Navigating the environment in pursuit of provisions exposes animals to several risks, including predation, yet it is essential for survival. Minimizing the time spent in danger by learning and recalling the location of reliable sources of food is therefore valuable. This ability is not as simple as it might sound: it requires that animals assess various food sources, associate the magnitude of rewards with the unique sensory cues defining each food location, and consolidate spatial memories of the most reliable locations for future recall. CA1 pyramidal neurons of the hippocampus play fundamental roles in spatial memory formation, encoding an animal's position through a map-like representation of the external environment (O'Keefe, 1976). In addition, hippocampal coding helps animals identify and memorize reward locations across changing environments to drive goal-oriented navigation (Gauthier and Tank, 2018; LeGates et al., 2018; Trouche et al., 2019). This complex function likely involves the integration of reward signals into the hippocampal circuit, with the ultimate goal of enhancing retention and stabilization of reward-salient memories.
The neural pathways bringing reward information to this brain structure are still not known, but neuromodulatory midbrain-hindbrain neurons, including noradrenergic, dopaminergic, and serotonergic systems, appear a priori to be good candidates for several reasons. First, they encode reward signals and reward predicting cues (Liu et al., 2014; Bouret and Richmond, 2015; Zhong et al., 2017). Second, they receive inputs from other ponto-medullary regions dictating specific physiological needs, such as hunger, thirst, and associated behavioral states (Watabe-Uchida et al., 2012; Pollak Dorocic et al., 2014; Schwarz et al., 2015). Third, they innervate the hippocampus via long-range projections (Takeuchi et al., 2016; Maddaloni et al., 2017). But previous work suggests that neither noradrenergic nor dopaminergic neurons transmit reward information to the hippocampus. For example, although noradrenergic neurons of the locus coeruleus drive selective attention to salient and novel environmental features, they do not encode reward signals per se (Kempadoo et al., 2016; Takeuchi et al., 2016). Furthermore, dopaminergic neurons of the ventral tegmental area show little involvement in the generation of reward-related spatial memories (Kempadoo et al., 2016; Takeuchi et al., 2016). On the other hand, serotonergic neurons of the median raphe nucleus (MRN), the principal source of serotonin to the hippocampus, affect memory formation (Teixeira et al., 2018) and promote positive reinforcement (Miliaressis, 1977). On this basis, serotonergic regulation of goal-directed memory circuits is a likely possibility.
In a recent article, Luchetti et al. (2020) investigated whether serotonergic fibers originating from the MRN carry reward-related signals to area CA1 of the hippocampus. To test this hypothesis, they used in vivo two-photon calcium imaging as a proxy to measure the activity of genetically identified serotonergic terminals in CA1 of mice exploring a virtual reality environment. By moving over a wheel positioned under a microscope, water-deprived mice could navigate a virtual linear track and intake water drops when stopping in a “reward zone” marked by unique visual cues. The amount of water received, up to three drops, was directly proportional to the time spent in the reward zone. At the end of the track, mice were teleported back to the starting point, ready for a new trial.
The authors recorded seven serotonergic axons (one per animal) over a period of 3-7 d, and found two distinct, mutually exclusive patterns of activity referred to as Type A and Type B. Type A fibers fired selectively during reward consumption, being silent when the mouse was passing or stopping at unrewarded positions. No activity was detected preceding the reward, suggesting that Type A fibers did not signal expectation for future rewards. The response magnitude of Type A fibers was proportional to the amount of reward ingested and gradually decreased over repeated trials, and thus provided the hippocampus with information about ongoing consumption and relative salience. Importantly, Type A fibers fired only if animals were water-deprived, arguing against potential reward-unrelated activity and, at the same time, suggesting integration of current physiological state. In contrast, Type B fibers were silent during reward delivery and consumption but ramped up their activity at the start of a locomotion bout. Calcium signals peaked right after locomotion onset and decayed when mice reached steady speed, suggesting an involvement in motor planning.
To assess whether the activity of Type A and B fibers influenced hedonic behavior, the authors used optogenetic inhibition to silence all CA1 serotonergic inputs when mice occupied the reward zone. They found a small but significant decrease in the number of rewards collected per trial, with consequent reduction in the time spent within the marked area. Collectively, these results indicate that MRN serotonergic neurons provide the hippocampus with at least two types of information: reward consumption and motor activity. Integration of these signals into the hippocampal circuits appears to be required for optimizing foraging strategies.
The hippocampus has long been considered a key region for spatial learning and memory. The main finding from Luchetti et al. (2020) contributes to a growing body of research probing the importance of the hippocampus in forming reward memories and guiding appetitive behaviors (Gauthier and Tank, 2018; LeGates et al., 2018; Trouche et al., 2019). A recent study has indeed shown that reward locations are specifically encoded by a small population of hippocampal neurons intermingled with CA1 pyramidal place cells (Gauthier and Tank, 2018). Unlike place cells, which typically remap randomly across changing environments, this pool of CA1 reward neurons stably signals reward-delivery locations across environments, likely representing the cellular basis of reward-salient spatial memories (Gauthier and Tank, 2018). Two other studies support hippocampal involvement in reward behavior by describing a CA1-to-Nucleus Accumbens (NAc) pathway required for recalling reward-related memories (LeGates et al., 2018; Trouche et al., 2019). Specifically, CA1-NAc synapses undergo LTP during contextual reward behavior. This synaptic plasticity is necessary and sufficient to generate conditioned place preference, as optogenetic-induced potentiation of CA1-NAc synapses promotes conditioned place preference, while their low-frequency stimulation or inhibition prevents it (LeGates et al., 2018; Trouche et al., 2019).
According to this framework, the median-raphe-to-CA1 reward-encoding serotonergic axons observed by Luchetti et al. (2020) might be the means by which reward-related information reaches neurons of CA1. Like many important discoveries, the idea that serotonin axons deliver reward information to the hippocampus sounds quite natural. Serotonin neurons, indeed, signal reward on different timescales (Liu et al., 2014; Cohen et al., 2015), they promote place preference (Wang et al., 2019), and they heavily innervate the hippocampus (Maddaloni et al., 2017). In addition, the overall connectivity of serotonin neurons (Pollak Dorocic et al., 2014) suggests that their reward-encoding properties might be shaped by internal states, such as hunger and thirst, on which the valence of any reward intrinsically depends. Supporting this hypothesis, Type A serotonergic fibers, described by Luchetti et al. (2020), signal water consumption only in thirsty mice and decrease their response as mice become sated. Nonetheless, memorizing food locations only when hungry or thirsty does not seem advantageous from an evolutionary point of view. It is likely that, in addition to a state-dependent serotonergic input, other neural pathways may promote state-independent memory formation of potential food sources, perhaps involving visual and olfactory cues associated with previous food consumption. The combination of these two pathways may ensure optimal foraging strategies.
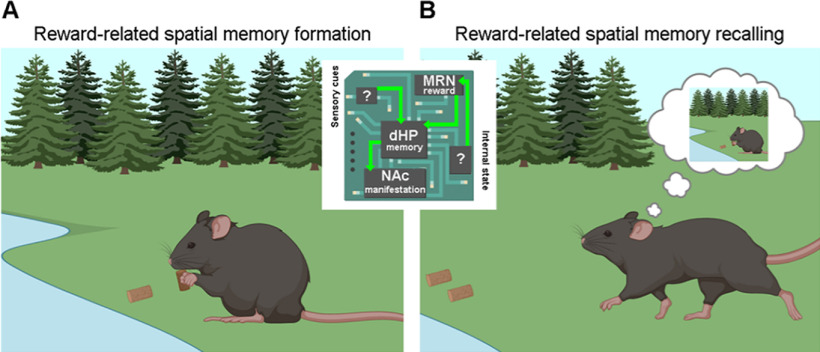
If reward-related serotonergic signaling in CA1 is involved in memory, as the work by Luchetti et al. (2020) suggests, it may trigger the formation, and the consequent behavioral manifestation, of reward-salient memories through a specialized MRN-CA1-NAc circuit (Fig. 1). At the synaptic level, serotonin release from Type A fibers during reward consumption may potentiate excitatory transmission at CA3-CA1 synapses, a form of plasticity thought to be important for memory formation. This synaptic mechanism may label as salient (reward-related) otherwise neutral spatial cues, and therefore induce the formation and consolidation of reward-associated memories in potentiated CA1 cells. These CA1 neurons may in turn promote recalling of reward memories through a NAc projection. Several studies support this hypothesis. (1) Serotonin inputs to CA1 play a permissive role in CA3-CA1 long-term synaptic potentiation and positively modulate spatial memory formation via activation of 5-HT4 receptors on CA1 cells (Teixeira et al., 2018). (2) Selective enhancement of serotonergic neurotransmission increases theta and gamma power in the hippocampus (Giorgi et al., 2017), which are positively associated with mnemonic functions (Winson, 1978). (3) Chronic treatment with the antidepressant fluoxetine, which elevates extracellular serotonin concentration, rescues reward behavior impairments caused by stress-induced weakening of CA1-to-NAc synapses (LeGates et al., 2018).
Figure 1.
Potential mechanisms through which reward-related spatial memories are formed and recalled. A, During consumption, appetitive signals coming from serotonergic neurons of the MRN reach CA1 hippocampal neurons, communicating reward ingestion and relative magnitude. CA1 hippocampal neurons in turn create a spatial memory trace by integrating sensory cues coming from the environment. B, A CA1-NAc pathway is then required for the expression of such reward memory.
Together, these findings raise intriguing questions regarding, for instance, the identity of CA1 cells targeted by MRN serotonergic neurons and the existence of additional reward routes to the hippocampus. They also suggest that MRN-CA1-NAc circuit dysfunction might contribute to anhedonia, a core symptom of several mood disorders involving inability to feel pleasure and lack of motivation. Further deciphering this circuit may therefore guide future translational works with the ultimate goal of elucidating the root basis of and improving therapies for such conditions. Leveraging serotonergic neuron transcriptomic heterogeneity, with hundreds of differentially expressed genes coding for ion channels and G-protein-coupled receptors across subtypes (Okaty et al., 2015), an exciting therapeutic approach entails the design of novel, more effective drugs that selectively target hippocampus-projecting MRN serotonergic neurons, without interfering with the function of other subtypes.
In conclusion, the work by Luchetti et al. (2020) shows the existence of a specialized serotonergic projection delivering reward information to the hippocampus. This pathway is required for forming and storing reward-related spatial memories that help animals to promptly recall reliable reward locations: not a dispensable skill in the ruthless competition for survival.
Footnotes
Editor's Note: These short reviews of recent JNeurosci articles, written exclusively by students or postdoctoral fellows, summarize the important findings of the paper and provide additional insight and commentary. If the authors of the highlighted article have written a response to the Journal Club, the response can be found by viewing the Journal Club at www.jneurosci.org. For more information on the format, review process, and purpose of Journal Club articles, please see http://jneurosci.org/content/jneurosci-journal-club.
Figure 1 was customized using www.Biorender.com illustrations.
The authors declare no competing financial interests.
References
- Bouret S, Richmond BJ (2015) Sensitivity of locus ceruleus to reward value for goal-directed actions. J Neurosci 35:4005–4014. 10.1523/JNEUROSCI.4553-14.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen JY, Amoroso MW, Uchida N (2015) Serotonergic neurons signal reward and punishment on multiple timescales. eLife 4:e06346 10.7554/eLife.06346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gauthier JL, Tank DW (2018) A dedicated population for reward coding in the hippocampus. Neuron 99:179–193.e7. 10.1016/j.neuron.2018.06.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giorgi A, Migliarini S, Galbusera A, Maddaloni G, Mereu M, Margiani G, Gritti M, Landi S, Trovato F, Bertozzi SM, Armirotti A, Ratto GM, De Luca MA, Tonini R, Gozzi A, Pasqualetti M (2017) Brain-wide mapping of endogenous serotonergic transmission via chemogenetic fMRI. Cell Rep 21:910–918. 10.1016/j.celrep.2017.09.087 [DOI] [PubMed] [Google Scholar]
- Kempadoo KA, Mosharov EV, Choi SJ, Sulzer D, Kandel ER (2016) Dopamine release from the locus coeruleus to the dorsal hippocampus promotes spatial learning and memory. Proc Natl Acad Sci USA 113:14835–14840. 10.1073/pnas.1616515114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeGates TA, Kvarta MD, Tooley JR, Francis TC, Lobo MK, Creed MC, Thompson SM (2018) Reward behaviour is regulated by the strength of hippocampus–nucleus accumbens synapses. Nature 564:258–262. 10.1038/s41586-018-0740-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Z, Zhou J, Li Y, Hu F, Lu Y, Ma M, Feng Q, Zhang J, Wang D, Zeng J, Bao J, Kim JY, Chen ZF, El Mestikawy S, Luo M (2014) Dorsal raphe neurons signal reward through 5-HT and glutamate. Neuron 81:1360–1374. 10.1016/j.neuron.2014.02.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luchetti A, Bota A, Weitemier A, Mizuta K, Sato M, Islam T, McHugh TJ, Tashiro A, Hayashi Y (2020) Two functionally distinct serotonergic projections into hippocampus. J Neurosci 40:4936–4944. 10.1523/JNEUROSCI.2724-19.2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maddaloni G, Bertero A, Pratelli M, Barsotti N, Boonstra A, Giorgi A, Migliarini S, Pasqualetti M (2017) Development of serotonergic fibers in the post-natal mouse brain. Front Cell Neurosci 11:202. 10.3389/fncel.2017.00202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miliaressis E. (1977) Serotonergic basis of reward in median raphe of the rat I. Pharmacol Biochem Behav 7:177–180. 10.1016/0091-3057(77)90204-0 [DOI] [PubMed] [Google Scholar]
- O'Keefe J. (1976) Place units in the hippocampus of the freely moving rat. Exp Neurol 51:78–109. [DOI] [PubMed] [Google Scholar]
- Okaty BW, Freret ME, Rood BD, Brust RD, Hennessy ML, deBairos D, Kim JC, Cook MN, Dymecki SM (2015) Multi-scale molecular deconstruction of the serotonin neuron system. Neuron 88:774–791. 10.1016/j.neuron.2015.10.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollak Dorocic I, Fürth D, Xuan Y, Johansson Y, Pozzi L, Silberberg G, Carlén M, Meletis K (2014) A whole-brain atlas of inputs to serotonergic neurons of the dorsal and median raphe nuclei. Neuron 83:663–678. 10.1016/j.neuron.2014.07.002 [DOI] [PubMed] [Google Scholar]
- Schwarz LA, Miyamichi K, Gao XJ, Beier KT, Weissbourd B, DeLoach KE, Ren J, Ibanes S, Malenka RC, Kremer EJ, Luo L (2015) Viral-genetic tracing of the input–output organization of a central noradrenaline circuit. Nature 524:88–92. 10.1038/nature14600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeuchi T, Duszkiewicz AJ, Sonneborn A, Spooner PA, Yamasaki M, Watanabe M, Smith CC, Fernández G, Deisseroth K, Greene RW, Morris RG (2016) Locus coeruleus and dopaminergic consolidation of everyday memory. Nature 537:357–362. 10.1038/nature19325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teixeira CM, Rosen ZB, Suri D, Sun Q, Hersh M, Sargin D, Dincheva I, Morgan AA, Spivack S, Krok AC, Hirschfeld-Stoler T, Lambe EK, Siegelbaum SA, Ansorge MS (2018) Hippocampal 5-HT input regulates memory formation and Schaffer collateral excitation. Neuron 98:992–1004.e4. 10.1016/j.neuron.2018.04.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trouche S, Koren V, Doig NM, Ellender TJ, El-Gaby M, Lopes-dos-Santos V, Reeve HM, Perestenko PV, Garas FN, Magill PJ, Sharott A, Dupret D (2019) A hippocampus-accumbens tripartite neuronal motif guides appetitive memory in space. Cell 176:1393–1406.e16. 10.1016/j.cell.2018.12.037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang HL, Zhang S, Qi J, Wang H, Cachope R, Mejias-Aponte CA, Gomez JA, Mateo-Semidey GE, Beaudoin GM, Paladini CA, Cheer JF, Morales M (2019) Dorsal raphe dual serotonin-glutamate neurons drive reward by establishing excitatory synapses on VTA mesoaccumbens dopamine neurons. Cell Rep 26:1128–1142.e7. 10.1016/j.celrep.2019.01.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watabe-Uchida M, Zhu L, Ogawa SK, Vamanrao A, Uchida N (2012) Whole-brain mapping of direct inputs to midbrain dopamine neurons. Neuron 74:858–873. 10.1016/j.neuron.2012.03.017 [DOI] [PubMed] [Google Scholar]
- Winson J. (1978) Loss of hippocampal theta rhythm results in spatial memory deficit in the rat. Science 201:160–163. 10.1126/science.663646 [DOI] [PubMed] [Google Scholar]
- Zhong W, Li Y, Feng Q, Luo M (2017) Learning and stress shape the reward response patterns of serotonin neurons. J Neurosci 37:8863–8875. 10.1523/JNEUROSCI.1181-17.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]