Abstract
Background
Isoprene (C5H8) is a clinically important breath metabolite. Although, hundreds of studies have reported differential expressions in isoprene exhalation as breath biomarker for diverse diseases, the substance couldn't enter to clinical practice as diagnostic marker. Moreover, many experimental/basic observations upon breath isoprene remained unrelated to the corresponding pathophysiological effects on its putative metabolic origin (i.e. mevalonate pathway). Here, we investigated the fundamental reason that hindered the rational interpretation and translation of this marker from basic to clinical science.
Methods
Via high-resolution mass-spectrometry based breathomics in 1026 human subjects, we discovered adults with significant deficiency (order of magnitude lower than the normal) and complete absence of breath isoprene. We prospectively applied real-time breathomics, quantitative gene expression analysis of the mevalonate pathway enzymes, lipid-profiling and hemodynamic monitoring on those isoprene deficient subjects and controls. Additionally, the subject with absence of isoprene was followed up throughout different phases of her womanhood.
Results
In contrast to convention, we witnessed that adults can live healthy without exhaling isoprene or with significant deficiency. This rare phenotype represents a recessive inheritance. Despite physio-metabolic changes during menstrual cycle (that is known to profoundly affect isoprene exhalation) and profoundly increased plasma cholesterol during pregnancy and after childbirth, isoprene remained absent. All genes of mevalonate pathway enzymes were normally expressed in all participants, without any down-regulation or compensatory up-regulation.
Conclusions
Absence/deficiency of isoprene despite normal lipid profiles and no mevalonate pathway malfunction disqualifies the long-believed metabolic origin of isoprene from cholesterol biosynthesis. Thus, clinical translation of breath isoprene expressions should not be generally attributed to corresponding pathophysiological effects onto mevalonate/cholesterol pathway. Our finding has refined and optimized the clinical interpretation of isoprene as biomarker in volatile metabolomics and breathomics. Future studies will address the correct metabolic origin of isoprene to imply this important marker to routine practice.
Keywords: Breath isoprene, Disease biomarker, Cholesterol biosynthesis, Mevalonate pathway, PTR-MS, Metabolic disorders, Volatile metabolomics, Breathomics
Breath isoprene, Disease biomarker, Cholesterol biosynthesis, Mevalonate pathway, PTR-MS, Metabolic disorders, Volatile metabolomics, Breathomics.
1. Introduction
Isoprene (C5H8) is the second most abundant (concentration mostly range: ~100–300 ppbV in adults) endogenous volatile organic compound (VOC) in human breath. Hundreds of clinical studies have randomly reported differential-expressions of exhaled isoprene concentrations as non-invasive biomarker to detect/monitor lung diseases [1,2], myocardial infraction [3], hypercholesterolemia [4], oxidative stress [5], cancers [6,7] chronic liver disease [8] and many other health conditions [9]. Moreover, relative changes in exhaled concentrations mirror various physio-metabolic effects [10,11,12,13,14]. Isoprene is believed to be a by-product of cholesterol biosynthesis [15,16], where mevalonic acid is deduced to non-sterol isoprenoid molecules [17]. According to literatures, absence of non-sterol isoprene is attributed to inherited errors (e.g. hyper immunoglobulinemia D syndrome, autoinflammatory periodic fever syndrome and mevalonic aciduria) in mevalonate pathway [18,19,20]. Breath isoprene deficiency in Duchenne muscle dystrophy patients supports its storage and washout from the muscle compartments [21].
Despite repeated claims to date, isoprene could not be translated to routine clinical practice as a disease specific biomarker. Most surprisingly, observed expressions of breath isoprene in many experimental/clinical studies (including our own) remained unexplained while considering the corresponding pathophysiological effects onto cholesterol biosynthesis (mevalonate pathway) and observed changes are randomly attributed to other effects such as endothelial cell damage, aging and oxidative stress, inflammation, ventilation and so on [22,23,24,25,26]. Despite previous doubts regarding the in vivo origin of isoprene [27,28,29], the fundamental truth remained unaddressed. This clearly summons the long-ignored investigation to address if we are addressing the correct biochemical origin while interpreting breath isoprene data as disease biomarker. As the answer to such fundamental mystery may hide within the rare cases of extremities (i.e. isoprene deficiency/absence), we started our search for such subjects, since 2013.
Until 2019, we have identified and quantified hundreds of VOCs (via high-resolution mass-spectrometry) within the exhaled breaths from >1000 humans, who belong to diverse demography. Eventually, we came across a few adults with significant isoprene deficiency and even without any traceable breath concentration. Looking at their health and lifestyle, we questioned the putative endogenous origin of breath isoprene [4,15,30] i.e. hypothesized since 1984 via an in vitro rat model [31]. In order to address that question, we have conducted a cross-disciplinary experiments involving real-time breathomics in parallel to lipid profiling and down-stream genetic analysis. Our present study will refine certain longstanding belief/conception upon the in vivo source of breath isoprene. This will offer rational interpretations of isoprene exhalations, which may translate this important breath biomarker towards clinical practice in the future.
2. Methods
2.1. Ethics
In accordance with the amended Declaration of Helsinki guidelines, an ethical approval (Approval number: A 2019-0040) from the Institutional Ethics Committee (University Medicine Rostock, Germany) and signed informed consent from all subjects were obtained prior to inclusion in the study. For breath sampling in infants, the consent of both parents was obtained.
2.2. Breath VOC screening in search of human subjects with deficiency and/or absence of breath isoprene
Since 2013 until 2019, we have enrolled 1026 subjects, who belong to diverse age, gender, BMI, ethnicity and health conditions within our breath test (i.e. real-time mass-spectrometry based). Demographic data are listed in Supplementary Table 1. In 2015, we spotted the first young and healthy woman (will be referred as ‘index’ within the manuscript) without any traceable breath isoprene. As her blood-relatives also turned out with significant breath isoprene deficiency, we also included her immediate family members (i.e. her parents, -sibling sister, infant daughter and her husband) into further experiments.
2.3. Measurement protocol for index, isoprene deficient subjects and controls
Breath VOC measurement were repeated three times (on three different days) in all subjects.
Knowing the possible up- or down-regulation of cholesterol homeostasis under endocrine changes (e.g. female sex-hormone milieu) [32,33] we measured index's breath compositions, lipid profile and hemodynamic parameters throughout the menstrual cycle (i.e. periods, mid-follicular, ovulation and mid-luteal phases), during her first pregnancy (monthly) and also after the successful child birth for any possible occurrence of isoprene or up- or down-regulation of any other endogenous substances.
The index, her family members (except the infant, due to ethical limitation) and four unrelated healthy adult controls (selected from the measured population) with normal breath isoprene levels, voluntarily provided blood for down-stream genetic analysis.
2.4. Study setup
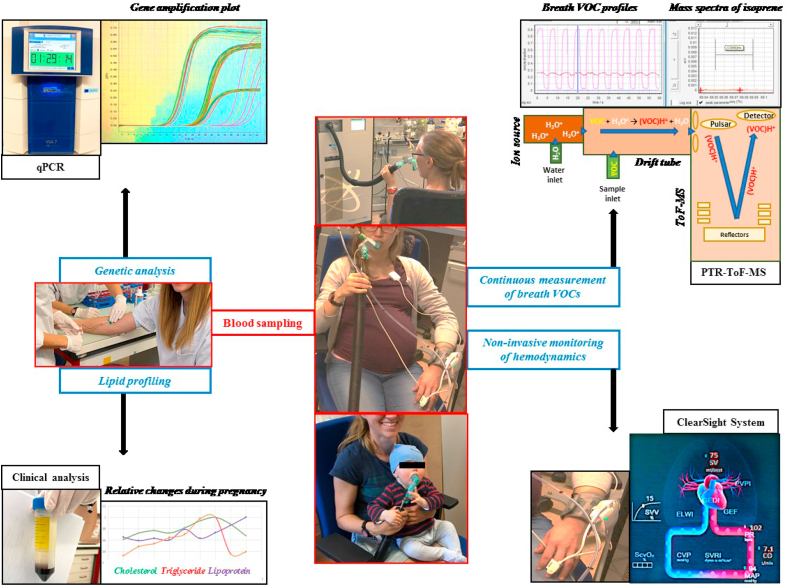
Figure 1 represents an overview of our study setup. Proton transfer reaction - time of flight - mass spectrometry (PTR-ToF-MS 8000; Ionicon Analytik GmbH, Innsbruck, Austria) based real-time analysis of breath VOCs along with the non-invasive hemodynamic monitoring were synchronised together. Venous blood samples were collected for lipid profiling and genetic analysis by the study physician.
Figure 1.
Schematic overview of the study setup. Continuous real-time breath analysis (via PTR-ToF-MS) and non-invasive hemodynamic monitoring (via ClearSight System – volume clamp method) were performed along with real-time gene expression analysis (via quantitative-PCR) and clinical lipid-profiling from collected blood samples. Moments from breath analysis of the index subject (before and during her pregnancy) and of her kid (daughter) are presented at the center.
2.5. Breath sampling and analysis
Subjects maintained a normal sitting posture [12] and performed oral breathing [34] via custom made Teflon-mouthpiece of 2.5 cm diameter [35]. The transfer-line of PTR-ToF-MS was connected to the sterile mouthpiece to record VOC concentrations for continuous breath-resolved analysis. After 1 min of metronome controlled paced breathing (respiratory rate = 12/min) subjects performed spontaneous breathing for five more minutes. This sampling protocol significantly reduces ventilatory variations and breath samples from 3rd minute (of spontaneous rhythm) onward were considered for analysis [36].
The working principles and pre-optimized experimental conditions of PTR-ToF-MS are described already [37]. Concisely, the soft ionisation of VOCs is based on a non-dissociative proton transfer reaction that ionises VOCs with relatively higher proton-affinity than water. Protonated VOCs are then detected via a high-resolution time-of-flight mass-spectrometer according to their mass to charge ratio. Our instrument can detect breath VOCs (including isoprene) up to low pptV range. For a detailed description of the method, see the Transparent Methods in Supplementary Material.
2.6. Blood sampling
Antecubital venous blood samples (20 ml/measurement/subject) were collected for the analysis of conventional lipid profiles and genetic analysis.
2.7. Lipid profiling
Total cholesterol, lipoproteins (a), high density lipoproteins (HDL), low density lipoproteins (LDL) and triglycerides were analysed via conventional clinical method.
2.8. Gene expression analysis
We checked the presence/absence of transcripts, which code for the enzymes of the mevalonate pathway. Here, we applied quantitative polymerase chain reaction (qPCR) upon the gene expressions of all principal enzymes of the mevalonate pathway. The regulating genes are acetyl-CoA acetyltransferase 2 (ACAT2), HMG-CoA synthase 1 -cytosolic (HMGCS1), HMG-CoA reductase (HMGCR), mevalonate kinase (MVK), phosphomevalonate kinase (PMVK), di-phosphomevalonate decarboxylase (MVD), isopentenyl-diphosphate delta isomerase 1 (IDI1), farnesyl-diphosphate synthase 1 (FDPS1), geranylgeranyl diphosphate synthase 1 (GGPS1), farnesyl-diphosphate farnesyltransferase 1 (FDFT1), lanosterol synthase (LSS), 7-dehydrocloesterol reductase (DHCR7). The working principles and optimised process of our qPCR is described before [38]. Primers used for gene expression analysis are listed in Supplementary Table 2. Relative gene expression is normalized to GAPDH (i.e. housekeeping gene) and calculated using the 2-ΔΔCt formula. Measurements were repeated thrice in all subjects. For a detailed description of the method, see the Transparent Methods in Supplementary Material.
2.9. Hemodynamic monitoring
We performed non-invasive measurement of cardiac output, stroke volume, pulse rate and mean arterial pressure etc. via our optimised volume clamp method [13] to track physiological effects during pregnancy. For a detailed description of the method, see the Transparent Methods in Supplementary Material.
2.10. Statistical analysis
Sample size was computed while designing this study. We applied analysis of variance (ANOVA) test by considering a minimum detectable difference (as reported clinical studies) in mean isoprene intensities of 40 cps, an expected standard deviation of 150 (i.e. varying between 100 – 200 according to age, gander, BMI health conditions etc.), 6 groups, a desired test-power of 0.99 and the alpha value of 0.005. Thus, the resulting sample size was 1020 (to represent relevance to the general population). In this study we have included 1026 subjects to detect less than 5% differences in exhaled isoprene concentrations up to low pptV levels.
Repeated measurements (thrice) of breath VOCs were performed in all subjects on different days. Mean values (as data are normally distributed) of expiratory and inspiratory isoprene concentrations were calculated over a minute of breath-resolved data in each measurement/subject.
Statistically significant differences in expiratory isoprene concentrations between different age groups (pre-school children, pre-adolescents, teenagers, adults and seniors) were judged via repeated measurement ANOVA on ranks (Friedman repeated measures analysis of variance on ranks, Shapiro-Wilk test for normal distribution and post hoc Student–Newman–Keuls method for pairwise multiple comparisons between all groups; p-value ≤0.005) in SigmaPlot (version 14) software. The adults are divided into three distinct cohorts depending on the normal levels (n = 532), absence (n = 01) and deficiency (n = 5) of exhaled isoprene. All groups were compared to each other and in Figure 3 we presented those referring to the group of adults with normal isoprene levels.
Figure 3.
Distribution of exhaled isoprene concentrations according to age groups. X-axis represents different age groups with number of measured subjects in each. Y-axis represents exhaled alveolar concentrations of isoprene (ppbV). The adults are presented into three distinct cohorts depending on the normal levels (n = 532), absence (n = 01) and deficiency (n = 5) of isoprene. Statistical significances of differences were tested by means of one-way repeated measurement-ANOVA on ranks (p-value ≤ 0.05). From all pairwise-multiple comparisons, statistically significant differences (with respect to adults with normal isoprene levels) are marked with blue-colored asterisk.
Statistically significant differences in expiratory and inspiratory concentrations (obtained via repeated measurements) were compared between the index subject, her parents, sibling-sister, infant daughter, husband and unrelated controls. A repeated measurement ANOVA on ranks (as indicated above) was applied for all pair-wise multiple comparisons.
3. Results
3.1. Alveolar isoprene exhalation in different groups
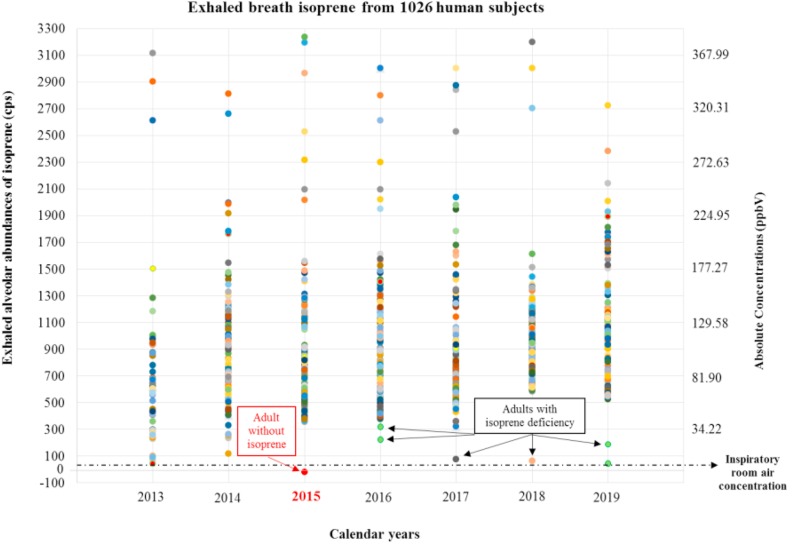
Figure 2 represents the exhaled alveolar isoprene concentrations from the entire study population of 1026 human subjects (aged between 01–90 years), measured from 2013 to 2019. Among them there were 7 pre-school children (3 males & 4 females; age: 0–05 years), 75 pre-adolescents (35 males & 40 females; age: 06–12 years), 193 teenagers (110 males & 83 females; age: 13–19 years), 538 adults (281 males & 257 females; age: 20–59 years) and 213 seniors (46 males & 167 females; age: 60–90 years).
Figure 2.
Exhaled alveolar isoprene concentrations from entire study population (1026 humans), measured since 2013 till 2019. X-axis represents calendar years since 2013. Y-axis represents exhaled alveolar concentrations of isoprene in all participants. Primary Y-axis stands for alveolar abundances in counts per second (cps) and secondary Y-axis stands for the absolute concentrations in parts-per-billion by volume (ppbV) i.e. obtained via standard calibration driven quantification. Each coloured dot represents the exhaled breath isoprene value (mean over a minute) from each subject. The horizontal dotted line represents the inspiratory (i.e. room air) concentration range of isoprene. The adult without isoprene (n = 01) and adults isoprene deficiency (n = 05) are also indicated clearly. Other data points at the low range (i.e. below 500 cps) are from pre-school kids and pre-adolescents.
Pre-school kids had exhaled low (near inspiratory) isoprene concentrations (mean ± SD) of 08.20 ± 2.74 ppbV. Concentrations in pre-adolescents ranged at 87.39 ± 60.25 ppbV and in teenagers ranged at 112.75 ± 70.16 ppbV. In seniors, exhaled isoprene concentrations ranged at 117.08 ± 42.78 ppbV.
Among the adults, exhaled isoprene concentrations in 532 adults were at the normal range of 137.52 ± 48.2 ppbV. Only the index woman (German ethnicity) did not exhale any detectable/quantifiable isoprene (with respect to the minimal inspiratory room-air concentrations) in her breath. Absolute isoprene concentrations (repeated measurements mean ± SD) in her isoprene deficient blood-relatives i.e. sibling sister, mother, father and infant daughter (<1 year old) were 19.87 ± 4, 27.55 ± 5, 39.25 ± 6 and 05 ± 2 ppbV, respectively. The same in her husband was 111.46 ± 15 ppbV. In 2017 and 2018, we spotted two other young and healthy women (27 & 24 years old) with deficient breath isoprene concentrations of 11.45 ± 3 ppbV (Spanish ethnicity) and 6.41 ± 2 ppbV (Columbian ethnicity), respectively. As they were in short-term study visit and soon moved to their home countries, we couldn't conduct any follow-up or genetic analysis. Surprisingly, neither the index nor the isoprene deficient subjects were suffering from any acute or chronic disease or any abnormality in cholesterol and lipid profile. Exhaled isoprene concentrations in four controls (02 males & 02 females, who were included in genetic analysis) were 114.52 ± 6.2, 144.51 ± 12.8, 176.70 ± 11.2 and 227.96 ± 10.4 ppbV, respectively.
Exhaled isoprene concentrations from the above-mentioned age cohorts are presented in Figure 3. Statistically significant differences are presented with respect to the group of adults (n = 532) with normal isoprene levels. Detailed results of statistical comparisons are listed in Supplementary Table 3.
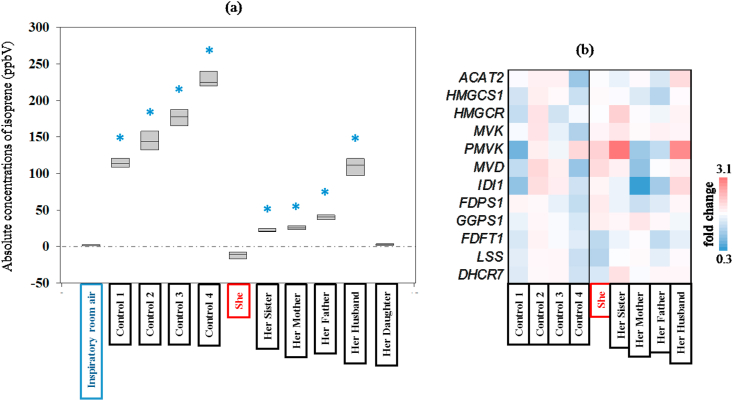
Differences between inspiratory and expiratory concentrations of breath isoprene from index, index family and unrelated healthy controls are presented in Figure 4 (a). No significant differences were found in the expiratory isoprene concentrations of index and her daughter with respect to inspiratory values. Detailed results of statistical comparisons are listed in Supplementary Table 4.
Figure 4.
Comparison of differences in exhaled alveolar isoprene concentrations in all subjects (a). Gene expression analysis of enzymes involved in cholesterol synthesis in all subjects (b). (a) Y-axis represents the absolute values of repeatedly measured isoprene concentrations (ppbV) in each subject's expiratory breath and in the corresponding inspiratory room air. X-axis represents study subjects and room air. Index subject is indicated as ‘She’ and rest of the family members are assigned in relation to her. Four healthy control subjects (unrelated to her) are also listed. Statistical significances were tested by means of one-way repeated measurement-ANOVA on ranks (p-value ≤ 0.05). From all pairwise-multiple comparisons, statistically significant differences (with respect to inspiratory room air) are marked with blue-colored asterisk. (b) Gene expression was determined by qPCR and subsequent ddCT determination relative to GAPDH. The mean of all controls was calculated and used as baseline value for fold change determination of controls and index family members. The regulating genes are acetyl-CoA acetyltransferase 2 (ACAT2), HMG-CoA synthase 1 -cytosolic (HMGCS1), HMG-CoA reductase (HMGCR), mevalonate kinase (MVK), phosphomevalonate kinase (PMVK), di-phosphomevalonate decarboxylase (MVD), isopentenyl-diphosphate delta isomerase 1 (IDI1), farnesyl-diphosphate synthase 1 (FDPS1), geranylgeranyl diphosphate synthase 1 (GGPS1), farnesyl-diphosphate farnesyltransferase 1 (FDFT1), lanosterol synthase (LSS), 7-dehydrocloesterol reductase (DHCR7).
3.2. Gene expression of mevalonate and cholesterol biosynthesis pathway enzymes
All genes analysed were expressed in control samples, index and all family members, indicating normal pathway functionality in Figure 4 (b). This is in line with the data obtained from blood lipid and cholesterol measurements. Compared to control samples the gene expression of the index subject was within normal range with enzymes responsible for the upper part of the cholesterol synthesis (ACAT2, HMGCS1, HMGCR, MVK, PMVK, MVD, IDI1, FDPS1, GGPS1) slightly increased (1.04-fold–1.77-fold). In contrast, FDFT1, LSS and DHCR7 were down-regulated (0.76-, 0.72- and 0.88-fold, respectively) but also within normal range. Her mother has a gene expression profile that is rather adverse, with upper pathway enzymes rather decreased and a slight up-regulation of downstream genes. The profile of the father resembles that of the mother while her husband demonstrates similarities with the index. Of note, the group of controls are quite heterogeneous with two persons (Control-1, male; Control-4, female) exhibiting overall low gene expression patterns and the other two (also one male, one female) a rather up-regulated cholesterol synthesis pathway. Detailed results of gene expression analysis are listed in the Supplementary Table 5.
Analysing the overall distribution, we could not detect a cluster or pattern separating controls and index family members. With all isoprene-deficient or -low family members well within the normal range and no clustering detected either within the family cohort or between family cohort and controls we conclude that aberrant cholesterol pathway gene expression is not a factor involved in absent isoprene synthesis.
3.3. Lipid profile, hemodynamics and VOC exhalation during womanhood of the subject without isoprene
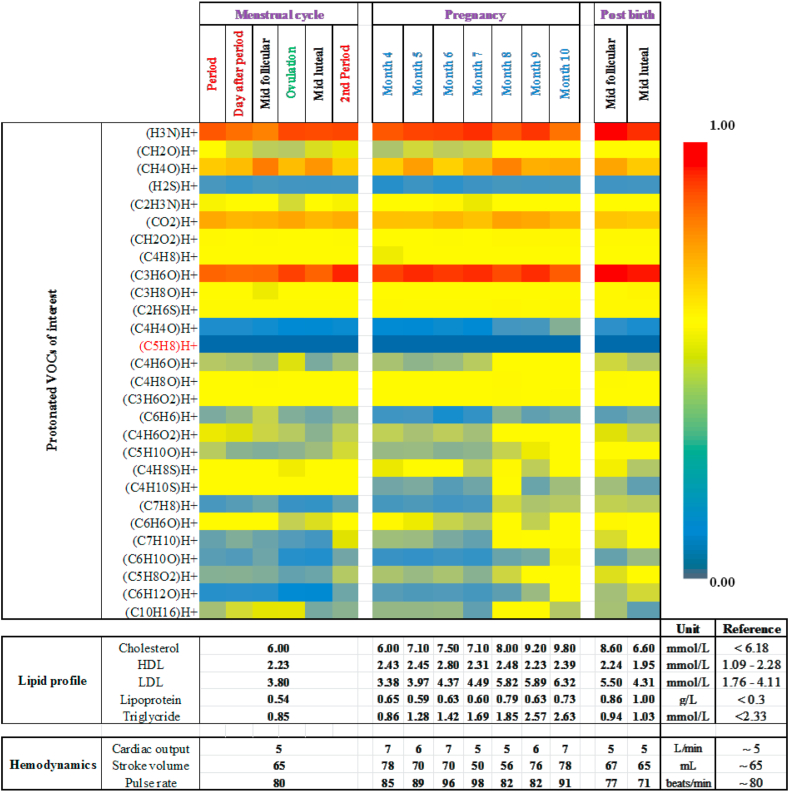
Changes in absolute values of lipid profile, hemodynamic parameters and relative changes in exhaled alveolar concentrations of 28 VOCs took place during her menstrual cycle phases, pregnancy and after child birth (Figure 5). Pronounced physiological and metabolic effects are observed on other breath VOCs during menstrual cycle and pregnancy. Despite profound fluctuation in cardiac output and increased cholesterol production during pregnancy, her isoprene remained absent.
Figure 5.
Heatmap of exhaled breath VOC compositions, lipid profile and hemodynamic values of the index subject during different phases of her womanhood. Normalized (onto maximum value) expression (i.e. Heatmap) of relative changes in exhaled VOC concentrations throughout her menstrual cycle (Period – day after period – mid follicular – ovulation phase – mid luteal – 2nd period), pregnancy (from 4th – 10th months) and the immediate follicular and luteal phases of the menstrual cycle after the successful child birth is presented as a heatmap. For VOCs, changes in colours from red to blue symbolise relative changes from higher to lower concentrations and vice versa (Please refer to the color scale). The sum formula of protonated isoprene [i.e. (C5H8)H+] is indicated in red color. Absolute values of lipid profile (cholesterol, HDL, LDL, lipoproteins and triglycerides) and hemodynamic parameters (cardiac output, stroke volume and pulse rate) along with corresponding healthy reference range (age, gender and BMI matched) are presented before, during and after pregnancy. In spite of pregnancy driven pronounced physiological and metabolic effects on many other VOCs, isoprene remained absent in the index.
In order to reduce confounding effects, only compounds with expiratory abundances significantly higher than the inspiratory room-air abundances (+standard deviations of room-air abundances) were selected for analysis. Among those, we selected 28 compounds in order to address a broad spectrum of chemical classes, physico-chemical properties and substance-specific physiological/metabolic effects. These substances reflect important aspects for human breathomics e.g. diverse origins (endogenous and blood borne, oral cavity, pre-exposure) and dependencies on physiological and metabolic changes and effects.
4. Discussion
In accordance with the previous evidences [24,39], a distinct dependency of isoprene exhalation on ageing is observed within our study population. In contrast to conventional knowledge [18,19], we witnessed that adults can live healthy without exhaling isoprene or with significant isoprene deficiency. Isoprene exhalations in index family indicate a recessive inheritance of this phenotype. Here, her isoprene deficient parents and sibling sister are carriers of the character whereas, tiny presence is quantified in her infant daughter (<1 year old) as her husband has normal levels (Please see Supplementary Figure. 1).
Any functional loss of one pathway member would probably result in compensatory upregulation of other enzymes. As this was not observed in gene expressions, we conclude that there are no functional aberrations in cholesterol synthesis. Very heterogenous gene expressions in controls and in persons without isoprene and with isoprene deficiency have indicated that mevalonate pathway gene expression does not influence isoprene synthesis and thereby, cholesterol biosynthesis is likely not the source of isoprene in human breath.
Since the inception of human breathomics, isoprene has been the substance of potential interest especially, in the field of non-invasive physiological and metabolic monitoring. Due to its low aqueous solubility and high volatility, isoprene mirrors the pulmonary ventilation-perfusion phenomenon [13]. Its systemic transport to alveolar capillary is cardiac output (lung perfusion determinant) dependent [12,40] and pulmonary elimination is based on intra-alveolar pressure gradient driven diffusion [35]. Simple physiological changes in hemodynamics and/or ventilation are immediately reflected in isoprene exhalation [11,12,34,40]. We observed diverse changes in breath isoprene levels throughout menstrual cycles – with/without administration of monthly oral contraceptive pills [14]. As physiological effects (ventilatory and hemodynamic) and metabolic changes (endocrine interplay) are readily observed in isoprene exhalation, this compound is supposed to fluctuate significantly during pregnancy. Despite pronounced physiological increase in her plasma cholesterol levels throughout gestation [41] and elevated cardiac output, isoprene did not appear (Figure 5). Exhaled alveolar concentrations of endogenous VOCs changed mainly because of pregnancy induced hormonal changes (causing homeostatic metabolic effects on in vivo origins of substances), obvious oxidative stress (embryonic development driven), changes in broncho-pulmonary gas-(VOCs)-exchange under increased cardiac output (counter-balancing oxygen- and nutrients demand of the foetus) and altered respiratory rate, tidal volume and minute ventilation (due to uplifted diaphragm of the mother). Here, we have restrained ourselves from discussing substance specific behaviour of VOCs as this is not the principal focus of this manuscript.
Literatures state that dimethylallyl pyrophosphate (DMAPP) is converted to isoprene by isoprene synthase enzyme in plants [42]. The enzyme is reasonably well studied in plants [43] but poorly characterized in microbes and animals. DMAPP undergoes non-enzymatic degradation in animal liver [31] but the reaction rate is too slow to constantly source breath isoprene. Although enzymes with multiple functional domains (catalytic sites with broad binding and conversion capabilities) can convert DMAPP to isoprene, bioinformatic sequence alignment (whole sequence/functional domain based) search for the plant isoprene synthase with human genes failed to find enzyme homologs. Consequently, the deficiency/absence of isoprene in healthy adults may indicate that the conversion of DMAPP to isoprene might not be essential for life. Seeing its emission from bacteria, marine algae, plants, animals and us it's hard to disregard the phylogenetic/evolutionary significance of its endogenous production [30,44]. As nature doesn't play dices, it's unlikely for any redundant biochemical pathway to sustain throughout the course of evolution. Although isoprene protects thermal damage of photosynthetic membranes in plants [42], the biological importance in animal or human is unknown. From a toxicological point of view, isoprene is a carcinogen by nature and prolonged exposure to inhaled isoprene has shown cancer growth in rodents [45,46,47]. Thus, isoprene exhalation might be a blood-breath excretory axis of hydrocarbon homeostasis in blood. Considering the muscle compartments as potential storage of isoprene [21], exercise increases its elimination [10] and thereby supports its benefit towards physiological detoxification.
Mevalonate kinase (MVK) enzyme deficiency is an ultra-rare autosomal recessive (gene locus: chromosome 12q24) inborn defect of metabolism (aka. Mevalonic aciduria) with an autoinflammatory phenotype [48,49] i.e. characterised by absence of isoprene. Allogenic bone marrow transplantation from heterogenous carrier of the mutant gene has shown symptomatic improvement in mevalonic aciduria patient [20]. In contract to that our index is healthy and all genes of the mevalonate pathway enzymes are transcribed despite the same recessive phenotype (i.e. absence of isoprene) as of mevalonic aciduria patients. Thus, one may assume that one of these multi-functional enzymatic sites is malfunctioning. Hence, she does not have isoprene but she doesn't suffer any consequences of the genetic abnormality. This has summoned future down-stream genome analysis (e.g. exome sequencing) to spot this healthy mutation in order to find the actual biochemical origin of isoprene in human. Our future work will address those investigations.
In volatile metabolomics and breathomics, clinical interpretations of differential-expressions/changes in endogenous VOC markers rely upon the primary effects of a pathophysiology onto the biochemical/metabolic origin of substances. Many clinical observations/results upon the effects of a disease onto cholesterol-genesis and isoprene exhalation remained unexplainable and are attributed to other phenomena such as endothelial damage [22], aging [39], inflammatory process [26], ventilatory effects [25] etc. For instance, we observed no relation (Pearson correlation based on 968 independent observations at p-value significance of ≤0.05. Please refer to the supplementary material of the corresponding article by Trefz et al.) between plasma cholesterol levels and exhaled isoprene concentrations within large cohort of healthy and diabetic children [23]. In line with those, our present finding is clearly reporting that the long anticipated in vivo origin of breath isoprene is incorrect. Thus, we have addressed a general misconception that hinders trustworthy bio-medical interpretation of this important biomarker in our field of omics.
5. Conclusions
We have pioneered that absence/deficiency of breath isoprene is not necessarily a pathological phenotype. The recessive pedigree and healthy manifestation of this phenotype along with no functional aberration in cholesterol- and/or mevalonate pathway genes have contradicted the conventionally hypothesised origin of isoprene in human breath. Our data shouts for future investigations to find the actual metabolic process/biochemical pathway of isoprene production. Otherwise, clinical interpretations of breath isoprene will be misleading and at random. Absence/suppression of true isoprene production process in our healthy index/index family have summoned further studies to track the possible presence of any compensatory mechanism in such individuals. Clinical translation of our basic findings may open up an unexplored frontier towards the limited clinical understanding of certain ultra-rare diseases as well as will trailblaze the rational interpretations of isoprene exhalation under various health conditions as reliable breath biomarkers in future.
5.1. Limitations of the study
While looking at the present limitations, despite screening >1000 subjects, we were able to find only one adult without isoprene and five adults with significant deficiency of isoprene and therefore, we could not run a rational statistical correlation analysis (between breath isoprene and gene-expression) within this study. However, our finding indicates a rare prevalence of this phenotype. Furthermore, considering the observed negligible amounts of exhaled isoprene in kids below the age of five years, it is hard to draw further conclusions onto observed concentrations of index's daughter before 2025.
Declarations
Author contribution statement
P. Sukul: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
J. Schubert and W. Miekisch: Conceived and designed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
A. Richter: Performed the experiments; Analyzed and interpreted the data; Wrote the paper.
Funding statement
This work was supported by FP7 People: Marie-Curie Actions (ITN-PIMMS) (287382), Inno-INDIGO-NCDs-CAPomics (BMBF 01DQ16010), Horizon 2020 Framework Programme (PCH-HEARTEN) (643694) and the European fund for regional development (EFRE).
Data availability statement
Data included in article/supplementary material/referenced in article.
Declaration of interests statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
Acknowledgements
We thank all the volunteers for participating in this study. Special thanks to Dr. Patricia Fuchs (RoMBAT, University Medicine Rostock, Germany) for supporting hemodynamic monitoring.
Appendix A. Supplementary data
The following is the supplementary data related to this article:
References
- 1.McGrath L.T., Patrick R., Mallon P., Dowey L., Silke B., Norwood W., Elborn S. Breath isoprene during acute respiratory exacerbation in cystic fibrosis. Eur. Respir. J. 2000;16:1065–1069. doi: 10.1034/j.1399-3003.2000.16f08.x. [DOI] [PubMed] [Google Scholar]
- 2.Schubert J.K., Müller W.P.E., Benzing A., Geiger K. Application of a new method for analysis of exhaled gas in critically ill patients. Intensive Care Med. 1998;24:415–421. doi: 10.1007/s001340050589. [DOI] [PubMed] [Google Scholar]
- 3.Mendis S., Sobotka P.A., Euler D.E. Expired hydrocarbons in patients with acute myocardial infarction. Free Radic. Res. 1995;23:117–122. doi: 10.3109/10715769509064026. [DOI] [PubMed] [Google Scholar]
- 4.Karl T., Prazeller P., Mayr D., Jordan A., Rieder J., Fall R., Lindinger W. Human breath isoprene and its relation to blood cholesterol levels: new measurements and modeling. J. Appl. Physiol. 2001;91:762–770. doi: 10.1152/jappl.2001.91.2.762. [DOI] [PubMed] [Google Scholar]
- 5.Pabst F., Miekisch W., Fuchs P., Kischkel S., Schubert J.K. Monitoring of oxidative and metabolic stress during cardiac surgery by means of breath biomarkers: an observational study. J. Cardiothorac. Surg. 2007;2:37. doi: 10.1186/1749-8090-2-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fuchs D., Jamnig H., Heininger P., Klieber M., Schroecksnadel S., Fiegl M., Hackl M., Denz H., Amann A. Decline of exhaled isoprene in lung cancer patients correlates with immune activation. J. Breath Res. 2012;6 doi: 10.1088/1752-7155/6/2/027101. [DOI] [PubMed] [Google Scholar]
- 7.Poli D., Carbognani P., Corradi M., Goldoni M., Acampa O., Balbi B., Bianchi L., Rusca M., Mutti A. Exhaled volatile organic compounds in patients with non-small cell lung cancer: cross sectional and nested short-term follow-up study. Respir. Res. 2005;6:71. doi: 10.1186/1465-9921-6-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Alkhouri N., Singh T., Alsabbagh E., Guirguis J., Chami T., Hanouneh I., Grove D., Lopez R., Dweik R. Isoprene in the exhaled breath is a novel biomarker for advanced fibrosis in patients with chronic liver disease: a pilot study. Clin. Transl. Gastroenterol. 2015;6:e112. doi: 10.1038/ctg.2015.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Salerno-Kennedy R., Cashman K.D. Potential applications of breath isoprene as a biomarker in modern medicine: a concise overview. Wien Klin. Wochenschr. 2005;117:180–186. doi: 10.1007/s00508-005-0336-9. [DOI] [PubMed] [Google Scholar]
- 10.King J., Kupferthaler A., Unterkofler K., Koc H., Teschl S., Teschl G., Miekisch W., Schubert J., Hinterhuber H., Amann A. Isoprene and acetone concentration profiles during exercise on an ergometer. J. Breath Res. 2009;3 doi: 10.1088/1752-7155/3/2/027006. [DOI] [PubMed] [Google Scholar]
- 11.King J., Kupferthaler A., Frauscher B., Hackner H., Unterkofler K., Teschl G., Hinterhuber H., Amann A., Högl B. Measurement of endogenous acetone and isoprene in exhaled breath during sleep. Physiol. Meas. 2012;33:413–428. doi: 10.1088/0967-3334/33/3/413. [DOI] [PubMed] [Google Scholar]
- 12.Sukul P., Trefz P., Kamysek S., Schubert J.K., Miekisch W. Instant effects of changing body positions on compositions of exhaled breath. J. Breath Res. 2015;9 doi: 10.1088/1752-7155/9/4/047105. [DOI] [PubMed] [Google Scholar]
- 13.Sukul P., Schubert J.K., Oertel P., Kamysek S., Taunk K., Trefz P., Miekisch W. FEV manoeuvre induced changes in breath VOC compositions: an unconventional view on lung function tests. Sci. Rep. 2016;6:28029. doi: 10.1038/srep28029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sukul P., Schubert J.K., Trefz P., Miekisch W. Natural menstrual rhythm and oral contraception diversely affect exhaled breath compositions. Sci. Rep. 2018;8:10838. doi: 10.1038/s41598-018-29221-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stone B.G., Besse T.J., Duane W.C., Evans C.D., DeMaster E.G. Effect of regulating cholesterol biosynthesis on breath isoprene excretion in men. Lipids. 1993;28:705–708. doi: 10.1007/BF02535990. [DOI] [PubMed] [Google Scholar]
- 16.Buszewski B., Kesy M., Ligor T., Amann A. Human exhaled air analytics: biomarkers of diseases. Biomed. Chromatogr. BMC. 2007;21:553–566. doi: 10.1002/bmc.835. [DOI] [PubMed] [Google Scholar]
- 17.Cerqueira N.M.F.S.A., Oliveira E.F., Gesto D.S., Santos-Martins D., Moreira C., Moorthy H.N., Ramos M.J., Fernandes P.A. Cholesterol biosynthesis: a mechanistic overview. Biochemistry. 2016;55:5483–5506. doi: 10.1021/acs.biochem.6b00342. [DOI] [PubMed] [Google Scholar]
- 18.Hoffmann G., Gibson K.M., Brandt I.K., Bader P.I., Wappner R.S., Sweetman L. Mevalonic aciduria — an inborn error of cholesterol and nonsterol isoprene biosynthesis. N. Engl. J. Med. 1986;314:1610–1614. doi: 10.1056/NEJM198606193142504. [DOI] [PubMed] [Google Scholar]
- 19.Haas D., Hoffmann G.F. 2009. Mevalonate Kinase Deficiency and Autoinflammatory Disorders. [DOI] [PubMed] [Google Scholar]
- 20.Neven B., Valayannopoulos V., Quartier P., Blanche S., Prieur A.-M., Debré M., Rolland M.-O., Rabier D., Cuisset L., Cavazzana-Calvo M., de Lonlay P., Fischer A. Allogeneic bone marrow transplantation in mevalonic aciduria. N. Engl. J. Med. 2007;356:2700–2703. doi: 10.1056/NEJMoa070715. [DOI] [PubMed] [Google Scholar]
- 21.King J., Mochalski P., Unterkofler K., Teschl G., Klieber M., Stein M., Amann A., Baumann M., isoprene Breath. Muscle dystrophy patients support the concept of a pool of isoprene in the periphery of the human body. Biochem. Biophys. Res. Commun. 2012;423:526–530. doi: 10.1016/j.bbrc.2012.05.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Trefz P., Obermeier J., Lehbrink R., Schubert J.K., Miekisch W., Fischer D.-C. Exhaled volatile substances in children suffering from type 1 diabetes mellitus: results from a cross-sectional study. Sci. Rep. 2019;9:15707. doi: 10.1038/s41598-019-52165-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Trefz P., Schmidt S.C., Sukul P., Schubert J.K., Miekisch W., Fischer D.-C. Non-invasive assessment of metabolic adaptation in paediatric patients suffering from type 1 diabetes mellitus. J. Clin. Med. 2019;8 doi: 10.3390/jcm8111797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kushch I., Arendacká B., Stolc S., Mochalski P., Filipiak W., Schwarz K., Schwentner L., Schmid A., Dzien A., Lechleitner M., Witkovský V., Miekisch W., Schubert J., Unterkofler K., Amann A. Breath isoprene--aspects of normal physiology related to age, gender and cholesterol profile as determined in a proton transfer reaction mass spectrometry study. Clin. Chem. Lab. Med. 2008;46:1011–1018. doi: 10.1515/CCLM.2008.181. [DOI] [PubMed] [Google Scholar]
- 25.Lirk P., Bodrogi F., Raifer H., Greiner K., Ulmer H., Rieder J. Elective haemodialysis increases exhaled isoprene. Nephrol. Dial. Transplant. 2003;18:937–941. doi: 10.1093/ndt/gfg049. [DOI] [PubMed] [Google Scholar]
- 26.T M.L., Robin P., Bernard S. Breath isoprene in patients with heart failure. Eur. J. Heart Fail. 2001;3:423–427. doi: 10.1016/s1388-9842(01)00128-3. [DOI] [PubMed] [Google Scholar]
- 27.King J., Koc H., Unterkofler K., Mochalski P., Kupferthaler A., Teschl G., Teschl S., Hinterhuber H., Amann A. Physiological modeling of isoprene dynamics in exhaled breath. J. Theor. Biol. 2010;267:626–637. doi: 10.1016/j.jtbi.2010.09.028. [DOI] [PubMed] [Google Scholar]
- 28.Turner C., Spanel P., Smith D. A longitudinal study of breath isoprene in healthy volunteers using selected ion flow tube mass spectrometry (SIFT-MS) Physiol. Meas. 2006;27:13–22. doi: 10.1088/0967-3334/27/1/002. [DOI] [PubMed] [Google Scholar]
- 29.Smith D., Španěl P., Enderby B., Lenney W., Turner C., Davies S.J. Isoprene levels in the exhaled breath of 200 healthy pupils within the age range 7–18 years studied using SIFT-MS. J. Breath Res. 2009;4 doi: 10.1088/1752-7155/4/1/017101. [DOI] [PubMed] [Google Scholar]
- 30.Kuzuyama T., Seto H. Diversity of the biosynthesis of the isoprene units. Nat. Prod. Rep. 2003;20:171–183. doi: 10.1039/b109860h. [DOI] [PubMed] [Google Scholar]
- 31.Deneris E.S., Stein R.A., Mead J.F. Invitro biosynthesis of isoprene from mevalonate utilizing a rat liver cytosolic fraction. Biochem. Biophys. Res. Commun. 1984;123:691–696. doi: 10.1016/0006-291x(84)90284-5. [DOI] [PubMed] [Google Scholar]
- 32.Metherall J.E., Waugh K., Li H. Progesterone inhibits cholesterol biosynthesis in cultured cells ACCUMULATION OF CHOLESTEROL PRECURSORS. J. Biol. Chem. 1996;271:2627–2633. doi: 10.1074/jbc.271.5.2627. [DOI] [PubMed] [Google Scholar]
- 33.Mumford S.L., Dasharathy S., Pollack A.Z., Schisterman E.F. Variations in lipid levels according to menstrual cycle phase: clinical implications. Clin. Lipidol. 2011;6:225–234. doi: 10.2217/clp.11.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sukul P., Oertel P., Kamysek S., Trefz P. Oral or nasal breathing? Real-time effects of switching sampling route onto exhaled VOC concentrations. J. Breath Res. 2017;11 doi: 10.1088/1752-7163/aa6368. [DOI] [PubMed] [Google Scholar]
- 35.Sukul P., Schubert J.K., Kamysek S., Trefz P., Miekisch W. Applied upper-airway resistance instantly affects breath components: a unique insight into pulmonary medicine. J. Breath Res. 2017;11 doi: 10.1088/1752-7163/aa8d86. [DOI] [PubMed] [Google Scholar]
- 36.Sukul P., Schubert J.K., Zanaty K., Trefz P., Sinha A., Kamysek S., Miekisch W. Exhaled breath compositions under varying respiratory rhythms reflects ventilatory variations: translating breathomics towards respiratory medicine. Sci. Rep. 2020;10:14109. doi: 10.1038/s41598-020-70993-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Trefz P., Schmidt M., Oertel P., Obermeier J., Brock B., Kamysek S., Dunkl J., Zimmermann R., Schubert J.K., Miekisch W. Continuous real time breath gas monitoring in the clinical environment by proton-transfer-reaction-time-of-flight-mass spectrometry. Anal. Chem. 2013;85:10321–10329. doi: 10.1021/ac402298v. [DOI] [PubMed] [Google Scholar]
- 38.Richter A., Sender S., Lenz A., Schwarz R., Hinz B., Knuebel G., Sekora A., Murua Escobar H., Junghanss C., Roolf C. Influence of Casein kinase II inhibitor CX-4945 on BCL6-mediated apoptotic signaling in B-ALL in vitro and in vivo. BMC Canc. 2020;20:184. doi: 10.1186/s12885-020-6650-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nelson N., Lagesson V., Nosratabadi A.R., Ludvigsson J., Tagesson C. Exhaled isoprene and acetone in newborn infants and in children with diabetes mellitus. Pediatr. Res. 1998;44:363–367. doi: 10.1203/00006450-199809000-00016. [DOI] [PubMed] [Google Scholar]
- 40.Sukul P., Trefz P., Schubert J.K., Miekisch W. Immediate effects of breath holding maneuvers onto composition of exhaled breath. J. Breath Res. 2014;8 doi: 10.1088/1752-7155/8/3/037102. [DOI] [PubMed] [Google Scholar]
- 41.Bartels Ä., O’Donoghue K. Cholesterol in pregnancy: a review of knowns and unknowns. Obstet. Med. 2011;4:147–151. doi: 10.1258/om.2011.110003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sharkey T.D. Isoprene synthesis by plants and animals. Endeavour. 1996;20:74–78. doi: 10.1016/0160-9327(96)10014-4. [DOI] [PubMed] [Google Scholar]
- 43.Sharkey T.D., Yeh S., Wiberley A.E., Falbel T.G., Gong D., Fernandez D.E. Evolution of the isoprene biosynthetic pathway in kudzu. Plant Physiol. 2005;137:700–712. doi: 10.1104/pp.104.054445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Harley P.C., Monson R.K., Lerdau M.T. Ecological and evolutionary aspects of isoprene emission from plants. Oecologia. 1999;118:109–123. doi: 10.1007/s004420050709. [DOI] [PubMed] [Google Scholar]
- 45.Melnick R.L., Sills R.C., Roycroft J.H., Chou B.J., Ragan H.A., Miller R.A. Inhalation toxicity and carcinogenicity of isoprene in rats and mice: comparisons with 1,3-butadiene. Toxicology. 1996;113:247–252. doi: 10.1016/0300-483x(96)03453-1. [DOI] [PubMed] [Google Scholar]
- 46.National Toxicology Program NTP toxicology and carcinogenesis studies of isoprene (CAS No. 78-79-5) in F344/N rats (inhalation studies) Natl. Toxicol. Progr. Tech. Rep. 1999;486:1–176. [PubMed] [Google Scholar]
- 47.Anderson D. Genetic and reproductive toxicity of butadiene and isoprene. Chem. Biol. Interact. 2001;135–136:65–80. doi: 10.1016/s0009-2797(01)00171-5. [DOI] [PubMed] [Google Scholar]
- 48.van der Burgh R., ter Haar N.M., Boes M.L., Frenkel J. Mevalonate kinase deficiency, a metabolic autoinflammatory disease. Clin. Immunol. 2013;147:197–206. doi: 10.1016/j.clim.2012.09.011. [DOI] [PubMed] [Google Scholar]
- 49.Zhang S. Natural history of mevalonate kinase deficiency: a literature review. Pediatr. Rheumatol. Online J. 2016;14 doi: 10.1186/s12969-016-0091-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data included in article/supplementary material/referenced in article.