Abstract
Background
Patients with classical Hodgkin lymphoma (cHL) relapsed or refractory (R/R) disease who relapse after or are ineligible for autologous stem cell transplantation have a poor prognosis. Recently, the anti-PD1 monoclonal antibodies nivolumab and pembrolizumab were approved by the US Food and Drug Administration (FDA; May 2016 and March 2017, respectively) as treatment options for R/R cHL patients.
Objective
In the absence of comparative clinical trials between these agents, this observational study was conducted to evaluate the healthcare resource utilization (HRU) of patients with cHL initiated on pembrolizumab compared to nivolumab in the USA.
Patients and Method
Healthcare insurance claims from Symphony Health’s IDV® (Integrated Dataverse) (July 2014–June 2018) were used in this retrospective study. The study population included adult patients with cHL initiated on pembrolizumab or nivolumab (index date). Inverse probability of treatment weighting was used to adjust for differences in patient characteristics between cohorts. All-cause and cHL-related hospitalizations and outpatient visits were measured during the observation (post-index) period and reported per patient-year (PPY). Rates of HRU were compared between cohorts using rate ratios (RRs).
Results
A total of 92 and 218 patients initiated on pembrolizumab and nivolumab, respectively, were included in the study population. After weighting, the mean age was similar at 55 years in both cohorts, while the proportion of females was lower in the pembrolizumab cohort (35.3%) compared to the nivolumab cohort (44.1%). Mean Quan–Charlson Comorbidity Index score was well balanced after weighting in the pembrolizumab and nivolumab cohorts (4.2 and 4.3, respectively). During the observation period, patients in the pembrolizumab cohort had significantly lower rates of all-cause hospitalizations (RR [95% CI] 0.33 [0.09–0.80]) and cHL-related hospitalizations (RR [95% CI] 0.14 [0.02–0.37]) than those in the nivolumab cohort. Rates of all-cause and cHL-related outpatient visits were not statistically different between patients in the pembrolizumab and nivolumab cohorts.
Conclusions
In this real-world study, adult cHL patients initiated on pembrolizumab had significantly lower rates of all-cause and cHL-related hospitalizations compared to patients initiated on nivolumab.
Key Points
| Patients with classical Hodgkin lymphoma (cHL) relapsed or refractory (R/R) disease who relapse after or are ineligible for stem cell transplantation have a poor prognosis. The recently approved anti-PD1 monoclonal antibodies nivolumab and pembrolizumab may address the unmet needs of patients with R/R cHL. |
| In the absence of comparative clinical trials between these agents, this observational study was conducted to evaluate the healthcare resource utilization (HRU) of patients with cHL initiated on pembrolizumab compared to nivolumab in the US. |
| This real-world study found that adult cHL patients initiated on pembrolizumab experienced significantly lower rates of all-cause and cHL-related hospitalizations compared to those initiated on nivolumab. |
Introduction
Hodgkin lymphoma (HL) is a lymphoid neoplasm of B cell origin characterized by the presence of multinucleated Reed-Sternberg cells surrounded by a distinctive immune infiltrate [1]. HL represents approximately 10% of all lymphoma diagnoses in the USA, with adults aged 20–34 years most frequently affected [2, 3]. HL can be classified either as classical Hodgkin lymphoma (cHL), which represents approximately 95% of HL cases, or as the rarer nodular lymphocyte-predominant Hodgkin lymphoma (NLPHL) [4].
Frontline therapy for cHL depends on the disease stage, but typically includes a combination of chemotherapy and radiation therapy [5]. While many patients may be cured with initial therapy, 15–22% of treated patients are refractory or eventually relapse [6, 7]. For these patients, salvage chemotherapy followed by autologous stem cell transplantation (ASCT) is the standard of care, resulting in cure rates of up to 50% [8]. However, not all patients are eligible for ASCT, including elderly patients for whom ASCT may substantially increase the risk of mortality, or patients who do not respond to salvage chemotherapy prior to ASCT [1, 8]. Patients who relapse after or are ineligible for ASCT reliably have a poor prognosis [1]. Subsequent treatment may include the CD30-directed antibody drug conjugate brentuximab vedotin (BV), which was approved by the US Food and Drug Administration (FDA) in August 2011 for the treatment of patients with cHL whose disease has progressed after ASCT or after two prior chemotherapy treatments for those who cannot receive ASCT [9]. BV has demonstrated improved outcomes and disease management in patients with cHL who have failed ASCT; however, a subset of treated patients eventually progress after treatment with BV [10, 11].
The anti-programmed cell death 1 (PD-1) monoclonal antibodies nivolumab and pembrolizumab were approved by the FDA in May 2016 and March 2017, respectively, as treatment options for patients with cHL who relapsed after three or more lines of prior therapy and/or ASCT [12, 13]. Approval was based on the single-arm clinical trials CHECKMATE-205 [14, 15] and CHECKMATE-039 [16] for nivolumab and KEYNOTE-087 [17] for pembrolizumab, which demonstrated overall response rates (ORRs) of 66–87% and 69%, respectively.
In addition to a poorer prognosis, relapsed cHL is also associated with higher healthcare resource utilization (HRU) and healthcare costs, with added costs for each additional line of therapy required [18, 19]. While both nivolumab and pembrolizumab have been associated with promising clinical outcomes, comparative studies have not been conducted between these two PD-1 inhibitors. Therefore, this retrospective study was conducted to evaluate the HRU among patients with cHL initiated on pembrolizumab compared to nivolumab in the USA.
Methods
Data Source
Healthcare insurance claims from the Symphony Health’s IDV® (Integrated Dataverse) from July 2014 to June 2018 were used. This large, nationally representative data source covers about 280 million lives annually and includes claims submitted to all payer types, including commercial plans, Medicare Part D, cash, assistance programs, and Medicaid. The data for study participants were de-identified and complied with the Health Insurance Portability and Accountability Act (HIPAA); therefore, no reviews by an institutional review board were required.
Study Design
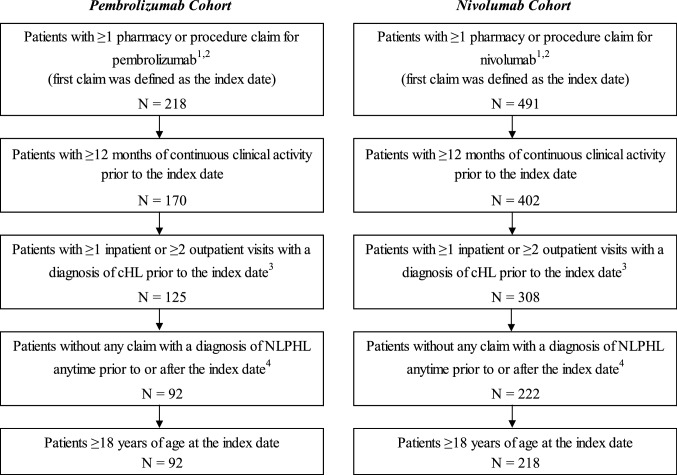
A retrospective cohort design was used to compare mutually exclusive pembrolizumab and nivolumab cohorts. Patients were included in the study population if they had at least one pharmacy or procedure claim for pembrolizumab or nivolumab (with the first dispensing or administration assigned as the index date), at least 12 months of continuous clinical activity prior to the index date, at least one hospitalization or two outpatient visits with a primary or secondary diagnosis of cHL (International Classification of Diseases, 9th Revision, Clinical Modification [ICD-9-CM]: 201.0x–201.2x, 201.5x–201.9x; International Classification of Diseases, 10th Revision, Clinical Modification [ICD-10-CM]: C81.1x–C81.9x) prior to the index date, and were at least 18 years of age at the index date (Fig. 1). Patients were excluded if they had a claim with a primary or secondary diagnosis of nodular lymphocyte-predominant Hodgkin lymphoma (NLPHL) at any time prior to or after the index date.
Fig. 1.
Sample selection. cHL classical Hodgkin lymphoma, GPI Generic Product Identifier, HCPCS Healthcare Common Procedure Coding System, ICD-9-CM International Classification of Diseases, 9th Revision, Clinical Modification, ICD-10-CM International Classification of Diseases, 10th Revision, Clinical Modification, NLPHL nodular lymphocyte-predominant Hodgkin lymphoma. 1 Patients who received both treatments and who initiated nivolumab prior to pembrolizumab approval or in the first months after were classified in the pembrolizumab cohort. Patients with an index treatment of pembrolizumab prior to nivolumab were considered as part of the pembrolizumab cohort (i.e., cohorts were mutually exclusive). 2 Pembrolizumab (GPI: 2135305300; HCPCS: J9271) and nivolumab (GPI: 2135304100; HCPCS: J9299) were identified from pharmacy and procedure claims. 3 Diagnosis of cHL in the primary or secondary position (ICD-9-CM: 201.0x–201.2x, 201.5x–201.9x; ICD-10-CM: C81.1x–C81.9x). 4 Diagnosis of NLPHL in the primary or secondary position (ICD-9-CM: 201.4x; ICD-10-CM: C81.0x)
Patients who initiated nivolumab (approval 17 May 2016) prior to pembrolizumab approval (15 March 2017) and then received the latter were classified in the pembrolizumab cohort since it was used while both treatments were approved. A sensitivity analysis was conducted excluding patients who received both pembrolizumab and nivolumab to remove the impact of any potential bias associated with receiving both treatments.
This study used an intent-to-treat (ITT) design whereby patients were not required to be treated throughout the observation period. The observation period (i.e., follow-up period) spanned from the index date up to the end of continuous clinical activity (defined as consecutive quarters with one or more pharmacy claim) or data availability, whichever occurred first. The baseline period was defined as the 12 months prior to the index date.
Study Outcomes
Study outcomes measured during the observation period included all-cause and cHL-related hospitalizations and outpatient visits. In addition, length of stay was also evaluated for all-cause and cHL-related hospitalizations. cHL-related HRU was identified based on claims with a primary or secondary diagnosis of cHL.
Statistical Analysis
To minimize potential confounding between the pembrolizumab and nivolumab cohorts, inverse probability of treatment weighting (IPTW) based on the propensity score (PS) was used to adjust for observed differences in baseline covariates between cohorts [20]. The propensity score was derived from multivariable logistic regression conditional on baseline covariates (i.e., measured during the baseline period or on the index date), including age, sex, region, type of insurance coverage, year of index date, Quan-Charlson Comorbidity Index (Quan-CCI) score [21], number of hospitalizations and outpatient visits, anti-cancer therapy use (which could account for differences in line of therapy), and Elixhauser and Diagnostic and Statistical Manual Of Mental Disorders, 5th edition (DSM-V) comorbidities (those with ≥ 10% prevalence in either cohort) [22, 23]. DSM-V comorbidities were included because HL has been shown to be associated with long-term psychiatric comorbidity, including anxiety and depression [24, 25]. Probability weights were calculated as 1/PS for the pembrolizumab cohort and 1/(1–PS) for the nivolumab cohort. Baseline characteristics were summarized using mean, standard deviation (SD), and median values for continuous variables, and relative frequencies and proportions for categorical variables.
Rates of HRU among weighted cohorts were calculated as the number of events divided by person-time of observation, to account for varying length of observation across patients, and were reported per person-year (PPY). Rates of HRU were compared between cohorts using rate ratios (RRs) from Poisson regression models with log-link; 95% confidence intervals (CIs) and P values were calculated using non-parametric bootstrap procedures with 999 replications.
All analyses were conducted using SAS Enterprise Guide Version 7.1 (SAS Institute, Cary, NC, USA).
Sensitivity Analyses
A sensitivity analysis excluding patients who received both pembrolizumab and nivolumab was conducted. Moreover, patients could have participated in a clinical trial since the study period overlaps with the approval dates for pembrolizumab and nivolumab (15 March 2017 and 17 May 2016, respectively). While clinical trial participation as measured by diagnosis and procedure codes (ICD-9-CM: V70.7; ICD-10-CM: Z00.6; Healthcare Common Procedure Coding System [HCPCS]: S9988, S9990, S9991, S9992, S9994, S9996) might be under-reported in claims, a sensitivity analysis was also conducted adjusting for enrollment in a clinical trial during baseline or on the index date to account for the difference in nivolumab and pembrolizumab FDA approval dates and potentially higher proportion of clinical trial patients in one cohort. PS and weights were re-calculated for both sensitivity analyses.
Results
Patient Characteristics
A total of 92 patients initiated on pembrolizumab and 218 patients initiated on nivolumab met the selection criteria and were included in the analysis (Table 1). Most baseline covariates were balanced between the weighted cohorts (i.e., standardized difference (std. diff.) < 20%). After weighting, the mean age was similar at 55 years in both cohorts, while the proportion of female patients was lower in the pembrolizumab cohort (35.3%) compared to the nivolumab cohort (44.1%; std. diff. = 17.9%). Approximately one-third of pembrolizumab and nivolumab patients received BV during baseline (29.9% and 35.3%, respectively; std. diff. = 11.7%). Of the 92 patients in the pembrolizumab cohort, six patients received nivolumab during the baseline period and one patient received nivolumab prior to the baseline period (i.e., more than 12 months prior to the index date).
Table 1.
Patient demographics and clinical characteristics
| Characteristics | Unweighted cohorts | Weighted cohortsa | ||||
|---|---|---|---|---|---|---|
| Pembrolizumab cohort | Nivolumab cohort | Std. diff. (%) | Pembrolizumab cohort | Nivolumab cohort | Std. diff. (%) | |
| (N = 92) | (N = 218) | (N = 92) | (N = 218) | |||
| Observation period,b days, mean ± SD (median) | 228.3 ± 174.0 (214) | 296.1 ± 222.5 (239) | 34.0 | 295.2 ± 236.5 (264) | 274.0 ± 213.0 (208) | 9.4 |
| Demographicsc | ||||||
| Age, years, mean ± SD (median) | 59.2 ± 16.0 (63) | 52.8 ± 18.5 (55) | 37.4 | 54.8 ± 18.7 (58) | 54.7 ± 18.3 (58) | 0.3 |
| Gender, female, n (%) | 37 (40.2) | 96 (44.0) | 7.7 | 32 (35.3) | 96 (44.1) | 17.9 |
| Year of index date,d n (%) | ||||||
| 2015 | 0 (0.0) | 3 (1.4) | 16.7 | 0 (0.0) | 3 (1.2) | 15.5 |
| 2016 | 7 (7.6) | 51 (23.4) | 44.7 | 26 (28.7) | 42 (19.2) | 22.3 |
| 2017 | 49 (53.3) | 104 (47.7) | 11.1 | 42 (45.6) | 107 (49.0) | 6.9 |
| 2018 | 36 (39.1) | 60 (27.5) | 24.8 | 24 (25.7) | 67 (30.6) | 10.8 |
| Region,c n (%) | ||||||
| South | 37 (40.2) | 98 (45.0) | 9.6 | 43 (46.9) | 95 (43.4) | 7.0 |
| Northeast | 22 (23.9) | 33 (15.1) | 22.3 | 13 (14.1) | 36 (16.6) | 7.1 |
| Midwest | 19 (20.7) | 52 (23.9) | 7.7 | 23 (25.2) | 49 (22.6) | 6.1 |
| West | 14 (15.2) | 33 (15.1) | 0.2 | 13 (13.9) | 36 (16.7) | 7.8 |
| Unknown | 0 (0.0) | 2 (0.9) | 13.6 | 0 (0.0) | 2 (0.7) | 12.2 |
| Insurance plan type,e,f n (%) | ||||||
| Commercial | 59 (64.1) | 149 (68.3) | 8.9 | 59 (64.0) | 145 (66.5) | 5.3 |
| Medicare | 25 (27.2) | 46 (21.1) | 14.2 | 26 (28.1) | 52 (23.9) | 9.6 |
| Medicaid | 3 (3.3) | 13 (6.0) | 12.9 | 2 (2.6) | 11 (5.0) | 12.9 |
| Government | 3 (3.3) | 8 (3.7) | 2.2 | 2 (1.8) | 8 (3.9) | 12.4 |
| Unknown | 2 (2.2) | 2 (0.9) | 10.2 | 3 (3.6) | 2 (0.7) | 19.8 |
| Baseline anti-cancer therapies,g n (%) | ||||||
| Any | 68 (73.9) | 188 (86.2) | 31.2 | 79 (85.7) | 184 (84.4) | 3.4 |
| Brentuximab Vedotin | 24 (26.1) | 86 (39.4) | 28.8 | 27 (29.9) | 77 (35.3) | 11.7 |
| Nivolumab | 6 (6.5) | – | – | 6 (6.4) | – | – |
| Ibrutinib | 0 (0.0) | 5 (2.3) | 21.7 | 0 (0.0) | 4 (2.1) | 20.5 |
| Pembrolizumab | – | 0 (0.0) | – | – | 0 (0.0) | – |
| Baseline all-cause HRU,g mean ± SD (median) | ||||||
| Hospitalizations | 1.47 ± 2.17 (1) | 1.44 ± 1.96 (1) | 1.1 | 1.56 ± 2.36 (1) | 1.49 ± 1.96 (1) | 3.1 |
| OP visits | 36.0 ± 29.9 (28) | 35.4 ± 25.5 (33) | 2.1 | 33.6 ± 26.6 (27) | 35.7 ± 25.8 (33) | 7.9 |
| Quan-CCI score,g mean ± SD (median) | 4.89 ± 2.72 (5) | 4.01 ± 2.64 (3) | 32.7 | 4.22 ± 2.64 (3) | 4.28 ± 2.75 (3) | 2.2 |
| Elixhauser comorbidities,g n (%) | ||||||
| Hypertension | 45 (48.9) | 72 (33.0) | 32.7 | 33 (36.2) | 83 (37.9) | 3.4 |
| Cardiac arrhythmias | 30 (32.6) | 58 (26.6) | 13.2 | 28 (30.6) | 59 (26.9) | 8.2 |
| Chronic pulmonary disease | 27 (29.3) | 42 (19.3) | 23.7 | 17 (18.9) | 47 (21.5) | 6.3 |
| Solid tumor without metastasis | 23 (25.0) | 30 (13.8) | 28.7 | 13 (14.2) | 35 (16.2) | 5.5 |
| Metastatic cancer | 22 (23.9) | 38 (17.4) | 16.1 | 19 (20.5) | 43 (19.6) | 2.1 |
| Diabetes | 18 (19.6) | 34 (15.6) | 10.4 | 16 (16.9) | 39 (17.8) | 2.2 |
| Coagulopathy | 17 (18.5) | 38 (17.4) | 2.7 | 13 (14.2) | 41 (19.0) | 13.1 |
| Valvular disease | 17 (18.5) | 26 (11.9) | 18.3 | 11 (12.4) | 28 (13.0) | 1.9 |
| Peripheral vascular disorders | 16 (17.4) | 24 (11.0) | 18.4 | 9 (9.6) | 28 (13.0) | 10.7 |
| Congestive heart failure | 15 (16.3) | 34 (15.6) | 1.9 | 15 (16.5) | 35 (16.2) | 0.8 |
| Liver disease | 12 (13.0) | 23 (10.6) | 7.7 | 12 (13.4) | 26 (11.7) | 5.2 |
| Weight loss | 11 (12.0) | 38 (17.4) | 15.5 | 13 (14.3) | 35 (16.0) | 4.7 |
| Obesity | 11 (12.0) | 19 (8.7) | 10.7 | 7 (7.3) | 21 (9.7) | 8.7 |
| Renal failure | 10 (10.9) | 18 (8.3) | 8.9 | 9 (9.8) | 23 (10.4) | 1.8 |
| Pulmonary circulation disorder | 10 (10.9) | 14 (6.4) | 15.9 | 5 (5.7) | 15 (6.7) | 4.0 |
| Deficiency anemias | 9 (9.8) | 25 (11.5) | 5.5 | 6 (6.2) | 24 (11.1) | 17.7 |
| DSM-V comorbidities,g n (%) | ||||||
| Depressive disorders | 17 (18.5) | 32 (14.7) | 10.2 | 13 (13.8) | 33 (15.0) | 3.7 |
| Substance-related and addictive disorders | 15 (16.3) | 18 (8.3) | 24.7 | 8 (8.5) | 22 (10.2) | 5.8 |
| Other conditions that may require a focus of clinical attentionh | 13 (14.1) | 21 (9.6) | 13.9 | 9 (9.5) | 25 (11.5) | 6.4 |
| Sleep–wake disorders | 10 (10.9) | 25 (11.5) | 1.9 | 8 (8.8) | 23 (10.6) | 6.0 |
| Anxiety disorders | 8 (8.7) | 35 (16.1) | 22.5 | 9 (10.2) | 30 (13.9) | 11.2 |
cHL classical Hodgkin Lymphoma, DSM-V Diagnostic and Statistical Manual of Mental Disorders, 5th edition, HRU healthcare resource utilization, IPTW inverse probability of treatment weighting, Quan-CCI Quan-Charlson Comorbidity Index, SD standard deviation, std. diff. standardized difference
aIPTW weights based on the propensity score were derived from a multivariable logistic model conditional on baseline covariates including age, sex, region, type of insurance coverage, year of index date, Quan-CCI, number of hospitalizations, number of outpatient visits, any anti-cancer therapy use, and all Elixhauser and DSM-V comorbidities with ≥ 10% prevalence in either cohort
bThe observation period spanned from the index date up to the end of data availability or end of clinical activity
cEvaluated at the index date
dThe index date was defined as the first dispensing or administration of either pembrolizumab or nivolumab
eEvaluated at the index date or during the 12-month baseline period (using the pharmacy claim closest to the index date)
fGeneral insurance plan type categories (commercial, Medicare, etc.) only. Government includes Veteran’s Administration and Medical Military (TRICARE)
gEvaluated during the 12-month baseline period
hThese conditions include relation problems, abuse and neglect, educational and occupational problems, housing and economic problems, other problems related to the social environment, other health-service encounters for counseling and medical advice, and other circumstances of personal history
Mean Quan-CCI score was well-balanced in the pembrolizumab and nivolumab cohorts (4.2 and 4.3, respectively; std. diff. = 2.2%). Among patients in the pembrolizumab and nivolumab cohorts, 36.2% and 37.9% had hypertension (std. diff. = 3.4%), and 30.6% and 26.9% had cardiac arrhythmias (std. diff. = 8.2%) at baseline, respectively. Moreover, among pembrolizumab and nivolumab initiators, 13.8% and 15.0% had depressive disorders (std. diff. = 3.7%), and 10.2% and 13.9% had anxiety disorders (std. diff. = 11.2%), respectively. The mean number of baseline hospitalizations (1.6 in the pembrolizumab cohort and 1.5 in the nivolumab cohort; std. diff. = 3.1%) was also well balanced after weighting.
The mean (median) observation period was 295 (264) days for patients in the pembrolizumab cohort and 274 (208) days for patients in the nivolumab cohort. Patients initiated on pembrolizumab received a mean (median) of 7.1 (6) infusions while those initiated on nivolumab received a mean (median) of 8.6 (6) infusions.
Hospitalizations
Patients in the pembrolizumab cohort had significantly lower rates of all-cause hospitalizations (0.46 PPY) compared to those in the nivolumab cohort (1.39 PPY), corresponding to 67% fewer hospitalizations PPY (RR [95% confidence interval (CI)]: 0.33 [0.09–0.80], P = 0.014; Fig. 2). The mean length of hospital stay was 3.5 days for the pembrolizumab cohort and 4.0 days for the nivolumab cohort (Table 2).
Fig. 2.
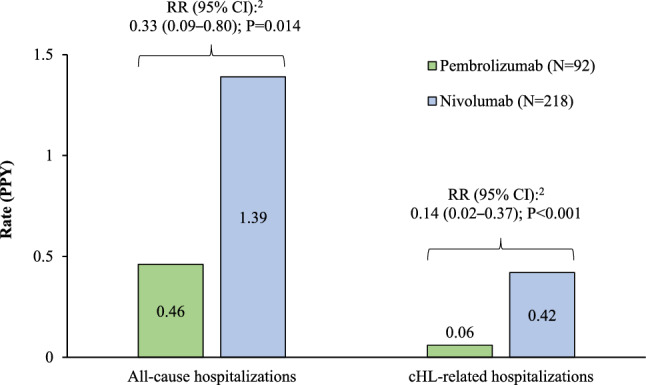
All-cause and cHL-related hospitalizations in the weighted1 pembrolizumab and nivolumab cohorts. CCI Charlson Comorbidity Index, cHL classical Hodgkin lymphoma, CI confidence interval, DSM-V Diagnostic and Statistical Manual of Mental Disorders, 5th edition, IPTW inverse probability of treatment weighting, PPY per person-year, RR rate ratio. 1 IPTW weights based on the propensity score were derived from a multivariable logistic model conditional on baseline covariates including age, sex, region, type of insurance coverage, year of index date, Quan-CCI, number of hospitalizations, number of outpatient visits, any anti-cancer therapy use, and all Elixhauser and DSM-V comorbidities ≥ 10% prevalence in either cohort. 2. Rate ratios were obtained from Poisson regression models with log-link; confidence intervals and p-values were calculated using non-parametric bootstrap procedure methods with 999 replications
Table 2.
HRU of patients with cHL in the weighted pembrolizumab and nivolumab cohorts
| Measures | Pembrolizumab cohort | Nivolumab cohort |
|---|---|---|
| (N = 92) | (N = 218) | |
| Observation perioda | ||
| Mean ± SD, days | 295.2 ± 236.5 | 274.0 ± 213.0 |
| Total person-years | 74.4 | 163.6 |
| All-cause HRU | ||
| Number of hospitalizations | 34 | 228 |
| LOS, days, mean [median] | 3.5 [3] | 4.0 [2] |
| Number of outpatient visits | 2,161 | 5,681 |
| cHL-related HRU | ||
| Number of hospitalizations | 4 | 68 |
| LOS, days, mean [median] | 6.4 [1] | 7.2 [4] |
| Number of outpatient visits | 1203 | 2933 |
cHL classical Hodgkin lymphoma, HRU healthcare resource utilization, LOS length of stay, SD standard deviation
aThe observation period spanned from the index date up to the end of data availability or end of clinical activity
Patients in the pembrolizumab cohort also had significantly lower rates of cHL-related hospitalizations (0.06 PPY) compared to those in the nivolumab cohort (0.42 PPY). for a RR of 0.14 (95% CI 0.02–0.37, P < 0.001; Fig. 2). The mean length of cHL-related hospital stay was 6.4 days for the pembrolizumab cohort and 7.2 days for the nivolumab cohort (Table 2). Similar results were observed when truncating follow-up at 12 months.
Outpatient Visits
The rates of all-cause outpatient visits were not significantly different between pembrolizumab initiators compared to nivolumab initiators (29.0 PPY vs. 34.7 PPY; RR [95% CI] 0.84 [0.56–1.11], P = 0.200; Fig. 3). Similarly, no significant difference was observed in the rates of cHL-related outpatient visits among the pembrolizumab and nivolumab cohorts (16.1 PPY vs. 17.9 PPY; RR [95% CI] 0.90 [0.47–1.42], P = 0.647; Fig. 3). Similar results were observed when truncating follow-up at 12 months.
Fig. 3.
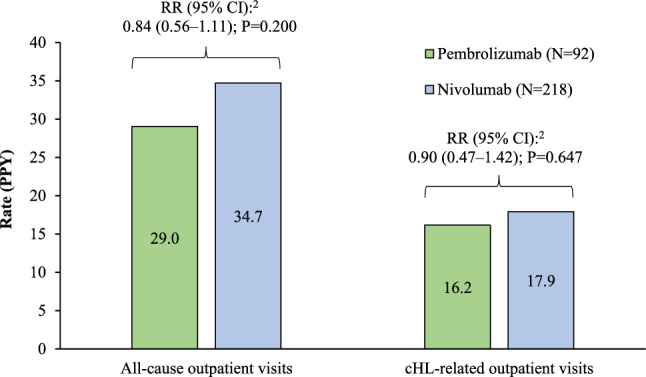
All-cause and cHL-related outpatient visits in the weighted1 pembrolizumab and nivolumab cohort. CCI Charlson Comorbidity Index, cHL classical Hodgkin lymphoma, CI confidence interval, DSM-V Diagnostic and Statistical Manual of Mental Disorders, 5th edition, IPTW inverse probability of treatment weighting, PPY per person-year, RR rate ratio. 1 IPTW weights based on the propensity score were derived from a multivariable logistic model conditional on baseline covariates including age, sex, region, type of insurance coverage, year of index date, Quan-CCI, number of hospitalizations, number of outpatient visits, any anti-cancer therapy use, and all Elixhauser and DSM-V comorbidities ≥ 10% prevalence in either cohort. 2 Rate ratios were obtained from Poisson regression models with log-link; confidence intervals and p-values were calculated using non-parametric bootstrap procedure methods with 999 replications
Sensitivity Analyses
Of the 92 patients in the pembrolizumab cohort, a total of seven patients had prior use of nivolumab and were excluded. Similar results were found after excluding these patients. Pembrolizumab initiators had significantly lower rates of all-cause hospitalizations (RR [95% CI] 0.29 [0.08–0.75], P = 0.020) and cHL-related hospitalizations (RR [95% CI] 0.09 [0.01–0.25], P = 0.002) compared to those in the nivolumab cohort. Patients in the pembrolizumab cohort also had significantly lower rates of all-cause outpatient visits (RR [95% CI] 0.78 [0.54–0.97], P = 0.022), though a non-significant difference in cHL-related outpatient visits was observed (RR [95% CI] 0.86 [0.44–1.36], P = 0.460). Similar results were found when adjusting for patients who had an encounter as part of a clinical trial (pembrolizumab: four patients (4.3%), nivolumab: ten patients (4.6%)) during the baseline period or on the index date (all-cause RR [95% CI] 0.33 [0.09–0.81], P = 0.014; cHL-related RR [95% CI] 0.12 [0.00–0.36], P < 0.001).
Discussion
This retrospective study based on real-world claims data from the Symphony Health database showed significant benefits of pembrolizumab compared to nivolumab on HRU. Adult cHL patients initiated on pembrolizumab had significantly lower rates of all-cause and cHL-related hospitalizations compared to those initiated on nivolumab. Similarly, fewer all-cause and cHL-related outpatient visits were also observed among patients in the pembrolizumab cohort, though the differences were not statistically significant. Similar trends in HRU were observed after excluding patients who received nivolumab prior to pembrolizumab.
Patients eligible for treatment with pembrolizumab or nivolumab represent a cHL population with a particular unmet need—those who have relapsed after three or more lines of prior therapy and/or ASCT [26, 27]. Patients who relapse after ASCT have poor outcomes, with one study reporting a median survival of only 26 months after ASCT failure [1, 28]. These patients may subsequently receive treatment with BV, but real-world studies have shown that 43–51% of BV-treated patients require additional systemic therapy following unsuccessful treatment [29, 30]. Additionally, patients with cHL who experience disease progression after BV have poor outcomes as well. In a retrospective analysis of institutional databases from a large US cancer center, patients who progressed after BV had a cumulative ORR of 33% to subsequent lines of treatment, and median progression-free survival (PFS) was only 3.5 months [10]. Notably, this study was conducted before the PD-1 era, and many of the included patients would now likely be eligible for treatment with PD-1 inhibitors.
In addition to poor clinical outcomes, patients eligible for treatment with pembrolizumab or nivolumab have a substantial economic burden. In a real-world study of patients with relapsed cHL (including 39% with prior stem-cell transplant), utilization of inpatient, emergency room (ER), and laboratory and radiology service was significantly higher compared to patients without relapsed cHL (i.e., proportion of patients who had an inpatient admission: 66.2% vs. 17.4%, P < 0.001; ER visit: 49.4% vs. 21.1%, P < 0.05; laboratory and radiology service: 100.0% vs. 93.4%, P < 0.05; and pharmacy prescription: 90.9% vs. 82.7%, P > 0.05) [18]. Additionally, Yasenchak et al. conducted a cost analysis among patients with cHL and found that each subsequent line of therapy (LOT) was associated with higher total healthcare costs (from $21,956 in LOT1 to $77,219 in LOT2, and $59,442 in LOT3) and even higher total healthcare costs (total cost per LOT increased seven- to eightfold compared to LOT1) among patients who received a stem cell transplant [19]. Taken together, the poor clinical and economic outcomes of patients with relapsed cHL highlight the substantial unmet need that may now be addressed with PD-1 inhibitors like pembrolizumab and nivolumab.
To our knowledge, the current study is the first to directly compare real-world outcomes associated with pembrolizumab and nivolumab treatment among patients with cHL. Comparisons of the two PD-1 inhibitors with regard to either clinical or economic outcomes are scarce in the literature. Indeed, the National Comprehensive Cancer Network® (NCCN) recommends individualized treatment for patients with relapsed or refractory cHL since there are no data to support a superior outcome with any of the available treatment modalities [5]. Of note, an indirect comparison of pembrolizumab (using data from KEYNOTE-087) versus standard of care was recently conducted by Keeping et al., which demonstrated a significantly prolonged PFS associated with pembrolizumab compared to standard of care (HR [95% CI] 5.00 [3.56–7.01]) [31]. However, the standard of care data was based on a study conducted prior to the FDA approval of nivolumab, and while 28 of 79 included patients were treated with investigational agents, the proportion receiving nivolumab was not disclosed [10]. With regard to the real-world outcomes of PD-1 inhibitors as a whole, a retrospective analysis of patients with relapsed or refractory cHL treated with a PD-1 inhibitor in US clinical practice was conducted by Bair et al., which identified 52 patients treated with nivolumab and one patient treated with pembrolizumab [32]. While a comparison between the two PD-1 inhibitors was not possible, the study identified an ORR of 68% for all patients, which is similar to the ORRs reported in the pivotal pembrolizumab and nivolumab clinical trials. With the lack of comparative trials to differentiate between pembrolizumab and nivolumab based on efficacy, the current study provides particularly important, albeit early, insight to differentiate between the two PD-1 inhibitors based on trends in HRU. Further research is warranted to identify the clinical drivers of the observed differences in HRU between pembrolizumab and nivolumab initiators.
The results of this study should be interpreted in light of some limitations. First, Symphony Health is a provider-based database, which may have resulted in a patient potentially being counted as multiple patients if seen by different doctors or offices. Although Symphony Health minimizes this discrepancy with a linking algorithm, it may still have missed extraneous patient IDs, resulting in underestimation of HRU at the patient level. Second, despite the adjustment for many covariates, there may have been residual confounding between the two cohorts due to unmeasured confounders. Furthermore, information on disease progression and survival are also not available in Symphony Health therefore, analyses regarding the contributions of HRU associated with progression and end-of-life care were not possible. Due to the ITT design, HRU associated with progression and end-of-life care can be attributed to pembrolizumab or nivolumab cohorts even though patients were not on treatment. Fourth, while cHL-related visits were identified by the presence of primary or secondary diagnosis codes, we do not know the reason for admission and cannot be certain these visits were truly cHL-related. Fifth, since the data source is provider-based, not insurance-based, the data source lacks patient eligibility files. While clinical activity was used to indicate eligibility, healthier patients who had not incurred any clinical services would not have been included in the study. Lastly, due to the nature of claims databases, coding inaccuracies or omissions in procedures and diagnoses could have occurred. However, the assumption is that these inaccuracies were randomly assigned and thus equally present in both cohorts.
Conclusions
In this real-world study, adult patients with cHL initiated on pembrolizumab had significantly lower rates of all-cause and cHL-related hospitalizations than those initiated on nivolumab. Additional research is warranted to further investigate these early trends in real-world HRU observed among patients with cHL initiating PD-1 inhibitors.
Acknowledgements
Medical writing assistance was provided by Christine Tam, an employee of Groupe d’analyse, Ltée, which received research funds from Merck & Co., Inc., to conduct this study.
Declarations
Funding
This study was funded by Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Kenilworth, NJ, USA.
Conflicts of interest/Competing interests
This study was funded by Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Kenilworth, NJ, USA. MR, XY, AN, SSS, and KDD are employees of Merck & Co., Inc. FL, GG, and SDM are employees of Groupe d’analyse, Ltée, a consulting company that has received research funds from Merck & Co., Inc., to conduct this study. MSD is an employee of Analysis Group, Inc., a consulting company that has received research funds from Merck & Co., Inc., to conduct this study.
Ethics approval
The data for study participants were de-identified and complied with the Health Insurance Portability and Accountability Act (HIPAA); therefore, no reviews by an institutional review board were required.
Consent to participate
The present investigation was based on administrative claims data and does not contain any procedures with human participants performed by any of the authors.
Consent for publication
All authors and persons named in the Acknowledgements section have given permission to be named in the article.
Availability of data and material (data transparency)
Claims data used in the present study are accessible through the Symphony Health’s IDV® (Integrated Dataverse) (from July 2014 to June 2018).
Code availability (software application or custom code)
All analyses were conducted using SAS Enterprise Guide Version 7.1 (SAS Institute, Cary, NC, USA).
Authors’ contributions
All authors were responsible for the study design and the interpretation of the study results. François Laliberté, Guillaume Germain, and Sean D. MacKnight were responsible for the data analysis. All authors reviewed the manuscript for intellectual content and approved the final draft. Medical writing assistance was provided by Christine Tam, an employee of Groupe d’analyse, Ltée, which received research funds from Merck & Co., Inc., to conduct this study.
References
- 1.Ansell SM. Hodgkin lymphoma: 2018 update on diagnosis, risk-stratification, and management. Am J Hematol. 2018;93(5):704–715. doi: 10.1002/ajh.25071. [DOI] [PubMed] [Google Scholar]
- 2.National Cancer Institute (NCI) Surveillance, Epidemiology, and End Results Program. Cancer Stat Facts: Hodgkin Lymphoma. 2019. https://seer.cancer.gov/statfacts/html/hodg.html. Accessed 15 Oct 2019.
- 3.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin. 2019;69(1):7–34. doi: 10.3322/caac.21551. [DOI] [PubMed] [Google Scholar]
- 4.Eichenauer DA, Andre M, Johnson P, Fossa A, Casasnovas O, Engert A. Controversies in the treatment of classical hodgkin lymphoma. HemaSphere. 2018;2(5):e149. doi: 10.1097/hs9.0000000000000149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.National Comprehensive Cancer Network® (NCCN®). NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®) Hodgkin Lymphoma2019. [DOI] [PubMed]
- 6.Moccia AA, Donaldson J, Chhanabhai M, Hoskins PJ, Klasa RJ, Savage KJ, et al. International Prognostic Score in advanced-stage Hodgkin’s lymphoma: altered utility in the modern era. J Clin Oncol. 2012;30(27):3383–3388. doi: 10.1200/JCO.2011.41.0910. [DOI] [PubMed] [Google Scholar]
- 7.Viviani S, Zinzani PL, Rambaldi A, Brusamolino E, Levis A, Bonfante V, et al. ABVD versus BEACOPP for Hodgkin’s lymphoma when high-dose salvage is planned. N Engl J Med. 2011;365(3):203–212. doi: 10.1056/NEJMoa1100340. [DOI] [PubMed] [Google Scholar]
- 8.Shanbhag S, Ambinder RF. Hodgkin lymphoma: a review and update on recent progress. CA Cancer J Clin. 2018;68(2):116–132. doi: 10.3322/caac.21438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Seattle Genetics, Inc. Highlights of Prescribing Information ADCETRIS (brentuximab vedotin). 2019. p. 1–12.
- 10.Cheah CY, Chihara D, Horowitz S, Sevin A, Oki Y, Zhou S, et al. Patients with classical Hodgkin lymphoma experiencing disease progression after treatment with brentuximab vedotin have poor outcomes. Ann Oncol. 2016;27(7):1317–1323. doi: 10.1093/annonc/mdw169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen R, Gopal AK, Smith SE, Ansell SM, Rosenblatt JD, Savage KJ, et al. Five-year survival and durability results of brentuximab vedotin in patients with relapsed or refractory Hodgkin lymphoma. Blood. 2016;128(12):1562–1566. doi: 10.1182/blood-2016-02-699850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Food and Drug Administration (FDA). Nivolumab (Opdivo) for Hodgkin Lymphoma. 2016. https://www.fda.gov/drugs/resources-information-approved-drugs/nivolumab-opdivo-hodgkin-lymphoma. Accessed 15 Oct 2019.
- 13.Food and Drug Administration (FDA). Pembrolizumab (KEYTRUDA) for classical Hodgkin lymphoma. 2017. https://www.fda.gov/drugs/resources-information-approved-drugs/pembrolizumab-keytruda-classical-hodgkin-lymphoma. Accessed 15 Oct 2019.
- 14.Armand P, Engert A, Younes A, Fanale M, Santoro A, Zinzani PL, et al. Nivolumab for relapsed/refractory classic hodgkin lymphoma after failure of autologous hematopoietic cell transplantation: extended follow-up of the multicohort single-arm phase II CheckMate 205 trial. J Clin Oncol. 2018;36(14):1428–1439. doi: 10.1200/JCO.2017.76.0793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Younes A, Santoro A, Shipp M, Zinzani PL, Timmerman JM, Ansell S, et al. Nivolumab for classical Hodgkin’s lymphoma after failure of both autologous stem-cell transplantation and brentuximab vedotin: a multicentre, multicohort, single-arm phase 2 trial. Lancet Oncol. 2016;17(9):1283–1294. doi: 10.1016/S1470-2045(16)30167-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ansell SM, Lesokhin AM, Borrello I, Halwani A, Scott EC, Gutierrez M, et al. PD-1 blockade with nivolumab in relapsed or refractory Hodgkin’s lymphoma. N Engl J Med. 2015;372(4):311–319. doi: 10.1056/NEJMoa1411087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen R, Zinzani PL, Fanale MA, Armand P, Johnson NA, Brice P, et al. Phase II study of the efficacy and safety of pembrolizumab for relapsed/refractory classic hodgkin lymphoma. J Clin Oncol. 2017;35(19):2125–2132. doi: 10.1200/JCO.2016.72.1316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bonafede M, Feliciano J, Cai Q, Noxon V, Princic N, Richhariya A, et al. Real-world analysis of cost, health care resource utilization, and supportive care in Hodgkin lymphoma patients with frontline failure. Clinicoecon Outcomes Res. 2018;10:629–641. doi: 10.2147/CEOR.S178649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yasenchak CA, Tseng WY, Yap M, Rembert D, Patt DA. Economic impact of disease progression following front-line therapy in classical Hodgkin lymphoma. Leuk Lymphoma. 2015;56(11):3143–3149. doi: 10.3109/10428194.2015.1030639. [DOI] [PubMed] [Google Scholar]
- 20.Austin PC. An introduction to propensity score methods for reducing the effects of confounding in observational studies. Multivar Behav Res. 2011;46(3):399–424. doi: 10.1080/00273171.2011.568786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Quan H, Sundararajan V, Halfon P, Fong A, Burnand B, Luthi JC, et al. Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative data. Med Care. 2005;43(11):1130–1139. doi: 10.1097/01.mlr.0000182534.19832.83. [DOI] [PubMed] [Google Scholar]
- 22.Elixhauser A, Steiner C, Harris DR, Coffey RM. Comorbidity measures for use with administrative data. Med Care. 1998;36(1):8–27. doi: 10.1097/00005650-199801000-00004. [DOI] [PubMed] [Google Scholar]
- 23.American Psychiatric Association . Diagnostic and statistical manual of mental disorders. 5. Arlington: American Psychiatric Publishing; 2003. [Google Scholar]
- 24.Daniels LA, Oerlemans S, Krol AD, Creutzberg CL, van de Poll-Franse LV. Chronic fatigue in Hodgkin lymphoma survivors and associations with anxiety, depression and comorbidity. Br J Cancer. 2014;110(4):868–874. doi: 10.1038/bjc.2013.779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Oerlemans S, Mols F, Nijziel MR, Zijlstra WP, Coebergh JW, van de Poll-Franse LV. The course of anxiety and depression for patients with Hodgkin’s lymphoma or diffuse large B cell lymphoma: a longitudinal study of the PROFILES registry. J Cancer Surviv. 2014;8(4):555–564. doi: 10.1007/s11764-014-0367-1. [DOI] [PubMed] [Google Scholar]
- 26.Bristol-Myers Squibb Company. Highlights of Prescribing Information OPDIVO (nivolumab). 2019. p. 1–31.
- 27.Merck & Co., Inc. Highlights of Prescribing Information KEYTRUDA® (pembrolizumab). 2019. p. 1–83.
- 28.Kewalramani T, Nimer SD, Zelenetz AD, Malhotra S, Qin J, Yahalom J, et al. Progressive disease following autologous transplantation in patients with chemosensitive relapsed or primary refractory Hodgkin’s disease or aggressive non-Hodgkin’s lymphoma. Bone Marrow Transpl. 2003;32(7):673–679. doi: 10.1038/sj.bmt.1704214. [DOI] [PubMed] [Google Scholar]
- 29.Shao C, Liu J, Zhou W, Raut MK, Monberg M, Cao X, et al. Treatment patterns, health care resource utilization, and costs in patients with relapsed/refractory Hodgkin lymphoma treated with brentuximab vedotin. Leuk Lymphoma. 2019;60(4):947–954. doi: 10.1080/10428194.2018.1508665. [DOI] [PubMed] [Google Scholar]
- 30.Szabo SM, Hirji I, Johnston KM, Juarez-Garcia A, Connors JM. Treatment patterns and costs of care for patients with relapsed and refractory Hodgkin lymphoma treated with brentuximab vedotin in the United States: a retrospective cohort study. PLoS One. 2017;12(10):e0180261. doi: 10.1371/journal.pone.0180261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Keeping S, Wu E, Chan K, Mojebi A, Ferrante SA, Balakumaran A. Pembrolizumab versus the standard of care for relapsed and refractory classical Hodgkin’s lymphoma progressing after brentuximab vedotin: an indirect treatment comparison. Expert Rev Hematol. 2018;11(6):503–511. doi: 10.1080/17474086.2018.1475226. [DOI] [PubMed] [Google Scholar]
- 32.Bair SM, Strelec LE, Feldman TA, Ahmed G, Armand P, Shah NN, et al. Outcomes and toxicities of programmed death-1 (PD-1) inhibitors in hodgkin lymphoma patients in the United States: a real-world. Multicenter retrospective analysis. Oncologist. 2019;24(7):955–962. doi: 10.1634/theoncologist.2018-0538. [DOI] [PMC free article] [PubMed] [Google Scholar]