Abstract
In the present study, Rhus vernicifera laccase (RvLac) was immobilized through covalent methods on the magnetic nanoparticles. Fe2O3 and Fe3O4 nanoparticles activated by 3-aminopropyltriethoxysilane followed with glutaraldehyde showed maximum immobilization yields and relative activity up to 81.4 and 84.3% at optimum incubation and pH of 18 h and 5.8, respectively. The maximum RvLac loading of 156 mg/g of support was recorded on Fe2O3 nanoparticles. A higher optimum pH and temperature of 4.0 and 45 °C were noted for immobilized enzyme compared to values of 3.5 and 40 °C for free form, respectively. Immobilized RvLac exhibited better relative activity profiles at various pH and temperature ranges. The immobilized enzyme showed up to 16-fold improvement in the thermal stability, when incubated at 60 °C, and retained up to 82.9% of residual activity after ten cycles of reuses. Immobilized RvLac exhibited up to 1.9-fold higher bisphenol A degradation efficiency potential over free enzyme. Previous reports have demonstrated the immobilization of RvLac on non-magnetic supports. This study has demonstrated that immobilization of RvLac on magnetic nanoparticles is very efficient especially for achieving high loading, better pH and temperature profiles, and thermal- and solvents-stability, high reusability, and higher degradation of bisphenol A.
Keywords: Bisphenol A, Enzyme immobilization, Magnetic nanoparticle, Reusability, Rhus vernicifera laccase, Stability
Introduction
Biocatalysts have been widely used for biotransformation applications such as industrial and environmental sectors [1, 2]. The enzymes as cell-free biocatalysts are founded more desirable to use in bioconversion reactions due to their high specificity towards substrate and reaction rate, easy in product separation, and tolerance towards higher substrate concentration, and solvents [3–5]. Primarily, the uses of the enzyme are limited due to their high cost and low stability. Various strategies have been employed to improve enzyme properties through the selection of suitable microbial sources, protein engineering, and immobilization [6–9]. The immobilization of biocatalysts in the form of either whole-cells or enzymes has been widely demonstrated to enhance their stability during biotransformation [10–14]. The enzyme’s properties such as activity and stability are significantly varied after immobilization on supports [11, 15]. Numerous methods have been evaluated for immobilization, including (1) adsorption on solid supports or polymers [16, 17], (2) encapsulation or entrapment within a polymeric matrix [10], or as metal-protein hybrids [18–20], (3) cross-linking through aggregation by cross-linker such as glutaraldehyde [21], and (4) covalent through functional groups binding [22, 23]. The extent of immobilization is largely dependent upon enzymes properties such as purity, size, surface charge, and their conformation, and the supports properties such as size, surface area, morphology, porous nature, and functional groups on their surface for the binding [21–24]. Additionally, the immobilization conditions such as a buffer (pH), temperature, and time of incubation are also proved effective in the course of immobilization [3, 25].
Various kinds of solid supports have been used for the immobilization of enzymes such as activated carbon [10], chitosan [26], rice straw [27], silica [23], tin oxide [3], and composite particles [17, 22]. For effective immobilize enzymes on supports, the loading of enzymes and high reusability are important aspects that are highly dependent on the structural properties of the supports such as surface area and their rigidity [11, 28]. A significant development in nanotechnology is leading to synthesize unique kinds of nanomaterials with controlled uniform morphology and high surface area that can positively influence the properties of the enzyme on immobilization [21, 23]. The magnetic nature of supports such as iron oxides (Fe2O3, and Fe3O4), and their composites have a beneficial influence of easy recovery or separation from the reaction by the simple use of the external magnetic field over non-magnetic supports [17, 21, 29].
Commercially available enzymes such as glucose oxidase [21], horseradish peroxidase [17], lipase [23], and laccase [29, 30] have been widely demonstrated for their immobilization on nanomaterials. Laccase is a multi-copper oxidase that catalyzes the oxidation of phenolics, and non-phenolics compounds [10, 31, 32]. Laccases are important enzymes due to their broad biotechnological applications: (1) biodegradation and bioremediation in (a) paper and pulp industry, (b) textile and food processing industry, (2) biosensor, and (3) biofuel production [8, 10, 21, 22, 33]. The immobilization of laccases is required for improving the process economy through enhanced activity, stability, and reusability [10, 28]. Rus vernicifera and bacterial laccases exhibit quite similar redox potential ~ 400 mV [34, 35]. In contrast, fungal laccases show higher redox potential between 470 and 810 [35]. Laccase immobilization on various kinds of supports has been established, including silica [24], kaolinites [10], membranes [36], and sepiolite [37]. Although numerous kinds of supports have been used to immobilize laccase, still there is a need for efficient supports to retain high loading, improved stability, and reusability for a potential application. Laccase from Trametes versicolor has been largely studied for immobilization [10, 24, 38]. A few reports have been noted for immobilization of R. vernicifera laccase (RvLac) on supports, including chitosan [26, 39], nylon membrane [16], sepiolite [37], and zirconium chloride [28]. In this study, RvLac immobilization on magnetic nanoparticles Fe2O3, and Fe3O4 functionally activated by 3-aminopropyltriethoxysilane (APTES) followed with glutaraldehyde was evaluated to improve loading and enzyme properties. The covalently immobilized RvLac on F3O4 magnetic nanoparticles showed improved stability and kinetic properties over free enzyme and exhibited high reusability. Further, the potential application of immobilized laccase was demonstrated for the degradation of bisphenol A.
Materials and Methods
Chemicals and Materials
Laccase (R. vemicifera), 2,2′-Azino-bis(3-ethylbenzothiazoline-6-sulfonic acid) (ABTS), APTES, glutaraldehyde, and fluorescein isothiocyanate (FITC) were procured from Sigma-Aldrich, USA. Fe2O3 (average size of 30–50 nm and surface area of 25–35 m2/g) and F3O4 (average size of 50–100 nm and surface area of 6–8 m2/g) particles were obtained from Nanostructured and Amorphous Materials, Inc., USA. All other chemicals and reagents from commercial suppliers were used of analytical grade.
Nanoparticles Functional Activation and Enzyme Immobilization
The functional modification of magnetic particles by APTES (2%, v/v) in toluene followed with glutaraldehyde (2%, v/v) in phosphate buffer (50 mM, pH 7) was carried out at room temperature as described previously [8, 40]. The immobilization of RvLac was performed through covalent methods on supports at an enzyme loading of 50 mg/g of supports at 4 °C for incubation up to 30 h with the agitation of 90 rpm. After enzyme immobilization, particles were recovered through centrifugation (Gyrozen 1580R, Republic of Korea) at 10,000 rpm for 10 min [41], and washed twice by a buffer. The concentration of protein was measured through the Bradford method in the supernatant [24]. Further, the influence of enzyme loading (50–300 mg/g of supports) was performed to improve immobilization. The immobilization yield (IY) and relative efficiency (RE) were calculated as follows: equation 1 [17], and equation 2 [22].
| 1 |
| 2 |
RvLac Activity Measurements
Enzyme activity was measured using the oxidation of ABTS through spectrophotometrically at 420 nm (ɛmax = 3.6 × 104/M × cm) [21]. Unit (U) of activity represents the amount of enzyme required to oxidize one µmol of ABTS per minute under standard conditions.
Characterization of Immobilized RvLac
The influence of process parameters pH [2.5–6.0: 2.5 (glycine–HCl), 3.0–3.5 (sodium-citrate), and 4.0–6.0 (sodium-acetate)] and temperature (25–70 °C) on the activity of free and immobilized RvLac was evaluated using ABTS. Kinetic parameters (Km and Vmax) were measured by varying ABTS concentrations 0.01–10.0 mM at optimum pH for free and immobilized RvLac in 50 mM buffer at 25 °C [17].
Stability and Reusability
The stability of the free or immobilized RvLac was evaluated at a higher temperature of 60 °C by measuring residual enzyme activity over different time intervals at optimum pH. Further, the reusability of immobilized RvLac was assessed up to ten cycles using ABTS. The immobilized enzyme was recovered through centrifugation at 10,000 rpm for 10 min and used to the next cycle of reaction. The initial RvLac activity was considered as 100%.
Solvents Stability and Degradation of Bisphenol A
The stability of free and immobilized RvLac on Fe2O3 nanoparticles was compared in various solvents (25%, v/v) based buffer reaction at optimum pH, including methanol, ethanol, propanol, acetone, and benzene for 4 h incubation at 25 °C. The bisphenol A degradation was evaluated in the flask with a working volume of 10 ml containing bisphenol A (50–125 µM) and free (pH 3.5, sodium-citrate) or immobilized RvLac (pH 4.0, sodium-acetate) on Fe2O3 (1 U/ml) in the buffer (50 mM) for 12 h of incubation at room temperature (25 °C) under shaking of 100 rpm. Thereafter, the reaction was halted by adding a few drops of concentrated hydrochloric acid. The residual bisphenol A amount was measured via 4-AAP coupled reaction spectrophotometrically (506 nm). The bisphenol A degradation efficiency was calculated as follows (Eq. 3) [42]:
| 3 |
Instrumental Measurements
All reactions assay absorption spectra were measured spectrophotometrically (Varian Cary 100 Bio UV–Vis spectrophotometer, USA) [43, 44]. The decomposition analysis of particles was measured using thermogravimetric analysis (TGA) [45]. Confocal laser scanning microscopy (CLSM) analysis was performed using FITC-labeled laccase immobilize on Fe2O3 nanoparticles by FV-1000 Olympus confocal microscope, Olympus, Japan [21]. All experiments were carried out in triplicate.
Results and Discussion
Immobilization of RvLac on Magnetic Nanoparticles
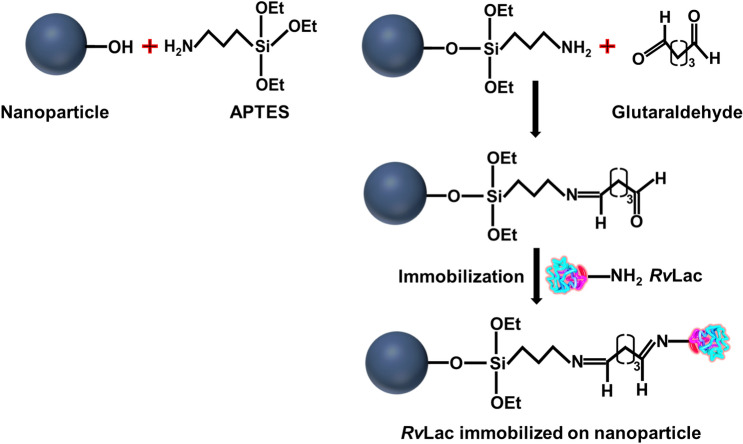
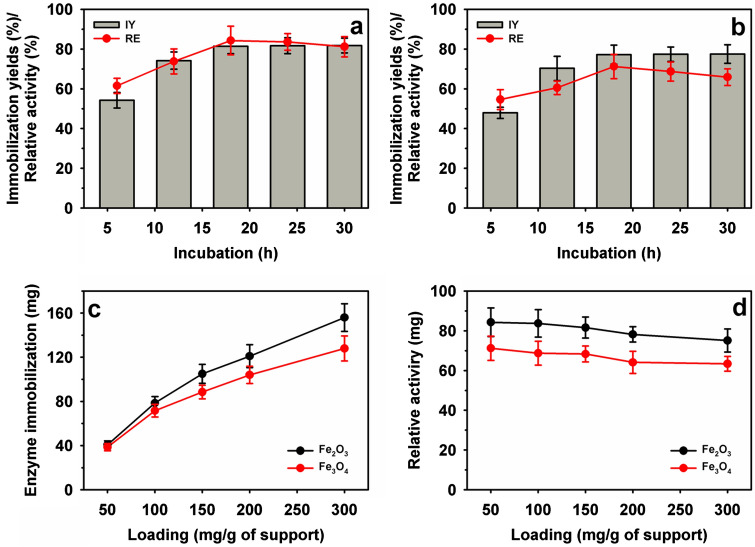
The illustration of the enzyme immobilization process on the magnetic particle is presented in Fig. 1. Immobilization of RvLac on magnetic supports occurs through covalent bonding between the basic amino acids such as lysine of enzyme and glutaraldehyde groups on the surface of the particles [22]. Initially, the immobilization profile was evaluated up to 30 h to measure the desirable incubation for the efficient immobilization of RvLac on functionally activated magnetic nanoparticles (Fig. 2a, b). The IY was increased with an increase in the incubation up to 18 h on nanoparticles. Thereafter, immobilization was stabilized up to 30 h of incubation with a maximum IY of 81.8 and 81.2% on Fe2O3 and Fe3O4 nanoparticles, respectively. The maximum RE of 84.3 (22.3 U/mg of protein) and 71.2% (18.8 U/mg of protein) were noted at the incubation of 18 h for Fe2O3 and Fe3O4 nanoparticles, respectively. A higher optimum IY and RE of 81.4 and 84.3% were observed on Fe2O3 for RvLac over the values of 77.2 and 71.2% on Fe3O4, respectively. Previously, up to ~ 200-fold lower IY (0.4%) was noted on chitosan for RvLac immobilization [39]. Similarly, RvLac immobilization on sepiolite and sepiolite modified with chitosan and copper showed significantly lower IY of 52, 55, and 62%, respectively [37]. A lower IY and RE of 56 and 36% were recorded on the chitosan microsphere [26]. These results suggested that the immobilization of RvLac is more efficient on magnetic particles over previous reports on sepiolite and chitosan [26, 39]. Previously, RvLac immobilization of magnetic (Fe2O3 or Fe3O4) particles has not been reported. A lower IY of 49.0 and 68.7% were shown on Fe3O4 chemically modified with glutaraldehyde and silica coating for laccase from T. versicolor [17, 38]. The loading of enzymes is an important criterion to improve process efficiency [5, 28]. Therefore, the concentration of enzyme was increased up to 300 mg/g of support for immobilization (Fig. 2c). The amount of enzyme was increased from 40.7 to 156 mg/g of support on Fe2O3 as compared from 38.6 to 128 mg/g of support on F3O4 nanoparticles on increasing concentration from 50 to 300 mg/g of supports. In contrast, the higher loading led to lower RE of 75.1 and 63.4% on Fe2O3 and F3O4 nanoparticles, respectively (Fig. 2d). Previously, a significantly lower loading of 32.3 mg/g of support was used for the immobilization of RvLac on zirconium chloride nanoparticles [28]. RvLac after immobilization on the nylon membrane exhibited much lower activity of ~ 2.8% over free enzyme (9.6 µmol/min) as a control [16]. Similarly, laccase immobilization on Fe3O4 and Fe3O4@MoS2 core–shell composite nanoparticles chemically modified with polyethyleneimine (PEI) showed lower 70 and 120 mg/g of supports, respectively [29]. In contrast, a significate lower loading up to 14.2 g/g of supports for T. versicolor laccase reported on graphene oxide/CuFe2O4 nanocomposites [46]. Similarly, RvLac immobilization noted more efficient over maximum loading of 17.3 and 82.6 mg/g of supports for Bacillus subtilis-derived laccase on Fe3O4 and magnetic carbon chemicals (MLC) particles, respectively [5]. Immobilization of enzymes on nanomaterials is highly influenced by particle size as well as surface area [17, 22, 24]. The higher immobilization of RvLac on Fe2O3 nanoparticles can be correlated with smaller size and ~ 4.4-fold higher surface area over Fe3O4 nanoparticles. The efficient immobilization of RvLac on Fe2O3 nanoparticles was confirmed by TGA measurements (Fig. 3). The high loading of RvLac on Fe2O3 nanoparticles correlated with a significant reduction of relative weight loss to 77.3% as compared to 89.4% for control particles. Further, the visualization of green fluorescence of FITC labeled laccase immobilized on Fe2O3 nanoparticles under CLSM analysis confirmed immobilization (Fig. 4).
Fig. 1.
Illustration of activation of magnetic nanoparticles by 3-aminopropyltriethoxysilane (APTE) followed with glutaraldehyde and immobilization of RvLac
Fig. 2.
Immobilization of RvLac on the magnetic nanoparticles: time profile on a Fe2O3 and b Fe3O4 nanoparticles, and c–d enzyme loading
Fig. 3.
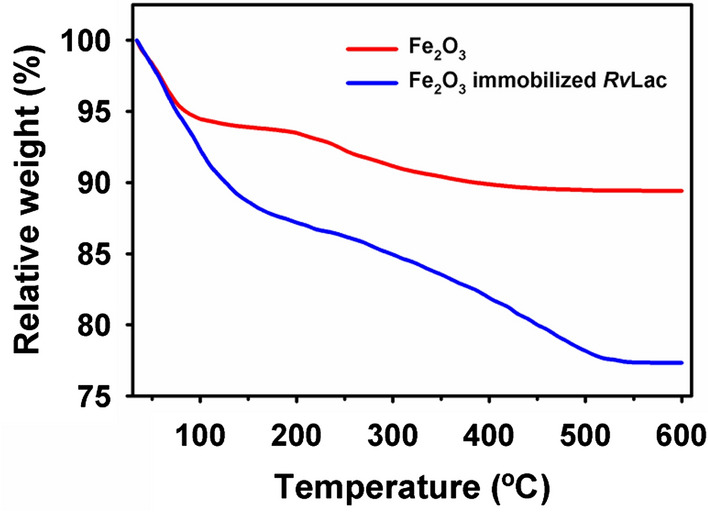
Thermogravimetric profiles pure and RvLac immobilized Fe2O3 nanoparticles
Fig. 4.
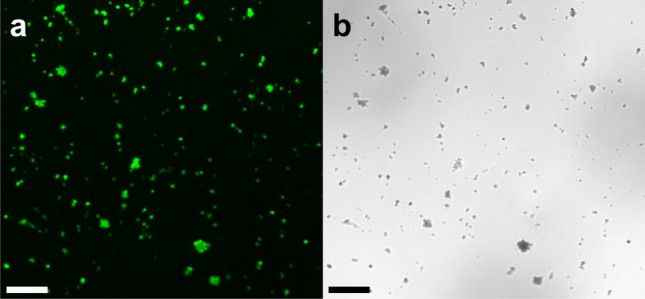
Confocal laser scanning microscopy images of immobilized RvLac on Fe2O3 nanoparticles in a green and b bright-field (scale bar is 0.5 µm)
Characterization of Immobilized RvLac
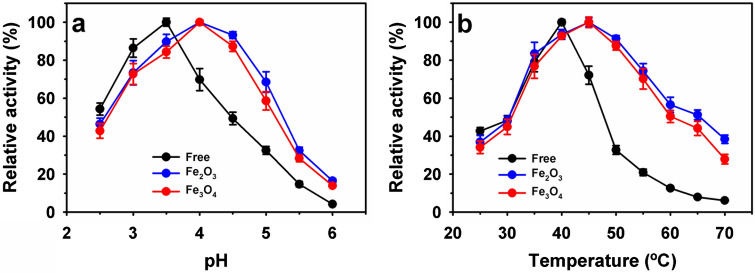
The physiological properties such as pH and temperature profiles of the enzymes are highly influenced after immobilization on the nanoparticles [3, 5, 37]. Therefore, the activity profiles of free and immobilized RvLac on magnetic nanoparticles were compared (Fig. 5a). The maximum activity of free RvLac of 26.4 U/mg of protein (100%) was noted at pH 3.5. At lower and higher pH of 2.5 and 6.0 free enzyme retained relative activity of 54.3 and 4.2%, respectively. After immobilization, a shift in pH optimum from 3.5 to 4.0 was recorded on both the nanoparticles. RvLac showed intermediate pH optima of 3.5 as compared to 3.0 for T. versicolor and 5.0 for B. subtilis towards ABTS [5, 17]. Immobilized RvLac exhibited a broader pH profile with a higher pH above 4.0 as compared to free form. At pH 6.0, immobilized RvLac on Fe2O3 and Fe3O4 particles revealed 3.9- and 3.3-fold higher relative activities to free enzyme (4.2%). In contrast, a similar pH optimum value of 7.0, 7.5, 7.5, and 7.5 noted for free and immobilized RvLac on chitosan [39], polypropylene membrane [36], nylon membrane [16], and zirconium chloride supports [28], respectively. Previously, a quite similar shift in pH profile was noted for Fe3O4@MoS2@PEI-facilitated laccase [29]. In contrast, T. versicolor laccase immobilized on Fe3O4 derived nanoparticles such as poly(amidoisophthalicacid)-coated (Fe@PA), cyclodextrin-anchored (Fe@-PA-CD), and chitosan-coated (Fe@PA-CD-Cs) supports exhibited quite similar pH profiles to free enzyme [47]. Similarly, B. subtilis-based laccase showed similar pH optima after immobilization on MLC [5].
Fig. 5.
Activity profiles of free and immobilized RvLac on the magnetic nanoparticles: a pH at 25 °C and b temperatures at optimum pH values
RvLac showed an optimum temperature of 40 °C with an activity of 61.8 U/mg of protein (100%) (Fig. 5b). At higher temperatures, a significant decrease in the relative activity to 12.6% and 6.1% noted at 60 and 70 °C, respectively. After immobilization, a shift in temperature from 40 to 45 °C was recorded on magnetic nanoparticles. At a temperature of 70 °C, immobilized RvLac on Fe2O3 and Fe3O4 particles retained a much higher relative activity of 38.4 and 27.9% as compared to a free enzyme (6.1%), respectively. A quite similar shift in temperature profile was noted after the immobilization of B. subtilis laccase on MLC supports [5]. In contract, immobilized RvLac on chitosan, and Zirconium chloride supports showed optimum temperature 45 and 40 °C, respectively, which were similar to their free forms of the enzyme [26, 28]. Also, no shift in the temperature optima was noted for T. versicolor laccase immobilized on various magnetic supports, including Fe@PA, Fe@-PA-CD, and Fe@PA-CD-Cs [47]. Overall, a shift in pH and temperature optima of immobilized RvLac might be associated with a strong binding of the enzyme to supports that can lead to desirable changes in confirmation of the enzyme [23, 32, 48].
The Km and Vmax (apparent) values of RvLac observed 1.69 mM and 68.1 µmol/min/mg protein for ABTS, respectively (Table 1). After immobilization, RvLac showed quite a similar substrate affinity (Km) value of 1.84 mM, and a Vmax value of 61.7 µmol/min/mg protein compared to free enzyme. Previously, T. versicolor laccase immobilized on Fe3O4 nanoparticles was showed 2.2-and 1.6-fold lower Km (0.06 mM) and Vmax (1140 µmol/min/mg protein) as compared to free enzyme towards ABTS, respectively [17]. In contrast, laccase was exhibited a 2.2-fold lower Vmax (26 mM/min) towered ABTS without a significant change in Km (1.8 mM) after immobilization on GO-CuFe2O4 particles [46]. RvLac immobilized on the nylon membrane was showed a 35.5-fold lower Vmax for quinone over free enzyme (9.58 µmol/min) [16]. In contrast, a quite similar kinetic parameter (Km and Vmax) was reported for free and immobilized B. subtilis laccase on MLC [5]. These reductions in kinetic parameters might be associated with the strong attachment between enzyme and support that results in diffusion limitation or undesirable conformational changes within enzyme after immobilization on these supports [16, 17, 46].
Table 1.
Kinetic parameters of free and immobilized RvLac
| RvLac | Km (mM) | Vmax (µmol/min/mg protein) |
|---|---|---|
| Free | 1.69 ± 0.41 | 68.1 ± 6.6 |
| Fe2O3 immobilized | 1.84 ± 0.40 | 61.7 ± 5.8 |
Thermal stability and Reusability of Immobilized RvLac
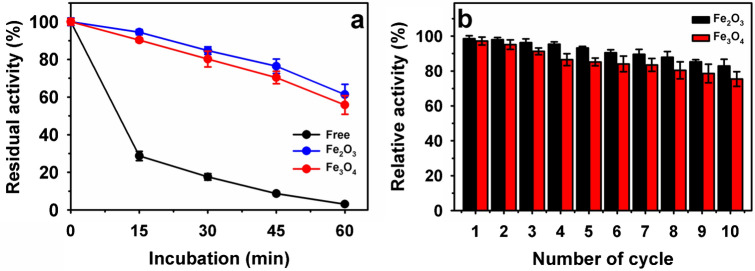
Immobilization of the enzymes on supports is primarily carried out to improve their stability over the free form of the enzyme. The gain in the stability might be not directly dependent on the immobilization methods or a kind of supports [21, 36, 39, 48]. Therefore, the thermal stability of covalently immobilized RvLac was compared to free enzyme at a high temperature of 60 °C for incubation up to 60 min (Fig. 6a). Free enzyme lost ~ 80% of residual activity within 30 min of incubation, whereas the residual activity decreased to 3.1% at a higher incubation of 60 min. On the other hand, immobilized RvLac showed more than 80% of the activity at 30 min of incubation at 60 °C. After 60 min of incubation, immobilized RvLac on Fe2O3 and Fe3O4 particles exhibited high residual activity of 61.3 and 55.8%, respectively. Previously, under similar conditions, RvLac immobilized on chitosan showed lower residual activity of 40.1% [39]. After immobilization on Fe2O3 and Fe3O4 particles, the thermal stability enhancement of 19.8- and 18.0-fold was noted at 60 °C as compared to the free enzyme. Previously, a significant lower enhancement of stability of ~ 2.7-fold was noted for immobilized RvLac on polypropylene membrane chemically modified with chromic acid at 60 °C for incubation of 150 min [36]. In contrast, B. subtilis-derived laccase exhibited a much lower improvement of 1.6-fold at a similar temperature [5]. RvLac showed half-life (t1/2) of ~ 13 min at 60 ºC as compared to t1/2 values of 12 min for T. versicolor [17], and ~ 20 min for bacterial laccase from γ-Proteobacterium JB [49]. The immobilized laccase on the chicken feather showed t1/2 activity of 134 min over free enzyme (109 min) at 60 °C [30]. In contrast, free and immobilized bacterial laccase from γ-Proteobacterium JB on nitrocellulose membrane showed similar temperature stability at 60 ºC [49].
Fig. 6.
a Thermal stability of free and immobilized RvLac on the magnetic nanoparticles at 60 °C and b reusability
Immobilization of enzymes on magnetic supports exhibits the benefits of easy separation using magnet over non-magnetic based supports [29]. The better reusability of immobilized enzymes primarily demonstrates the beneficial influence contribution toward the process economy [47, 48]. The reusability of RvLac immobilized on magnetic nanoparticles was evaluated for the oxidation of ABTS (Fig. 6b). After five- and ten-cycles of reuses, Fe2O3 immobilized RvLac retained residual activity of 93.2, and 82.9%, respectively. Under similar conditions, RvLac immobilized Fe3O4 showed slightly lower residual activity of 85.2 and 75.4%, respectively. The decline in residual activity towards successive cycles might be associated with partial inactivation of enzyme or leaching [19, 21]. Previously, Fe3O4@MoS2@PEI immobilized laccase showed lower residual activity of 62.0% after ten cycles of reuses [29]. Magnetic nanoparticles Fe@PA, Fe@-PA-CD, and Fe@PA-CD-Cs immobilized T. versicolor laccase retained significantly lower residual activity up to ~ 40.0% [47]. In contrast, metal-protein hybrids using cooper and zinc metal ions and laccase showed much lower reusability of 16.5 and 43.7% after ten cycles of reuses, respectively [42]. Here, the lower reusability associated with structural instability of metal-protein hybrids during the process over better structural rigidity maintained by nanoparticles that led to high reusability to magnetic nanoparticles based enzyme immobilization system. The cumulative relative activity of 918% retained by covalently immobilized RvLac on Fe2O3 after ten cycles of reuses, these results indicated that immobilization will be 7.7-fold cost-effective compared to uses of free enzyme activity. Under similar conditions, 2.4-fold cost–benefit was estimated for Fe3O4 nanoparticles immobilized laccase from T. versicolor through adsorption [17]. Overall, RvLac immobilized on Fe2O3 nanoparticles exhibited better IY, RE, and reusability than previous reports of laccase immobilization on various matrices (Table 2).
Table 2.
Immobilization of laccase on different matrices
| Matrix | Method | Immobilization yield (%) | Relative efficiency (%) | Reusability (%) | References |
|---|---|---|---|---|---|
| Chitosan microsphere | Adsorption | 70.0 | 45.0 | – | [26] |
| Graphene oxide/CuFe2O4 | Covalent | 14.2a | 88.0 | 80.0 | [46] |
| Nylon membrane | Covalent | –b | 2.8 | – | [16] |
| Water–soluble chitosan | Adsorption | 56.0 | 30.0 | 80.0 | [26] |
| Zirconium chloride | Adsorption | 32.3b | – | – | [28] |
| Fe3O4 | Adsorption | 49.0 | 45.3 | 21.3 | [17] |
| Adsorption | 4.7 | 80.0 | – | [29] | |
| Fe3O4@MoS2 | Adsorption | 8.0 | 90.0 | 62.0 | [29] |
| SrFe12O19 | Covalent | 66.5 | 42.7 | – | [22] |
| Y3Fe5O12 | Covalent | 68.7 | 46.9 | – | |
| Fe2O3 | Covalent | 81.4 | 84.3 | 82.9 | This study |
aAmount of enzyme immobilization in mg/g of support
bNot available or applicable
Solvent Stability and Bisphenol A degradation
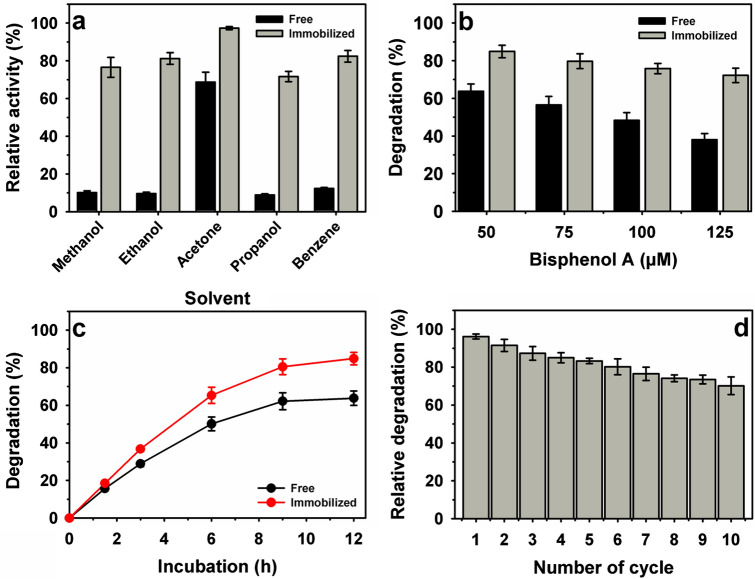
The phenolics compounds exhibit low solubility in water and require a solvent system in a few cases. Laccase shows a wide range of specificity towards phenolics substrate [17, 50]. After incubation in various solvents, RvLac exhibited a significant decline in the residual activity to the ranges of 8.9–68.7% with the order propanol > ethanol > methanol > benzene > acetone (Fig. 7a). Immobilized RvLac on Fe2O3 nanoparticles retained superior residual activity in the presence of solvents and showed higher solvents tolerance up to 8.5-fold as compared with the free enzyme. This result suggested that immobilized RvLac can be potentially applied for a solvent-based reaction system [21, 39]. Bisphenol A is a commonly used substrate in the industrial process, including plastics and resins [21]. The generation of a large quantum of bisphenol A as waste material is leading to environmental problems due to their toxicity towards aquatic fauna [5, 42]. The RvLac resulted in the degradation efficiency of 63.8% at bisphenol A concentration of 50 μM (Fig. 7b). Thereafter, an increase in the concentration up to 125 μM led to a decline in degradation efficiency to 38.1%. In contrast, immobilized RvLac on Fe2O3 nanoparticles showed significantly higher bisphenol A degradation efficiency of 84.9 and 72.2% at a concentration of 50 and 125 μM, respectively. After the immobilization of RvLac on Fe2O3 nanoparticles, bisphenol A degradation efficiency improved up to 1.9-fold. Previously, lower bisphenol A degradation of 53.0 and 68% reported for T. versicolor laccase immobilized on magnetic nanoparticles Fe@PA and Fe@-PA-CD, respectively [47]. Similarly, a lower enhancement of 50% was noted for B. subtilis-derived laccase immobilized on magnetic carbon nanocarriers [5]. In contrast, 1.3- and 1.5-fold higher degradation of bisphenol A over copper and zinc-based metal-protein hybrids, respectively [42]. Remarkably, a low enhancement of ~ 3.0% was recorded by Fe3O4@MoS2@PEI immobilized laccase over free enzyme [29]. The degradation of bisphenol A increased sharply up to 9 h of incubation followed by stabilization at 12 h (Fig. 7c). After ten cycles of reuses, immobilized RvLac retained a high relative degradation efficiency of 70.2% (Fig. 7d). In contrast, a low bisphenol A degradation efficiency of 13.0% was maintained for fungal laccase from T. versicolor immobilized on Fe@-PA-CD [47]. On the other hand, ~ 60% bisphenol A degradation was recorded for magnetic carbon nanocarriers immobilized bacterial laccase from B. subtilis under similar recycling conditions [5].
Fig. 7.
Free and immobilized RvLac on Fe2O3 nanoparticles: a solvent stability (25%, v/v), b bisphenol A degradation, 50 µM bisphenol A degradation c time profile, and d immobilized enzyme reusability for incubation of 6 h each cycle
Conclusion
In conclusion, this study reports covalent immobilization of RvLac on magnetic nanoparticles Fe2O3 and Fe3O4 modified with APTES followed by glutaraldehyde. The RvLac immobilization was established more efficiently on F2O3 nanoparticles due to smaller size and high surface area compared to Fe3O4 nanoparticles. After immobilization, the enzyme exhibited better relative activity profiles at high pH and temperatures, and significantly improved thermostability as compared to free form. Immobilized RvLac on Fe2O3 particles retained high reusability and showed higher bisphenol A degradation. Previous studies have reported RvLac immobilization on non-magnetic supports. This developed magnetic nanoparticles-based biocatalyst can be used efficiently for other kinds of potential biotechnological applications.
Acknowledgements
This research was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Science, ICT & Future Planning (2019R1F1A1063131, 2020H1D3A2A01060467, 2017R1A2B3011676). This work was also supported by KU Research Professor Program of Konkuk University.
Compliance with Ethical Standards
Conflict of interest
The authors declare no conflict of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Shukla P. Synthetic biology perspectives of microbial enzymes and their innovative applications. Indian J Microbiol. 2019;59:401–409. doi: 10.1007/s12088-019-00819-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Singh DN, Sood U, Singh AK, Gupta V, Shakarad M, Rawat CD, Lal R. Genome sequencing revealed the biotechnological potential of an obligate thermophile Geobacillus thermoleovorans strain RL isolated from hot water spring. Indian J Microbiol. 2019;59:351–355. doi: 10.1007/s12088-019-00809-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Anwar MZ, Kim DJ, Kumar A, Patel SKS, Otari S, Mardina P, Jeong JH, Sohn JH, Kim JH, Park JT, Lee JK. SnO2 hollow nanotubes: a novel and efficient support matrix for enzyme immobilization. Sci Rep. 2017;7:15333. doi: 10.1038/s41598-017-15550-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lee J-K, Patel SKS, Sung BH, Kalia VC. Biomolecules from municipal and food industry wastes: an overview. Bioresour Technol. 2020;298:122346. doi: 10.1016/j.biortech.2029.122346. [DOI] [PubMed] [Google Scholar]
- 5.Zhang C, You S, Liu Y, Wang C, Yan Q, Qi W, Su R, He Z. Construction of luffa sponge-based magnetic carbon nanocarriers for laccase immobilization and its application in the removal of bisphenol A. Bioresour Technol. 2020;305:123085. doi: 10.1016/j.biortech.2020.123085. [DOI] [PubMed] [Google Scholar]
- 6.Gao H, Li J, Sivakumar D, Kim T-S, Patel SKS, Kalia VC, Kim I-W, Zhang Y-W, Lee J-K. NADH oxidase from Lactobacillus reuteri: a versatile enzyme for oxidized cofactor regeneration. Int J Biol Macromol. 2019;123:629–636. doi: 10.1016/j.ijbiomac.2018.11.096. [DOI] [PubMed] [Google Scholar]
- 7.Kondaveeti S, Patel SKS, Woo J, Wee JH, Kim S-Y, Al-Raoush RI, Kim I-W, Kalia VC, Lee J-K. Characterization of cellobiohydrolases from Schizophyllum commune KMJ820. Indian J Microbiol. 2019;60:160–166. doi: 10.1007/s12088-019-00843-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kumar V, Patel SKS, Gupta RK, Otari SV, Gao H, Lee JK, Zhang L. Enhanced saccharification and fermentation of rice straw by reducing the concentration of phenolic compounds using an immobilized enzyme cocktail. Biotechnol J. 2019;14:1800468. doi: 10.1002/biot.201800468. [DOI] [PubMed] [Google Scholar]
- 9.Panday D, Patel SKS, Singh R, Kumar P, Thakur V, Chand D. Solvent-tolerant acyltransferase from Bacillus sp. APB-6: purification and characterization. Indian J Microbiol. 2019;59:500–507. doi: 10.1007/s12088-019-00836-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fernández-Fernández M, Sanromán MÁ, Moldes D. Recent developments and applications of immobilized laccase. Biotechnol Adv. 2013;31:1808–1825. doi: 10.1016/j.biotechadv.2012.02.013. [DOI] [PubMed] [Google Scholar]
- 11.Kim T-S, Patel SKS, Selvaraj C, Jung W-S, Pan C-H, Kang YC, Lee J-K. A highly efficient sorbitol dehydrogenase from Gluconobacter oxydans G624 and improvement of its stability through immobilization. Sci Rep. 2016;6:33438. doi: 10.1038/srep33438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Patel SKS, Kumar V, Mardina P, Li J, Lestari R, Kalia VC, Lee J-K. Methanol production from simulated biogas mixtures by co-immobilized Methylomonas methanica and Methylocella tundrae. Bioresour Technol. 2018;263:25–32. doi: 10.1016/j.biortech.2018.04.096. [DOI] [PubMed] [Google Scholar]
- 13.Patel SKS, Kalia VC, Joo JB, Kang YC, Lee J-K. Biotransformation of methane into methanol by methanotrophs immobilized on coconut coir. Bioresour Technol. 2020;297:122433. doi: 10.1016/j.biortech.2019.122433. [DOI] [PubMed] [Google Scholar]
- 14.Patel SKS, Shanmugam R, Kalia VC, Lee J-K. Methanol production by polymer-encapsulated methanotrophs from simulated biogas in the presence of methane vector. Bioresour Technol. 2020;304:123022. doi: 10.1016/j.biortech.2020.123022. [DOI] [PubMed] [Google Scholar]
- 15.Otari SV, Patel SKS, Kim S-Y, Haw JR, Kalia VC, Kim I-W, Lee J-K. Copper ferrite magnetic nanoparticles for the immobilization of enzyme. Indian J Microbiol. 2019;59:105–108. doi: 10.1007/s12088-018-0768-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Durate D, Casadio R, Martelli L, Tasco G, Portaccio M, Luca PD, Bencivenga U, Rossi S, Martino SD, Grano V, Diano N, Mita DG. Isothermal and non-isothermal bioreactors in the detoxification of waste waters polluted by aromatic compounds by means of immobilised laccase from Rhus vernicifera. J Mol Catal B Enzym. 2004;27:191–206. doi: 10.1016/j.molcatb.2003.11.008. [DOI] [Google Scholar]
- 17.Patel SKS, Choi SH, Kang YC, Lee J-K. Eco-friendly composite of Fe3O4-reduced graphene oxide particles for efficient enzyme immobilization. ACS Appl Mater Inter. 2017;9:2213–2222. doi: 10.1021/acsami.6b05165. [DOI] [PubMed] [Google Scholar]
- 18.Kumar A, Kim I-W, Patel SKS, Lee J-K. Synthesis of protein-inorganic nanohybrids with improved catalytic properties using Co3(PO4)2. Indian J Microbiol. 2018;58:100–104. doi: 10.1007/s12088-017-0700-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kumar A, Patel SKS, Mardan B, Pagolu R, Lestari R, Jeong S-H, Kim T, Haw JR, Lim S-Y, Kim I-W, Lee J-K. Immobilization of xylanase using a protein-inorganic hybrid system. J Microbiol Biotechnol. 2018;28:638–644. doi: 10.4014/jmb.1710.10037. [DOI] [PubMed] [Google Scholar]
- 20.Patel SKS, Gupta RK, Kumar V, Mardina P, Lestari R, Kalia VC, Choi M-S, Lee J-K. Influence of metal ions on the immobilization of β-glucosidase through protein-inorganic hybrids. Indian J Microbiol. 2019;59:370–374. doi: 10.1007/s12088-019-0796-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Patel SKS, Choi SH, Kang YC, Lee J-K. Large-scale aerosol-assisted synthesis of biofriendly Fe2O3 yolk-shell particles: a promising support for enzyme immobilization. Nanoscale. 2016;8:6728–6738. doi: 10.1039/C6NR00346J. [DOI] [PubMed] [Google Scholar]
- 22.Patel SKS, Anwar MZ, Kumar A, Otari SV, Pagolu RT, Kim S-Y, Kim I-W, Lee J-K. Fe2O3 yolk-shel particle-based laccase biosensor for efficient detection of 2,6-dimethoxyphenol. Biochem Eng J. 2018;132:1–8. doi: 10.1016/j.bej.2017.12.013. [DOI] [Google Scholar]
- 23.Kumar A, Park GD, Patel SKS, Kondaveeti S, Otari S, Anwar MZ, Kalia VC, Singh Y, Kim SC, Cho B-K, Sohn J-H, Kim DR, Kang YC, Lee J-K. SiO2 microparticles with carbon nanotube-derived mesopores as an efficient support for enzyme immobilization. Chem Eng J. 2019;359:1252–1264. doi: 10.1016/j.cej.2018.11.052. [DOI] [Google Scholar]
- 24.Patel SKS, Kalia VC, Choi JH, Haw JR, Kim IW, Lee JK. Immobilization of laccase on SiO2 nanocarriers improves its stability and reusability. J Microbiol Biotechnol. 2014;24:639–647. doi: 10.4014/jmb.1401.01025. [DOI] [PubMed] [Google Scholar]
- 25.Patel SKS, Otari SV, Kang YC, Lee JK. Protein-inorganic hybrid system for efficient his-tagged enzymes immobilization and its application in L-xylulose production. RSC Adv. 2017;7:3488–3494. doi: 10.1039/c6ra24404a. [DOI] [Google Scholar]
- 26.Yang WY, Min DY, Wen SX, Jin L, Rong L, Tetsuo M, Chen Bo. Immobilization and characterization of laccase from Chinese Rhus vernicifera on modified chitosan. Process Biochem. 2006;41:1378–1382. doi: 10.1016/j.procbio.2006.01.018. [DOI] [Google Scholar]
- 27.Otari SV, Patel SKS, Kalia VC, Lee J-K. One-step hydrothermal synthesis of magnetic rice straw for effective lipase immobilization and its application in esterification reaction. Bioresour Technol. 2020;302:122887. doi: 10.1016/j.biortech.2020.122887. [DOI] [PubMed] [Google Scholar]
- 28.Lu R, Miyakoshi T. Studies on acetone powder and purified Rhus laccase immobilized on zirconium chloride for oxidation of phenols. Enzyme Res. 2012;2012:375309. doi: 10.1155/2012/375309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ran F, Zou Y, Xu Y, Liu X, Zhang H. Fe3O4@MoS2@PEI-facilitated enzyme tethering for efficient removal of persistent organic pollutants in water. Chem Eng J. 2019;375:121947. doi: 10.1016/j.cej.2019.121947. [DOI] [Google Scholar]
- 30.Suman SK, Patnam PL, Ghosh S, Jain SL. Chicken feather derived novel support material for immobilization of laccase and its application in oxidation of veratryl alcohol. ACS Sustain Chem Eng. 2019;7:3464–3474. doi: 10.1021/acssuschemeng.8b05679. [DOI] [Google Scholar]
- 31.Kondaveeti S, Pagolu R, Patel SKS, Kumar A, Bisht A, Dad D, Kalia VC, Kim I-W, Lee J-K. Bioelectrochemical detoxification of phenolic compounds during enzymatic pre-treatment of rice straw. J Microbiol Biotechnol. 2019;29:1760–1768. doi: 10.4014/jmb.1909.09042. [DOI] [PubMed] [Google Scholar]
- 32.Sharma KK, Kuhad RC. Laccase: enzyme revisited and function redefined. Indian J Microbiol. 2008;48:309. doi: 10.1007/s12088-008-0028-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gupta V, Capalash N, Gupta N, Sharma P. Bio-prospecting laccases in the bacterial diversity of activated sludge from pulp and paper industry. Indian J Microbiol. 2017;57:75–82. doi: 10.1007/s12088-016-0624-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Singh G, Bhalla A, Kaur P, Capalash N, Sharma P. Laccase from prokaryotes: a new source for an old enzyme. Rev Environ Sci Biotechnol. 2011;10:309–326. doi: 10.1007/s11157-011-9257-4. [DOI] [Google Scholar]
- 35.Singh G, Kaur K, Puri S, Sharma P. Critical factors affecting laccase-mediated biobleaching of pulp in paper industry. Appl Microbiol Biotechnol. 2015;99:155–164. doi: 10.1007/s00253-014-6219-0. [DOI] [PubMed] [Google Scholar]
- 36.Georgieva S, Godjevargova T, Portaccio M, Lepore M, Mita DG. Advantages in using non-isothermal bioreactors in bioremediation of water polluted by phenol by means of immobilized laccase from Rhus vernicifera. J Mol Catal B Enzym. 2008;55:177–184. doi: 10.1016/j.molcatb.2008.03.011. [DOI] [Google Scholar]
- 37.Olshansky Y, Masaphy S, Root RA, Rytwo G. Immobilization of Rhus vernicifera laccase on sepiolite; effect of chitosan and copper modification on laccase adsorption and activity. Appl Clay Sci. 2018;152:143–147. doi: 10.1016/j.clay.2017.11.006. [DOI] [Google Scholar]
- 38.Mogharabi-Manzari M, Heydari M, Sadeghian-Abadi S, Yousefi-Mokri M, Faramarzi MA. Enzymatic dimerization of phenylacetylene by laccase immobilized on magnetic nanoparticles via click chemistry. Biocatal Biotransform. 2019;37:455–465. doi: 10.1080/10242422.2019.1611788. [DOI] [Google Scholar]
- 39.Wan Y-Y, Lu R, Akiyama K, Okamoto K, Honda T, Du Y-M, Yoshida T, Miyakoshi T, Knill CJ, Kennedy JF. Effects of lacquer polysaccharides, glycoproteins and isoenzymes on the activity of free and immobilised laccase from Rhus vernicifera. Int J Biol Macromol. 2010;47:76–81. doi: 10.1016/j.ijbiomac.2010.03.016. [DOI] [PubMed] [Google Scholar]
- 40.Patel SKS, Gupta RK, Kondaveeti S, Otari SV, Kumar A, Kalia VC, Lee J-K. Conversion of biogas to methanol by methanotrophs immobilized on chemically modified chitosan. Bioresour Technol. 2020;315:123791. doi: 10.1016/j.biortech.2020.123791. [DOI] [PubMed] [Google Scholar]
- 41.Kondaveeti S, Patel SKS, Pagolu R, Li J, Kalia VC, Choi M-S, Lee J-K. Conversion of simulated biogas to electricity: sequential operation of methanotrophic reactor effluents in microbial fuel cell. Energy. 2019;189:116309. doi: 10.1016/j.energy.2019.116309. [DOI] [Google Scholar]
- 42.Patel SKS, Choi H, Lee J-K. Multimetal-based inorganic–protein hybrid system for enzyme immobilization. ACS Sustain Chem Eng. 2019;7:13633–13638. doi: 10.1021/acssuschemeng.9b02583. [DOI] [Google Scholar]
- 43.Patel SKS, Kim J-H, Kalia VC, Lee J-K. Antimicrobial activity of amino-derivatized cationic polysaccharides. Indian J Microbiol. 2019;59:96–99. doi: 10.1007/s12088-018-00764-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Patel SKS, Ray S, Prakash J, Wee JH, Kim S-Y, Lee J-K, Kalia VC. Co-digestion of biowastes to enhance biological hydrogen process by defined mixed bacterial cultures. Indian J Microbiol. 2019;59:154–160. doi: 10.1007/s12088-018-00777-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Patel SKS, Jeon MS, Gupta RK, Jeon Y, Kalia VC, Kim SC, Cho B-K, Kim DR, Lee J-K. Hierarchical macro-porous particles for efficient whole-cell immobilization: application in bioconversion of greenhouse gases to methanol. ACS Appl Mater Interfaces. 2019;11:18968–18977. doi: 10.1021/acsami.9b03420. [DOI] [PubMed] [Google Scholar]
- 46.Rouhani S, Rostami A, Salimi A, Pourshiani O. Graphene oxide/CuFe2O4 nanocomposite as a novel scaffold for the immobilization of laccase and its application as a recyclable nanobiocatalyst for the green synthesis of arylsulfonyl benzenediols. Biochem Eng J. 2018;133:1–11. doi: 10.1016/j.bej.2018.01.004. [DOI] [Google Scholar]
- 47.Tarasi R, Alipour M, Gorgannezhad L, Imanparast S, Yousefi-Ahmadipour A, Ramezani A, Ganjali MR, Shafiee A, Faramarzi MA, Khoobi M. Laccase immobilization onto magnetic β-cyclodextrin-modified chitosan: improved enzyme stability and efficient performance for phenolic compounds elimination. Macromol Res. 2018;26:755–762. doi: 10.1007/s13233-018-6095-z. [DOI] [Google Scholar]
- 48.Patel SKS, Otari SV, Li J, Kim DR, Kim SC, Cho B-K, Kalia VC, Kang YC, Lee J-K. Synthesis of cross-linked protein-metal hybrid nanoflowers and its application in repeated batch decolorization of synthetic dyes. J Hazard Mater. 2018;347:442–450. doi: 10.1016/j.jhazmat.2018.01.003. [DOI] [PubMed] [Google Scholar]
- 49.Singh G, Bhalla A, Capalash N, Sharma P. Characterization of immobilized laccase from γ-proteobacterium JB: approach towards the development of biosensor for the detection of phenolic compounds. Indian J Sci Technol. 2010;3:48–53. doi: 10.17485/ijst/2010/v3i1/29643. [DOI] [Google Scholar]
- 50.Angural S, Rana M, Sharma A, Warmoota R, Puri N, Gupta N. Combinatorial biobleaching of mixedwood pulp with lignolytic and hemicellulolytic enzymes for paper making. Indian J Microbiol. 2020;60:383–387. doi: 10.1007/s12088-020-00867-6. [DOI] [PMC free article] [PubMed] [Google Scholar]