Introduction
Pyoderma gangrenosum (PG) is a rare neutrophilic cutaneous disorder characterized by single or multiple inflammatory nodules, papules, or pustules, which rapidly ulcerate with undermined borders, tenderness, and cribriform scarring upon healing.1 It is a challenging condition to identify, given initial misdiagnosis and mismanagement with physical debridement, propensity for superinfection, and rarity.2 Furthermore, once the correct diagnosis is recognized, therapeutic challenges include promptly implementing an effective treatment to prevent rapid progression with concomitant morbidity and distress to the patient. Treatment options include topical and intralesional steroids, systemic glucocorticoids, conventional immunosuppressants, biologics, and intravenous immunoglobulin.3 We present the novel use of guselkumab, a monoclonal antibody targeting interleukin (IL)-23, as an effective and safe treatment option for severe and recalcitrant PG.
Case report
A 60-year-old woman presented to the dermatology clinic with a 1-year history of lower-leg ulcers. The patient's clinical history was significant for a monoclonal gammopathy of undetermined significance and type 2 diabetes. She was previously followed at a wound care center for the management of presumed venous stasis ulcers. The patient failed treatments, including local wound care with silver sulfadiazine, compression wraps, and clobetasol ointment. Physical examination revealed a purulent ulceration with jagged undermined violaceous borders on the left lower leg. A bacterial culture was performed, and the patient was prescribed ciprofloxacin and triamcinolone cream for presumed superinfection and inflammation, respectively. Despite treatment, the ulceration on her left leg enlarged significantly with surrounding edema. Intralesional steroid injections were administered, and a class I topical steroid was prescribed without improvement. Tissue was sampled and sent for both histology and culture to exclude occult infection or malignancy. Pathology revealed ulceration with underlying acute and chronic inflammation and tissue necrosis. These features along with a negative culture were not diagnostic, but consistent with PG.2 In light of her history of monoclonal gammopathy of undetermined significance along with the clinical presentation, the ulcer was diagnosed as PG.3
Biologic therapy was chosen for management, given the large size of the ulcer, the rapid progression of disease, medication safety profile, and availability of product samples from pharmaceutical companies in the office. Biologics were the preferred treatment choice for immune modulation, given her history of gammopathy and favorable side effect profile.3 Furthermore, newer biologics such as ustekinumab, which targets IL-12/23, have emerging case studies demonstrating support for the treatment of PG.4,5
Ustekinumab manufacturer product samples were readily available in-office and administered initially. Shortly thereafter, the ulceration deepened, exuding a strong odor with exposure of tendons, ligaments, and muscle, prompting hospital admission. A Pseudomonas superinfection was identified and treated with intravenous antibiotics. Ustekinumab administration was temporarily suspended focusing on local wound care. Following stabilization, ustekinumab was restarted. The patient experienced rapid improvement with minimal pain and nearly complete re-epithelialization.
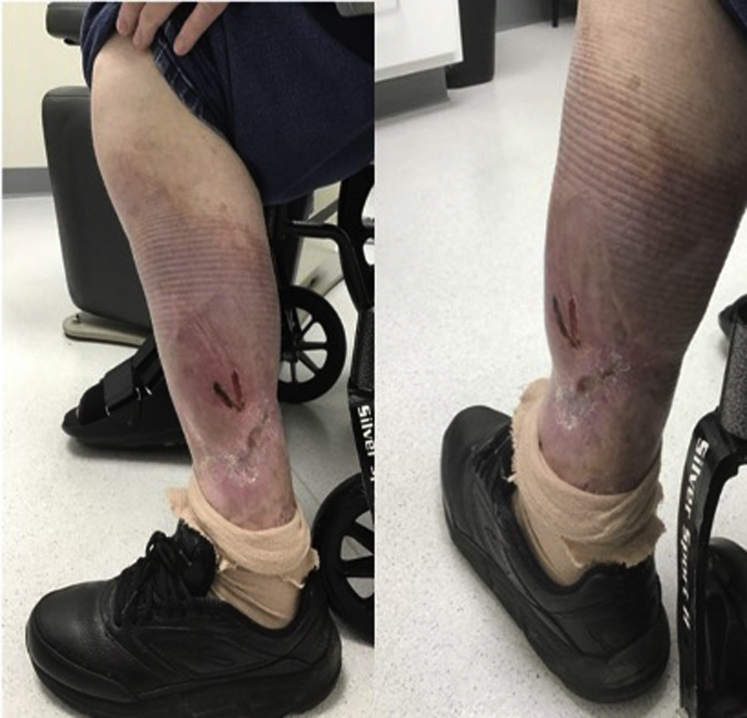
Sixteen months later, a relapse occurred while the patient still received ustekinumab with ulceration and associated pain. Assuming development of neutralizing autoantibodies against ustekinumab, the patient was placed on and failed both adalimumab and infliximab sequentially. The ulcer expanded to approximately 60% of her left lower leg circumference with re-exposure of tendon and muscle (Fig 1). The patient was then administered intravenous immunoglobulin by her oncologist for potential management of both the monoclonal gammopathy of undetermined significance and PG. In addition, prednisone was instituted which arrested disease progression.
Fig 1.
Severe PG involving the left lower extremity with muscle and tendon exposure in May 2019 when ustekinumab was initiated. PG, Pyoderma gangrenosum.
Given the previous response to an IL-12/23 antagonist and failure of 2 tumor necrosis factor alfa inhibitors, we speculated that inhibiting IL-23 with guselkumab might halt and even reverse disease progression. Within weeks of starting guselkumab at 100 mg monthly dosing, the ulcer bed began retreating from underlying exposed muscles and tendons. Within 3 months, the ulcer was virtually non-existent. Re-epithelialization was notable over 95%of the original ulcer. The response was found to be durable, with continued remission of her PG after 15 months (Fig 2).
Fig 2.
Severe PG involving the left lower extremity with muscle and tendon exposure in May 2019 after failing ustekinumab, adalimumab, and infliximab. PG, Pyoderma gangrenosum.
Discussion
PG should always be included in the differential diagnosis of a chronic ulcer that does not heal with conventional therapies. The Su et al criteria, The Delphi Consensus of International Experts, and PARACELSUS (Progressing disease, Assessment of relevant differential diagnoses, Reddish-violaceous wound border, Amelioration by immunosuppressant drugs, Characteristically irregular shape of ulceration, Extreme pain, Localization of lesion at the site of the trauma, Suppurative inflammation in histopathology, Undermined wound borders, and Systemic disease associated) score are helpful diagnostic tools.3 Criteria for diagnosis comprises the exclusion of other similar presenting disorders such as venous stasis ulcers, infectious processes, and malignancy.2,3 Additional diagnostic criteria includes a typical clinical presentation, histopathology findings, improvement with immunosuppressants, and a history of pathergy.3 The presence of an associated typical systemic disease, such as inflammatory bowel disease, rheumatoid arthritis, monoclonal gammopathy, or hematologic malignancy is helpful for diagnosis.3 Once a diagnosis is made, treatment presents a challenge because defined management is lacking in the literature.2
Treatment modalities focus on local wound care, infection control, avoidance of pathergy, pain management, topical or intralesional steroids, oral immunosuppressants, and biologics.3 Case reports and expert opinions have mentioned prednisone, cyclosporine, methotrexate, mycophenolate, and azathioprine as treatment options.3 Regarding biologic management, only one randomized, double-blinded, placebo-controlled study has been performed, analyzing the efficacy of the biologic infliximab versus placebo, with support for the use of 5 mg/kg dosing regimen of infliximab.6
More recently, the focus on PG treatment has been newer biologics. Genova et al4 evaluated a woman with PG on the lower extremity, which had failed topical therapy in addition to oral steroids. Upon evaluation of the tissue on biopsy, a marked increase in expression of IL23A was found when compared with healthy tissue. This prompted a trial of ustekinumab, which targets IL-12/23. Following 14 weeks of treatment, the lesion had healed completely. Since then, there have been other studies evaluating ustekinumab with promising results for the treatment of recalcitrant PG.5 In this case, the patient initially responded well to ustekinumab, but presumably developed neutralizing autoantibodies after 16 months of use. Fortunately, her PG responded to IL-23 blockade with guselkumab, a monoclonal antibody targeting IL-23 and currently indicated for the treatment of moderate to severe plaque psoriasis and psoriatic arthritis.
This case demonstrates a common conundrum among providers treating patients with recalcitrant PG. Case reports have identified IL-1/IL-6 antagonists, janus kinase-signal transducer and activator of transcription inhibitors, and phosphodiesterase type 4 inhibitors, but none have evaluated guselkumab despite the significance of IL-23 expression.3 PG can be a severely debilitating disease, and the need for evidence-based treatment is crucial. A larger trial analyzing the effect of IL-23 targeting biologics for the treatment of PG would be helpful in further defining the role of this medication within the existing treatment algorithm.
Conflicts of interest
None disclosed.
Footnotes
Funding sources: None.
IRB approval status: Not applicable.
References
- 1.Schadt C. Pyoderma gangrenosum: Pathogenesis, clinical features, and diagnosis. In: Post T, ed. UpToDate. Waltham, MA: UpToDate;Available at: www.uptodate.com. Accessed August 8, 2020.
- 2.George C., Deroide F., Rustin M. Pyoderma gangrenosum - a guide to diagnosis and management. Clin Med (Lond) 2019;19(3):224–228. doi: 10.7861/clinmedicine.19-3-224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fletcher J., Alhusayen R., Alavi A. Recent advances in managing and understanding pyoderma gangrenosum. F1000Res. 2019;8 doi: 10.12688/f1000research.19909.1. F1000 Faculty Rev-2092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Guenova E., Teske A., Fehrenbacher B. Interleukin 23 expression in pyoderma gangrenosum and targeted therapy with ustekinumab. Arch Dermatol. 2011;147(10):1203–1205. doi: 10.1001/archdermatol.2011.168. [DOI] [PubMed] [Google Scholar]
- 5.Vallerand I.A., Hardin J. Ustekinumab for the treatment of recalcitrant pyoderma gangrenosum: a case report. SAGE Open Med Case Rep. 2019;7 doi: 10.1177/2050313X19845206. 2050313X19845206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brooklyn T.N., Dunnill M.G.S., Shetty A. Infliximab for the treatment of pyoderma gangrenosum: a randomised, double blind, placebo controlled trial. Gut. 2006;55(4):505–509. doi: 10.1136/gut.2005.074815. [DOI] [PMC free article] [PubMed] [Google Scholar]