Abstract
Male reproductive dysfunction is one of the common complications of diabetes mellitus that causes infertility. This study was designed to investigate the protective effect of Momordica cymbalaria (M. cymbalaria) extracts on diabetes mediated reproductive toxicity in male Wistar rats. The induction of diabetes was performed using a single intraperitoneal injection of alloxan (120 mg/kg). Skin and seed extracts (250 and 500 mg/kg) of M. cymbalaria were orally administered to alloxan-induced diabetic male rats for 28 days. Postprandial blood glucose (PBG) levels were recorded at 7-day interval for four weeks. The effects of the treatment on blood glucose, weight of reproductive organs, sperm count, testosterone levels, antioxidant capacity, and histomorphology were evaluated. Treatment with the above extracts of M. cymbalaria significantly (p < 0.05) improved the reproductive parameters as well as the antioxidant levels superoxide dismutase (SOD) and glutathione-s-transferase (GST) in the diabetic rats. Also, oral treatment with M. cymbalaria extracts significantly reduced the PBG and malondialdehyde (MDA) levels. Further, it revived the histomorphology of reproductive organs in diabetic rats. Interestingly, skin extract at a dose of 500 mg/kg was found to be more efficient in elevating the level of testosterone and sperm count in the diabetic rats. Based on the results, it is clear that M. cymbalaria not only regulates the postprandial blood glucose levels but also improves the reproductive health in the diabetic state.
Keywords: Male infertility, Diabetes mellitus, Anti-diabetic, Momordica cymbalaria, Antioxidant activity, Reproductive health
Introduction
Diabetes mellitus is a chronic multifactorial disease, characterized by prolonged hyperglycemia that arises from the inability to produce insulin or utilize it properly, which leads to the alterations in carbohydrate, fat, and protein metabolism. Due to factors like lifestyle changes, stress, obesity, and physical inactivity, the incidence of diabetes have been rising in men of reproductive age (Agbaje et al. 2007; Lascar et al. 2018). Even though it is well known that diabetes mellitus generates several severe complications, male infertility is one among them which remains a major challenge due to its negative impact on the social–cultural and emotional space of an individual. Shreds of evidence suggest that the increased incidence of diabetes mellitus has been strongly linked with reducing birth rates and infertility (Lutz 2006; Hamilton and Ventura 2006; Barkabi-Zanjani et al. 2020). If this increasing pattern in the occurrence of diabetes continues, this will end up turning more men as infertile earlier to and at the childbearing stage in the next few years (Maresch et al. 2017).
It has been reported that diabetes mellitus can affect the right functioning of male reproductive organs at various levels by interfering with spermatogenesis, sperm maturation, and linked reproductive processes. Several epidemiological surveys show that about 51% of the diabetic population had suffered from various stages of reproductive complications (La et al. 2009; Jiang et al. 2014; Maiorino et al. 2014; Shi et al. 2017). Numerous studies have demonstrated the decline in reproductive functions using diabetic male animal models (Ballester et al. 2004; Scarano et al. 2006; Amaral et al. 2006). Specifically, persistent hyperglycemia has been recognized as a significant factor of diabetes mellitus which leads to the excessive production of free radicals and suppression of antioxidant enzymes in vital organs of the human system (Agarwal et al. 2014). As a consequence, proper metabolic functioning of major organs namely the liver, heart, kidney, and testis will get deteriorated (Abtahi-Evari et al. 2017; Rowley et al. 2017). Diabetes mellitus has been proposed to cause male fertility through factors such as oxidative stress, sperm nuclear DNA fragmentation, hypogonadism, immotility of sperms, and generation of enzymatic glycation end products (Condorelli et al. 2018). Furthermore, it perturbs the mitochondrial bioenergy generation, thus contributing to mitochondrial DNA deletions and alterations in mitochondrial DNA copy number which exaggerates the damage to a greater extent as the mitochondrial DNA is devoid of protective histones, antioxidant-rich cytoplasm, and DNA repair mechanisms compared with the genomic DNA (Wu et al. 2018). The rising prevalence of infertility in men caused by diabetes has received much attention because of its effect on physical and psychological well-being.
Accumulating evidence suggests that antioxidant therapy can decrease the oxidative stress created during the diabetic condition as well as increase the fertility rate in men (Gharagozloo et al. 2011; Adewoyin et al. 2017). So far, hormones and anti-diabetic drugs are the main medications available for the treatment of reproductive dysfunction in males but they are associated with many side effects at the endocrine level. Concomitantly, the cost of treatment poses a great public challenge in developing countries. Meanwhile, natural products are widely preferred as they are easily available and free of harmful side effects (Ajazuddin et al. 2014). Therefore, it is necessary to find a natural alternative with protective effects against diabetic-induced reproductive dysfunction that may improve the reproductive health of diabetic men. Momordica cymbalaria Hook. F (family, Cucurbitaceae) is a perennial climber mostly distributed in southern areas of India, namely Tamil Nadu, Andhra Pradesh, Karnataka, Madhya Pradesh, and Maharashtra. This commonly consumed vegetable has been reported for anti-diabetic, cardioprotective, hepatoprotective, and nephroprotective activities (Abbirami et al. 2019a, b). Our previous studies revealed the in vitro anti-diabetic, antioxidant, and in vivo anti-diabetic properties of M. cymbalaria (Elangovan et al. 2019). As M. cymbalaria is well reputed for its anti-diabetic activity, we hypothesize that it could also assist in ameliorating the male reproductive dysfunction due to its anti-oxidative and anti-diabetic properties. Hence, the present study was designed to examine the effect of M. cymbalaria on male reproductive system of alloxan-induced diabetic rats.
Materials and methods
Chemicals
Alloxan monohydrate was purchased from Sigma Co. (St. Louis, MO, USA). Glibenclamide (Daonil®) was obtained from Emcure Pharmaceuticals, India. All other reagents used in the study were of analytical grade.
Preparation of plant extracts
M. cymbalaria was procured from the local market of Sivakasi (Tamil Nadu, India), taxonomically identified by the Department of Botany, St Joseph’s College, Tiruchirappalli. A voucher with specimen number, SJCBOT2561, has been deposited at the same institute. Lyophilized samples of skin and seed were ground into a fine powder using an electric grinder and then extracted with ethanol and methanol, respectively. 10 g of plant sample was extracted using 100 ml of aforesaid solvents in a Soxhlet apparatus for 8 h. To obtain the crude extracts of the vegetable parts, the solvent extracts were subjected to evaporation under reduced pressure at 55 ºC using the rotary evaporator (Buchi R-210, Flawil, Switzerland) and the crude extracts were used for the animal study.
Experimental animals
Male albino Wistar rats (150—200 g) were procured from Kerala Veterinary and Animal Sciences University, Thrissur, Kerala and housed in polypropylene cages and maintained under standard laboratory conditions (temperature 20 ± 2 °C, relative humidity 60–70% and 12 h dark–light cycles) with free access to standard rat feed (Sai Durga feeds and food stocks, Chennai, India) and water. All animals were acclimatized to laboratory conditions for 7 days before the commencement of the experiment. Before the commencement of the study, the experimental protocols were approved by the Institutional Animal Ethical Committee of Bharathidasan University (BDU/IAEC/P16/2018/07.08.2018), Tiruchirappalli, Tamil Nadu, India.
Acute oral toxicity study
The acute toxicity study of the ethanol skin and methanol seed extracts of M. cymbalaria was performed by following the Organization of Economic Cooperation and Development (OECD) guidelines No. 423 (OECD 2001). The animals were fasted overnight before the experiment but had free access to water. M. cymbalaria extracts were administered by oral gavage in an increasing concentration of up to 2000 mg/kg b.w. All the animals were carefully observed for the first four hours and then every day over 14 days for signs of toxicity such as mortality, salivation, drowsiness, restlessness, writhing, convulsions, urination, asthenia, and defecation after oral administration of the extracts.
Induction of diabetes mellitus
After acclimatization, diabetes was induced in overnight fasted male rats by a single intraperitoneal injection of freshly prepared alloxan monohydrate (120 mg/kg b.w.) dissolved in ice-cold physiological saline (0.9% NaCl). The fasting blood glucose level was determined after 72 h of alloxan injection using the single-touch glucometer by collecting blood from the tail of all experimental rats. Experimental rats with fasting blood glucose level above 200 mg/dl were considered as diabetic and chosen for the study (Hamzah et al. 2018).
Experimental design
The male rats were divided into seven groups with each group consisting of six animals. Except for Group 1, which served as normal control, all other groups were comprised of diabetic rats. Group 2 served as diabetic control, received alloxan (120 mg/kg b.w. i.p.). Groups 3 and 4 received skin ethanol extract of M. cymbalaria (250 mg/kg and 500 mg/kg b.w. p.o., respectively). Groups 5 and 6 received seed methanol extract of M. cymbalaria (250 mg/kg and 500 mg/kg b.w. p.o., respectively) and Group 7 received the standard drug, Glibenclamide (0.6 mg/kg b.w. p.o.) daily for 28 days.
Postprandial blood glucose
Postprandial blood glucose levels were monitored on the 0, 7th, 14th, 21st, and 28th days of the study. Blood was collected via tail vein puncture and glucose levels were measured by glucometer (Johnson & Johnson, U.S.A).
Sample collection
On the last day of the experiment, rats were fasted overnight and anesthetized through intraperitoneal administration of pentobarbital (45 mg/kg b.w). Whole blood was collected through cardiac puncture and kept at room temperature for sedimentation. The serum obtained after centrifugation at 3,000 rpm for 10 min was stored at – 20 °C for biochemical analysis. The reproductive organs (testis and epididymis) were excised immediately, rinsed in cold phosphate buffer (0.1 M, pH 7.4), weighed, and subsequently processed for carrying out the antioxidant and histological assessments.
Determination of sperm count and testosterone
Sperm count was carried out according to the method demonstrated by Han et al. (2019) with slight modifications. The right epididymal tissues of the rats were dissected and minced with surgical scissors in 2 ml of ice-cold saline (0.9% NaCl) followed by incubation in a water bath at 37 °C for 5 min. Diluting medium (50 g of sodium bicarbonate in 10 ml of 40% formalin) at a ratio of 1:100 was used to dilute the highly concentrated semen sample obtained from the experimental rats. Semen sample (15 µl) was loaded into a Neubauer counting chamber and the number of sperms in the central square was counted under a light microscope (400× magnification). Serum testosterone level was measured using standard protocols prescribed in the ELISA kit, Monobind Inc, USA (Product code–3725-300).
Antioxidants and malondialdehyde (MDA) levels in reproductive organs
The testicular and epididymal tissues were immediately collected after euthanasia for the analysis of MDA and antioxidant levels. Subsequently, tissue samples were chopped with surgical scissors and homogenized, respectively in ice-cold phosphate buffer (0.1 M, pH 7.4) using glass homogenizer. The homogenate was centrifuged at 10,000 rpm for 10 min at 4 ºC and the supernatant was subsequently used for the analysis. The antioxidant enzyme, superoxide dismutase was estimated according to the method of Pinto et al. (2018), and the absorbance of the enzyme at 420 nm, was expressed as U/mg protein. Also, the antioxidant activity of Glutathione S-transferase was conducted by following the method of Abarikwu et al. (2018) and expressed as units/mg protein. The absorbance of the reaction product was read at 340 nm and was expressed as U/ mg protein. Further, the lipid peroxidation assay was performed as previously described by Mohamed et al. (2018), and the metabolic end-product malondialdehyde expressed in nmol/ mg protein using the thiobarbituric acid test (TBA-test).
Histopathological evaluations
Reproductive organs were removed and washed in ice-cold saline solution and immediately fixed in 10% neutral buffered formalin solution. Five micron thick sections were cut and stained with hematoxylin and eosin and observed for possible histopathological observations. Histological evaluations were evaluated with a light microscope at 400× magnification. To measure the testicular injury, the degree of histological alterations were numerically evaluated by the mean Johsen score (MJS) ranged from 1 to 10 as formerly described by Johnsen (1970). Table 3. Shows the criteria for the histological assessment of the testicular injury.
Table 3.
Johnsen’s testicular score for histological assessment
| Score | Histological description |
|---|---|
| 10 | Full spermatogenesis and perfect tubules |
| 9 | Disorganized spermatogenesis; slightly impaired spermatogenesis |
| 8 | A few spermatozoa present |
| 7 | No spermatozoa but many spermatids present |
| 6 | No spermatozoa, only a few spermatids present |
| 5 | No spermatozoa or spermatids but many spermatocytes present |
| 4 | Only a few spermatocytes present |
| 3 | No spermatocytes only spermatogonia present |
| 2 | No germ cells but only Sertoli cells present |
| 1 | No germ cells and no Sertoli cells present |
Statistical analysis
All data were expressed as mean ± standard deviation. Statistical package for social sciences (SPSS) version 25 (IBM Corporation, Armonk, NY, USA) was used to analyze the data. Statistical analyses were performed using one-way analysis of variance (ANOVA) followed by Duncan's multiple range test. All graphical representations were executed using GraphPad Prism version 6.0 (GraphPad Software, Inc., La Jolla, CA, USA). Values of p < 0.05 were considered significant.
Results
Acute oral toxicity study
The animals treated with doses up to 2000 mg/kg of M. cymbalaria did not show any symptom of toxic reaction or lethality during the experimental period. Acute toxicity studies revealed that the M. cymbalaria extracts were safe and non-toxic up to 2000 mg/kg of body weight. Hence, the doses 250 and 500 mg/kg b.w. were selected for the present study.
Measurement of reproductive organs weight
Reproductive organ weights such as testis and epididymis of the experimental animals were given in Table 1. Treatment of skin extracts at concentrations 250 and 500 mg/kg, increased the weight of the right and left testes in the diabetic rats (Groups 3 and 4) when compared to the diabetic control group. Moreover, the testis weight of the skin extract-treated group was similar to that of the control group. On the other hand, the testes weights of the seed extract-treated groups (Groups 5 and 6) were lower than the lower dose of the skin extract-treated group (Group 3) but higher than the diabetic control group. Besides, the epididymal weights of the untreated diabetic control rats were significantly lower than the normal control and M.cymbalaria extract-treated diabetic rats. However, all M.cymbalaria extract-treated diabetic groups showed a significant increase (p < 0.05) in the weight of epididymis than the standard drug, Glibenclamide-treated group 7.
Table 1.
Effect of M. cymbalaria extracts on reproductive organ weights in alloxan-induced diabetic male rats
| *Group | Organ weight (g) | |||
|---|---|---|---|---|
| Right testis | Left testis | Right epididymis | Left epididymis | |
| Group 1 | 1.437 ± 0.14a | 1.4290 ± 0.09a | 0.8547 ± 0.02a | 0.7407 ± 0.02a |
| Group 2 | 1.262 ± 0.06b | 1.2003 ± 0.08b,c | 0.6123 ± 0.06d | 0.5280 ± 0.01c |
| Group 3 | 1.4067 ± 0.06a,b | 1.3640 ± 0.05a | 0.7100 ± 0.01b,c | 0.7107 ± 0.03a |
| Group 4 | 1.3013 ± 0.06a,b | 1.3227 ± 0.08a,b | 0.6743 ± 0.02b,c,d | 0.7080 ± 0.04a |
| Group 5 | 1.3567 ± 0.05a,b | 1.1960 ± 0.08b,c | 0.7300 ± 0.04b | 0.6643 ± 0.05a,b |
| Group 6 | 1.2967 ± 0.00a,b | 1.0683 ± 0.07c | 0.6403 ± 0.01c,d | 0.7173 ± 0.00a |
| Group 7 | 1.3847 ± 0.09a,b | 1.2897 ± 0.06a,b | 0.6037 ± 0.07d | 0.6303 ± 0.07b |
*Group 1 – Normal control; Group 2 – Diabetic control; Group 3 – Skin extract (250 mg/kg b.w.); Group 4 – Skin extract (500 mg/kg b.w.); Group 5 – Seed extract (250 mg/kg b.w.); Group 6 – Seed extract (500 mg/kg b.w.); Group 7 – Glibenclamide (0.6 mg/kg). The results are presented as mean ± standard deviation (n = 6). Values with different superscripts (a–d) are significantly different from each other (p < 0.05)
Effect of M.cymbalaria on postprandial blood glucose (PBG) levels
As presented in Table 2, postprandial glucose levels recorded were significantly higher in Group 2, i.e., untreated diabetic group compared with the control group (p < 0.05). A stable PBG level was observed in Group 1 animals throughout the experimental period. In Group 2 (diabetic control group), the PBG level was observed as 358 mg/dl on the 28th day of the study. The PBG level after the treatment with skin extract 500 mg/kg (Group 4) was reduced to 85 mg/dl and further to 73 mg/dl at the 21st and 28th days of the study, respectively. Meanwhile, seed extract at a concentration of 500 mg/kg also significantly decreased the PBG level (89 mg/dl), which was slightly lower than the skin extract-treated groups (Groups 3 and 4) and higher than the untreated diabetic group. However, PBG level reduced by the skin extract-treated group at the 28th day (Group 3—81.33 mg/dl) was lesser than the standard drug, Glibenclamide-treated Group 7, which showed 86 mg/dl at the 28th day of the experiment Fig. 1.
Table 2.
Effect of M. cymbalaria extracts on postprandial blood glucose levels in alloxan-induced diabetic male rats
| *Group | Postprandial blood glucose (mg/dl) | ||||
|---|---|---|---|---|---|
| Day 0 | Day 7 | Day 14 | Day 21 | Day 28 | |
| Group 1 | 105.33 ± 4.0f | 79.00 ± 2.0d | 129.66 ± 5.7f | 95.33 ± 3.5e | 101.66 ± 5.1c |
| Group 2 | 450.66 ± 2.5b | 469.33 ± 4.7a | 368 ± 2.0a | 381 ± 2.0a | 358 ± 2.0a |
| Group 3 | 439.33 ± 1.5c | 414.33 ± 4.5b | 338 ± 3.0b | 146.33 ± 5.5d | 81.33 ± 4.1c,d,e |
| Group 4 | 419.33 ± 3.5d | 269.33 ± 3.0c | 190.33 ± 6.5e | 85 ± 8.8e | 73 ± 5.1e |
| Group 5 | 475.33 ± 4.5a | 418.33 ± 3.0b | 366.33 ± 4.5a | 305 ± 6.0b | 173 ± 4.0b |
| Group 6 | 422.00 ± 3.0d | 268.00 ± 7.0c | 223.00 ± 6.02d | 213 ± 7.2c | 89 ± 7.5d |
| Group 7 | 395.00 ± 7.5e | 410.66 ± 5.5b | 236.66 ± 6.0c | 141.66 ± 5.6d | 86 ± 4.5d |
*Group 1 – Normal control; Group 2 – Diabetic control; Group 3 – Skin extract (250 mg/kg b.w.); Group 4 – Skin extract (500 mg/kg b.w.); Group 5 – Seed extract (250 mg/kg b.w.); Group 6 – Seed extract (500 mg/kg b.w.); Group 7 – Glibenclamide (0.6 mg/kg). The results are presented as mean ± standard deviation (n = 6). Values with different superscripts (a–f) are significantly different from each other (p < 0.05)
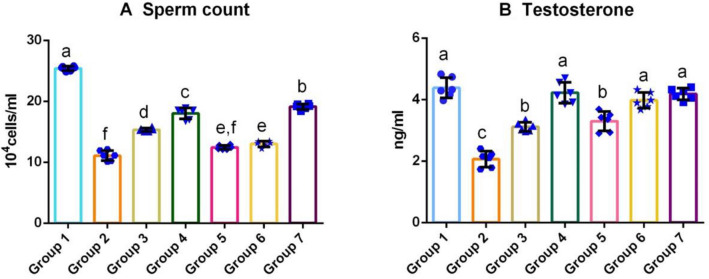
Fig. 1.
Effect of M. cymbalaria extracts on sperm count (a) and testosterone levels (b) in the testicular tissue of alloxan-induced diabetic rats. Group 1 – Normal control; Group 2 – Diabetic control; Group 3 – Skin extract (250 mg/kg b.w.); Group 4 – Skin extract (500 mg/kg b.w.); Group 5 – Seed extract (250 mg/kg b.w.); Group 6 – Seed extract (500 mg/kg b.w.); Group 7 – Glibenclamide (0.6 mg/kg). Bars represent mean ± standard deviation (n = 6). Bars with different superscripts (a–f) differ significantly from each other (p < 0.05)
Effect of M.cymbalaria on sperm count
As presented in Fig. 2a, sperm count was significantly decreased in the untreated diabetic group in comparison with the control (Group 1). Similar to the results of testosterone levels, the sperm count was observed to be greater in the skin extract-treated group (Group 4) than other M.cymbalaria extract-treated groups. Further, the sperm count of the skin extract-treated group (Group 3) was higher than both the seed extract-treated groups (Groups 5 and 6). At the same time, the seed extract-treated group (Group 4) showed a higher number of sperm cells when compared to the untreated diabetic group (Group 2).
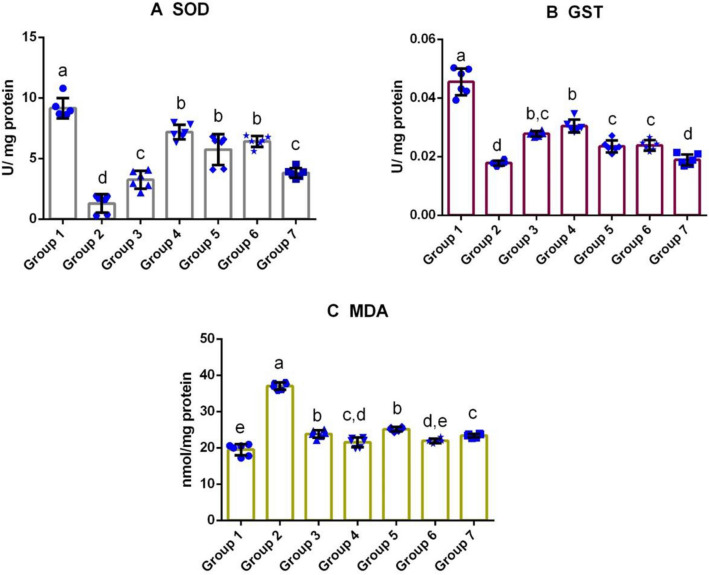
Fig. 2.
Effect of M. cymbalaria extracts on antioxidant enzymes and lipid peroxidation in testicular tissue of alloxan-induced diabetic rats. a The effect of M. cymbalaria extracts on the level of superoxide dismutase (SOD), b The effect of M. cymbalaria extracts on the level of glutathione S-transferase (GST) and c The effect of M. cymbalaria extracts on malondialdehyde (MDA) content. Group 1 – Normal control; Group 2 – Diabetic control; Group 3 – Skin extract (250 mg/kg b.w.); Group 4 – Skin extract (500 mg/kg b.w.); Group 5 – Seed extract (250 mg/kg b.w.); Group 6 – Seed extract (500 mg/kg b.w.); Group 7 – Glibenclamide (0.6 mg/kg). Bars represent mean ± standard deviation (n = 6). Bars with different superscripts (a–e) differ significantly from each other (p < 0.05)
Effect of M.cymbalaria on testosterone levels
The untreated diabetic rats (Group 2) showed a significant decrease in the testosterone level than all other experimental groups. The testosterone level of Group 4 was comparable with that of the animals treated with the standard drug, Glibenclamide (Group 7). M.cymbalaria extract-treated groups showed a considerable increase in the testosterone levels when compared with the untreated diabetic group (Fig. 2b).
Effect of M.cymbalaria on malondialdehyde levels
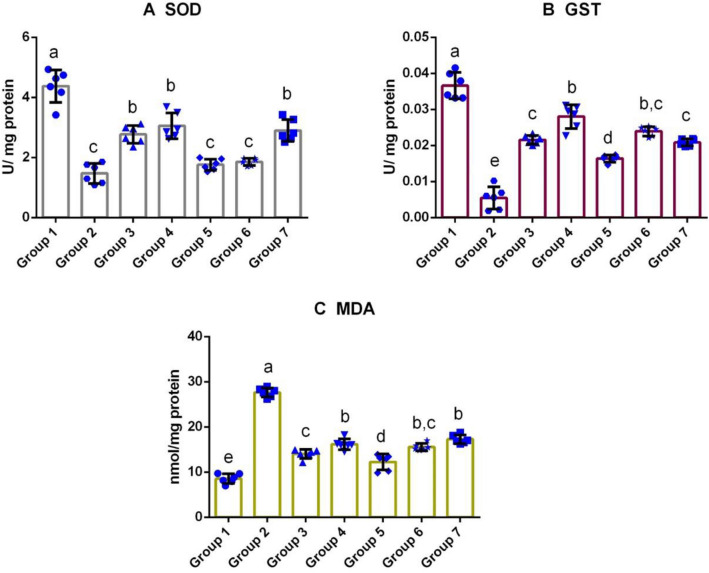
Significantly increased level of MDA was recorded in the testis and epididymal tissues of the untreated diabetic group. Treatment with a higher dose of the seed and skin extracts, significantly reduced the MDA concentration in the testis of the Groups 4 and 6, whereas the testis of the rats treated with Glibenclamide showed slightly elevated levels of MDA than the Group 1. No significant difference was detected between the groups that received the lower doses of extracts. Interestingly, the epididymis of rats treated with the lower dose of M. cymbalaria seed and skin extracts showed a lower level of lipid peroxidation than other M. cymbalaria-treated diabetic groups. The observed MDA level in the skin extract (Group 4)-treated group was statistically similar to the standard drug, Glibenclamide treated, Group 7. Whereas the untreated diabetic group exhibited a higher amount of lipid peroxidation among the experimental groups (Figs. 2c and 3c).
Fig. 3.
Effect of M. cymbalaria extracts on antioxidant enzymes and lipid peroxidation in epididymal tissue of alloxan-induced diabetic rats. a The effect of M. cymbalaria extracts on the level of superoxide dismutase (SOD), b The effect of M. cymbalaria extracts on the level of glutathione S-transferase (GST) and c The effect of M. cymbalaria extracts on malondialdehyde (MDA) content. Group 1 – Normal control; Group 2 – Diabetic control; Group 3 – Skin extract (250 mg/kg b.w.); Group 4 – Skin extract (500 mg/kg b.w.); Group 5 – Seed extract (250 mg/kg b.w.); Group 6 – Seed extract (500 mg/kg b.w.); Group 7 – Glibenclamide (0.6 mg/kg). Bars represent mean ± standard deviation (n = 6). Bars with different superscripts (a–e) differ significantly from each other (p < 0.05)
Effect of M.cymbalaria on SOD levels
Figures 2a and 3a illustrate the assessment of SOD in the experimental rats. The study revealed a significant decline in the SOD level of the testicular tissue in the untreated diabetic rats (Group 2) when compared to the control rats, Group 1. Administration of the animals with the high dose of skin and seed extracts (Groups 4 and 6) reversed the oxidative stress significantly towards the level of the control group and also there was no significant difference in the SOD levels between those two groups. Among the experimental groups, Group 2 showed a decrease in the level of SOD than all other experimental groups. In the epididymal tissue analyses, skin extract at a dose of 500 mg/kg exhibited a significantly higher level of SOD than the other extract-treated groups (Groups 3, 5, 6, and 7) but it was lower than the control group (Group 1). In addition, SOD level of the lower dose of the skin extract-treated group (Group 3) was comparable with that of the standard drug-treated group (Group 7). However, no significant difference was observed among the following groups, Groups 1, 5, and 6.
Effect of M.cymbalaria on GST levels
As shown in Figs. 2b and 3b, the GST levels were found to be decreased in the testicular and epididymal tissues of Group 2 (diabetic control). In contrast, both the lower and higher doses of the skin extract (Groups 3 and 4) showed a significant increase in the GST levels, both in the testicular and epididymal tissues. In the case, testicular tissues, the GST level of the group administered with seed extract (500 mg/kg) was greater than the lower dose of seed extract-treated group (250 mg/kg) and the Glibenclamide-treated group. A similar pattern of GST levels was observed in the epididymal tissues of the experimental rats.
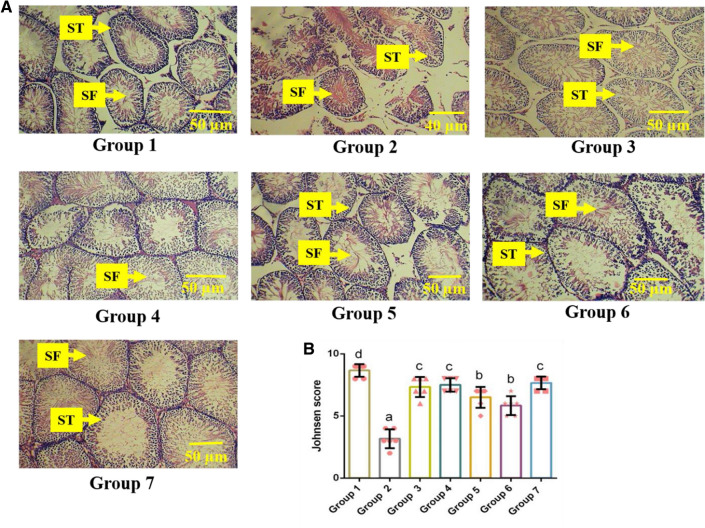
Histopathology of testes and epididymis
Figure 4a shows the alterations in the testicular histoarchitecture of the experimental groups. Given the testicular histology, Group 1 showed a normal histological arrangement of seminiferous tubules and healthy spermatozoa flagella. Conversely, the histological section of the testis from Group 2 showed tubular disorganization, severe damage in spermatozoa flagella, atrophy of germinal epithelial cells, and deteriorations in seminiferous tubules. Among the extract-treated groups, skin extracts at a concentration of 500 and 250 mg/kg reduced the atrophic condition created by diabetes. Similarly, the histomorphology of the testicular tissue section of the Glibenclamide administered group was comparable with the control group. Restoration in the histological section was observed in the seed extract-treated groups (Groups 5 and 6) with slight degenerations in the germinal cells concerning the testicular tissues. Figure 5 represents the histology of epididymis from the experimental groups. In H&E staining, the epididymis of the control group presented a clear view of normal columnar epithelium and densely arranged spermatozoa. In contrast, Group 2 showed highly distorted architecture with few germinal cells, reduced sperm density, deformed connective tissue, and cellular debris, compared to other diabetic groups. The groups treated with seed extracts (Groups 5 and 6) showed mild disruptions in the epithelium and a moderate sperm density with fewer sperms in the lumen. Concurrently, Groups 4 and 5, treated with skin extracts portrayed normal structural integrity with clearly defined spermatozoa and columnar epithelium that was on par with the control and Glibenclamide-treated groups. To further assess the impact of M. cymbalaria extracts on reproductive organs, the Johnsen score system was implemented. The changes in the spermatogenesis in the testicular tissue among the experimental groups were analyzed using the Johnsen score as presented in Table 3. The Johnsen score for Group 1 (8.66 ± 0.51) was found to be significantly (p < 0.05) higher than the diabetic groups. The scores of M. cymbalaria extracts and Glibenclamide-treated rats were significantly higher than the scores of the diabetic control rats (3.16 ± 0.75). On the other hand, there was no significant difference in the score between the lower (Group 3) and higher doses of skin-treated groups (Group 4). A similar fashion was observed in the seed extract-treated groups (Groups 5 and 6). Furthermore, the score of skin extract (7.50 ± 0.54) at a concentration of 500 mg/kg was greater than the other M. cymbalaria extract-treated groups (Fig. 4b).
Fig. 4.
Effect of M. cymbalaria extracts on histological alterations (H& E staining) in the testicular tissue of experimental animals (observation under 400X magnification). Group 1 – Representative sample of testicular section showing typical testicular architecture with normally organized germ cell layers of seminiferous tubules (ST) and spermatozoa flagella (SF) in the normal control group; Group 2 – Representative sample of testicular section showing cellular debris, degeneration and disarrangement of germ cells in the diabetic control; Group 3 – Representative sample of testicular section showing complete spermatogenesis, normal cluster of interstitial cells and germ cells in the skin extract (250 mg/kg b.w.)-treated group; Group 4 – Representative sample of testicular section showing normal alignment of spermatozoa flagella (SF) intact and matured germ cells in the skin extract (500 mg/kg b.w.)-treated group; Group 5 – Representative sample of testicular section showing complete spermatogenesis and enlarged interstitial space in the seed extract (250 mg/kg b.w.)-treated group; Group 6 – Representative sample of testicular section showing intact and matured germ cells with mild disorganization in the seed extract (500 mg/kg b.w.) treated; Group 7 – Representative sample of testicular section showing normally structured germ cell layers of seminiferous tubules (ST) and spermatozoa flagella (SF) in the Glibenclamide (0.6 mg/kg)-treated group. b Johnsen’s score in the testicular tissues of the experimental animals. Bars represent mean ± standard deviation (n = 6). Bars with different superscripts (a–d) differ significantly from each other (p < 0.05)
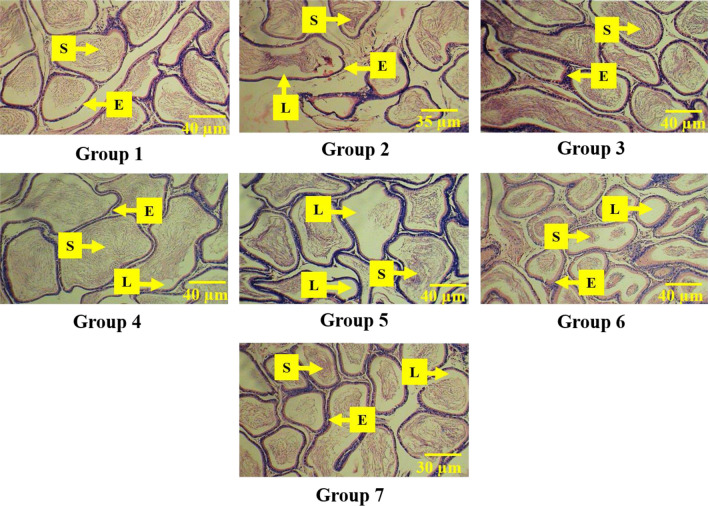
Fig. 5.
a Effect of M. cymbalaria extracts on histological alterations (H& E staining) in the epididymal tissue of experimental animals (observation under 400X magnification). Group 1 – Representative sample of epididymal section showing a typical arrangement of spermatozoa (S), epithelium (E) and lumen (L) of the normal control group; Group 2 – Representative sample of epididymal section showing severe damage and inflammatory infiltrate in the diabetic control group; Group 3 – Representative sample of epididymal section showing pronounced amount of spermatozoa (S) and well-defined epithelial layer in the skin extract (250 mg/kg b.w.)-treated group; Group 4 – Representative sample of epididymal section showing great quantity of spermatozoa (S) with intact intercellular spaces in the skin extract (500 mg/kg b.w.)-treated group; Group 5 – Representative sample of epididymal section showing thick lining of epithelial cells (E) in the seed extract (250 mg/kg b.w.) treated; Group 6 – Representative sample of epididymal section showing moderate recovery with empty lumen (L) and enlarged intercellular spaces in the seed extract (500 mg/kg b.w.)-treated group; Group 7 – normal arrangement of spermatozoa (S) and epithelial cells (E) in the Glibenclamide (0.6 mg/kg)-treated group
Discussion
Even though the anti-diabetic and antioxidant properties of M. cymbalaria in ameliorating diabetes have been confirmed by our previous study (Elangovan et al. 2019), its ability to defend the oxidative stress generated in the male reproductive organs as a consequence of diabetes remained unexplored. Therefore, the anti-diabetic and antioxidant effects of M. cymbalaria skin and seed extracts against diabetes-mediated reproductive damage in male rats were explored in this study. Diabetes mellitus is one of the most common metabolic disorders characterized by increased blood sugar which affects multiple organs such as the heart, retina, and reproductive system in both genders of animals and humans (Adedara et al. 2019; Shoorei et al. 2019). Most importantly, the main complication of diabetes has been reported to be its effect on the male reproductive system, with numerous medical studies documenting a reduction in androgens level, sperm count, motility, viability, and abnormal sperm morphology (Wankeu-Nya et al. 2019).
Many studies have reported that hyperglycemia plays a vital role in reproductive dysfunction mediated by oxidative stress. Hence, hyperglycemia-induced oxidative stress has been considered as a significant factor affecting the testicular function through the excessive production of reactive oxygen species (ROS) that disrupt the ability of the body to counteract free radicals by either enzymatic or non-enzymatic antioxidants (Akinyemi et al. 2015; Oguntibeju et al. 2020). Therefore, mitigation of postprandial glucose has been viewed as a significant factor to neutralize the oxidative stress and secondary complications in diabetes mellitus (Smolders et al. 2017). Since oxidative stress serves as a key factor in promoting the reproductive dysfunction in testis and epididymis, antioxidants could be used to counteract those deleterious effects (Shrilatha and Muralidhara 2007). To investigate the reproductive damage caused by diabetes, alloxan was used to induce diabetes in the experimental male rats. It is a sugar analog, which is known to cause diabetes mellitus by generating reactive species like superoxide and hydrogen peroxide radicals as well as inhibiting the predominantly expressed glucose sensor glucokinase, in the liver and pancreatic beta cells (Fujieda et al. 2018; Lin et al. 2019).
In this study, the observed increase in the postprandial blood glucose level in alloxan-induced male experimental rats confirmed the diabetic condition. Particularly, a stable diabetic state was observed in the alloxan (120 mg/kg)-induced untreated diabetic group throughout the experimental period. On the other hand, administration of skin extracts remarkably reduced the postprandial hyperglycemia in the alloxan-induced diabetic rats. Various studies have documented that enzyme inhibitors such as α-glucosidase and α-amylase could predominantly decrease the postprandial increase of blood glucose and associated complications in diabetes mellitus (Jang et al. 2018; Pradeep and Sreerama 2018; Wu et al. 2019). Moreover, this is in congruence with our previous study that demonstrated the α-amylase [IC50 value of skin—185.1 µg/ml; IC50 value of seed—231 µg/ml] and α-glucosidase [IC50 value of skin—245.9 µg/ml; IC50 value of seed—224.4 µg/ml] inhibitory activities of M. cymbalaria at five different concentrations (50– 250 µg/ml). The inhibitory potential of M. cymbalaria extracts against the enzymes was compared with the IC50 value of the reference standard, acarbose [α-amylase—79.79 µg/ml; α- glucosidase—104.3 µg/ml] (Abbirami et al. 2019a, b).
Similarly, Marella et al. (2016) stated the effect of M.cy protein, a 17-kDa anti-hyperglycemic protein, isolated from the aqueous fruit extract of M. cymbalaria on the enzymes, α- glucosidase and aldose reductase. The IC50 was detected to be 0.16 mg/ml and 0.22 mg/ml for α-glucosidase and aldose reductase, respectively. Furthermore, a study by Firdous et al. (2009) investigated the anti-diabetic activity of saponin fraction obtained from the root of M. cymbalaria using Streptozotocin (STZ)–Nicotinamide (240 mg/kg, i.p)-induced diabetic mice. In this study, treatment with the saponin fraction (175 mg/kg b.w., p.o/30 days), decreased the blood glucose level (107.6 ± 2.51 mg/dl) which was slightly higher than the reference compound, metformin (90.46 ± 1.14 mg/dl) at a concentration of 350 mg/kg and lower than the diabetic control (163.41 ± 2.12 mg/dl). Interestingly, the results of the present work positively correlate with the above-mentioned studies suggesting the ability of M. cymbalaria to regulate the postprandial glucose elevation of glucose levels in the diabetic state.
A decrease in the organ weight, sperm count, testosterone level, and increase in the sperm deformities are the common symptoms of diabetic-mediated reproductive impairment (Laleethambika et al. 2019). Opuwari and Monsees (2020) reported that a reduction of testicular weight is a reflective parameter for the interpretation of male gonadal toxicity. The results of the current study showed that there was a significant decrease in the weights of testis and epididymis in the diabetic control group when compared with the control group. Administration of M. cymbalaria extracts improved the weights of the reproductive organs of the diabetic rats. Our outcomes were consistent with a study by Li et al. (2019) that documented the effect of vitexin, a major bioactive flavonoid isolated from Trigonella foenum-graecum in regulating the weight of reproductive organs in STZ-induced diabetic mice. Three different concentrations of vitexin (10, 20, and 40 mg/mg) for a period of 62 days reversed the weight of the reproductive organs of diabetic animals. Apart from this, many flavonoids namely hesperetin, quercetin, curcumin, catalpol (Kanter et al. 2012; Rashid and Sil 2015; Samie et al. 2018; Jiao et al. 2020) have been shown to revive the reproductive organ weights in diabetic rats. Likewise, in this study, the administration of M. cymbalaria improved the weight of reproductive organs in the experimental rats. This may be due to the flavonoid content of M. cymbalaria as reported by our previous study (Abbirami et al. 2019a, b).
Additionally, sperm count has been considered an indispensable parameter in the assessment of the proper functioning of the reproductive system. It has been reported that reactive oxygen species destruct the biochemical structures of the cell membranes as they own a high affinity to polyunsaturated fatty acids (PUFA). Sperm cells contain an enormous amount of PUFA which makes them susceptible to the free radical attack. In a diabetic state, the amplified oxidative stress increases the lipid peroxidation of PUFA which in turn causes DNA damage in the sperm cell membrane (Shooeri et al. 2019). This is supported by diabetic-mediated depletion in the sperm count in the diabetic control group and restoration of sperm count in the M. cymbalaria skin extract-treated groups in the present study. In connection with the decreased MDA content in the M. cymbalaria extract-treated diabetic groups, it is clear that the administration of M. cymbalaria might have protected the sperm cells from lipid peroxidation Besides, some other studies have also reported the antioxidant effects of M. cymbalaria in oxidative stress parameters on various tissues of diabetic rats such as dorsal root ganglion (DRG) neurons, pancreas, liver, and kidney tissue in earlier studies (Koneri et al. 2014; Marella et al. 2016; Elangovan et al. 2019). Therefore, the increment of sperm count in the extract-treated groups may be connected with the free radical scavenging ability of M. cymbalaria.
In the context of male infertility developed by diabetes, it has been demonstrated that testosterone level plays an important role in the spermatogenesis (Rato et al. 2015; Choubey et al. 2020). Additionally, testosterone is one of the chief products of androgen synthesis that acts as a biomarker for testicular damage. In continuation of the sperm count, the level of the reproductive hormone, testosterone was determined in all experimental groups. The results of the present study indicated a significant decrease in the testosterone level in the diabetic control rats as compared to the normal control rats. Our outcomes are in line with the formerly published studies that reported the induction of diabetes by alloxan in male rats leads to a decrease in the testosterone level (Ghlissi et al. 2013; Ojewale et al. 2014; Sebai et al. 2015; Akinola et al. 2015; Ahmed et al. 2020). It has also been documented that a decline in the function of two important cells, namely Leydig and Sertoli is linked with a decrease in the production of insulin (Minaz et al. 2019). In this study, treatment with M. cymbalaria extracts improved the testosterone level in the diabetic rats which could be due to the anti-diabetic effect of M. cymbalaria as published by us previously (Elangovan et al. 2019).
Our biochemical findings showed that the sperm count and testosterone levels were significantly lower in the diabetic control group, whereas significantly higher in the diabetic groups treated with M. cymbalaria. Notably, the skin extract at a concentration of 500 mg/kg was found to be more potent in increasing the sperm count and testosterone level in the experimental animals. This might be due to the presence of bioactive compounds in M. cymbalaria that could stimulate the hypothalamic–pituitary axis endocrine activity followed by the secretion of testosterone in the Leydig cell (Maneesh et al. 2006). Even though several factors that have been reported to influence the development of diabetes mellitus, oxidative stress is undeniably one among them. Hyperglycemia-mediated free radical damage is now viewed as the triggering factor for the generation of diabetes-related complications (Abdullah et al. 2018; Maremanda et al. 2020). In diabetes, oxidative stress is driven by many pathways, for example, in the polyol pathway, production of advanced glycation end products (AGE) and protein kinase C down-regulates the activity of antioxidant enzymes inside the system causing damage to major organs (Rains et al. 2011; Vlassara et al. 2013). Accumulating shreds of evidence suggest that this oxidative stress can cause DNA damage in the testicular and epithelial tissues which in turn can contribute to the poor quality of sperms. Moreover, it leads to the irregular secretion of gonadotropins, abnormal spermatogenesis, defective semen quality, and anomalous apoptosis in testes (Alves et al. 2013; Muratori et al. 2015; Roessner et al. 2012). Also, DNA damage in sperm is known to be associated with low embryo quality, decreased embryo implantation rates, greater miscarriage rates, and health complications in the next generation (Ammar et al. 2019; Ni et al. 2019).
Furthermore, MDA is an important marker of lipid peroxidation that plays a crucial role in male infertility (Karimi et al. 2011; La et al. 2009). Additionally, the biochemical processes like lipid peroxidation, protein glycation reactions, and auto-oxidation of glucose molecules induced by hyperglycemia result in the excessive formation of free radicals like superoxide, hydroxyl, and peroxyl radicals and exhaustion of the endogenous antioxidants such as SOD and GST that convert the reactive radicals into harmless non-radical products (La Vignera et al. 2019). Hence, the damage in reproductive tissues, as a consequence of diabetes, is mainly due to either the disturbance in pro-oxidant–antioxidant balance or an immoderate amount of oxidative stress produced by free radicals. In the current study, MDA content was noticed to be elevated and the level of SOD and GST were reduced in the reproductive tissues (testis and epididymis) of the diabetic control group when compared with the control group. Treatment with skin and seed extracts of M. cymbalaria significantly reduced diabetes-induced lipid peroxidation and elevated the activity of antioxidant enzymes such as SOD and GST in the treated diabetic groups.
Many studies have reported that antioxidant enzymes can transform free radicals into the neutralized products through their scavenging properties in male reproductive organs (Seven et al. 2020). However, the outcomes of the current work were in agreement with a study that showed the treatment with methanolic leaf extract of Mallotus roxburghianus alleviated alloxan-induced diabetic reproductive toxicity, decreased the level of MDA, and increased the activity of SOD and GST (Roy et al. 2015). According to our previous study, the maximum quantity of total phenolics and flavonoids can be extracted from the skin when ethanol is used as an extraction solvent. In contrast, the maximum quantity of the same compounds can be extracted when methanol is used as an extraction solvent for seeds (Abbirami et al. 2019a, b). Apparently, the phenolic and flavonoid compounds in M. cymbalaria might have contributed to the antioxidant activity in the present study. Besides, extract of the plant M. cymbalaria, a natural inhibitor of free radicals, is reported to scavenge 2,2-diphenyl-1-picrylhydrazyl (DPPH), 2,2′-azino-bis-3-ethylbenzotiazolin-6-sulfonic acid (ABTS), NO, and increase endogenous antioxidants such as catalase (CAT), glutathione peroxidase (GPx), and SOD (Swamy et al. 2015; Marella et al. 2016; Abbirami et al. 2019a, b). Therefore, inhibition of oxidative stress is, possibly, responsible for the mitigation of the damage caused by diabetes in the reproductive organs of the male diabetic rats.
Further, the role of M.cymbalaria extracts in the morphological alterations of the reproductive organs was confirmed by the histological assessments. Several animal studies have reported the negative impact of diabetes on the testicular and epididymal tissues in the form of atrophy, necrosis, and degenerative changes. Similar abnormal alterations were recorded in the alloxan-induced untreated diabetic control group of the current study (Ghanbari et al. 2015). Among the treated diabetic groups, skin extract (250 and 500 mg/kg)-treated groups showed a noticeable recovery from the diabetic-mediated reproductive injury and oxidative stress. On the other hand, the seed extract (250 and 500 mg/kg)-treated groups showed a moderate recovery of testicular and epididymal tissues. Further, the same trend reflected in the results of the histological assessment of testicular injury through the Johnsen score system. Moreover, a molecular docking study by Abbirami et al. 2019a described the interaction between the compounds identified via GC–MS analysis of M. cymbalaria and the diabetic target, peroxisome proliferator-activated receptor gamma (PPARγ). The results of the aforementioned study revealed the active compounds namely 2-methoxy-4-vinyl phenol, guaiacol, carbinoxamine maleate, 4 N ethylcytosine, and methyl cinnamate of M. cymbalaria based on their binding affinity towards PPARγ. Since this study categorized the volatile compounds of M. cymbalaria, other phytochemicals like phenolics, flavonoids, terpenoids, and saponins and their mechanism of action should be investigated to identify the compounds responsible for its anti-diabetic effects.
Though some earlier studies have reported the anti-diabetic activity of M. cymbalaria, this is the first study to investigate the active part of the plant that is responsible for attenuating male infertility in alloxan-induced diabetic rats. Treatment with skin extracts (250 and 500 mg/kg) significantly improved the sperm count, testosterone level, antioxidant enzymes, and decreased lipid peroxidation in testicular and epididymal tissues in comparison with the seed extracts (250 and 500 mg/kg) of M. cymbalaria. Taken together, the skin of the popularly consumed vegetable M.cymbalaria is the active part that can be considered as a potential natural source to attenuate diabetic-mediated reproductive dysfunction.
Conclusion
The present data showed that Momordica cymbalaria administration can revive testicular damage by improving the levels of testosterone and antioxidants in alloxan-induced diabetic mellitus. Hence, this plant could be considered as a source of herbal remedies to treat male infertility associated with diabetes mellitus. However, further research is needed to find the various molecular mechanisms behind the positive effects of M. cymbalaria on diabetic-mediated reproductive impairment.
Acknowledgments
The authors are thankful to UGC-SAP, MHRD-RUSA and DST-FIST for providing the instrumental facility to perform this research work.
Author contributions
EA performed the animal studies, interpreted the data and wrote the manuscript. DS undertook the histological examination of the reproductive organs. SA participated in the statistical analysis. RS and EA have conducted all in vivo experiments. LDK and RG carried out extract preparations as well as assisted in the animal studies. TS designed and supervised the work. All authors read and approved and the manuscript.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflicts of interest.
Ethics approval
The experimental design was approved by Institutional Animal Ethical Committee (Registration No.779/PO/Re/S/03/ CPCSEA) and the study was performed according to the guidelines of the Committee for the Purpose of Control and Supervision of Experiments on Animals (CPCSEA).
References
- Abarikwu SO, Oruitemeka S, Uwadileke IA, Omeodu SI, Okoye NF, Mgbudom-Okah CJ, Ohanador R. Oral administration of cadmium depletes intratesticular and epididymal iron levels and inhibits lipid peroxidation in the testis and epididymis of adult rats. J Trace Elem Med Bio. 2018;48:213–223. doi: 10.1016/j.jtemb.2018.04.011. [DOI] [PubMed] [Google Scholar]
- Abbirami E, Dinesh Kumar L, Guna R, Gayathri N, Sivasudha T. Alpha amylase, alpha glucosidase inhibition and profiling of volatile compounds of biologically active extracts from Momordica cymbalaria (Hook, Fenzl) skin and seeds. AJEAT. 2019;8(1):67–73. [Google Scholar]
- Abbirami E, Selvakumar M, Dinesh Kumar L, Guna R, Sivasudha T. Identification of novel drug-like compounds from Momordica cymbalaria as PPAR-γ agonists: A molecular docking study. AJEAT. 2019;8:71–74. [Google Scholar]
- Abdullah KM, Alam MM, Iqbal Z, Naseem I. Therapeutic effect of vitamin B 3 on hyperglycemia, oxidative stress and DNA damage in alloxan induced diabetic rat model. Biomed Pharmacother. 2018;105:1223–1231. doi: 10.1016/j.biopha.2018.06.085. [DOI] [PubMed] [Google Scholar]
- Abtahi-Evari SH, Shokoohi M, Abbasi A, Rajabzade A, Shoorei H, Kalarestaghi H. Protective effect of Galega officinalis extract on Streptozotocin induced kidney damage and biochemical factor in diabetic rats. CJMB. 2017;4:108–114. [Google Scholar]
- Adedara IA, Okpara ES, Busari EO, Omole O, Owumi SE, Farombi EO. Dietary protocatechuic acid abrogates male reproductive dysfunction in streptozotocin-induced diabetic rats via suppression of oxidative damage, inflammation and caspase-3 activity. Eur J Pharmacol. 2019;849:30–42. doi: 10.1016/j.ejphar.2019.01.033. [DOI] [PubMed] [Google Scholar]
- Adewoyin M, Ibrahim M, Roszaman R, Isa M, Alewi N, Rafa A, Anuar M. Male infertility: the effect of natural antioxidants and phytocompounds on seminal oxidative stress. Diseases (Basel, Switzerland) 2017;5(1):9. doi: 10.3390/diseases5010009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agarwal A, Mulgund A, Alshahrani S, Assidi M, Abuzenadah AM, Sharma R, Sabanegh E. Reactive oxygen species and sperm DNA damage in infertile men presenting with low level leukocytospermia. Reprod Biol Endocrin. 2014;12(1):126. doi: 10.1186/1477-7827-12-126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agbaje IM, Rogers DA, McVicar CM, McClure N, Atkinson AB, Mallidis C, Lewis SEM. Insulin dependent diabetes mellitus: implications for male reproductive function. Hum Reprod. 2007;22(7):1871–1877. doi: 10.1093/humrep/dem077. [DOI] [PubMed] [Google Scholar]
- Ahmed AA, Mollica A, Stefanucci A, Tayrab E, Ahmed H, Essa MEA (2020) Gum Arabic improves the reproductive capacity through upregulation of testicular glucose transporters (GLUTs) mRNA expression in Alloxan induced diabetic rat. Bioact Carbohydr Diet 22:1–10
- Ajazuddin A, Alexander A, Qureshi L, Kumari P, Vaishnav M, Sharma S, Saraf S. Role of herbal bioactives as a potential bioavailability enhancer for Active Pharmaceutical Ingredients. Fitoterapia. 2014;97:1–14. doi: 10.1016/j.fitote.2014.05.005. [DOI] [PubMed] [Google Scholar]
- Akinola OB, Dosumu OO, Sanusi SA, Ajayi TF, Olajide TH. PPAR-γ agonist pioglitazone improves semen quality and testicular histomorphometrics with partial reversal of hyperglycaemia in alloxan-induced diabetic rats. Middle East Fertil Soc J. 2015;20(4):271–279. [Google Scholar]
- Akinyemi AJ, Adedara IA, Thome GR, Morsch VM, Rovani MT, Mujica LKS, Duarte T, Duarte M, Oboh G, Schetinger MRC. Dietary supplementation of ginger and turmeric improves reproductive function in hypertensive male rats. Toxicol Rep. 2015;2:1357–1366. doi: 10.1016/j.toxrep.2015.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alves M, Martins A, Rato L, Moreira P, Socorro S, Oliveira P. Molecular mechanisms beyond glucose transport in diabetes related male infertility. BBA Mol Basis Dis. 2013;1832:626–35. doi: 10.1016/j.bbadis.2013.01.011. [DOI] [PubMed] [Google Scholar]
- Amaral S, Moreno AJ, Santos MS, Seiça R, Ramalho-Santos J. Effects of hyperglycemia on sperm and testicular cells of Goto-Kakizaki and streptozotocin-treated rat models for diabetes. Theriogenology. 2006;66(9):2056–2067. doi: 10.1016/j.theriogenology.2006.06.006. [DOI] [PubMed] [Google Scholar]
- Ammar O, Haouas Z, Hamouda B, Hamdi H, Hellara I, Jlali A, Cheikh HB, Mehdi M. Relationship between sperm DNA damage with sperm parameters, oxidative markers in teratozoospermic men. Eur J Obstet Gyn R B. 2019;233:70–75. doi: 10.1016/j.ejogrb.2018.12.003. [DOI] [PubMed] [Google Scholar]
- Ballester J, Munoz MC, Dominguez J, Rigau T, Guinovart JJ, Rodriguez-Gil JE. Insulin-dependent diabetes affects testicular function by FSH-and LH-linked mechanisms. J Androl. 2004;25(5):706–719. doi: 10.1002/j.1939-4640.2004.tb02845.x. [DOI] [PubMed] [Google Scholar]
- Barkabi-Zanjani S, Ghorbanzade V, Aslani M, Ghalibafsabbaghi A, Chodari L. Diabetes mellitus and the impairment of male reproductive function: Possible signaling pathways. Diabetes Metab Syndr. 2020;14(5):1307–1314. doi: 10.1016/j.dsx.2020.07.031. [DOI] [PubMed] [Google Scholar]
- Choubey M, Ranjan A, Bora PS, Krishna A. Protective role of adiponectin against testicular impairment in high-fat diet/streptozotocin-induced type 2 diabetic mice. Biochimie. 2020;168:41–52. doi: 10.1016/j.biochi.2019.10.014. [DOI] [PubMed] [Google Scholar]
- Condorelli RA, La Vignera S, Mongioi LM, Alamo A, Calogero AE. Diabetes mellitus and infertility: different pathophysiological effects in type 1 and type 2 on sperm function. Front Endocrinol. 2018;9:268. doi: 10.3389/fendo.2018.00268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elangovan A, Subramanian A, Durairaj S, Ramachandran J, Lakshmanan DK, Ravichandran NG, Thilagar S. Antidiabetic and hypolipidemic efficacy of skin and seed extracts of Momordica cymbalaria on alloxan induced diabetic model in rats. J Ethnopharmacol. 2019;241:111989. doi: 10.1016/j.jep.2019.111989. [DOI] [PubMed] [Google Scholar]
- Firdous M, Koneri R, Sarvaraidu CH, Shubhapriya KH. NIDDM antidiabetic activity of saponins of Momordica cymbalaria in streptozotocin-nicotinamide NIDDM mice. JCDR. 2009;3(1):1460–1465. [Google Scholar]
- Fujieda H, Kogami M, Sakairi M, Kato N, Makino M, Takahashi N, Miyazawa T, Harada S, Yamashita T. Discovery of a potent glucokinase activator with favorable liver and pancreas distribution pattern for the treatment of type 2 diabetes mellitus. Eur J Med Chem. 2018;156:269–294. doi: 10.1016/j.ejmech.2018.06.060. [DOI] [PubMed] [Google Scholar]
- Ghanbari E, Nejati V, Najafi G, Khazaei M, Babaei M. Study on the effect of royal jelly on reproductive parameters in streptozotocin-induced diabetic rats. I J Fertil Steril. 2015;9:113–120. doi: 10.22074/ijfs.2015.4215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gharagozloo P, Aitken RJ. The role of sperm oxidative stress in male infertility and the significance of oral antioxidant therapy. Hum Reprod. 2011;26(7):1628–1640. doi: 10.1093/humrep/der132. [DOI] [PubMed] [Google Scholar]
- Ghlissi Z, Atheymen R, Boujbiha MA, Sahnoun Z, Makni Ayedi F, Zeghal K, El Feki A, Hakim A. Antioxidant and androgenic effects of dietary ginger on reproductive function of male diabetic rats. Int J Food Sci Nutr. 2013;64(8):974–978. doi: 10.3109/09637486.2013.812618. [DOI] [PubMed] [Google Scholar]
- Hamilton BE, Ventura SJ. Fertility and abortion rates in the United States, 1960–2002. Int J Androl. 2006;29:34–45. doi: 10.1111/j.1365-2605.2005.00638.x. [DOI] [PubMed] [Google Scholar]
- Hamzah RU, Lawal AR, Madaki FM, Erukainure OL. Methanolic extract of Celosia argentea var crista leaves modulates glucose homeostasis and abates oxidative hepatic injury in diabetic rats. Comp Clin Path. 2018;27(4):1065–1071. doi: 10.1007/s00580-018-2702-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han XX, Jiang YP, Liu N, Wu J, Yang JM, Li YX, Sun M, Sun T, Zheng P, Yu JQ. Protective effects of Astragalin on spermatogenesis in streptozotocin-induced diabetes in male mice by improving antioxidant activity and inhibiting inflammation. Biomed Pharmacother. 2019;110:561–570. doi: 10.1016/j.biopha.2018.12.012. [DOI] [PubMed] [Google Scholar]
- Jang JH, Park JE, Han JS. Scopoletin inhibits α-glucosidase in vitro and alleviates postprandial hyperglycemia in mice with diabetes. Eur J Pharmacol. 2018;834:152–156. doi: 10.1016/j.ejphar.2018.07.032. [DOI] [PubMed] [Google Scholar]
- Jiang X, Bai Y, Zhang Z, Xin Y, Cai L. Protection by sulforaphane from type 1 diabetes-induced testicular apoptosis is associated with the up-regulation of Nrf2 expression and function. Toxicol Appl Pharmacol. 2014;279(2):198–210. doi: 10.1016/j.taap.2014.06.009. [DOI] [PubMed] [Google Scholar]
- Jiao N, Chen Y, Zhu Y, Wang W, Liu M, Ding W, Lv G, Lu J, Yu B, Xu H. Protective effects of catalpol on diabetes mellitus-induced male reproductive damage via suppression of the AGEs/RAGE/Nox4 signaling pathway. Life Sci. 2020;256:116736. doi: 10.1016/j.lfs.2019.116736. [DOI] [PubMed] [Google Scholar]
- Johnsen SG. Testicular biopsy score count - a method for registration of spermatogenesis in human testes: normal values and results in 335 hypogonadal males. Hormones. 1970;1:2–25. doi: 10.1159/000178170. [DOI] [PubMed] [Google Scholar]
- Kanter M, Aktas C, Erboga M. Protective effects of quercetin against apoptosis and oxidative stress in streptozotocin-induced diabetic rat testis. Food Chem Toxicol. 2012;50(3–4):719–725. doi: 10.1016/j.fct.2011.11.051. [DOI] [PubMed] [Google Scholar]
- Karimi J, Goodarzi MT, Tavilani H, Khodadadi I, Amiri I. Relationship between advanced glycation end products and increased lipid peroxidation in semen of diabetic men. Diabetes Res Clin. 2011;91:61–66. doi: 10.1016/j.diabres.2010.09.024. [DOI] [PubMed] [Google Scholar]
- Koneri RB, Samaddar S, Simi SM, Rao ST. Neuroprotective effect of a triterpenoid saponin isolated from Momordica cymbalaria Fenzl in diabetic peripheral neuropathy. Indian J Pharmacol. 2014;46(1):76. doi: 10.4103/0253-7613.125179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- La Vignera S, Condorelli RA, Vicari E, Dagata R, Salemi M, Calogero AE. High levels of lipid peroxidation in semen of diabetic patients. Andrologia. 2019;44:565–570. doi: 10.1111/j.1439-0272.2011.01228.x. [DOI] [PubMed] [Google Scholar]
- La VS, Calogero AE, Condorelli R, Lanzafame F, Giammusso B, Vicari E. Andrological characterization of the patient with diabetes mellitus. Minerva Endocrinol. 2009;34(1):1–9. [PubMed] [Google Scholar]
- Laleethambika N, Anila V, Manojkumar C, Muruganandam I, Giridharan B, Ravimanickam T, Balachandar V. Diabetes and sperm DNA damage: efficacy of antioxidants. SN Comprehen Clin Med. 2019;1(1):49–59. [Google Scholar]
- Lascar N, Brown J, Pattison H, Barnett AH, Bailey CJ, Bellary S. Type 2 diabetes in adolescents and young adults. Lancet Diabetes Endo. 2018;6(1):69–80. doi: 10.1016/S2213-8587(17)30186-9. [DOI] [PubMed] [Google Scholar]
- Li ZM, Liu N, Jiang YP, Yang JM, Zheng J, Sun M, Li YX, Sun T, Wu J, Yu JQ. Vitexin alleviates streptozotocin-induced sexual dysfunction and fertility impairments in male mice via modulating the hypothalamus–pituitary–gonadal axis. Chem Biol Interact. 2019;297:119–129. doi: 10.1016/j.cbi.2018.10.013. [DOI] [PubMed] [Google Scholar]
- Lin DC, Huang CY, Ting WH, Lo FS, Lin CL, Yang HW, Chang TY, Lin CH, Tzeng YW, Yang WS, Juang YL, Lee YJ. Mutations in glucokinase and other genes detected in neonatal and type 1B diabetes patient using whole exome sequencing may lead to disease-causing changes in protein activity. BBA-Mol Basis Dis. 1865;1865(2):428–433. doi: 10.1016/j.bbadis.2018.11.013. [DOI] [PubMed] [Google Scholar]
- Lutz W. Fertility rates and fufture population trends: will Europe's birth rate recover or continue to decline? Int J Androl. 2006;29:25–33. doi: 10.1111/j.1365-2605.2005.00639.x. [DOI] [PubMed] [Google Scholar]
- Maiorino M, Bellastella G, Esposito K. Diabetes and sexual dysfunction: current perspectives. Diabet Metab Synd Ob. 2014;7(7):95–105. doi: 10.2147/DMSO.S36455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maneesh M, Jayalakshmi H, Singh TA, Chakrabarti A. Impaired hypothalamic pituitary- gonadal axis function in men with diabetes mellitus. Indian J Clin Biochem. 2006;21:165–168. doi: 10.1007/BF02913088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marella S, Maddirela DR, Kumar EGTV, Tilak TK, Badri KR, Chippada A. Mcy protein, a potential antidiabetic agent: evaluation of carbohydrate metabolic enzymes and antioxidant status. Int J Biol Macromol. 2016;86:481–488. doi: 10.1016/j.ijbiomac.2016.01.062. [DOI] [PubMed] [Google Scholar]
- Maremanda KP, Srivalliputturu SB, Jena G. Zinc deficient diet exacerbates the testicular and epididymal damage in type 2 diabetic rat: Studies on oxidative stress-related mechanisms. Reprod Biol. 2020;20(2):191–201. doi: 10.1016/j.repbio.2020.03.002. [DOI] [PubMed] [Google Scholar]
- Maresch CC, Stute DC, Alves MG, Oliveira PF, de Kretser DM, Linn T. Diabetes-induced hyperglycemia impairs male reproductive function: a systematic review. Human Reprod Update. 2017;24(1):1–20. doi: 10.1093/humupd/dmx033. [DOI] [PubMed] [Google Scholar]
- Minaz N, Razdan R, Hammock BD, Mujwar S, Goswami SK. Impact of diabetes on male sexual function in streptozotocin-induced diabetic rats: Protective role of soluble epoxide hydrolase inhibitor. Biomed Pharmacother. 2019;115:108897. doi: 10.1016/j.biopha.2019.108897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohamed MZ, Hafez HM, Zenhom NM, Mohammed HH. Cilostazol alleviates streptozotocin-induced testicular injury in rats via PI3K/Akt pathway. Life Sci. 2018;198:136–142. doi: 10.1016/j.lfs.2018.02.038. [DOI] [PubMed] [Google Scholar]
- Muratori M, Tamburrino L, Marchiani V, Cambi M, Azzari B, Forti G, Baldi E. Investigation on the origin of sperm DNA fragmentation: role of apoptosis, immaturity and oxidative stress. Mol Med. 2015;21:109–122. doi: 10.2119/molmed.2014.00158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ni W, Pan C, Pan Q, Fei Q, Huang X, Zhang C (2019) Methylation levels of IGF2 and KCNQ1 in spermatozoa from infertile men are associated with sperm DNA damage. Andrologia 51:1–8 [DOI] [PubMed]
- OECD (Organization for Economic Cooperation and Development) (2001) Guidelines for testing of chemicals: acute oral toxicity–acute toxic class method. Guideline No. 423
- Oguntibeju OO, Aboua Y, Kachepe P. Possible therapeutic effects of vindoline on testicular and epididymal function in diabetes-induced oxidative stress male Wistar rats. Heliyon. 2020;6(4):e03817. doi: 10.1016/j.heliyon.2020.e03817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ojewale AO, Olaniyan OT, Faduyile FA, Odukanmi OA, Oguntola JA, Dare BJ (2014) Testiculo protective effects of ethanolic roots extract of Pseudocedrela kotschyi on alloxan induced testicular damage in diabetic rats. J Adv Med Med Res 4(1):548–563
- Opuwari C, Monsees T. Green tea consumption increases sperm concentration and viability in male rats and is safe for reproductive, liver and kidney health. Sci Rep. 2020;10(1):1–14. doi: 10.1038/s41598-020-72319-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinto C, Rodriguez-Galdon B, Cestero JJ, Macias P (2018) Processed tomatoes improves the antioxidant status of carbon tetrachloride-intoxicated rat tissues. Eur Food Res Technol 244:1843–1852
- Pradeep PM, Sreerama YN. Phenolic antioxidants of foxtail and little millet cultivars and their inhibitory effects on α-amylase and α-glucosidase activities. Food Chem. 2018;247:46–55. doi: 10.1016/j.foodchem.2017.11.103. [DOI] [PubMed] [Google Scholar]
- Rains JL, Jain SK. Oxidative stress, insulin signaling, and diabetes. Free Radic Biol Med. 2011;50(5):567–575. doi: 10.1016/j.freeradbiomed.2010.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rashid K, Sil PC. Curcumin enhances recovery of pancreatic islets from cellular stress induced inflammation and apoptosis in diabetic rats. Toxicol Appl Pharmacol. 2015;282(3):297–310. doi: 10.1016/j.taap.2014.12.003. [DOI] [PubMed] [Google Scholar]
- Rato L, Alves MG, Duarte AI, Santos MS, Moreira PI, Cavaco JE, Oliveira PF. Testosterone deficiency induced by progressive stages of diabetes mellitus impairs glucose metabolism and favors glycogenesis in mature rat Sertoli cells. Int J Biochem Cell Biol. 2015;66:1–10. doi: 10.1016/j.biocel.2015.07.001. [DOI] [PubMed] [Google Scholar]
- Roessner C, Paasch U, Kratzsch J, Grunewald GHJS, S, Sperm apoptosis signalling in diabetic men. Reprod Biomed Online. 2012;25(3):292–299. doi: 10.1016/j.rbmo.2012.06.004. [DOI] [PubMed] [Google Scholar]
- Rowley WR, Bezold C, Arikan Y, Byrne E, Krohe S. Diabetes 2030: insights from yesterday, today, and future trends. Popul Health Manag. 2017;20(1):6–12. doi: 10.1089/pop.2015.0181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy VK, Chenkual L, Gurusubramanian G. Protection of testis through antioxidant action of Mallotus roxburghianus in alloxan-induced diabetic rat model. J Ethnopharmacol. 2015;176:268–280. doi: 10.1016/j.jep.2015.11.006. [DOI] [PubMed] [Google Scholar]
- Samie A, Sedaghat R, Baluchnejadmojarad T, Roghani M. Hesperetin, a citrus flavonoid, attenuates testicular damage in diabetic rats via inhibition of oxidative stress, inflammation, and apoptosis. Life Sci. 2018;210:132–139. doi: 10.1016/j.lfs.2018.08.074. [DOI] [PubMed] [Google Scholar]
- Scarano WR, Messias AG, Oliva SU, Klinefelter GR, Kempinas WG. Sexual behaviour, sperm quantity and quality after short-term streptozotocin-induced hyperglycaemia in rats. Int J Androl. 2006;29(4):482–488. doi: 10.1111/j.1365-2605.2006.00682.x. [DOI] [PubMed] [Google Scholar]
- Sebai H, Selmi S, Rtibi K, Gharbi N, Sakly M. Protective effect of Lavandula stoechas and Rosmarinus officinalis essential oils against reproductive damage and oxidative stress in alloxan-induced diabetic rats. J Med Food. 2015;18(2):241–249. doi: 10.1089/jmf.2014.0040. [DOI] [PubMed] [Google Scholar]
- Seven I, Tatli Seven P, Gul Baykalir B, Parlak Ak T, Ozer Kaya S, Yaman M. Bee glue (propolis) improves reproductive organs, sperm quality and histological changes and antioxidant parameters of testis tissues in rats exposed to excess copper. Andrologia. 2020;52(4):e13540. doi: 10.1111/and.13540. [DOI] [PubMed] [Google Scholar]
- Shi GJ, Li ZM, Zheng J, Chen J, Han XX, Wu J, Li GY, Chang Q, Li YX, Yu JQ. Diabetes associated with male reproductive system damages: onset of presentation, pathophysiological mechanisms and drug intervention. Biomed Pharmacother. 2017;90:562–574. doi: 10.1016/j.biopha.2017.03.074. [DOI] [PubMed] [Google Scholar]
- Shoorei H, Khaki A, Khaki AA, Hemmati AA, Moghimian M, Shokoohi M. The ameliorative effect of carvacrol on oxidative stress and germ cell apoptosis in testicular tissue of adult diabetic rats. Biomed Pharmacother. 2019;111:568–578. doi: 10.1016/j.biopha.2018.12.054. [DOI] [PubMed] [Google Scholar]
- Shrilatha B. Early oxidative stress in testis and epididymal sperm in streptozotocin-induced diabetic mice: its progression and genotoxic consequences. Reprod Toxicol. 2007;23(4):578e87. doi: 10.1016/j.reprotox.2007.02.001. [DOI] [PubMed] [Google Scholar]
- Smolders L, Mensink RP, Plat J. An acute intake of theobromine does not change postprandial lipid metabolism, whereas a high-fat meal lowers chylomicron particle number. Nutr Res. 2017;40:85–94. doi: 10.1016/j.nutres.2017.03.007. [DOI] [PubMed] [Google Scholar]
- Swamy MK, Akhtar MS, Mohanty SK, Sinniah UR. Synthesis and characterization of silver nanoparticles using fruit extract of Momordica cymbalaria and assessment of their in vitro antimicrobial, antioxidant and cytotoxicity activities. Spectrochimica Acta Molec Biomolec Spectrosc. 2015;151:939–944. doi: 10.1016/j.saa.2015.07.009. [DOI] [PubMed] [Google Scholar]
- Vlassara H, Striker GE. Advanced glycation endproducts in diabetes and diabetic complications. Endocrin Metab Clinic. 2013;42:697–719. doi: 10.1016/j.ecl.2013.07.005. [DOI] [PubMed] [Google Scholar]
- Wankeu-Nya M, Watcho P, Deeh Defo PB, Ngadjui E, Nguelefack TB, Kamtchouing P, Kamanyi A. Aqueous and ethanol extracts of Dracaena arborea (Wild) Link (Dracaenaceae) alleviate reproductive complications of diabetes mellitus in rats. Andrologia. 2019;51(10):e13381. doi: 10.1111/and.13381. [DOI] [PubMed] [Google Scholar]
- Wu X, Ding H, Hu X, Pan J, Liao Y, Gong D, Zhang G. Exploring inhibitory mechanism of gallocatechin gallate on a-amylase and a-glucosidase relevant to postprandial hyperglycemia. J Funct Foods. 2018;48:200–209. [Google Scholar]
- Wu H, Huffman AM, Whitcomb BW, Josyula S, Labrie S, Tougias E, Rahil T, Sites CK, Pilsner JR. Sperm mitochondrial DNA measures and semen parameters among men undergoing fertility treatment. Reprod Biomed Online. 2019;38(1):66–75. doi: 10.1016/j.rbmo.2018.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]