with more sinuous folds and the surface becomes more hydrophilic thus creating a hostile and durable environment against S. aureus [89]. The N-containing groups formed by N-PIII can be grafted onto the interface between PEEK and bone cells and the surface free energy change encourages more osteoblasts to adhere, proliferate, and differentiate into bone tissues as confirmed by the elevated activity of the alkaline phosphatase (ALP) in cells on the N-PIII PEEK [90]. Different from grafting N-containing groups on polymeric substrates, N-PIII produces a TiN film (<1 μm) on bio-inert Ti–6Al–4V. The TiN film is corrosion resistant and supports the growth of bone cells but not bacteria. Together with the enhanced mechanical strength, the N-PIII treated Ti–6Al–4V sample has large potential in dental implants and prostheses [91].
Nitrogen PIII can be combined with other techniques to produce synergistic effects. Zheng et al. have prepared TiO2 coatings on Ti surface by micro-arc oxidation and doped the samples with nitrogen by N-PIII. Incorporation of nitrogen enhances photocatalysis by shifting the absorbing region from UV-light to visible light so that N–TiO2 at the interface can utilize visible light to kill bacteria [145]. While excessive release of Ag nanoparticles from PE poses toxicity to normal cells, combined Ag and N2 PIII using a reduced amount of Ag produces an interface with excellent surface roughness, hydrophilicity, protein adhesion, cytocompatibility, and bacterial resistance [100]. Oxygen PIII and nitrogen PIII have also been performed in concert to form C O and C NH C–NH2 groups on the surface of poly(butylene succinate) to elevate the antibacterial ability [92].
4.2.5. Nonmetallic species: H2O
The elemental distributions in coatings are critical to the functionalities. When the affinity between oxygen and substrate is higher than that between the metal and oxygen, an oxide coating doped with the metallic species can be constructed. Polak et al. have used H2O PIII to produce an oxide layer on Ti and compared with oxygen PIII, the use of H2O prevents the formation of water-insoluble copper oxide or deep incorporation of copper atoms in titanium oxide. Consequently, the O2–Cu PIII sample contains copper oxide deep below the surface of titanium oxide, while H2O–Cu PIII produces metallic copper inside the titanium oxide simultaneously. The hybrid interface exhibits good biocompatibility stemming from oxidized titanium and controlled release of antibacterial copper ions [93].
4.2.6. Other elements
The elements mentioned above are the common elements utilized for plasma-activated biomedical implants and the biomedical effects have been verified by many studies. In some other cases, researchers are attempting to carry out multiple ion implantation with a complex plasma system to fulfill more than one functionality. For example, Ti–O/Ti–N film with enhanced wear resistance and blood compatibility can be realized by a combination of plasma immersion ion implantation and reactive plasma nitriding/oxidation and the composite film [101]. With CH4 and C2H2 as the carbon source and Fe14Nd2B as the metal source, radiofrequency thermal plasma is used to generate magnetic nanoparticles, the biomedical applications of which are promising [146]. Hopefully, the versatile plasma-based technology can be tailored to work with more elements to aid the implementation of more surface modifications.
5. Conclusion and outlook
Plasmas, as the fourth state of matter and composed of energetic and reactive species, constitute a powerful tool to improve the physical and biochemical characteristics of biomaterials and biomedical implants. Classified by temperature, plasmas can be divided into non-thermal and thermal ones. The mild operation conditions for non-thermal plasma make it a great tool to treat heat-sensitive abiotic/biotic surfaces forming direct plasma-based interfaces. Among these, atmospheric-pressure non-thermal plasmas have attracted much attention from the field of plasma medicine due to their convenience and versatility. As is described above, therapeutic effects including surface sterilization, wound healing, blood coagulation, and skin diseases/cancer treatment can all be achieved by atmospheric-pressure non-thermal plasma. On the heels of the anticipated advance in materials design and therapeutic measures, better immune therapy and tumor treatment with portable devices are envisioned.
Thermal plasma and low-pressure non-thermal, which require harsher work environment but generate more potent cohesion, are not suitable for directly treating biotic surfaces but are widely implemented in surface modification of artificial biomaterials and biomedical implants constructing indirect plasma-materials-cells interfaces. The indirect surfaces are then directed for different biomedical scenarios and the ensuing surface interactions see the corresponding efficacy. The surface roughness and chemical composition greatly affect the corrosion and bacterial resistance and proper performance can be realized by choosing appropriate plasma-based techniques including etching, ion implantation, and deposition. Etching in most cases is chosen for cleaning but it can also be employed to create the desired surface morphology with assistance with lithography. Ion implantation and coating deposition introduce different elements such as metal elements (Ag and Zn) and nonmetal species (O, N, H2O) improving the antibacterial ability or creating micro/nano-structures for better biochemical properties. Usually, Ag tends to make an antibacterial surface and Zn works for promoting osteogenesis. Non-metal species are commonly used to generate activated surfaces on polymers or composites fulfilling further requirements. Given the cocktail peculiarity of plasma, it is now widely recognized that the mixture of the components in the complex system contributes to the therapeutic effects. But the in-depth mechanisms about how plasma-activated surfaces realize the functions will be more precisely clarified in cooperation with diversified disciplines, which will help provide certain directions on how to choose parameters for specific functionality. For instance, the verified antibacterial factor of electron transfer indicates that plasma treatment that brings more electrical interactions at the interface is expected to kill more bacteria. As the technology continues to evolve and deeper understanding of the reactions at the interfaces are obtained, we believe that novel biomaterials and implants with multiple functionalities will be created and welcomed in biomedical engineering and clinical science.
Declaration of competing interest
There is no conflict of interest.
Acknowledgements
This work was supported by City University of Hong Kong Strategic Research Grant (SRG) No. 7005264, Guangdong - Hong Kong Technology Cooperation Funding Scheme (TCFS) No. GHP/085/18SZ (CityU 9440230), and Hong Kong Research Grants Council General Research Funds (GRF) No. CityU 11205617.
Footnotes
Peer review under responsibility of KeAi Communications Co., Ltd.
Contributor Information
Guomin Wang, Email: guomiwang2-c@my.cityu.edu.hk.
Paul K. Chu, Email: paul.chu@cityu.edu.hk.
References
- 1.Stoodley P., et al. Biofilms as complex differentiated communities. 2002;56:187–209. doi: 10.1146/annurev.micro.56.012302.160705. [DOI] [PubMed] [Google Scholar]
- 2.Ahn C., An B.S., Jeung E.B. Streptozotocin induces endoplasmic reticulum stress and apoptosis via disruption of calcium homeostasis in mouse pancreas. Mol. Cell. Endocrinol. 2015;412:302–308. doi: 10.1016/j.mce.2015.05.017. [DOI] [PubMed] [Google Scholar]
- 3.Ikeda K., et al. UCP1-independent signaling involving SERCA2b-mediated calcium cycling regulates beige fat thermogenesis and systemic glucose homeostasis. Nat. Med. 2017;23:1454–1465. doi: 10.1038/nm.4429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ryskamp D.A., et al. The polymodal ion channel transient receptor potential vanilloid 4 modulates calcium flux, spiking rate, and apoptosis of mouse retinal ganglion cells. J. Neurosci. 2011;31:7089–7101. doi: 10.1523/JNEUROSCI.0359-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mallilankaraman K., et al. MCUR1 is an essential component of mitochondrial Ca2+ uptake that regulates cellular metabolism. Nat. Cell Biol. 2012;14:1336–1343. doi: 10.1038/ncb2622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang M., et al. Design of biomimetic and bioactive cold plasma-modified nanostructured scaffolds for enhanced osteogenic differentiation of bone marrow-derived mesenchymal stem cells. Tissue Eng. 2014;20:1060–1071. doi: 10.1089/ten.TEA.2013.0235. [DOI] [PubMed] [Google Scholar]
- 7.Park C., et al. Enhanced osseointegration ability of poly(lactic acid) via tantalum sputtering-based plasma immersion ion implantation. ACS Appl. Mater. Interfaces. 2019;11:10492–10504. doi: 10.1021/acsami.8b21363. [DOI] [PubMed] [Google Scholar]
- 8.Metelmann H.-R., et al. Clinical experience with cold plasma in the treatment of locally advanced head and neck cancer. Clinical Plasma Medicine. 2018;9:6–13. doi: 10.1016/j.cpme.2017.09.001. [DOI] [Google Scholar]
- 9.Yan D., Sherman J.H., Keidar M. Cold atmospheric plasma, a novel promising anti-cancer treatment modality. Oncotarget. 2017;8:15977–15995. doi: 10.18632/oncotarget.13304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Qing Y.a., et al. Potential antibacterial mechanism of silver nanoparticles and the optimization of orthopedic implants by advanced modification technologies. Int. J. Nanomed. 2018;13:3311–3327. doi: 10.2147/ijn.s165125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chappuis V., et al. Long-Term outcomes of dental implants with a titanium plasma-sprayed surface: a 20-year prospective case series study in partially edentulous patients. Clin. Implant Dent. Relat. Res. 2013;15:780–790. doi: 10.1111/cid.12056. [DOI] [PubMed] [Google Scholar]
- 12.Adipurnama I., et al. Surface modification and endothelialization of polyurethane for vascular tissue engineering applications: a review. Biomater Sci. 2016;5:22–37. doi: 10.1039/c6bm00618c. [DOI] [PubMed] [Google Scholar]
- 13.Nanbu K. Probability theory of electron-molecule, ion-molecule, molecule-molecule, and Coulomb collisions for particle modeling of materials processing plasmas and cases. IEEE Trans. Plasma Sci. 2000;28:971–990. doi: 10.1109/27.887765. [DOI] [Google Scholar]
- 14.Williams E.R., Geotis S.G., Bhattacharya A.B. A radar study of the plasma and geometry of lightning. J. Atmos. Sci. 1989;46:1173–1185. doi: 10.1175/1520-0469(1989)046<1173:arsotp>2.0.co;2. [DOI] [Google Scholar]
- 15.Hewish A., Symonds M.D. Radio investigation of the solar plasma. Planet. Space Sci. 1969;17:313–320. doi: 10.1016/0032-0633(69)90064-6. [DOI] [Google Scholar]
- 16.Blixt E.M., Semeter J., Ivchenko N. Optical flow analysis of the aurora borealis. Geosci. Rem. Sens. Lett. IEEE. 2006;3:159–163. doi: 10.1109/lgrs.2005.860981. [DOI] [Google Scholar]
- 17.Terasawa T.-o., Saiki K. Growth of graphene on Cu by plasma enhanced chemical vapor deposition. Carbon. 2012;50:869–874. doi: 10.1016/j.carbon.2011.09.047. [DOI] [Google Scholar]
- 18.Wang C.L., et al. Low-temperature plasma-enhanced atomic layer deposition of tin oxide electron selective layers for highly efficient planar perovskite solar cells. J. Mater. Chem. 2016;4:12080–12087. doi: 10.1039/c6ta04503k. [DOI] [Google Scholar]
- 19.Kim H., et al. Plasma-enhanced atomic layer deposition of ultrathin oxide coatings for stabilized lithium-sulfur batteries. Advanced Energy Materials. 2013;3:1308–1315. doi: 10.1002/aenm.201300253. [DOI] [Google Scholar]
- 20.Petitpas G., et al. A comparative study of non-thermal plasma assisted reforming technologies. Int. J. Hydrogen Energy. 2007;32:2848–2867. doi: 10.1016/j.ijhydene.2007.03.026. [DOI] [Google Scholar]
- 21.Wasa K., Hayakawa S. Noyes Publications; 1992. Handbook of Sputter Deposition Technology: Principles, Technology, and Applications. [Google Scholar]
- 22.Torstrick F.B., et al. Porous PEEK improves the bone-implant interface compared to plasma-sprayed titanium coating on PEEK. Biomaterials. 2018;185:106–116. doi: 10.1016/j.biomaterials.2018.09.009. [DOI] [PubMed] [Google Scholar]
- 23.Ke D., et al. Compositionally graded doped hydroxyapatite coating on titanium using laser and plasma spray deposition for bone implants. Acta Biomater. 2019;84:414–423. doi: 10.1016/j.actbio.2018.11.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hussein M.A., Suryanarayana C., Al-Aqeeli N. Fabrication of nano-grained Ti–Nb–Zr biomaterials using spark plasma sintering. Mater. Des. 2015;87:693–700. doi: 10.1016/j.matdes.2015.08.082. [DOI] [Google Scholar]
- 25.Chakravarty D., et al. 3D porous graphene by low-temperature plasma welding for bone implants. Adv. Mater. 2016;28:8959–8967. doi: 10.1002/adma.201603146. [DOI] [PubMed] [Google Scholar]
- 26.Rakhmyanov K., Rakhmyanov A., Zhuravlev A. Advantages of high-precision plasma cutting for processing bimetallic compositions. Appl. Mech. Mater. 2014;698:294–298. doi: 10.4028/www.scientific.net/AMM.698.294. [DOI] [Google Scholar]
- 27.Laroussi M. Low-temperature plasmas for medicine? IEEE Trans. Plasma Sci. 2009;37:714–725. doi: 10.1109/tps.2009.2017267. [DOI] [Google Scholar]
- 28.Scholtz V., et al. Nonthermal plasma--A tool for decontamination and disinfection. Biotechnol. Adv. 2015;33:1108–1119. doi: 10.1016/j.biotechadv.2015.01.002. [DOI] [PubMed] [Google Scholar]
- 29.Isbary G., et al. A first prospective randomized controlled trial to decrease bacterial load using cold atmospheric argon plasma on chronic wounds in patients. Br. J. Dermatol. 2010;163:78–82. doi: 10.1111/j.1365-2133.2010.09744.x. [DOI] [PubMed] [Google Scholar]
- 30.Schmidt A., et al. Nrf2 signaling and inflammation are key events in physical plasma-spurred wound healing. Theranostics. 2019;9:1066–1084. doi: 10.7150/thno.29754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Alkawareek M.Y., et al. Application of atmospheric pressure nonthermal plasma for the in vitro eradication of bacterial biofilms. FEMS Immunol. Med. Microbiol. 2012;65:381–384. doi: 10.1111/j.1574-695X.2012.00942.x. [DOI] [PubMed] [Google Scholar]
- 32.Napartovich A.P. Plasma Polym. 2001;6:1–14. doi: 10.1023/a:1011313322430. [DOI] [Google Scholar]
- 33.Chu P. Plasma-surface modification of biomaterials. Mater. Sci. Eng. R Rep. 2002;36:143–206. doi: 10.1016/s0927-796x(02)00004-9. [DOI] [Google Scholar]
- 34.Tamarack R. Czarnik,MD. EBULLISM at 1 MILLION FEET: Surviving Rapid/Explosive Decompression.
- 35.Patiño A., et al. Surface and bulk cotton fibre modifications: plasma and cationization. Influence on dyeing with reactive dye. Cellulose. 2011;18:1073–1083. doi: 10.1007/s10570-011-9554-7. [DOI] [Google Scholar]
- 36.Joshi S.G., et al. Control of methicillin-resistant Staphylococcus aureus in planktonic form and biofilms: a biocidal efficacy study of nonthermal dielectric-barrier discharge plasma. Am. J. Infect. Contr. 2010;38:293–301. doi: 10.1016/j.ajic.2009.11.002. [DOI] [PubMed] [Google Scholar]
- 37.Krajcarz D. Comparison metal water jet cutting with laser and plasma cutting. Procedia Engineering. 2014;69:838–843. doi: 10.1016/j.proeng.2014.03.061. [DOI] [Google Scholar]
- 38.Wu A.S., et al. Porcine intact and wounded skin responses to atmospheric nonthermal plasma. J. Surg. Res. 2013;179:e1–e12. doi: 10.1016/j.jss.2012.02.039. [DOI] [PubMed] [Google Scholar]
- 39.Moreau M., Orange N., Feuilloley M.G. Non-thermal plasma technologies: new tools for bio-decontamination. Biotechnol. Adv. 2008;26:610–617. doi: 10.1016/j.biotechadv.2008.08.001. [DOI] [PubMed] [Google Scholar]
- 40.Bundscherer L., et al. Impact of non-thermal plasma treatment on MAPK signaling pathways of human immune cell lines. Immunobiology. 2013;218:1248–1255. doi: 10.1016/j.imbio.2013.04.015. [DOI] [PubMed] [Google Scholar]
- 41.Ke Z., Huang Q. Haem-assisted dityrosine-cross-linking of fibrinogen under non-thermal plasma exposure: one important mechanism of facilitated blood coagulation. Sci. Rep. 2016;6:26982. doi: 10.1038/srep26982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Golkowski M., et al. Hydrogen-peroxide-enhanced nonthermal plasma effluent for biomedical applications. IEEE Trans. Plasma Sci. 2012;40:1984–1991. doi: 10.1109/tps.2012.2200910. [DOI] [Google Scholar]
- 43.Kang S.U., et al. Nonthermal plasma induces head and neck cancer cell death: the potential involvement of mitogen-activated protein kinase-dependent mitochondrial reactive oxygen species. Cell Death Dis. 2014;5 doi: 10.1038/cddis.2014.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fridman G., et al. Applied Plasma Medicine. Plasma Processes and Polymers. 2008;5:503–533. doi: 10.1002/ppap.200700154. [DOI] [Google Scholar]
- 45.Akishev Y., et al. Atmospheric-pressure, nonthermal plasma sterilization of microorganisms in liquids and on surfaces. Pure Appl. Chem. 2008;80:1953–1969. doi: 10.1351/pac200880091953. [DOI] [Google Scholar]
- 46.Walsh J.L., Kong M.G. Room-temperature atmospheric argon plasma jet sustained with submicrosecond high-voltage pulses. Appl. Phys. Lett. 2007;91:221502. doi: 10.1063/1.2817965. [DOI] [Google Scholar]
- 47.Daeschlein G., et al. Skin decontamination by low-temperature atmospheric pressure plasma jet and dielectric barrier discharge plasma. J. Hosp. Infect. 2012;81:177–183. doi: 10.1016/j.jhin.2012.02.012. [DOI] [PubMed] [Google Scholar]
- 48.Reuter S., et al. From RONS to ROS: tailoring plasma jet treatment of skin cells. IEEE Trans. Plasma Sci. 2012;40:2986–2993. doi: 10.1109/tps.2012.2207130. [DOI] [Google Scholar]
- 49.Liu M., et al. Recent developments in polydopamine: an emerging soft matter for surface modification and biomedical applications. Nanoscale. 2016;8:16819–16840. doi: 10.1039/c5nr09078d. [DOI] [PubMed] [Google Scholar]
- 50.Vasilev K., et al. Tailoring the surface functionalities of titania nanotube arrays. Biomaterials. 2010;31:532–540. doi: 10.1016/j.biomaterials.2009.09.074. [DOI] [PubMed] [Google Scholar]
- 51.Li M., et al. Surface modification of silicone for biomedical applications requiring long-term antibacterial, antifouling, and hemocompatible properties. Langmuir. 2012;28:16408–16422. doi: 10.1021/la303438t. [DOI] [PubMed] [Google Scholar]
- 52.Mo S., et al. Dimensional-dependent antibacterial behavior on bioactive micro/nano polyetheretherketone (PEEK) arrays. Chem. Eng. J. 2020;392:123736. doi: 10.1016/j.cej.2019.123736. [DOI] [Google Scholar]
- 53.Donnelly V.M., Kornblit A. Plasma etching: yesterday, today, and tomorrow. J. Vac. Sci. Technol.: Vacuum, Surfaces, and Films. 2013;31 doi: 10.1116/1.4819316. [DOI] [Google Scholar]
- 54.Bräuer G., et al. Magnetron sputtering – milestones of 30 years. Vacuum. 2010;84:1354–1359. doi: 10.1016/j.vacuum.2009.12.014. [DOI] [Google Scholar]
- 55.Horprathum M., et al. Dynamic in situ spectroscopic ellipsometric study in inhomogeneous TiO2 thin-film growth. J. Appl. Phys. 2010;108 doi: 10.1063/1.3457839. [DOI] [Google Scholar]
- 56.Feng Y., et al. The double-wire feed and plasma arc additive manufacturing process for deposition in Cr-Ni stainless steel. J. Mater. Process. Technol. 2018;259:206–215. doi: 10.1016/j.jmatprotec.2018.04.040. [DOI] [Google Scholar]
- 57.Ueda M., et al. Plasma immersion ion implantation with auxiliary heating: application to SS304 stainless steel. Phys. Status Solidi. 2008;5:977–980. doi: 10.1002/pssc.200778303. [DOI] [Google Scholar]
- 58.Ueda M., et al. New possibilities of plasma immersion ion implantation (PIII) and deposition (PIII&D) in industrial components using metal tube fixtures. Surf. Coating. Technol. 2017;312:37–46. doi: 10.1016/j.surfcoat.2016.08.067. [DOI] [Google Scholar]
- 59.Lu T., Qiao Y., Liu X. Surface modification of biomaterials using plasma immersion ion implantation and deposition. Interface Focus. 2012;2:325–336. doi: 10.1098/rsfs.2012.0003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bilek M.M.M. Biofunctionalization of surfaces by energetic ion implantation: review of progress on applications in implantable biomedical devices and antibody microarrays. Appl. Surf. Sci. 2014;310:3–10. doi: 10.1016/j.apsusc.2014.03.097. [DOI] [Google Scholar]
- 61.Daeschlein G., et al. Skin and wound decontamination of multidrug-resistant bacteria by cold atmospheric plasma coagulation. J Dtsch Dermatol Ges. 2015;13:143–150. doi: 10.1111/ddg.12559. [DOI] [PubMed] [Google Scholar]
- 62.Kim B., et al. Effect of atmospheric pressure plasma on inactivation of pathogens inoculated onto bacon using two different gas compositions. Food Microbiol. 2011;28:9–13. doi: 10.1016/j.fm.2010.07.022. [DOI] [PubMed] [Google Scholar]
- 63.Liao X., et al. Lethal and sublethal effect of a dielectric barrier discharge atmospheric cold plasma on Staphylococcus aureus. J. Food Protect. 2017;80:928–932. doi: 10.4315/0362-028X.JFP-16-499. [DOI] [PubMed] [Google Scholar]
- 64.Panngom K., et al. Non-thermal plasma treatment diminishes fungal viability and up-regulates resistance genes in a plant host. PloS One. 2014;9 doi: 10.1371/journal.pone.0099300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Joshi S.G., et al. Nonthermal dielectric-barrier discharge plasma-induced inactivation involves oxidative DNA damage and membrane lipid peroxidation in Escherichia coli. Antimicrob. Agents Chemother. 2011;55:1053–1062. doi: 10.1128/AAC.01002-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Han L., et al. Bacterial inactivation by high-voltage atmospheric cold plasma: influence of process parameters and effects on cell leakage and DNA. J. Appl. Microbiol. 2014;116:784–794. doi: 10.1111/jam.12426. [DOI] [PubMed] [Google Scholar]
- 67.Vandamme M., et al. ROS implication in a new antitumor strategy based on non-thermal plasma. Int. J. Canc. 2012;130:2185–2194. doi: 10.1002/ijc.26252. [DOI] [PubMed] [Google Scholar]
- 68.Panngom K., et al. Preferential killing of human lung cancer cell lines with mitochondrial dysfunction by nonthermal dielectric barrier discharge plasma. Cell Death Dis. 2013;4:e642. doi: 10.1038/cddis.2013.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Keidar M., et al. Cold plasma selectivity and the possibility of a paradigm shift in cancer therapy. Br. J. Canc. 2011;105:1295–1301. doi: 10.1038/bjc.2011.386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Volotskova O., et al. Targeting the cancer cell cycle by cold atmospheric plasma. Sci. Rep. 2012;2:636. doi: 10.1038/srep00636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Cheng X., et al. Synergistic effect of gold nanoparticles and cold plasma on glioblastoma cancer therapy. J. Phys. Appl. Phys. 2014;47:335402. doi: 10.1088/0022-3727/47/33/335402. [DOI] [Google Scholar]
- 72.Chen G., et al. Transdermal cold atmospheric plasma-mediated immune checkpoint blockade therapy. Proc. Natl. Acad. Sci. U. S. A. 2020;117:3687–3692. doi: 10.1073/pnas.1917891117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Li Y., et al. Effects of atmospheric-pressure non-thermal bio-compatible plasma and plasma activated nitric oxide water on cervical cancer cells. Sci. Rep. 2017;7:45781. doi: 10.1038/srep45781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kalghatgi S.U., et al. Mechanism of blood coagulation by nonthermal atmospheric pressure dielectric barrier discharge plasma. IEEE Trans. Plasma Sci. 2007;35:1559–1566. doi: 10.1109/tps.2007.905953. [DOI] [Google Scholar]
- 75.Bekeschus S., et al. Human mononuclear cell survival and proliferation is modulated by cold atmospheric plasma jet. Plasma Process. Polym. 2013;10:706–713. doi: 10.1002/ppap.201300008. [DOI] [Google Scholar]
- 76.Emmert S., et al. Atmospheric pressure plasma in dermatology: ulcus treatment and much more. Clinical Plasma Medicine. 2013;1:24–29. doi: 10.1016/j.cpme.2012.11.002. [DOI] [Google Scholar]
- 77.Ueda M., et al. Histological and nuclear medical comparison of inflammation after hemostasis with non-thermal plasma and thermal coagulation. Plasma Process. Polym. 2015;12:1338–1342. doi: 10.1002/ppap.201500099. [DOI] [Google Scholar]
- 78.Shi Y., et al. Characterization of soot inside a diesel particulate filter during a nonthermal plasma promoted regeneration step. Appl. Therm. Eng. 2019;150:612–619. doi: 10.1016/j.applthermaleng.2019.01.015. [DOI] [Google Scholar]
- 79.Nasruddin, et al. Cold plasma on full-thickness cutaneous wound accelerates healing through promoting inflammation, re-epithelialization and wound contraction. Clinical Plasma Medicine. 2014;2:28–35. doi: 10.1016/j.cpme.2014.01.001. [DOI] [Google Scholar]
- 80.Gan L., et al. Cold atmospheric plasma ameliorates imiquimod-induced psoriasiform dermatitis in mice by mediating antiproliferative effects. Free Radic. Res. 2019;53:269–280. doi: 10.1080/10715762.2018.1564920. [DOI] [PubMed] [Google Scholar]
- 81.Arjunan K.P., et al. Non-thermal dielectric barrier discharge plasma induces angiogenesis through reactive oxygen species. J. R. Soc. Interface. 2012;9:147–157. doi: 10.1098/rsif.2011.0220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Mei S., et al. Antibacterial effects and biocompatibility of titanium surfaces with graded silver incorporation in titania nanotubes. Biomaterials. 2014;35:4255–4265. doi: 10.1016/j.biomaterials.2014.02.005. [DOI] [PubMed] [Google Scholar]
- 83.Li J., et al. Antimicrobial activity and cytocompatibility of Ag plasma-modified hierarchical TiO2 film on titanium surface. Colloids Surf. B Biointerfaces. 2014;113:134–145. doi: 10.1016/j.colsurfb.2013.08.030. [DOI] [PubMed] [Google Scholar]
- 84.Wang G., et al. Antibacterial effects of titanium embedded with silver nanoparticles based on electron-transfer-induced reactive oxygen species. Biomaterials. 2017;124:25–34. doi: 10.1016/j.biomaterials.2017.01.028. [DOI] [PubMed] [Google Scholar]
- 85.Cao H., et al. Electron storage mediated dark antibacterial action of bound silver nanoparticles: smaller is not always better. Acta Biomater. 2013;9:5100–5110. doi: 10.1016/j.actbio.2012.10.017. [DOI] [PubMed] [Google Scholar]
- 86.Harrasser N., et al. Antibacterial efficacy of titanium-containing alloy with silver-nanoparticles enriched diamond-like carbon coatings. Amb. Express. 2015;5:77. doi: 10.1186/s13568-015-0162-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Zhao Y., et al. Balancing the osteogenic and antibacterial properties of titanium by codoping of Mg and Ag: an in vitro and in vivo study. ACS Appl. Mater. Interfaces. 2015;7:17826–17836. doi: 10.1021/acsami.5b04168. [DOI] [PubMed] [Google Scholar]
- 88.Jin G., et al. Osteogenic activity and antibacterial effect of zinc ion implanted titanium. Colloids Surf. B Biointerfaces. 2014;117:158–165. doi: 10.1016/j.colsurfb.2014.02.025. [DOI] [PubMed] [Google Scholar]
- 89.Morozov I.A., et al. Structural-mechanical and antibacterial properties of a soft elastic polyurethane surface after plasma immersion N2(+) implantation. Mater Sci Eng C Mater Biol Appl. 2016;62:242–248. doi: 10.1016/j.msec.2016.01.062. [DOI] [PubMed] [Google Scholar]
- 90.Gan K., et al. Bioactivity and antibacterial effect of nitrogen plasma immersion ion implantation on polyetheretherketone. Dent. Mater. 2016;32:e263–e274. doi: 10.1016/j.dental.2016.08.215. [DOI] [PubMed] [Google Scholar]
- 91.Huang H.-H., et al. Nitrogen plasma immersion ion implantation treatment to enhance corrosion resistance, bone cell growth, and antibacterial adhesion of Ti-6Al-4V alloy in dental applications. Surf. Coating. Technol. 2019;365:179–188. doi: 10.1016/j.surfcoat.2018.06.023. [DOI] [Google Scholar]
- 92.Wang H., et al. Rat calvaria osteoblast behavior and antibacterial properties of O(2) and N(2) plasma-implanted biodegradable poly(butylene succinate) Acta Biomater. 2010;6:154–159. doi: 10.1016/j.actbio.2009.07.026. [DOI] [PubMed] [Google Scholar]
- 93.Polak M., et al. Oxygen and water plasma-immersion ion implantation of copper into titanium for antibacterial surfaces of medical implants. Adv. Eng. Mater. 2010;12:B511–B518. doi: 10.1002/adem.200980048. [DOI] [Google Scholar]
- 94.Liu J., et al. In situ plasma fabrication of ceramic-like structure on polymeric implant with enhanced surface hardness, cytocompatibility and antibacterial capability. J. Biomed. Mater. Res. 2016;104:1102–1112. doi: 10.1002/jbm.a.35652. [DOI] [PubMed] [Google Scholar]
- 95.Hu H., et al. Antibacterial activity and increased bone marrow stem cell functions of Zn-incorporated TiO2 coatings on titanium. Acta Biomater. 2012;8:904–915. doi: 10.1016/j.actbio.2011.09.031. [DOI] [PubMed] [Google Scholar]
- 96.Qiao Y., et al. Stimulation of bone growth following zinc incorporation into biomaterials. Biomaterials. 2014;35:6882–6897. doi: 10.1016/j.biomaterials.2014.04.101. [DOI] [PubMed] [Google Scholar]
- 97.Yang C.H., et al. Effect of oxygen plasma immersion ion implantation treatment on corrosion resistance and cell adhesion of titanium surface. Clin. Oral Implants Res. 2011;22:1426–1432. doi: 10.1111/j.1600-0501.2010.02132.x. [DOI] [PubMed] [Google Scholar]
- 98.Shiau D.-K., et al. Enhancing the blood response and antibacterial adhesion of titanium surface through oxygen plasma immersion ion implantation treatment. Surf. Coating. Technol. 2019;365:173–178. doi: 10.1016/j.surfcoat.2018.05.029. [DOI] [Google Scholar]
- 99.Poulsson A.H., et al. Osseointegration of machined, injection moulded and oxygen plasma modified PEEK implants in a sheep model. Biomaterials. 2014;35:3717–3728. doi: 10.1016/j.biomaterials.2013.12.056. [DOI] [PubMed] [Google Scholar]
- 100.Zhang W., et al. Enhanced cytocompatibility of silver-containing biointerface by constructing nitrogen functionalities. Appl. Surf. Sci. 2015;349:327–332. doi: 10.1016/j.apsusc.2015.05.012. [DOI] [Google Scholar]
- 101.Leng Y.X., et al. Fabrication of Ti–O/Ti–N duplex coatings on biomedical titanium alloys by metal plasma immersion ion implantation and reactive plasma nitriding/oxidation. Surf. Coating. Technol. 2001;138:296–300. doi: 10.1016/s0257-8972(00)01172-5. [DOI] [Google Scholar]
- 102.Ellinas K., et al. “Mesh-assisted” colloidal lithography and plasma etching: a route to large-area, uniform, ordered nano-pillar and nanopost fabrication on versatile substrates. Microelectron. Eng. 2011;88:2547–2551. doi: 10.1016/j.mee.2010.12.073. [DOI] [Google Scholar]
- 103.Ellinas K., Tserepi A., Gogolides E. From superamphiphobic to amphiphilic polymeric surfaces with ordered hierarchical roughness fabricated with colloidal lithography and plasma nanotexturing. Langmuir. 2011;27:3960–3969. doi: 10.1021/la104481p. [DOI] [PubMed] [Google Scholar]
- 104.Lademann O., et al. Antisepsis of the follicular reservoir by treatment with tissue-tolerable plasma (TTP) Laser Phys. Lett. 2011;8:313–317. doi: 10.1002/lapl.201010123. [DOI] [Google Scholar]
- 105.Heinlin J., et al. Plasma medicine: possible applications in dermatology. J Dtsch Dermatol Ges. 2010;8:968–976. doi: 10.1111/j.1610-0387.2010.07495.x. [DOI] [PubMed] [Google Scholar]
- 106.Thiyagarajan M. A portable Atmospheric air plasma device for biomedical treatment applications. J. Med. Dev. Trans. ASME. 2013;7 doi: 10.1115/1.4023498. [DOI] [Google Scholar]
- 107.Rajan M.T., Wilkins A., Phung B. Atmospheric pressure cold plasma application for hospital sterilization. 2017. 1-1. [DOI]
- 108.Liao X., Liu D., Ding T. Nonthermal plasma induces the viable-but-nonculturable state in Staphylococcus aureus via metabolic suppression and the oxidative stress response. Appl. Environ. Microbiol. 2020;86 doi: 10.1128/AEM.02216-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Lu H., et al. Bacterial inactivation by high-voltage atmospheric cold plasma: influence of process parameters and effects on cell leakage and DNA. J. Appl. Microbiol. 2014;116:784–794. doi: 10.1111/jam.12426. [DOI] [PubMed] [Google Scholar]
- 110.Volotskova O., et al. Targeting the cancer cell cycle by cold atmospheric plasma. Sci. Rep. 2012;2 doi: 10.1038/srep00636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Eghtedari M., et al. Engineering of hetero-functional gold nanorods for the in vivo molecular targeting of breast cancer cells. Nano Lett. 2009;9:287–291. doi: 10.1021/nl802915q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Sen C.K., Packer L. Antioxidant and redox regulation of gene transcription. Faseb. J. 1996;10:709–720. doi: 10.1096/fasebj.10.7.8635688. [DOI] [PubMed] [Google Scholar]
- 113.Droge W. Free radicals in the physiological control of cell function. Physiol. Rev. 2002;82:47–95. doi: 10.1152/physrev.00018.2001. [DOI] [PubMed] [Google Scholar]
- 114.Suschek C.V., Opländer C. The application of cold atmospheric plasma in medicine: the potential role of nitric oxide in plasma-induced effects. Clinical Plasma Medicine. 2016;4:1–8. doi: 10.1016/j.cpme.2016.05.001. [DOI] [Google Scholar]
- 115.von Woedtke T., Metelmann H.R., Weltmann K.D. Clinical plasma medicine: state and perspectives ofin VivoApplication of cold atmospheric plasma. Contrib. Plasma Phys. 2014;54:104–117. doi: 10.1002/ctpp.201310068. [DOI] [Google Scholar]
- 116.Schmidt A., Bekeschus S. Redox for repair: cold physical plasmas and Nrf2 signaling promoting wound healing. Antioxidants. 2018;7:146. doi: 10.3390/antiox7100146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Bernhardt T., et al. Plasma medicine: applications of cold atmospheric pressure plasma in dermatology. Oxidative Medicine and Cellular Longevity. 2019;(2019):1–10. doi: 10.1155/2019/3873928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Rajasekaran P., et al. Characterization of dielectric barrier discharge (DBD) on mouse and histological evaluation of the plasma-treated tissue. Plasma Process. Polym. 2011;8:246–255. doi: 10.1002/ppap.201000122. [DOI] [Google Scholar]
- 119.Stoffels E., Sakiyama Y., Graves D.B. Cold atmospheric plasma: charged species and their interactions with cells and tissues. IEEE Trans. Plasma Sci. 2008;36:1441–1457. doi: 10.1109/tps.2008.2001084. [DOI] [Google Scholar]
- 120.von Woedtke T., et al. Plasmas for medicine. Phys. Rep. 2013;530:291–320. doi: 10.1016/j.physrep.2013.05.005. [DOI] [Google Scholar]
- 121.Guillemot F., et al. Ti4+ to Ti3+ conversion of TiO2 uppermost layer by low-temperature vacuum annealing: interest for titanium biomedical applications. J. Colloid Interface Sci. 2002;255:75–78. doi: 10.1006/jcis.2002.8623. [DOI] [PubMed] [Google Scholar]
- 122.Nogueira R.D., et al. Evaluation of surface roughness and bacterial adhesion on tooth enamel irradiated with high intensity lasers. Braz. Dent. J. 2017;28:24–29. doi: 10.1590/0103-6440201701190. [DOI] [PubMed] [Google Scholar]
- 123.Lorenzetti M., et al. The influence of surface modification on bacterial adhesion to titanium-based substrates. ACS Appl. Mater. Interfaces. 2015;7:1644–1651. doi: 10.1021/am507148n. [DOI] [PubMed] [Google Scholar]
- 124.Selim S.A., et al. Chemical composition, antimicrobial and antibiofilm activity of the essential oil and methanol extract of the Mediterranean cypress (Cupressus sempervirens L.) BMC Compl. Alternative Med. 2014;14:179. doi: 10.1186/1472-6882-14-179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Schwitalla A., Muller W.D. PEEK dental implants: a review of the literature. J. Oral Implantol. 2013;39:743–749. doi: 10.1563/AAID-JOI-D-11-00002. [DOI] [PubMed] [Google Scholar]
- 126.Catauro M., Bollino F., Papale F. Preparation, characterization, and biological properties of organic-inorganic nanocomposite coatings on titanium substrates prepared by sol-gel. J. Biomed. Mater. Res. 2014;102:392–399. doi: 10.1002/jbm.a.34721. [DOI] [PubMed] [Google Scholar]
- 127.Kim C.S., et al. Proteomic analysis of the biological response of MG63 osteoblast-like cells to titanium implants. Odontology. 2014;102:241–248. doi: 10.1007/s10266-013-0115-4. [DOI] [PubMed] [Google Scholar]
- 128.Minagar S., et al. Cell response of anodized nanotubes on titanium and titanium alloys. J. Biomed. Mater. Res. 2013;101:2726–2739. doi: 10.1002/jbm.a.34575. [DOI] [PubMed] [Google Scholar]
- 129.Ouyang L., et al. Influence of sulfur content on bone formation and antibacterial ability of sulfonated PEEK. Biomaterials. 2016;83:115–126. doi: 10.1016/j.biomaterials.2016.01.017. [DOI] [PubMed] [Google Scholar]
- 130.Zhao Y., et al. Cytocompatibility, osseointegration, and bioactivity of three-dimensional porous and nanostructured network on polyetheretherketone. Biomaterials. 2013;34:9264–9277. doi: 10.1016/j.biomaterials.2013.08.071. [DOI] [PubMed] [Google Scholar]
- 131.Agarwal A., et al. Surfaces modified with nanometer-thick silver-impregnated polymeric films that kill bacteria but support growth of mammalian cells. Biomaterials. 2010;31:680–690. doi: 10.1016/j.biomaterials.2009.09.092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Lee D., Cohen R.E., Rubner M.F. Antibacterial properties of Ag nanoparticle loaded multilayers and formation of magnetically directed antibacterial microparticles. Langmuir. 2005;21:9651–9659. doi: 10.1021/la0513306. [DOI] [PubMed] [Google Scholar]
- 133.Fiedler J., et al. Copper and silver ion implantation of aluminium oxide-blasted titanium surfaces: proliferative response of osteoblasts and antibacterial effects. Int. J. Artif. Organs. 2011;34:882–888. doi: 10.5301/ijao.5000022. [DOI] [PubMed] [Google Scholar]
- 134.Zhao Y., et al. Balancing the osteogenic and antibacterial properties of titanium by codoping of Mg and Ag: an in vitro and in vivo study. ACS Appl. Mater. Interfaces. 2015;7:17826–17836. doi: 10.1021/acsami.5b04168. [DOI] [PubMed] [Google Scholar]
- 135.Seo H.J., et al. Zinc may increase bone formation through stimulating cell proliferation, alkaline phosphatase activity and collagen synthesis in osteoblastic MC3T3-E1 cells. Nutr Res Pract. 2010;4:356–361. doi: 10.4162/nrp.2010.4.5.356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Wang X., et al. Zinc-containing apatite layers on external fixation rods promoting cell activity. Acta Biomater. 2010;6:962–968. doi: 10.1016/j.actbio.2009.08.038. [DOI] [PubMed] [Google Scholar]
- 137.Ovesen J., et al. The positive effects of zinc on skeletal strength in growing rats. Bone. 2001;29:565–570. doi: 10.1016/s8756-3282(01)00616-0. [DOI] [PubMed] [Google Scholar]
- 138.Rizwan M., et al. Surface modification of valve metals using plasma electrolytic oxidation for antibacterial applications: a review. J. Biomed. Mater. Res. 2018;106:590–605. doi: 10.1002/jbm.a.36259. [DOI] [PubMed] [Google Scholar]
- 139.Fang J., et al. Biocompatibility and antibacterial properties of zinc-ion implantation on titanium. J. Hard Tissue Biol. 2014;23:35–44. doi: 10.2485/jhtb.23.35. [DOI] [Google Scholar]
- 140.Puleo D. Understanding and controlling the bone–implant interface. Biomaterials. 1999;20:2311–2321. doi: 10.1016/s0142-9612(99)00160-x. [DOI] [PubMed] [Google Scholar]
- 141.Zhao X., Yang J., You J. Surface modification of TiO2 coatings by Zn ion implantation for improving antibacterial activities. Bull. Mater. Sci. 2016;39:285–291. doi: 10.1007/s12034-015-1127-1. [DOI] [Google Scholar]
- 142.Finney L.A., O'Halloran T.V. Transition metal speciation in the cell: insights from the chemistry of metal ion receptors. Science. 2003;300:931–936. doi: 10.1126/science.1085049. [DOI] [PubMed] [Google Scholar]
- 143.Mändl S., Rauschenbach B. Improving the biocompatibility of medical implants with plasma immersion ion implantation. Surf. Coating. Technol. 2002;156:276–283. doi: 10.1016/s0257-8972(02)00085-3. [DOI] [Google Scholar]
- 144.Yang C.H., et al. Oxygen plasma immersion ion implantation treatment enhances the human bone marrow mesenchymal stem cells responses to titanium surface for dental implant application. Clin. Oral Implants Res. 2015;26:166–175. doi: 10.1111/clr.12293. [DOI] [PubMed] [Google Scholar]
- 145.Zheng L., Qian S., Liu X.-y. Induced antibacterial capability of TiO2 coatings in visible light via nitrogen ion implantation. Trans. Nonferrous Metals Soc. China. 2020;30:171–180. doi: 10.1016/s1003-6326(19)65189-7. [DOI] [Google Scholar]
- 146.Bystrzejewski M., et al. Carbon encapsulated magnetic nanoparticles for biomedical applications: thermal stability studies. Biomol. Eng. 2007;24:555–558. doi: 10.1016/j.bioeng.2007.08.006. [DOI] [PubMed] [Google Scholar]