Abstract
Microbial communities are catalysts that drive the operation of microbial fuel cells (MFCs). In this study, the use of a defined co-culture of Escherichia coli and Pseudomonas aeruginosa towards improved power generation in MFCs is described. The co-culture has been initially evaluated for substrate consumption, biofilm formation and microbial electron transfer activity. The co-culture gave an enhanced power density of 190.44 mW m−2, while E. coli and P. aeruginosa as pure cultures generated lesser power densities of 139.24 and 158.76 mW m−2 respectively. The photosynthetic alga Chlorella vulgaris was then inoculated in the cathode chamber. Co-cultures in the presence of C. vulgaris improved the mean power density from 175 mW m−2 to 248 mW m−2, a 41.7% rise. A synergistic effect was observed when the co-cultures were coupled with C. vulgaris. Combining co-cultures with photosynthetic MFCs offers a lot of promise in studying mechanisms and expanding the nature of applications.
Keywords: Chlorella vulgaris, Co-culture, Escherichia coli, Microbial fuel cell, Pseudomonas aeruginosa, Power generation
Chlorella vulgaris, Co-culture,Escherichia coli, Microbial fuel cell, Pseudomonas aeruginosa, Power generation.
1. Introduction
Microbial fuel cells (MFCs) are bioelectrochemical systems (BES) that use microbes as catalysts to oxidise organic matter for current generation [1]. In these systems, electrons are generated via microbial oxidation of the substrate provided as the fuel. The electrons are transferred to the anode via various mechanisms, pass through an external load and combine with a cathodic electron acceptor [2]. Electrochemically active bacteria constitute the most important component of MFCs, since the fundamental process of generating current depends on their oxidative capability. A wide range of environments host electroactive bacteria, including extreme ones [3]. Commonly used inocula in MFCs like wastewater, soil sediment and anaerobic sludge naturally contain electroactive bacteria, which can be enriched during MFC operation. Studies on the microbial community composition in MFCs have revealed several groups of microbes that are mutually dependent on each other to perform activities like breakdown of complex organic substrates [4, 5, 6]. In these cases, however, the complexity of the microbial community makes it difficult to understand their community dynamics and the roles that each species plays in substrate oxidation and electron transfer. It is difficult to steer a complex microbial community towards achieving specific biotechnological applications. Due to these considerations, the use of defined co-cultures has recently attracted interest from researchers [7]. A co-culture system contains two or more different types of microbial species. Co-cultures make it possible to study the interactions between microbial species that drive MFC operation, while at the same time providing scope for improving technological applications.
Photosynthetic microbial fuel cells hold great potential towards generating carbon-neutral bioenergy from wastewater via photosynthetic microorganisms. In photosynthetic MFCs, sunlight is converted into electricity via the mechanism of a MFC [8]. In addition to electricity generation, these systems provide advantages of removing carbon dioxide from atmosphere and treatment of wastes. The concept of photosynthetic MFCs has attracted interest from researchers as early as 1964 in the development of MFC technology [9]. MFCs containing photosynthetic bacteria in the cathode have been used to generate oxygen as the terminal electron acceptor. The algal species Chlorella vulgaris has particularly been of considerable interest in photosynthetic MFCs. C. vulgaris is a photoautolithotroph., and derives its energy from light and carbon fixation of inorganic carbon dioxide [10]. It is known to be present naturally in many wastewaters and can assimilate nitrogen and phosphorus from wastewater, making it an ideal contender for use in MFCs for bioremediation [10, 11, 12]. In addition, use of C. vulgaris in the cathode chamber can reduce operational costs since there is no need for external aeration.
In this manuscript, considering the above factors, a defined co-culture of Escherichia coli and Pseudomonas aeruginosa has been studied to evaluate their performance in MFCs. The co-culture has been studied for biofilm formation, substrate utilization and microbial electron transfer activity in order to evaluate its suitability to adapt to anodic conditions. The co-culture in the anode has then been integrated with C. vulgaris as the photosynthetic alga in the cathode chamber to enhance power generation. The performance of the co-culture has also been compared with pure cultures of P. aeruginosa and E. coli. The study describes the integration of defined co-cultures with a photosynthetic MFC in order to enhance the performance and increase the robustness of the system for important applications.
2. Experimental procedures
2.1. Growth curve and biofilm formation
E. coli (DH5α strain; ATCC reference 35218), P. aeruginosa (ATCC reference 27853) and a co-culture containing both the microorganisms (1:1 inoculum ratio) were used for experiments. The nutrient medium consisted of (per litre) CH3COONa, 1.00 g; NH4Cl, 0.31 g; NaH2PO4·H2O, 5.38 g; Na2HPO4, 8.66 g; KCl, 0.13 g, vitamin solution (12.5 ml) and trace mineral solution (12.5 ml) (pH 7) [4]. Inoculation of the nutrient medium was done with overnight cultures of E. coli and P. aeruginosa (1% v/v). Growth curves were recorded for both the microorganisms immediately after inoculation by measuring absorbance values at 600 nm at periodic intervals of 1 hour using a UV-VIS spectrophotometer (SpectraMax M5, Molecular Devices, USA). The mean generation time for E. coli and P. aeruginosa was calculated as per the formula mentioned below:
where k is the growth constant, N(t) is the bacterial population at time t, N (0) the bacterial population at time zero, and is the generation time [13]. Consumption of acetate (carbon source) in the nutrient medium as growth progressed was estimated for the microbial cultures. Acetate concentration was estimated at 450 nm using a colorimetric assay kit as per the manufacturer's instructions (Sigma-Aldrich). Biofilm formation was determined using the microtiter dish biofilm formation assay as follows [14]. The bacterial cultures were diluted 1:100 times and 100 μl of the diluted cultures were each pipetted into 96 well flat-bottom microtiter plate. The microtiter plate was incubated at 37 °C for 6 h. After incubation, the supernatants were discarded. The walls of the microtiter plate were washed with phosphate buffered saline (PBS). To stain the biofilms, 200 μl of 0.1% crystal violet was added in each of the wells. After staining with crystal violet for a period of 30 min, the solution was discarded and the wells were washed with PBS. The stained crystal violet was solubilised with 200μl of 30% acetic acid and incubated for 30 min at room temperature. This solution was then transferred into a fresh microtiter plate and biofilm formation was estimated by measuring absorbance at 595 nm using a UV-VIS spectrophotometer (SpectraMax M5, Molecular Devices, USA).
2.2. Dye reduction-based electron-transfer activity monitoring (DREAM) assay
Pure cultures of E. coli and P. aeruginosa and the co-culture were subjected to the DREAM assay to estimate microbial electron transfer activity. The assay was performed as per Aiyer et al. [15] as follows. Two ml of the microbial culture was taken in a cuvette, to which methylene blue (0.2 μl; final concentration of 50 mg ml−1) was added. Immediately after methylene blue addition, absorbance was recorded at 660 nm at 10 s intervals for a period of 1 min using a UV-VIS spectrophotometer (SpectraMax M5, Molecular Devices, USA). The cuvette was closed using a lid while estimating absorbance in order to minimise oxygen interference.
2.3. Construction of the microbial fuel cell (MFC)
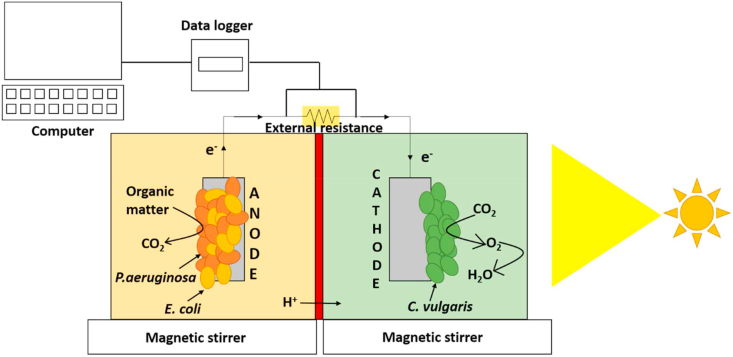
Two-chambered microbial fuel cells were constructed from glass bottles. The anode and cathode chambers had working volumes of 150 ml each. Carbon cloth was used as the electrode across both chambers. The anode and cathode had projected surface areas of 1 cm2 and 6 cm2 respectively. Nafion 117 was used as the proton exchange membrane (PEM) and had a total surface area of 7 cm2. Prior to using the PEM in the MFC, the membrane was pre-treated with 3% H2O2 solution for 1 hour at 60 °C, followed by immersion in 0.5M H2SO4 for 1 hour at 60 °C. The electrodes were connected by titanium wires. A data acquisition unit (LabJack U12, USA) connected to a computer was used for recording data from the MFCs.
2.4. Inoculation and operation of the MFC
E. coli, P. aeruginosa and a co-culture of both these microorganisms were inoculated separately in the anode chambers of different MFCs. Initially, the catholyte consisted of tap water sparged continuously with oxygen. The anode and the cathode chambers were stirred continuously using magnetic stirrers. After inoculation, the MFCs were operated in open circuit mode for a period of 90 min. After a stable OCV was obtained, polarization and power density curves were recorded for the MFCs using a range of resistors (100Ω - 3.3kΩ). Current (I) was calculated as per Ohm's Law (I = V/R). Current density was calculated by dividing the current generated by the projected surface area of the anode electrode. Power (P) was calculated as the product of cell voltage and current, while power density was calculated by dividing the power generated by the projected surface area of the anode. Internal resistance was computed from the slope of the polarization curves. The MFC was operated at the resistance that gave the maximum power density.
C. vulgaris (ATCC reference 30821) was introduced in the cathode chamber of the MFC containing the co-culture on the 11th day of operation. Prior to inoculation in MFCs, C. vulgaris was grown in 2-litre flasks with a nutrient medium adapted as per Gouveia et al. [16] containing (per litre) KNO3, 1.250g; KH2PO4, 1.250g; MgSO4.7H2O, 1.000g; CaCl2, 0.084g; H3BO3, 0.111g; FeSO4, 0.050g; ZnSO4, 0.088g; MnCl2, 0.014g; MOO3, 0.007g; CuSO4, 0.016g; Co(NO3)2.6H2O, 0.005g; Fe-EDTA, 0.5g. When an absorbance of 0.6 was reached for the algal culture at 540 nm, the algal biomass was harvested by centrifugation (10000 rpm, 20 min, 4 °C) [16, 17]. The algal biomass was inoculated in the cathode chambers so as to obtain 2 g l−1 of biomass dry weight in the cathode compartment. The MFCs were run in fed batch mode with addition of fresh anolyte and catholyte solutions periodically. The cathode was exposed to sunlight, and voltage was recorded continuously for day-night cycles using the data logging system. Dissolved oxygen (DO) levels in the catholyte were measured with a dissolved oxygen probe (Hach HQ40d). All MFCs were operated at room temperature (30 ± 3 °C). Each experiment was performed in duplicate and the mean values have been reported. Figure 1 depicts a schematic diagram of the MFC setup used.
Figure 1.
Schematic representation of the MFC.
3. Results
3.1. Growth and biofilm formation
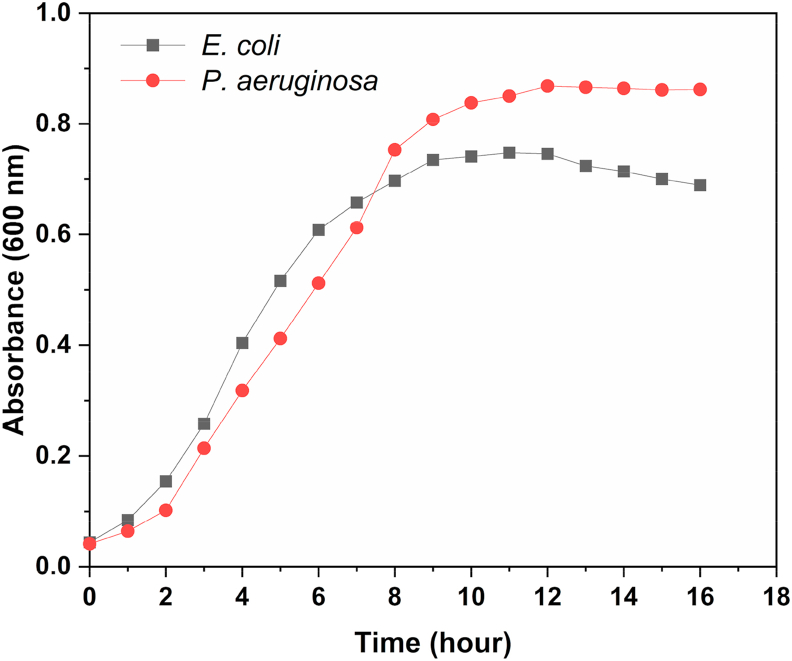
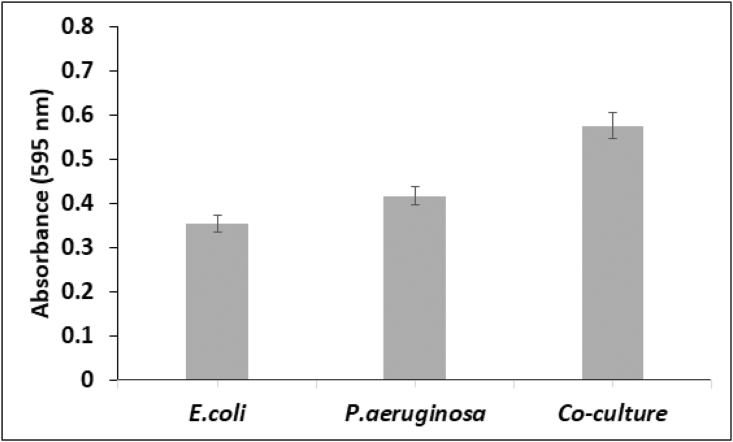
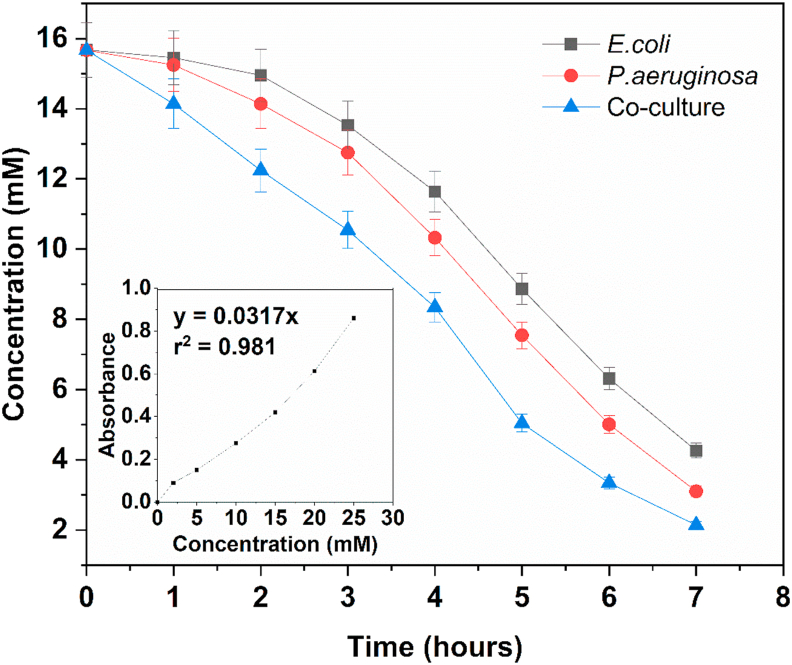
Initial experiments were aimed at studying the growth characteristics of the microbial cultures. Figure 2 depicts the growth curves of E. coli and P. aeruginosa. The cultures attained exponential phase after a brief lag phase. Onset of stationary phase was observed 8 h after inoculation. The mean generation time of E. coli and P. aeruginosa was estimated to be 24.18 min and 27.92 min respectively. Biofilm formation was determined in the wells of a microtiter plate based on staining the bacterial cells of the biofilm with crystal violet. Absorbance values were recorded at 595 nm (Figure 3). Biofilm formation was also determined for the co-culture of E. coli and P. aeruginosa. The extent of biofilm formation was highest for the co-culture, whereas E. coli gave the smallest biofilm mass. Figure 4 demonstrates the consumption of acetate provided as the carbon source. The rate of consumption of acetate was maximum for the co-culture, indicating that it was effective at metabolising the substrates provided. Consumption of acetate increased as the cultures progressed from lag phase to exponential stage, where the bacterial cells multiply by geometric progression. Onset of the stationary phase coincided with reduced levels of acetate in the medium. In the acetate standard curve, a linear relationship was observed between absorbance at 450 nm and concentration of acetate (inset, Figure 4).
Figure 2.
Growth curves of E. coli and P. aeruginosa.
Figure 3.
Biofilm formation observed for E. coli, P. aeruginosa and a co-culture of both the microbes.
Figure 4.
Consumption of acetate over time for E. coli, P. aeruginosa, and the co-culture. Inset: Standard curve of acetate demonstrating linear relation between absorbance and acetate concentration.
3.2. DREAM assay to estimate microbial electron transfer activity
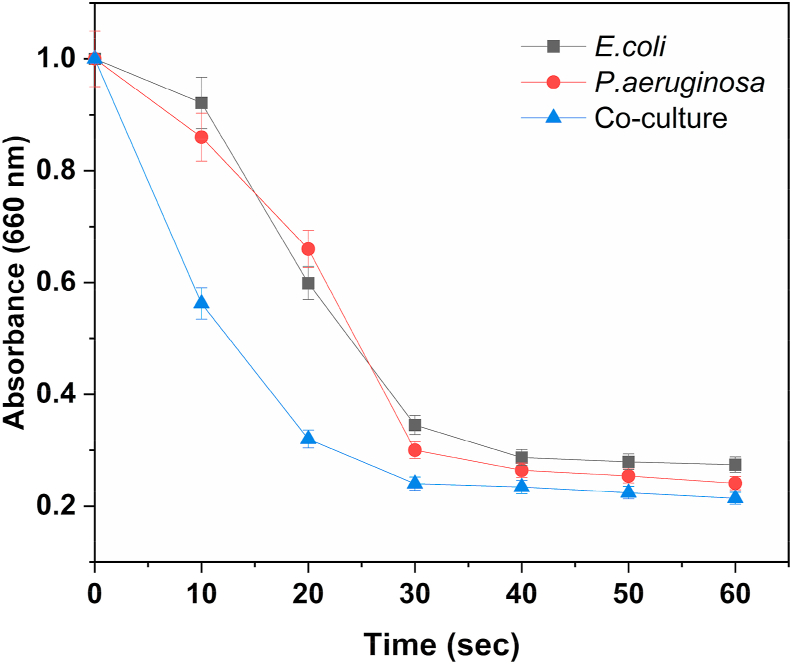
The DREAM assay provides an estimate of the microbial electron transfer activity of a bacterial sample. It is based on reduction of the redox dye methylene blue to its colourless analog. Reduction of the dye is measured at an absorbance of 660 nm. The results of DREAM assay performed on the cultures are presented in Figure 5. The co-culture demonstrated maximum electron transfer activity, as can be seen by the sharp decrease in A660 values after addition of methylene blue. E. coli and P. aeruginosa reduced methylene blue to a lesser extent. Nutrients provided as substrates to the bacteria are oxidised, leading to generation of electrons. These electrons are transferred to methylene blue which acts as the terminal electron acceptor, reducing it to a colourless state. The extent of dye reduction is thus directly dependent on microbial metabolic activity. The cuvette was kept closed with a cap during the assay to eliminate interference from oxygen. The DREAM assay is a rapid, cost-effective and simple way to determine microbial electron transfer activity of bacterial samples. It can be used as a suitable alternative to expensive electrochemical methods. This assay has previously been used to determine microbial electron transfer activities of samples used as inocula in microbial fuel cells [15, 18]. It has also been employed for estimating antibiotic resistance among different bacteria [19].
Figure 5.
Reduction profiles of E. coli, P. aeruginosa and the co-culture obtained with the DREAM assay.
3.3. Power generation in the MFC
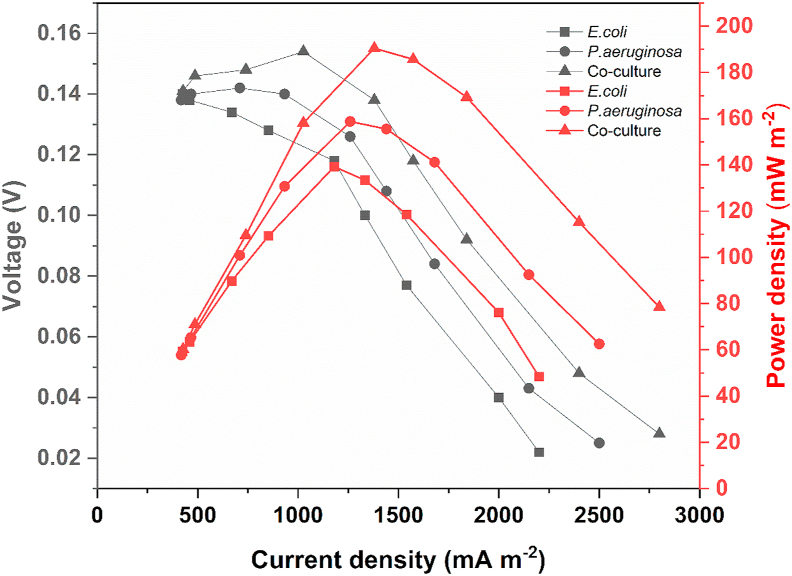
Polarization (grey) and power density curves (red) are depicted in Figure 6. Performance of the co-culture was significantly better as compared to E. coli and P. aeruginosa in terms of power output. The co-culture generated a maximum power density of 190.44 mW m−2, as against power densities of 139.24 mW m−2 and 158.76 mW m−2 obtained with E. coli and P. aeruginosa cultures respectively. Maximum power densities for all the three cultures was obtained at an external resistance of 1000 Ω. When the MFC was operated with sterile medium in the anode chamber, no generation of power was observed. The polarization curves indicate the differences in electrochemical losses for each of the three cultures used as anolytes. Lowered losses were observed for the co-culture as compared to E. coli and P. aeruginosa. A sharp drop in current density values in the region of ohmic polarization indicate that ohmic losses were predominant for all cultures.
Figure 6.
Polarization and power density curves recorded for E. coli, P. aeruginosa and the co-culture. Polarization curves are marked in grey, while power density curves are shown in red.
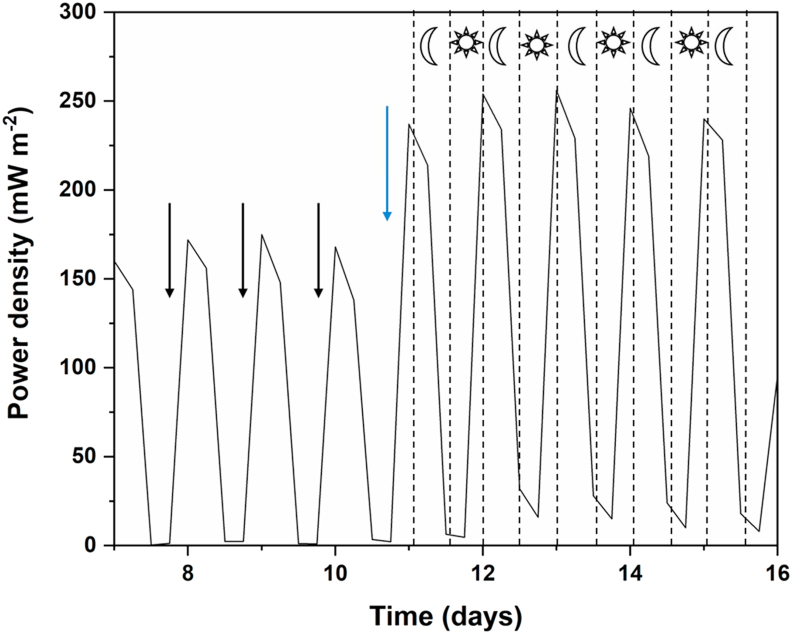
Since the best performance was observed with the use of co-culture, further experiments were done with the co-culture. The MFC was operated at the external resistance of 1000 Ω since it produced the maximum power output. After successive cycles of enrichment with the nutrient medium, C. vulgaris was introduced in the cathode chamber on day 11 of MFC operation (Figure 7). The results clearly indicate that C. vulgaris boosted the performance of MFCs in conjunction with the co-culture at the anode. Introduction of C. vulgaris in the MFC increased the average power output from 175 mW m−2 to 248 mW m−2, an increase of 41.7%. The cathode chamber was exposed to natural sunlight when C. vulgaris was introduced. As a result, the power density produced was in consonance with the natural day-night cycles. Power generation increased during the day, when photosynthetic activity of C. vulgaris peaked and decreased during night time due to cessation of photosynthesis. This result is in agreement with several other studies using C. vulgaris in MFCs that report the dependence of voltage on light [20, 21, 22]. Oxidants are required continuously in the cathode chamber for operation of the MFC. During daytime, photosynthetic activity from C. vulgaris ensured generation of oxygen, with the generated voltage being proportional to the algal oxygen-generating ability. During night, respiratory activity exhibited by the alga consumed oxygen and reduced current generation. Dissolved oxygen levels in the catholyte varied according to the diurnal cycles. Higher DO levels were observed during the day (0.84 mg l−1) due to photosynthetic release, while lower DO levels at night (0.25 mg l−1) resulted due to respiratory consumption. The oxygen released due to photosynthetic activity of C. vulgaris served as the electron acceptor in cathode. Similar results with C. vulgaris have been reported previously when it was used in the cathode chamber [22, 23]. A synergistic effect on power generation was thus observed when the co-culture in the anode chamber was combined with C. vulgaris in the cathode chamber. A lowering of internal resistance was observed with C. vulgaris, while current density and power density increased. Table 1 provides information on the electrochemical parameters observed before and after the introduction of C. vulgaris in the MFC.
Figure 7.
Power density obtained for the co-culture over time. Initially, the cathode compartment did not contain C. vulgaris (till day 10). The black arrows indicate medium replenishments. C. vulgaris was introduced in the cathode chamber of the MFC on day 11 (blue arrow). The sun and moon symbols represent power generation of the MFC during daytime and night time respectively.
Table 1.
Electrochemical parameters obtained during operation of the co-culture MFC with and without C. vulgaris.
| Electrochemical parameter | Without C. vulgaris |
With C. vulgaris |
|---|---|---|
| Pmax (mW m−2) | 190.44 | 256.04 |
| Imaz (mA m−2) | 1380 | 1600 |
| Vmax (V) | 0.138 | 0.160 |
| Rint (kΩ) | 2.1 | 1.6 |
| OCV (V) | 0.430 | 0.444 |
Pmax: Maximum power density; Imax: Maximum current density; Vmax: Maximum voltage; Rint: Internal resistance; OCV: Open circuit voltage.
4. Discussion
Since bacteria are the catalysts driving operation of MFCs, it is crucial to take into account the bacterial community that is being used. Several bacterial species have been used either as pure cultures or mixed cultures for power generation in MFCs. While pure cultures of electricigens help to facilitate understanding of electron transfer mechanisms, there are several limitations associated with their use. Pure cultures require a strict control of operating parameters, and need a defined nutrient medium for optimal growth [7]. It is also difficult to use pure cultures in scaling-up studies. The use of mixed cultures overcomes some of these problems due to their inherent resilience and flexibility. A mixed culture community has been defined as a “multi-species assemblage, in which organisms live together in a contiguous environment and interact with each other.” [24] Mixed cultures can produce power densities similar to even greater than pure cultures of electricigens [25, 26]. This can perhaps be attributed to synergistic interactions between different community members which results in better adaptation of the consortium to stress conditions. Also, a mixed culture is better adapted to utilize complex substrates like wastewater that a pure culture may not be able to do so. Syntrophic microbial species are known to exist in mixed cultures [27]. These species may not be actively involved in electricity generation but are definitely known to assist electrogenic bacteria in degrading substrates. However, contrasting reports of higher power densities by Shewanella and Geobacter pure cultures as compared to mixed cultures have also been reported [28, 29]. Despite these advantages a mixed culture provides, however, there are certain drawbacks observed with their use. Mixed culture communities are extremely complex in their makeup, making it difficult for researchers to understand their electrochemical behaviour and mechanisms of electron transport. While mixed bacterial communities that develop in MFCs have been characterized to some extent, it is not entirely possible to identify every organism that contributes to current generation [7]. Also, it is difficult to predict the evolution of community ecology of a mixed culture in response to changes in operating conditions. Often, this results in several species of non-electrogenic organisms that have a detrimental effect on performance of the MFC. The use of defined co-cultures provides an excellent alternative that combines the advantages of mixed and pure cultures in the operation of MFCs. Co-cultures provide opportunities to study the interactions occurring between different microorganisms. They also expand the range of nutrients that can be metabolized in MFCs.
P. aeruginosa serves an excellent candidate to design binary co-cultures. P. aeruginosa is a gram-negative, facultative anaerobe, and is known to secrete several electron mediators that aid in the transfer of electrons to the anode [30]. Among these are phenazine-based metabolites like pyocyanin, phenazine-1-carboxylic acid and phenazine-1-carboxamide that boost current generation [31, 32]. A co-culture of P. aeruginosa and E. coli can produce higher power in a MFC than individual pure cultures due to synergistic effects. In MFCs, the syntrophic relation between E. coli and P. aeruginosa could be characterised as an “obligately mutualistic metabolism”, where the producer is critically dependent on the consumer [33]. The two microbes differ in their metabolic pathways, with E. coli predominantly using glucose as its preferred carbon source. However, in certain cases, utilisation of glucose becomes energetically expensive and E. coli shifts to anaerobic mode of metabolism, a phenomenon called as “overflow metabolism”, where it secretes acetate in the medium [34]. Since E. coli grows at a faster rate than P. aeruginosa, it is likely that the metabolites secreted by E. coli are utilised as substrates by P. aeruginosa. The importance of such a mechanism for current generation was demonstrated in P. aeruginosa, which emphasised the importance of quorum sensing in enhancing performance of MFCs [35]. An advantage of using E. coli is that it can remove traces of oxygen from the anode chamber, thus maintaining anaerobic conditions effectively in the anode for stable operation. This would also minimise the power required for sparging the anode with N2 gas. Previous studies have demonstrated the syntrophic relation of P. aeruginosa in co-cultures to be based on metabolite-based mutualism [36, 37]. The combined respiration of E. coli and P. aeruginosa in the co-culture resulted in improved biofilm formation and substrate utilization (Figures 3 and 4). In MFCs, this association is beneficial in adapting to anaerobic metabolism in order to use the anode as the terminal electron acceptor.
When C. vulgaris was introduced in the MFC as the photosynthetic organism, sunlight acted as its main energy source and the nutrient medium provided acted as the source of electrons. The net Gibbs free energy of the photo-electrochemical reaction is negative, while the electromotive force is positive, implying that electricity generation is feasible [38]. Table 2 compares the dissolved oxygen and power density generated by photosynthetic MFCs using C. vulgaris as the photosynthetic alga. Though the range of values is quite large due to differences in design and operational parameters, a common trend that emerged is that higher levels of dissolved oxygen are obtained during the day, which leads to a peak in power production. One such study compared power generation with and without the presence of C. vulgaris in the cathode [10]. Power produced in the presence of C. vulgaris was nearly double (34.2 ± 10.0 mW m−2) compared to that generated without the alga (15.6 ± 9.7 mW m−2). The increased power generation reported in this study is due to the synergy between co-cultures and C. vulgaris.
Table 2.
Comparison of power densities and dissolved oxygen obtained with photosynthetic MFCs using C. vulgaris.
| Type of MFC | Microbes used |
Power Density (mW/m2) | Dissolved oxygen (mg/l) |
Reference | ||
|---|---|---|---|---|---|---|
| Anode | Cathode | Day | Night | |||
| Two chambered | Co-culture (P. aeruginosa + E. coli) | C. vulgaris | 248 with C. vulgaris; 190 without C. vulgaris | 0.84 | 0.25 | This study |
| Single chambered | Mixed photosynthetic consortium | 72.96 | 0.45 | 0.11 | [38] | |
| Two chambered | Mixed consortium | C. vulgaris | 62.7 | --- | --- | [16] |
| Two chambered | Anaerobic consortium | C. vulgaris | 327 | --- | --- | [41] |
| Two chambered | Mixed consortium | C. vulgaris | 14.40 | 12 | 2 | [42] |
| Two chambered | Mixed consortium | C. vulgaris | 34.2 with C. vulgaris; 15.6 without C. vulgaris |
86.34 | 5.75 | [10] |
| Two chambered | Anaerobic consortium | C. vulgaris | 187 | 4.5 | 1.2 | [43] |
A close relation exists between the dissolved oxygen levels and current generation in a photosynthetic MFC. Higher levels of oxygen due to photosynthesis during the day leads to a rise in current output. It has been established that oxygen concentration in the presence of alga is significantly higher as compared to that obtained by bubbling oxygen without alga [10]. With time, the dissolved oxygen levels in the catholyte improve as the algae acclimatise to the operating conditions. As oxygen concentration increases due to photosynthesis, more oxygen diffuses onto the cathode surface, enhancing the reduction reaction and ultimately leading to a lowering of electrochemical losses. Formation of an algal biofilm on the cathode has also been reported to reduce ohmic and charge transfer resistance losses in MFCs by acting as a direct mediator to transfer electrons [39]. Though algal biofilm formation was not estimated in this study, this could be the reason for reduced losses observed with C. vulgaris (Table 1).
It must also be noted that dissolved oxygen is affected by temperature. In this study, the MFCs were operated at a temperature of around 30 °C, which is close to the optimum temperature. Juang et al [40] reported that when temperature is increased from to 27 °C–31.2 °C, the dissolved oxygen concentration in the cathode chamber concomitantly increased from 3.7 to 6.8 mg l−1.
Light intensities too play a crucial role in performance of the system. Light has no effect on the internal resistance of the system, and can be regulated accordingly to optimise the system for power generation [39]. An appropriate light intensity, whether natural or artificial, promotes photosynthetic activity and enhances oxygen availability for reduction at the cathode [44]. Bazdar et al. [44] have stated that light can influence performance of photosynthetic MFCs in four ways, viz. 1) a lag phase where increasing light intensity does not improve performance; 2) a light limiting phase where increasing light intensity improves performance, 3) a light saturating phase where increasing light intensity does not improve performance, and 4) a light inhibition phase, where increasing light intensity decreases performance. For C. vulgaris, a light intensity of 500–6500 lux has been reported to be in optimum range [44, 45].
The advantages photosynthetic MFCs provide include treatment of biodegradable wastes by bacteria in the anode along with carbon dioxide, phosphorus and nitrogen removal (via fixation) in the cathode. In order to boost efficiency, organic matter from the anode compartment could be transferred to the cathode compartment to serve as nutrients for the alga. This scenario also ensures that the organic wastes are subjected to anaerobic and aerobic conditions, and could improve treatment efficiency. The algal biomass in the cathode compartment needs to be carefully monitored by optimising light and nutrient levels. Using the alga in the cathode chamber not only ensures oxygen supply for completing the reduction reaction, but also produces biomass that could be used as feed to the microbes in the anode chamber. Under controlled stress conditions, carotenoids can be secreted by C. vulgaris [16]. These products are high-value compounds with antioxidant properties, and have potential applications in medicine and pharmaceuticals. The algal biomass and its extracts could also be used for generation of biofuel and biogas through anaerobic digestion [46].
5. Conclusions
A co-culture of E. coli and P. aeruginosa utilised substrates efficiently and demonstrated an improved electron transfer activity as compared to the pure cultures. The co-culture also produced significantly better current output. After introduction of C. vulgaris in the cathode, the power output of the MFC improved by 41.7%. The power output in the presence of C. vulgaris followed a diurnal pattern. Operating the MFC in fed-batch mode was beneficial in terms of power output. An integrated bioelectrochemical system with defined co-cultures and acclimatised photosynthetic organisms could be the next logical undertaking towards bettering performance.
Declarations
Author contribution statement
Kartik S. Aiyer: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Funding statement
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Data availability statement
Data included in article/supp. material/referenced in article
Declaration of interests statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
Acknowledgements
The author is grateful to the Central Research Instruments Facility of the Sri Sathya Sai Institute of Higher Learning for providing research facilities. The author dedicates this work to Bhagawan Sri Sathya Sai Baba, Founder Chancellor of the Sri Sathya Sai Institute of Higher Learning.
References
- 1.Slate A.J., Whitehead K.A., Brownson D.A.C., Banks C.E. Microbial fuel cells: an overview of current technology. Renew. Sustain. Energy Rev. 2019;101:60–81. [Google Scholar]
- 2.Patil S.A., Hägerhäll C., Gorton L. Advances in Chemical Bioanalysis. Springer International Publishing; 2012. Electron transfer mechanisms between microorganisms and electrodes in bioelectrochemical systems; pp. 71–129. [Google Scholar]
- 3.Chabert N., Amin Ali O., Achouak W. All ecosystems potentially host electrogenic bacteria. Bioelectrochemistry. 2015;106:88–96. doi: 10.1016/j.bioelechem.2015.07.004. [DOI] [PubMed] [Google Scholar]
- 4.Beecroft N.J., Zhao F., Varcoe J.R., Slade R.C.T., Thumser A.E., Avignone-Rossa C. Dynamic changes in the microbial community composition in microbial fuel cells fed with sucrose. Appl. Microbiol. Biotechnol. 2012;93:423–437. doi: 10.1007/s00253-011-3590-y. [DOI] [PubMed] [Google Scholar]
- 5.Pepè Sciarria T., Arioli S., Gargari G., Mora D., Adani F. Monitoring microbial communities’ dynamics during the start-up of microbial fuel cells by high-throughput screening techniques. Biotechnol. Reports. 2019;21 doi: 10.1016/j.btre.2019.e00310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stratford J.P., Beecroft N.J., Slade R.C.T., Grüning A., Avignone-Rossa C. Anodic microbial community diversity as a predictor of the power output of microbial fuel cells. Bioresour. Technol. 2014;156:84–91. doi: 10.1016/j.biortech.2014.01.041. [DOI] [PubMed] [Google Scholar]
- 7.Cao Y., Mu H., Liu W., Zhang R., Guo J., Xian M., Liu H. Electricigens in the anode of microbial fuel cells: pure cultures versus mixed communities. Microb. Cell Factories. 2019;18:39. doi: 10.1186/s12934-019-1087-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rosenbaum M., He Z., Angenent L.T. Light energy to bioelectricity: photosynthetic microbial fuel cells. Curr. Opin. Biotechnol. 2010;21:259–264. doi: 10.1016/j.copbio.2010.03.010. [DOI] [PubMed] [Google Scholar]
- 9.Berk R.S., Canfield J.H. Bioelectrochemical energy conversion. Appl. Microbiol. 1964;12:10–12. doi: 10.1128/am.12.1.10-12.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Commault A.S., Laczka O., Siboni N., Tamburic B., Crosswell J.R., Seymour J.R., Ralph P.J. Electricity and biomass production in a bacteria- Chlorella based microbial fuel cell treating wastewater. J. Power Sources. 2017;356:299–309. [Google Scholar]
- 11.Palmer C.M. A composite rating of algae tolerating organic pollution. J. Phycol. 1969;5:78–82. doi: 10.1111/j.1529-8817.1969.tb02581.x. [DOI] [PubMed] [Google Scholar]
- 12.González L.E., Cañizares R.O., Baena S. Efficiency of ammonia and phosphorus removal from a colombian agroindustrial wastewater by the microalgae Chlorella vulgaris and Scenedesmus dimorphus. Bioresour. Technol. 1997;60:259–262. [Google Scholar]
- 13.Maier R.M., Pepper I.L. Environmental Microbiology. Elsevier; 2015. Bacterial growth; pp. 37–56. [Google Scholar]
- 14.Aiyer K.S., Vijayakumar B.S. An improvised microtiter dish biofilm assay for non-invasive biofilm detection on microbial fuel cell anodes and studying biofilm growth conditions. Braz. J. Microbiol. 2019;50:769–775. doi: 10.1007/s42770-019-00091-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Aiyer K.S., Rai R., Vijayakumar B.S. Dye reduction-based electron-transfer activity monitoring assay for assessing microbial electron transfer activity of microbial fuel cell inocula. J. Environ. Sci. 2020;96:171–177. doi: 10.1016/j.jes.2020.04.037. [DOI] [PubMed] [Google Scholar]
- 16.Gouveia L., Neves C., Sebastião D., Nobre B.P., Matos C.T. Effect of light on the production of bioelectricity and added-value microalgae biomass in a Photosynthetic Alga Microbial Fuel Cell. Bioresour. Technol. 2014;154:171–177. doi: 10.1016/j.biortech.2013.12.049. [DOI] [PubMed] [Google Scholar]
- 17.Lakshmidevi R., Gandhi N.N., Muthukumar K. Carbon neutral electricity production from municipal solid waste landfill leachate using algal-assisted microbial fuel cell. Appl. Biochem. Biotechnol. 2020;191:852–866. doi: 10.1007/s12010-019-03160-5. [DOI] [PubMed] [Google Scholar]
- 18.Aiyer K.S., Vijayakumar B.S. Screening sediment samples used as anolytes in microbial fuel cells for microbial electron transfer activity using DREAM assay. Biotechnol. Lett. 2019;41:979–985. doi: 10.1007/s10529-019-02704-3. [DOI] [PubMed] [Google Scholar]
- 19.Aiyer K.S., Rai R., Vijayakumar B.S. Assessing activity of antimicrobial agents and screening antibiotic-resistant bacteria through DREAM assay. Appl. Biochem. Biotechnol. 2019;188:1158–1167. doi: 10.1007/s12010-019-02981-8. [DOI] [PubMed] [Google Scholar]
- 20.Xiao L., Young E.B., Berges J.A., He Z. Integrated photo-bioelectrochemical system for contaminants removal and bioenergy production. Environ. Sci. Technol. 2012;46:11459–11466. doi: 10.1021/es303144n. [DOI] [PubMed] [Google Scholar]
- 21.Liu L., Choi S. Self-sustaining, solar-driven bioelectricity generation in micro-sized microbial fuel cell using co-culture of heterotrophic and photosynthetic bacteria. J. Power Sources. 2017;348:138–144. [Google Scholar]
- 22.Wu X., Song T., Zhu X., Wei P., Zhou C.C. Construction and operation of microbial fuel cell with Chlorella vulgaris biocathode for electricity generation. Appl. Biochem. Biotechnol. 2013;171:2082–2092. doi: 10.1007/s12010-013-0476-8. [DOI] [PubMed] [Google Scholar]
- 23.Wang X., Feng Y., Liu J., Lee H., Li C., Li N., Ren N. Sequestration of CO2 discharged from anode by algal cathode in microbial carbon capture cells (MCCs) Biosens. Bioelectron. 2010;25:2639–2643. doi: 10.1016/j.bios.2010.04.036. [DOI] [PubMed] [Google Scholar]
- 24.Konopka A. What is microbial community ecology? ISME J. 2009;3:1223–1230. doi: 10.1038/ismej.2009.88. [DOI] [PubMed] [Google Scholar]
- 25.Engel C.E.A., Schattenberg F., Dohnt K., Schröder U., Müller S., Krull R. Long-term behavior of defined mixed cultures of Geobacter sulfurreducens and Shewanella oneidensis in bioelectrochemical systems. Front. Bioeng. Biotechnol. 2019;7:60. doi: 10.3389/fbioe.2019.00060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sydow A., Krieg T., Mayer F., Schrader J., Holtmann D. Electroactive bacteria—molecular mechanisms and genetic tools. Appl. Microbiol. Biotechnol. 2014;98:8481–8495. doi: 10.1007/s00253-014-6005-z. [DOI] [PubMed] [Google Scholar]
- 27.Chae K.-J., Choi M.-J., Lee J.-W., Kim K.-Y., Kim I.S. Effect of different substrates on the performance, bacterial diversity, and bacterial viability in microbial fuel cells. Bioresour. Technol. 2009;100:3518–3525. doi: 10.1016/j.biortech.2009.02.065. [DOI] [PubMed] [Google Scholar]
- 28.Nevin K.P., Richter H., Covalla S.F., Johnson J.P., Woodard T.L., Orloff a L., Jia H., Zhang M., Lovley D.R. Power output and columbic efficiencies from biofilms of Geobacter sulfurreducens comparable to mixed community microbial fuel cells. Environ. Microbiol. 2008;10:2505–2514. doi: 10.1111/j.1462-2920.2008.01675.x. [DOI] [PubMed] [Google Scholar]
- 29.Nimje V.R., Chen C.-Y., Chen H.-R., Chen C.-C., Huang Y.M., Tseng M.-J., Cheng K.-C., Chang Y.-F. Comparative bioelectricity production from various wastewaters in microbial fuel cells using mixed cultures and a pure strain of Shewanella oneidensis. Bioresour. Technol. 2012;104:315–323. doi: 10.1016/j.biortech.2011.09.129. [DOI] [PubMed] [Google Scholar]
- 30.Schmitz S., Rosenbaum M.A. Boosting mediated electron transfer in bioelectrochemical systems with tailored defined microbial cocultures. Biotechnol. Bioeng. 2018;115:2183–2193. doi: 10.1002/bit.26732. [DOI] [PubMed] [Google Scholar]
- 31.Venkataraman A., Rosenbaum M.A., Perkins S.D., Werner J.J., Angenent L.T. Metabolite-based mutualism between Pseudomonas aeruginosa PA14 and Enterobacter aerogenes enhances current generation in bioelectrochemical systems. Energy Environ. Sci. 2011;4:4550. [Google Scholar]
- 32.Pham T.H., Boon N., De Maeyer K., Höfte M., Rabaey K., Verstraete W. Use of Pseudomonas species producing phenazine-based metabolites in the anodes of microbial fuel cells to improve electricity generation. Appl. Microbiol. Biotechnol. 2008;80:985–993. doi: 10.1007/s00253-008-1619-7. [DOI] [PubMed] [Google Scholar]
- 33.Dolfing J. Syntrophy in microbial fuel cells. ISME J. 2014;8:4–5. doi: 10.1038/ismej.2013.198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pinhal S., Ropers D., Geiselmann J., De Jong H. Acetate metabolism and the inhibition of bacterial growth by acetate. J. Bacteriol. 2019;201:1–19. doi: 10.1128/JB.00147-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Venkataraman A., Rosenbaum M., Arends J.B.A., Halitschke R., Angenent L.T. Quorum sensing regulates electric current generation of Pseudomonas aeruginosa PA14 in bioelectrochemical systems. Electrochem. Commun. 2010;12:459–462. [Google Scholar]
- 36.Qiao Y.J., Qiao Y., Zou L., Wu X.S., Liu J.H. Biofilm promoted current generation of Pseudomonas aeruginosa microbial fuel cell via improving the interfacial redox reaction of phenazines. Bioelectrochemistry. 2017;117:34–39. doi: 10.1016/j.bioelechem.2017.04.003. [DOI] [PubMed] [Google Scholar]
- 37.Islam M.A., Ethiraj B., Cheng C.K., Yousuf A., Khan M.M.R. An insight of synergy between Pseudomonas aeruginosa and Klebsiella variicola in a microbial fuel cell. ACS Sustain. Chem. Eng. 2018;6:4130–4137. [Google Scholar]
- 38.Chandra R., Venkata Subhash G., Venkata Mohan S. Mixotrophic operation of photo-bioelectrocatalytic fuel cell under anoxygenic microenvironment enhances the light dependent bioelectrogenic activity. Bioresour. Technol. 2012;109:46–56. doi: 10.1016/j.biortech.2011.12.135. [DOI] [PubMed] [Google Scholar]
- 39.Saba B., Christy A.D., Yu Z., Co A.C. Sustainable power generation from bacterio-algal microbial fuel cells (MFCs): an overview. Renew. Sustain. Energy Rev. 2017;73:75–84. [Google Scholar]
- 40.Juang D.F., Lee C.H., Hsueh S.C. Comparison of electrogenic capabilities of microbial fuel cell with different light power on algae grown cathode. Bioresour. Technol. 2012;123:23–29. doi: 10.1016/j.biortech.2012.07.041. [DOI] [PubMed] [Google Scholar]
- 41.Huarachi-Olivera R., Dueñas-Gonza A., Yapo-Pari U., Vega P., Romero-Ugarte M., Tapia J., Molina L., Lazarte-Rivera A., Pacheco-Salazar D.G., Esparza M. Bioelectrogenesis with microbial fuel cells (MFCs) using the microalga Chlorella vulgaris and bacterial communities. Electron. J. Biotechnol. 2018;31:34–43. [Google Scholar]
- 42.González del Campo A., Cañizares P., Rodrigo M.A., Fernández F.J., Lobato J. Microbial fuel cell with an algae-assisted cathode: a preliminary assessment. J. Power Sources. 2013;242:638–645. [Google Scholar]
- 43.Liu T., Rao L., Yuan Y., Zhuang L. Bioelectricity generation in a microbial fuel cell with a self-sustainable photocathode. Sci. World J. 2015;2015 doi: 10.1155/2015/864568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bazdar E., Roshandel R., Yaghmaei S., Mardanpour M.M. The effect of different light intensities and light/dark regimes on the performance of photosynthetic microalgae microbial fuel cell. Bioresour. Technol. 2018;261:350–360. doi: 10.1016/j.biortech.2018.04.026. [DOI] [PubMed] [Google Scholar]
- 45.He H., Zhou M., Yang J., Hu Y., Zhao Y. Simultaneous wastewater treatment, electricity generation and biomass production by an immobilized photosynthetic algal microbial fuel cell. Bioproc. Biosyst. Eng. 2014;37:873–880. doi: 10.1007/s00449-013-1058-4. [DOI] [PubMed] [Google Scholar]
- 46.Ayala-Parra P., Liu Y., Field J.A., Sierra-Alvarez R. Nutrient recovery and biogas generation from the anaerobic digestion of waste biomass from algal biofuel production. Renew. Energy. 2017;108:410–416. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data included in article/supp. material/referenced in article



 @kartik_s_aiyer
@kartik_s_aiyer