Abstract
Articular cartilage regeneration is one of the challenges faced by orthopedic surgeons. Microcarrier applications have made great advances in cartilage tissue engineering in recent years and enable cost-effective cell expansion, thus providing permissive microenvironments for cells. In addition, microcarriers can be loaded with proteins, factors, and drugs for cartilage regeneration. Some microcarriers also have the advantages of injectability and targeted delivery. The application of microcarriers with these characteristics can overcome the limitations of traditional methods and provide additional advantages. In terms of the transformation potential, microcarriers have not only many advantages, such as providing sufficient and beneficial cells, factors, drugs, and microenvironments for cartilage regeneration, but also many application characteristics; for example, they can be injected to reduce invasiveness, transplanted after microtissue formation to increase efficiency, or combined with other stents to improve mechanical properties. Therefore, this technology has enormous potential for clinical transformation. In this review, we focus on recent advances in microcarriers for cartilage regeneration. We compare the characteristics of microcarriers with other methods for repairing cartilage defects, provide an overview of the advantages of microcarriers, discuss the potential of microcarrier systems, and present an outlook for future development.
Translational potential of this article
We reviewed the advantages and recent advances of microcarriers for cartilage regeneration. This review could give many scholars a better understanding of microcarriers, which can provide doctors with potential methods for treating patients with cartilage injure.
Keywords: Microcarriers, Biomaterials, Cartilage, Tissue engineering, Delivery, Cells
Introduction
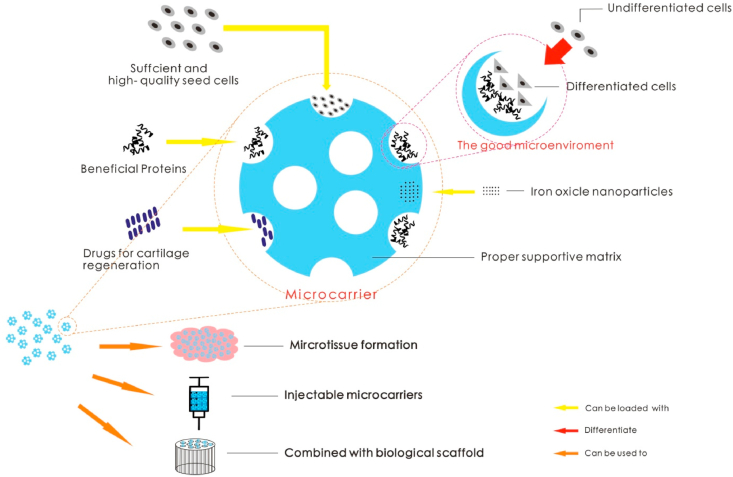
Articular cartilage plays a vital role in the human body. However, because of the avascular nature of articular cartilage, its intrinsic healing ability is limited. Thus, the management of articular cartilage defects is a challenge for doctors [1]. If left untreated, joint cartilage damage can easily cause or exacerbate osteoarthritis (OA) [2]. OA is one of the most common degenerative joint diseases, and it is characterized by limited joint mobility and pain. Current methods for clinically treating cartilage damage include microfracture and surgical lavage [3]. The classic repair techniques in clinical treatment include bone marrow stimulation techniques, synthetic or metal-based techniques and pharmacologically stimulated techniques. However, the application of these technologies often has limiting factors, such as the inability to repair large-area defects, high revision surgery rates, and poor treatment effects [4]. In recent decades, tissue engineering (TE) technology has developed rapidly [[5], [6], [7], [8]], thus providing new methods for cartilage defect repair. In the field of TE designed to repair cartilage defects, many advances have been made in the use of microcarriers for repair [9,10]. A microcarrier is a microparticle approximately 100–300 microns in diameter that can be loaded with other substances, such as cells, cytokines or drugs [11,12]. Microcarriers have unique advantages as cartilage damage repair materials (see Fig. 1 for the schematic diagram) and thus provide some alternative ideas for the clinical treatment of OA caused by cartilage damage.
Figure 1.
Applications of microcarriers.
The PubMed database was searched for relevant papers. As previously mentioned, a microcarrier is a kind of particle that can be loaded with cells, drugs or other material. Microcarriers are called microspheres, microbeads, and microparticles in some studies. Therefore, our search strategy included these four keywords, and then the searched documents were individually judged to determine whether they focused on microcarriers. The search strategy included the following keywords ((microcarriers OR microspheres OR microparticles OR microbeads OR microcapsules) AND (cartilage)) AND (((damage OR defects) AND (repair OR treatment)) OR regeneration). The relevant literature from the six years prior to January 2020 was obtained. Then, we checked whether all papers related to the field of study were included and read and evaluated these papers and their references carefully. We found that the literature obtained after searching all included preclinical studies. In this review, we outline the advantages and characteristics of various types of microcarriers in the field of cartilage damage repair as well as the prospects for the widespread use of microcarriers in the future, and we compare the microcarrier applications with traditional treatment methods.
Characteristics of traditional methods and microcarrier methods for treating cartilage damage
With increasing age, the function and quality of cartilage will worsen. From a spatial perspective, cartilage damage usually progresses from the articular cartilage surface to the subchondral bone, thus leading to the development of OA [13]. OA poses an enormous economic and social burden [14]. Degenerative joint damage is associated with the extent of the cartilage loss. Studies have reported that cartilage defects are prone to progression in patients with OA who have had symptoms for more than 2 years [15].
Therefore, the treatment of articular cartilage defects is particularly important. Some commonly used traditional techniques for cartilage defect treatment are as follows:
Commonly Used Traditional Techniques.
- ●
-
●
Biological tissue to fill cartilage defect repair sites [18];
- ●
-
●
Metal or other man-made material repair [21];
- ●
-
●
Drug to stimulate cartilage regeneration [24].
Microfracture technology is one of the most commonly used techniques for repairing cartilage damage among BMS approaches, and it is performed to repair bone marrow defects by migrating bone marrow-derived mesenchymal stem cells (BMSCs) from the bone marrow to the surface of the cartilage defect via fracture drilling. However, the cartilage potential of this kind of stem cell in elderly individuals is greatly reduced [25]. If the state of the disease is added, then the repair ability will be greatly weakened. This method has a limited therapeutic effect for patients with large-scale cartilage damage. Therefore, “vibrant” BMSCs are the core of microfracture technology approaches to repair cartilage.
Autologous cartilage transplantation is a representative way to repair cartilage damage by using biological tissue to fill cartilage defects [25]. Osteochondral transplantation is an effective method for treating mutual friction damage of knee joint cartilage. The rate of reoperation and failure is high for this approach, although the clinical symptoms of patients with successful transplantations are significantly improved. However, in addition to the high failure rate of surgery, autologous cartilage transplantation itself is a method of “robbing Peter to pay Paul” via the repair of damaged cartilage through the destruction of healthy cartilage. Fresh osteochondral allografts are often used to rescue young patients with severe articular cartilage degenerative diseases [26]. Although allogeneic cartilage transplantation has been reported to effectively alleviate the symptoms of cartilage damage such as pain, the donor source is very limited, which makes the application of this technology extremely limited.
Finding an effective way to repair damaged cartilage before the onset of OA has become one a global issue. Cartilage TE technology is emerging as a new treatment, and it has brought hope to those in need of treatment for this common injury. Cartilage TE involves culturing and expanding cartilage seed cells in vitro with the participation of some cell differentiation and proliferation factors, coculturing a certain amount of seed cells with biological scaffold materials, and reshaping them into areas with damaged cartilage to form new cartilage tissue integrated with body tissues to repair damaged cartilage.
In the field of TE, microcarriers [11] are a class of functional small particles with diameters of approximately 100–300 microns. Currently, these particles are composed of a variety of materials with good biocompatibility. Microcarriers are proper supportive matrices capable of enhancing seed cells in bioreactor systems [27].
Compared with the traditional methods mentioned above, the use of functional TE microcarriers to repair cartilage damage can not only integrate and capitalize on the advantages of traditional methods but also avoid some of the limitations of traditional methods.
Characteristics compared with those of traditional technology.
-
✓
One of the important advantages of microcarriers is that they can be loaded with a large number of seed cells, which also pushes the method of cell treatment for cartilage damage to a new level [28]. Compared with BMSCs released by BMS, more BMSCs can be loaded on microcarriers. If electrospray fabrication is adopted, a large number of BMSCs can be encapsulated in theory [29,30].
-
✓
Microcarriers made of high-quality biomaterials fully exploit the traditional method of filling cartilage defects with biomaterials. For example [31], alginate-based microcarriers may be similar to the extracellular matrix (ECM), even without seed cells.
-
✓
Broadly speaking, microcarriers are also artificial materials. Notably, this method is not limited by the source of the implants. Excellent microcarriers with chondrocyte are dynamically cultured to form tissue-engineered microtissues that can mimic cartilage tissue. The microtissue [32] is similar to the cartilage tissue provided by the cartilage transplantation method. The application of microcarriers has greatly broadened the treatment methods for traditional artificial material implantation.
-
✓
Some drugs, such as glucosamine sulfate and chondroitin sulfate, are very effective in treating cartilage damage [33]. Microcarriers can act as a porter for site-specific drug delivery [34]. These effective drugs are loaded on microcarriers with biological compatibility, nonallergenic properties, and biodegradability and thus are well-suited for the treatment of cartilage diseases [35].
Overview of the advantages of microcarriers in the treatment of cartilage defects
The goal of TE is to develop biological alternatives to restore the function of damaged tissue. Current TE strategies for cartilage regeneration rely heavily on synthetic or natural implants. Seed cells, effective cell modification and proper supportive matrices are the three foundations of TE [36]. In the field of TE for the repair of cartilage damage, microcarrier applications have enormous potential because they have advantages in all three aspects. The following summarizes the advantages of microcarriers from these three aspects.
Sufficient and high-quality seed cells
Sufficient and high-quality seed cells are one of the important elements of TE [37]. Microcarriers can be loaded with seed cells and deliver the seed cells to the damaged site to complete the repair [38].
BMSCs have been extensively studied [[39], [40], [41], [42]], and a number of scholars are committed to their application in cartilage regeneration. BMSCs have been demonstrated to have immunomodulatory properties, multilineage potential [43] and tissue homing properties. Moreover, notably, even BMSCs isolated from patients with advanced OA have chondrogenic ability and can synthesize cartilage-specific ECM [44]. Reports [30,32,45,46] have noted that a large number of BMSCs can be carried by microcarriers and successfully differentiated into chondrocyte for cartilage TE. These experiments indicated that microcarriers containing BMSCs have the potential to be used in cartilage defect repair in vitro and in vivo.
Human adipose-derived stem cells (hADSCs) are a type of mesenchymal stem cells (MSCs) with chondrogenic differentiation ability [47]. Previous studies [48,49] have reported that the amplification of ADSCs on microcarriers followed by implantation has great benefits for cartilage regeneration. One study [50] proved that microcarriers support cartilage regeneration because microcarriers that degrade too quickly facilitate the production of immature bone-like tissue. Another study [51] explored the importance of the cell density and differentiation status of MSCs in microspheres for cartilage regeneration. This study shows that regardless of the differentiation status of MSCs, a locally high cell density is conducive to cartilage repair and the differentiation status of MSCs has a crucial effect on regeneration. In another experiment [52] investigating treatments with rabbit OA models, the combined use of hADSCs and transforming growth factor-β (TGF-β) 3 microspheres promoted cartilage regeneration better than microspheres containing only one material.
The role of chondrocyte in cartilage regeneration is clear [48]. Some microcarriers deliver seed cells that are chondrocyte [42,53], and chondrocyte produced in dynamic microcarriers and tissue culture plates have similar yields [28].
Moreover, a unique advantage is that the seed cells carried by microcarriers can stay in the defect area for a relatively long time. A color-enhanced overlay of the image from one study [32] showed that the cells or their progeny cells carried by the microcarriers were still detectable at week 6.
Microenvironment
The microenvironment is another important factor in TE. Microcarriers can provide a good microenvironment that is conducive to cartilage defect repair, such as a unique culture method that promotes cell growth; provides an appropriate matrix to promote cell adhesion and proliferation; can mimic cartilage; and allows for the addition of cytokines to promote chondrogenic differentiation. The microenvironment provided by microcarriers can also affect some protein levels [54,55], and it can indicate how seed cells integrate into the tissue matrices and then utilize these cells to successfully restore the function of the damaged area.
For cultured chondrocyte, genetic analysis results show that a dynamic microcarrier culture can maintain the chondrocyte phenotype while expanding chondrocyte. The dynamic microcarrier amplification method on chondrocyte has shown that compared to monolayer amplification, it can improve general hyaline cartilage protein levels, such as Col2 and Agg [28].
The microenvironment provided by certain biological materials can promote cell proliferation and adhesion, such as gelatin [56]. Studies [30,57] have reported that microcarriers containing gelatin can promote cell proliferation and have the potential to repair cartilage damage.
Some cytokines, such as TGF-β1, can induce MSC chondrogenic differentiation [58]. Research by Agata Zykwinska confirms that microcarriers based on marine exopolysaccharide (EPS) can encapsulate TGF-β1, which has potential in future applications for repairing cartilage defects [59]. These microcarriers based on EPS act as reservoirs of growth factors to retain them in a targeted location and can induce regenerative responses. Because these microcarriers based on EPS can mimic the function of glycosaminoglycans (GAGs), they can prevent the loaded growth factor from being destroyed by some enzymes.
In addition to TGF, ECM protein is also one of the key factors in promoting cartilage regeneration [31]. The microenvironment maintained by high-quality biomaterials also has characteristics similar to natural ECM, such as alginate hydrogel for cartilage regeneration. Biomaterial-based approaches, such as the use of preformed hydrogel matrices, are often supplemented with biochemical incentives to maintain chondrogenic differentiation while providing mechanical stability. Alginate is an excellent material for making microcarriers. A previous study [60] clarified that alginate hydrogel microspheres provided superior natural ECM in chondrocyte cultures compared to monolayer cultures with or without the addition of TGF-β1. As an artificial ECM implanted in the area of cartilage defects, high-quality microcarriers should also resemble the features of natural ECM as much as possible in their internal structure.
The purpose of the application of matrix-assisted strategies is to create a good microenvironment for cell growth [61,62]. In Ramkumar’s scientific research [46], BMSCs were embedded in modular microspheres composed of agarose (AG) and collagen type II (COL-II) at different concentrations. COL-II can promote chondrocyte cell proliferation, ECM deposition and tissue wound healing [63]; therefore, microspheres prepared with COL-II are designed to mimic proteoglycan and some protein components of the cartilage ECM. The 80–100 micron microspheres the authors prepared do not exactly match the size of the defined microcarriers but produced effects similar to those of lineage-specific differentiation of embedded BMSCs, which can be maintained in culture. Importantly, microbeads prepared with COL-II promote the chondrogenic phenotype; in addition, these microspheres are very robust and support cell viability. Similarly, scholars [64] have applied a cell-free COL-I plug with poly (l-glutamic acid) (PLGA) microspheres containing bone morphogenetic protein-7 to successfully repair knee joint cartilage defects in Goettingen minipigs. In another study [65], Paulomi Ghosh’s team encapsulated decellularized cartilage in microspheres. The advantage of this encapsulation is that decellularized cartilage itself has a large number of proteins, cytokines, GAGs, and TGF that are beneficial to chondrocyte, and when combined with microspheres, these materials can provide a good microenvironment for chondrocyte. Moreover, it was proven that the protein carried by this microsphere retains its function after filament production by melt extrusion, which shows that filaments containing chondroinductive microspheres can be produced via this method to better repair cartilage.
In summary, various components that are beneficial to cartilage defect repair can be integrated in microcarriers to provide a microenvironment that is beneficial to the growth, differentiation, and proliferation of seed cells and promotes the secretion of cartilage-related proteins.
Proper supportive matrices
Proper supportive matrices are another extremely important condition in cartilage regeneration TE. Ordinary microcarriers are generally divided into synthetic microcarriers and natural microcarriers. Synthetic microcarriers include polyacrylamide microcarriers, glucose microcarriers, polystyrene microcarriers, etc. However, their biocompatibility is not good and almost all of them lack cell recognition sites, thus affecting cell growth to a certain extent [66]. Therefore, scientists are now using natural polymers as materials for preparing microcarriers that facilitate good biocompatibility, such as chitosan, gelatin, alginate, and others [53,60,67]. Different kinds of microcarriers constructed by researchers do not have the same properties as supportive matrices.
A research report in 2018 [32] stated that microcarriers by crocodile dialdehyde bacterial cellulose with DL-allo-hydroxylysine and complexing chitosan can induce cell proliferation, regulate cell function, and promote cell growth and migration. Hydroxylysine is an amino acid with excellent properties, such as low immunogenicity, suitable biodegradability, and good compatibility [68]. Hydroxylysine is an abundant and important component of type II collagen. Especially in cell culture, hydroxylysine promotes cell differentiation and chondrogenesis [69]. In addition, the material composition of the microcarriers also contains oxidized bacterial cellulose, which can accurately mimic the structural characteristics of the natural ECM and has sufficient mechanical strength [70].
Moreover, other researchers have combined natural materials with synthetic materials to make microcarriers. Researchers produced PLGA microspheres and chitosan polyelectrolyte complex (PEC) microspheres and compared the cartilage damage repair abilities of the two types of microspheres [53]. Chondrocyte-loaded PEC microspheres produced more cartilage matrix than chitosan microspheres.
If biomaterials have potential application value in repairing cartilage defects, they must be able to promote cell growth and differentiation, have degradability and have sufficient mechanical properties. Many scholars have performed ongoing research towards achieving these goals. Some scholars [71] have reported that porous PLGA microcarriers can induce MSC chondrogenesis. The biocompatible scaffolds prepared by E Filová’s team [72] show improved biomechanical properties, and they developed a porous scaffold of poly-epsilon-caprolactone (PCL) with incorporated chitosan microparticles. The microparticles constructed via PCL have advantages in mechanical strength and porosity for cartilage regeneration, and PCL microspheres can better repair cartilage if they contain chitosan, with the repair effect positively related to the concentration of chitosan.
In addition to the three key factors of TE described above, microcarriers have many other unique advantages.
Microtissue formation
Studies [73] have shown that host-implant integration and graft maturation will be better improved if the scaffolds are continuously cultured in a perfusion culture system with exogenous stimulation prior to implantation.
In the construction of tissue-engineered cartilage, the ECM secreted by microcarrier-loaded cells binds each of these particles together, which can accelerate the formation of tissue-engineered cartilage [74]. Microcarriers containing seed cells are transplanted to a custom bioreactor for dynamic culture [32]. After the microcarriers secrete the ECM, microtissue can be obtained [75]. Moreover, the microtissue has another benefit because functional microtissue can be easily placed at the site of cartilage defects by implantation, which prevents cell damage and loss caused by the digestion of the cells before being transferred from a monolayer culture in a flask [76].
Injectable microcarriers
Most TE technology for treating articular cartilage defects requires opening the joint cavity to place a biological scaffold, which inevitably causes great damage to the surrounding tissues of the joint [77]. However, due to its unique size advantage, microcarriers can be localized to cartilage defect sites that contain biologically active microtissues or seed cells by injection, which can reduce trauma caused by large-scale incisions [[78], [79], [80]].
However, for injectable microcarriers, one problem that cannot be ignored is how to affix the microcarriers to the cartilage defect for a long time after implantation for cartilage regeneration. Part of the studies [32] used bioprotein glue for adhesion fixation. Although this method is not inappropriate, the injected microspheres need to be accurately placed at the tissue defect site, which creates certain difficulties for clinical surgery. Notably, researchers have developed magnetic actuation microspheres [81], with a body composed of PLGA and a surface coated with magnetic nanoparticles. This microcarrier contains cells that can pinpoint the cartilage defect site by a magnetic field. Additional research has focused on this magnetically actuated microsphere [82]. Researchers also prepared Janus microspheres based on alginate for encapsulating MSCs in one compartment and iron oxide nanoparticles (IONPs) in another. Because the toxicity of IONPs to cells is controversial [83], the cells in this microsphere are separated from the IONPs, which can prohibit potential damage to cells. In addition, the high load capacity of such microspheres to IONPs results in easier movement after magnetization, which is another advantage. These magnetic microcarriers are of great significance for cartilage regeneration.
Combined with a biological scaffold
Microcarriers with cells can also be used in conjunction with biological scaffolds for cartilage regeneration [84,85]. PLGA microspheres combined with collagen/silk fibroin composite scaffolds can effectively repair cartilage and promote integration between the repaired cartilage and surrounding normal tissues [84]. The combination of cold atmospheric plasma (CAP)-modified electrospun scaffolds and embedded microspheres used as a TE material has been reported. CAP and microspheres can synergistically promote stem cell growth and improve the chondrogenic differentiation of BMSCs [85].
Similarly, another report [86] described similar stents. Compared with the abovementioned collagen/silk fibroin scaffold, this microcarrier also contains hyaluronic acid, which presents the following advantages: ability to regulate the surrounding environment, maintain structural integrity [87], and produce anti-inflammatory and analgesic effects [88]. In addition, these researchers also inserted the components of pilose antler polypeptides (PAPs), which can promote cartilage healing, into PLGA microspheres without cells [89]. The authors demonstrated that PAP microspheres with this scaffold can promote cartilage regeneration.
Drug loading
In addition to the factors and proteins described above and the ability of microspheres to deliver seed cells, microspheres can also be effective for drug delivery. Scholars have used sustained-release microspheres to deliver BMP-2 and TGF-3 to repair cartilage in pigs [90]. Chondroitin sulfate is an most important drug that has been shown to contribute to cartilage regeneration [91]. A long-term, large animal study [92] clarified that chondroitin sulfate-containing microspheres have a better effect on cartilage regeneration than microfracture techniques.
This drug delivery system is often placed at the cartilage defect site by injection, which is convenient and presents low invasiveness. Studies [93,94] have also reported the use of microcarriers as transporters for an injectable drug delivery system. These studies use injectable microspheres that reduce cartilage degradation to treat OA, which is notable in cartilage regeneration. The authors [93] impregnated PLGA microspheres with fluvastatin, a drug with anabolic and anticatabolic effects on chondrocyte, to reduce cartilage degradation in rabbits and achieved excellent results. They [94] infused tumor necrosis factor-alpha stimulated gene-6, a protein that inhibits plasmin, into PLGA degradable 10 wt% heparin microparticles to treat OA in rats and achieved positive results.
In summary, microcarriers have considerable advantages for cartilage regeneration. These microcarriers differ in seed cells, proteins, factors and drugs, matrices and other aspects. The microcarriers related to cartilage regeneration that were developed in recent years (these microcarriers were reported in articles screened by the aforementioned methods) are summarized in Table 1.
Table 1.
Recent examples of microcarriers for cartilage regeneration (Research reported from January 2014 to January 2020).
| Matrix | Cells | Drugs/proteins/factors/others | Composite other scaffolds | Animals (cartilage regeneration in vivo) | Refs. |
|---|---|---|---|---|---|
| a agarose and collagen type II | BMSCs | / | / | / | [46] |
| PLGA | ADSCs | TGF-β3 | / | rabbits | [52] |
| aPLGA | MC3T3-E1 preosteoblasts and ATDC5 prechondrocytes | BMP-7 | multichannel biphasic calcium phosphate granule-hyaluronic acid-gelatin | / | [95] |
| dialdehyde bacterial cellulose - DL-allo-hydroxylysine - chitosan | BMSCs | / | / | mice | [32] |
| Alginate | ADSCs | / | / | rabbits | [50] |
| Collagen | BMSCs | / | / | rabbits | [51] |
| PLGA | BMSCs | / | collagen-silk fibroin | rabbits | [84] |
| aPLGA | BMSCs | bovine serum albumin and TGF-β1 | ε-caprolactone | / | [85] |
| a chitosan and ECM | chondrocyte | / | / | / | [78] |
| a PLGA | BMSCs | decellularized cartilage and chondroitin sulfate | / | / | [42] |
| a PLGA and chitosan polyelectrolyte | chondrocyte | / | / | / | [53] |
| a poly (lactic acid) | MSCs | decellularized cartilage matrix | / | / | [65] |
| PLGA | BMSCs | pilose antler polypeptides | silk fibroin/collagen/hyaluronic acid | rabbits | [86] |
| a PCL | chondrocyte | Chitosan | / | / | [72] |
| PLGA | / | chondroitin sulfate and β-tricalcium phosphate | / | sheep | [92] |
| PLAG, collagen, chitosan and hyaluronic acid sodium | BMSCs | kartogenin and polylysine-heparin sodium nanoparticles containing TGF-β1 | / | rabbits | [96] |
| a collagen | hMSCs | TGF-β3 | / | / | [38] |
| PCL and the hydroxyapatite | MSCs | / | / | rabbits | [97] |
| PLGA | / | BMP-7 | / | minipigs | [64] |
| PLGA | chondrocyte | / | poly (vinyl alcohol) hydrogel | rabbits | [98] |
| a gelatin and alginate | BMSCs | / | PCL | / | [30] |
| Alginate | ADSCs | / | / | sheep | [29] |
| a poly (lactic acid) | BMSCs | decellularized cartilage matrix | poly (caprolactone) filaments | / | [99] |
| a glycosaminoglycan | ADSCs | TGF-β1 | / | / | [59] |
| a PLGA and decellularized cartilage | BMSCs | TGF-β | / | / | [100] |
| PLGA | BMSCs | BMP-2 | PLGA thin polymeric membrane | rabbits | [101] |
| a chitosan | chondrocyte | / | / | / | [102] |
| a PLGA | BMSCs | / | collagen/chitosan/hyaluronic acid sodium | / | [103] |
| a polyurethane | BMSCs | chemokines SDF-1, and Y27632 | polyurethane | / | [104] |
| PLLA/chitosan | BMSCs | / | poly (l-lactic acid) (PLLA) membrane | rabbits | [105] |
| a methacrylated hyaluronic acid | BMSCs | decellularized cartilage/devitalized cartilage | / | / | [106] |
| PLGA | / | BMP-2 and TGF-β1 | segmented polyurethane | rabbits | [107] |
| Chitosan | BMSCs | BMP-2 and TGF-β3 | demineralized bone matrix | pigs | [90] |
| a alginate | chondrocyte | arginine-glycine-aspartic acid | / | / | [79] |
| a PLGA | MSCs | TGF-β3 and SOX9 | / | / | [54] |
| alginate-PCEC | chondrocyte | / | / | rabbits | [80] |
| alginate-PLGA | BMSCs | BMP-2 or TGFβ1 | / | rabbits | [108] |
| a poly (l-lactic acid) | ASCs | TGF-β3, collagen II, magnetic nanoparticles | / | / | [109] |
| a PLGA | MSCs | magnetic nanoparticles | / | / | [81] |
| a PLLA-based copolymers with acrylic | BMSCs | cytomodulin, BMP-2 | / | / | [110] |
| attapulgite, collagen I | / | naringin, TGF-β1 | / | rabbit | [111] |
| a agarose | MSCs | chitosan-chondroitin sulfate | / | / | [112] |
| a PLGA | MSCs | BSA, TGF-β3 | / | / | [113] |
| a PLGA | bone marrow cells | IGF-I | / | / | [114] |
| a PLGA | MSCs | BMP-7 and TGF-β3, heparin and Tetronic 1107 | / | / | [115] |
| a chitosan | MSCs | Kartogenin | / | / | [116] |
| a gelatin | ADSCs | TGF-β1 | / | / | [57] |
| a PCL | chondrocyte | / | / | / | [117] |
| a poly (l-lactic acid) | ADSCs | collagen II-TGF-β3 | / | / | [49] |
| a gelatin | ADSCs | TGF-β1 | / | / | [118] |
| a poly (alanine ethyl ester-co-glycine ethyl ester) phosphazene | BMSCs | TGF-β1 or IGF-I | demineralized bone matrix | / | [119] |
| a PLGA-nanohydroxyapatite | BMSCs or chondrocyte | / | / | / | [120] |
| a gelatin | / | IL-4 and IL-13 | / | / | [121] |
| a alginate | ADSCs | / | / | / | [122] |
| Gelatin | / | IGF-1 and BMP-2 | / | rabbits | [55] |
| a poly-l-arginine | chondrocyte | C-type natriuretic peptide | / | / | [123] |
| PLGA | chondrocyte | cartilaginous hydrogel | hydrogel conjugated to PLGA | rabbits | [124] |
| a PCL | MSCs (bone marrow, cord blood, fetal and Wharton’s jelly) | / | / | / | [125] |
| a PLGA | BMSCs | chondroitin sulfate and tricalcium phosphate | / | / | [45] |
| a hyaluronan | MSCs | TGF-β3 | / | / | [126] |
| PLGA | / | Dexamethasone | / | canine models | [127] |
| a collagen | chondrocyte | Insulin | / | / | [128] |
| a PLGA | ADSCs | TGF-β1 | / | / | [128] |
| a alginate | ADSCs | iron oxide nanoparticles | / | / | [82] |
PCEC: amphiphilic poly(ε-caprolactone)-b-poly(ethylene glycol)-b-poly(ε-caprolactone)
BMP: bone morphogenetic protein
ASC: adipose stem cell
BSA: bovine serum albumin
IGF-I: insulin-like growth factor I
Studies without animal models indicated
Prospects for the application of microcarriers in the treatment of cartilage defects
The microenvironment provided by microcarriers composed of different materials is crucial for repairing cartilage defects. As mentioned earlier [59], microcarriers based on EPS with GAG-like functions can effectively encapsulate growth factors that promote cartilage regeneration. Moreover, many studies [32] have confirmed that microcarriers with a structure more similar to that of the natural ECM of cartilage tissue present better cartilage defect repair results. Moreover, a study [78] has shown that microcarriers containing 1% ECM of cartilage tissue have higher chondrocyte attachment. Therefore, we suspect that if a natural ECM can be directly produced in microcarriers that present appropriate pore sizes, mechanical properties and porosity characteristics, the protection of growth factors associated with the microenvironment will more easily promote cartilage regeneration. In the future, additional research in this direction would be welcome.
Choosing appropriate materials to prepare microcarriers is also one of the important conditions for effectively repairing cartilage damage. The appropriate biomaterial has good biocompatibility and other advantages. For instance, alginate hydrogel microspheres can effectively increase ECM protein expression [60]. However, few studies have investigated whether microcarriers prepared by composite materials can effectively promote the growth of seed cells. In a study by Yichi Xu in 2019 [30], alginate-gelatin microspheres were produced that can promote cell growth more than microspheres of a single alginate material can. However, such microspheres have not been tested in vivo. If the results of in vivo experiments prove its effectiveness, then the argument that this microsphere repairs cartilage damage will be more reliable. As previously mentioned, nanofibrous cellulosic material can provide a natural nanofibrous structure similar to a natural ECM. The microcarriers of this structure made with oxidized bacterial cellulose have sufficient mechanical strength to prevent the microstrand from collapse due to the contraction forces of the loaded cells [129]. Whether other nanofibrous cellulosic materials can better mimic a natural ECM requires further research by scientific researchers.
We found that microcarriers made from natural materials still have a shortcoming: if the volume of the microcarriers is too large, then the diffusion of oxygen and nutrients will be insufficient [130], which may lead to the death of deep seed cells. Therefore, the volume of microcarriers is also very important for cell proliferation. Another factor for the diffusion of oxygen and nutrients is the porosity of the microcarriers. Porosity and volume have become the key factors affecting seed cell proliferation. The future development direction of microcarriers should be the identification of appropriate porosities and volumes, which will play an important role in TE.
The cell source is also an important issue in the application of microcarriers. Chondrocyte-bearing microcarriers can certainly treat cartilage defects [53]. However, to avoid rejection, these chondrocyte are often isolated from cartilage tissue in patients. Even though more chondrocyte can be proliferated in vitro, the cartilage in the area of the cell source must be damaged. Compared to cartilage damage, adipose tissue damage is more easily accepted. ADSCs also present chondrogenic differentiation [47] and thus can be used as seed cells. Future applications of microcarriers in this direction will have clinical significance.
Notably, injectable microcarriers can create a minimally invasive surgical method for cartilage regeneration. More importantly, the study and development of magnetic microcarriers has greatly transcended the application limitations of injectable microcarriers [32,82]. This kind of magnetic microcarrier not only is implanted into the cartilage damage site at one time similar to traditional TE materials but also can be injected multiple times because only a magnetic field is required to target joint cartilage regeneration. If this aspect is thoroughly studied, then it will open a new chapter in cartilage regeneration.
Although some microcarriers have been proven to have excellent repair effects based on in vivo and in vitro experiments, reliable clinical studies have not bene performed to research their ability to repair human articular cartilage damage. Hopefully, orthopedic surgeons seeking a method of quickly relieving the pain of cartilage injury patients will focus on the advances of microcarriers in repairing cartilage and confirm their clinical transformation ability through clinical studies in the future.
Declaration of competing interest
Author Sida Liao, Author Haoye Meng, Author Junkang Li, Author Jun Zhao, Author Yichi Xu, Author Aiyuan Wang, Author Wenjing Xu, Author Jiang Peng, and Author Shibi Lu declare that they have no conflicts of interest.
Acknowledgments
This study was supported by the National Key Research and Development Program of China (2016YFC1102104) and the National Natural Science Foundation of China (Grant No. 81972047).
Footnotes
The work was performed at Chinese PLA General Hospital
Contributor Information
Haoye Meng, Email: menghaoye@126.com.
Junkang Li, Email: 1582302320@qq.com.
Jun Zhao, Email: 15801208995@163.com.
Yichi Xu, Email: xuyichi0333@hotmail.com.
Aiyuan Wang, Email: wangaiyuan301@126.com.
Wenjing Xu, Email: wenjingkitty@163.com.
Jiang Peng, Email: pengjiang301@126.com.
Shibi Lu, Email: lushibi301@126.com.
References
- 1.Krishnan Y., Grodzinsky A.J. Cartilage diseases. Matrix Biol : journal of the International Society for Matrix Biology. 2018;71-72:51–69. doi: 10.1016/j.matbio.2018.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Doherty M, Muthuri SG, McWilliams DF, Zhang W. History of knee injuries and knee osteoarthritis: a meta-analysis of observational studies. Osteoarthritis Cartilage. 2011;19(11):1286–1293. doi: 10.1016/j.joca.2011.07.015. [DOI] [PubMed] [Google Scholar]
- 3.Wascher DC, Richter DL, Schenck RC Jr, Treme G. Knee articular cartilage repair and restoration techniques: a review of the literature. Sports health. 2016;8(2):153–160. doi: 10.1177/1941738115611350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cain EL, Clancy WG. Treatment algorithm for osteochondral injuries of the knee. Clin Sports. 2001;20(2):321–342. doi: 10.1016/s0278-5919(05)70309-4. [DOI] [PubMed] [Google Scholar]
- 5.Schloo B, Vacanti CA, Langer R, Vacanti JP. Synthetic polymers seeded with chondrocyte provide a template for new cartilage formation. Plast Reconstr Surg. 1991;88(5):753–759. doi: 10.1097/00006534-199111000-00001. [DOI] [PubMed] [Google Scholar]
- 6.Mikos AG, Holland TA, Bodde EW, Cuijpers VM, Baggett LS, Tabata V. Degradable hydrogel scaffolds for in vivo delivery of single and dual growth factors in cartilage repair. Osteoarthritis Cartilage. 2007;15(2):187–197. doi: 10.1016/j.joca.2006.07.006. [DOI] [PubMed] [Google Scholar]
- 7.Basiri A., Farokhi M, Azami M, Ebrahimi-Barough S, Mohamadnia A, Rashtbar M. A silk fibroin/decellularized extract of Wharton’s jelly hydrogel intended for cartilage tissue engineering. Progress in biomaterials. 2019;8(1):31–42. doi: 10.1007/s40204-019-0108-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gao J, Dennis JE, Solchaga LA, Awadallah AS, Goldberg VM, Caplan AI. Tissue-engineered fabrication of an osteochondral composite graft using rat bone marrow-derived mesenchymal stem cells. Tissue Eng. 2001;7(4):363–371. doi: 10.1089/10763270152436427. [DOI] [PubMed] [Google Scholar]
- 9.Xie Y, Gong Y, Hong Y, Gao C, Shen J. Collagen-coated polylactide microspheres as chondrocyte microcarriers. Biomaterials. 2005;26(32):6305–6313. doi: 10.1016/j.biomaterials.2005.03.038. [DOI] [PubMed] [Google Scholar]
- 10.Choi Y S, Park S N, Suh H. Adipose tissue engineering using mesenchymal stem cells attached to injectable PLGA spheres. Biomaterials. 2005;26(29):5855–5863. doi: 10.1016/j.biomaterials.2005.02.022. [DOI] [PubMed] [Google Scholar]
- 11.van Wezel A L. Growth of cell-strains and primary cells on micro-carriers in homogeneous culture. Nature. 1967;216(5110):64–65. doi: 10.1038/216064a0. [DOI] [PubMed] [Google Scholar]
- 12.Senuma Y, Franceschin S, Hilborn JG, Tissières P, Bisson I, Frey P. Bioresorbable microspheres by spinning disk atomization as injectable cell carrier: from preparation to in vitro evaluation. Biomaterials. 2000;21(11):1135–1144. doi: 10.1016/s0142-9612(99)00276-8. [DOI] [PubMed] [Google Scholar]
- 13.Jiang Y, Tuan RS. Origin and function of cartilage stem/progenitor cells in osteoarthritis. Nat Rev Rheumatol. 2015;11(4):206–212. doi: 10.1038/nrrheum.2014.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Woolf A D, Pfleger B. Burden of major musculoskeletal conditions. Bull World Health Organ. 2003;81(9):646–656. [PMC free article] [PubMed] [Google Scholar]
- 15.Ding C, Davies-Tuck ML, Wluka AE, Wang Y, Teichtahl AJ, Jones G. The natural history of cartilage defects in people with knee osteoarthritis. Osteoarthritis Cartilage. 2008;16(3):337–342. doi: 10.1016/j.joca.2007.07.005. [DOI] [PubMed] [Google Scholar]
- 16.Gomoll AH. Microfracture and augments. J Knee Surg. 2012;25(1):9–15. doi: 10.1055/s-0031-1299654. [DOI] [PubMed] [Google Scholar]
- 17.Chen G, Guzmán-Morales J, Lafantaisie-Favreau CH, Hoemann CD. Subchondral chitosan/blood implant-guided bone plate resorption and woven bone repair is coupled to hyaline cartilage regeneration from microdrill holes in aged rabbit knees. Osteoarthritis Cartilage. 2014;22(2):323–333. doi: 10.1016/j.joca.2013.12.011. [DOI] [PubMed] [Google Scholar]
- 18.De Young AJ, Meric G, Gracitelli GC, Görtz S, Bugbee WD. Fresh osteochondral allograft transplantation for bipolar reciprocal osteochondral lesions of the knee. Am J Sports Med. 2015;43(3):709–714. doi: 10.1177/0363546514562549. [DOI] [PubMed] [Google Scholar]
- 19.Minas T, Bryant T, Gomoll AH, Solhpour S, Rosenberger R, Probst C. Autologous chondrocyte implantation for joint preservation in patients with early osteoarthritis. Clin Orthop Relat Res. 2010;468(1):147–157. doi: 10.1007/s11999-009-0998-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kon E, Filardo G, Condello V, Collarile M, Di Martino A, Zorzi C. Second-generation autologous chondrocyte implantation: results in patients older than 40 years. Am J Sports Med. 2011;39(8):1668–1675. doi: 10.1177/0363546511404675. [DOI] [PubMed] [Google Scholar]
- 21.Neri MP, Di Martino A, Kon E, Perdisa F, Sessa A, Filardo G. Surgical treatment of early knee osteoarthritis with a cell-free osteochondral scaffold: results at 24 months of follow-up. Injury. 2015:S33–S38. doi: 10.1016/S0020-1383(15)30052-8. [DOI] [PubMed] [Google Scholar]
- 22.Nejadnik H, Hui JH, Feng Choong EP, Tai BC, Lee EH. Autologous bone marrow-derived mesenchymal stem cells versus autologous chondrocyte implantation: an observational cohort study. Am J Sports Med. 2010;38(6):1110–1116. doi: 10.1177/0363546509359067. [DOI] [PubMed] [Google Scholar]
- 23.Remberger K, Kaul G, Cucchiarini M, Kohn D, Madry H. Failed cartilage repair for early osteoarthritis defects: a biochemical, histological and immunohistochemical analysis of the repair tissue after treatment with marrow-stimulation techniques. Knee Surg Sports Traumatol Arthrosc : official journal of the ESSKA. 2012;20(11):2315–2324. doi: 10.1007/s00167-011-1853-x. [DOI] [PubMed] [Google Scholar]
- 24.Marsetyo AF, Lubis AMT, Siagian C, Wonggokusuma E, Setyohadi B. Comparison of glucosamine-chondroitin sulfate with and without methylsulfonylmethane in grade I-II knee osteoarthritis: a double blind randomized controlled trial. Acta medica Indonesiana. 2017;49(2):105–111. [PubMed] [Google Scholar]
- 25.Smeriglio P, Lai JH, Dhulipala L, Behn AW, Goodman SB, Smith RL. Comparative potential of juvenile and adult human articular chondrocyte for cartilage tissue formation in three-dimensional biomimetic hydrogels. Tissue Eng. 2015;21:147–155. doi: 10.1089/ten.TEA.2014.0070. [DOI] [PubMed] [Google Scholar]
- 26.Locht R C, Gross AE, Langer F. Late osteochondral allograft resurfacing for tibial plateau fractures. J Bone Jt Surg Am Vol. 1984;66(3):328–335. [PubMed] [Google Scholar]
- 27.Park J, Kwon S, Hwang NS, Kang BJ. Clinical application of bone morphogenetic protein-2 microcarriers fabricated by the cryopolymerization of gelatin methacrylate for the treatment of radial fracture in two dogs. In vivo. 2018;32(3):575–581. doi: 10.21873/invivo.112278. (Athens, Greece) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Han J, Tee CA, Yang Z, Yin L, Wu Y, Lee EH. Improved zonal chondrocyte production protocol integrating size-based inertial spiral microchannel separation and dynamic microcarrier culture for clinical application. Biomaterials. 2019;220:119409. doi: 10.1016/j.biomaterials.2019.119409. [DOI] [PubMed] [Google Scholar]
- 29.Gümüşkaya B, Bozkurt M, Aşık MD, Gürsoy S, Türk M, Karahan S. Autologous stem cell-derived chondrocyte implantation with bio-targeted microspheres for the treatment of osteochondral defects. J Orthop Surg Res. 2019;14(1):394. doi: 10.1186/s13018-019-1434-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Xu Y, Peng J, Richards G, Lu S. Optimization of electrospray fabrication of stem cell-embedded alginate-gelatin microspheres and their assembly in 3D-printed poly(ε-caprolactone) scaffold for cartilage tissue engineering. Journal of orthopaedic translation. 2019;18:128–141. doi: 10.1016/j.jot.2019.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Krishnan Y, Grodzinsky AJ. Cartilage diseases. Matrix Biol : journal of the International Society for Matrix Biology. 2018:51–69. doi: 10.1016/j.matbio.2018.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang Y, Yuan X, Yu K, Meng H, Zheng Y, Peng J. Fabrication of nanofibrous microcarriers mimicking extracellular matrix for functional microtissue formation and cartilage regeneration. Biomaterials. 2018;171:118–132. doi: 10.1016/j.biomaterials.2018.04.033. [DOI] [PubMed] [Google Scholar]
- 33.Jerosch J. Effects of glucosamine and chondroitin sulfate on cartilage metabolism in OA: outlook on other nutrient partners especially omega-3 fatty acids. International journal of rheumatology. 2011;2011:969012. doi: 10.1155/2011/969012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Li W, Ding Y, Yu S, Yao Q. Multifunctional chitosan-45S5 bioactive glass-poly(3-hydroxybutyrate-co-3-hydroxyvalerate) microsphere composite membranes for guided tissue/bone regeneration. ACS Appl Mater Interfaces. 2015;7(37):20845–20854. doi: 10.1021/acsami.5b06128. [DOI] [PubMed] [Google Scholar]
- 35.Chen C, Xia C, Mei S, Zhu R, Pang Y, Wang J. The amelioration of cartilage degeneration by photo-crosslinked GelHA hydrogel and crizotinib encapsulated chitosan microspheres. Oncotarget. 2017;8(18):30235–30251. doi: 10.18632/oncotarget.15750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bakhshandeh B, Zarrintaj P, Oftadeh MO, Keramati F, Fouladiha H, Sohrabi-Jahromi S. Tissue engineering; strategies, tissues, and biomaterials. Biotechnol Genet Eng Rev. 2017;33(2):144–172. doi: 10.1080/02648725.2018.1430464. [DOI] [PubMed] [Google Scholar]
- 37.Vinatier C, Guicheux J. Cartilage tissue engineering: from biomaterials and stem cells to osteoarthritis treatments. Annals of physical and rehabilitation medicine. 2016;59(3):139–144. doi: 10.1016/j.rehab.2016.03.002. [DOI] [PubMed] [Google Scholar]
- 38.Mathieu M, Vigier S, Labour MN, Jorgensen C, Belamie E, Noël D. Induction of mesenchymal stem cell differentiation and cartilage formation by cross-linker-free collagen microspheres. Eur Cell Mater. 2014;28:82–96. doi: 10.22203/ecm.v028a07. discussion 96-7. [DOI] [PubMed] [Google Scholar]
- 39.Chu CQ, Zhang X, Wu S, Naccarato T, Prakash-Damani M, Chou Y. Regeneration of hyaline-like cartilage in situ with SOX9 stimulation of bone marrow-derived mesenchymal stem cells. PloS One. 2017;12(6) doi: 10.1371/journal.pone.0180138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Li Z, Wang Z, Ba R, Wei J, Zhao Y, Wu W. Angiogenic potential of human bone marrow-derived mesenchymal stem cells in chondrocyte brick-enriched constructs promoted stable regeneration of craniofacial cartilage. Stem cells translational medicine. 2017;6(2):601–612. doi: 10.5966/sctm.2016-0050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ishihara K, Nakayama K, Akieda S, Matsuda S, Iwamoto Y. Simultaneous regeneration of full-thickness cartilage and subchondral bone defects in vivo using a three-dimensional scaffold-free autologous construct derived from high-density bone marrow-derived mesenchymal stem cells. J Orthop Surg Res. 2014;9:98. doi: 10.1186/s13018-014-0098-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gupta V, Tenny KM, Barragan M, Berkland CJ, Detamore MS. Microsphere-based scaffolds encapsulating chondroitin sulfate or decellularized cartilage. J Biomater Appl. 2016;31(3):328–343. doi: 10.1177/0885328216655469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mosca JD, Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284(5411):143–147. doi: 10.1126/science.284.5411.143. (New York, N.Y.) [DOI] [PubMed] [Google Scholar]
- 44.Learmonth I, Kafienah W, Mistry S, Dickinson SC, Sims TJ, Hollander AP. Three-dimensional cartilage tissue engineering using adult stem cells from osteoarthritis patients. Arthritis Rheum. 2007;56(1):177–187. doi: 10.1002/art.22285. [DOI] [PubMed] [Google Scholar]
- 45.Gupta V, Mohan N, Berkland CJ, Detamore MS. Microsphere-based scaffolds carrying opposing gradients of chondroitin sulfate and tricalcium phosphate. Frontiers in bioengineering and biotechnology. 2015;3:96. doi: 10.3389/fbioe.2015.00096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Daley EL, Tiruvannamalai Annamalai R, Mertz DR, Stegemann JP. Collagen Type II enhances chondrogenic differentiation in agarose-based modular microtissues. Cytotherapy. 2016;18(2):263–277. doi: 10.1016/j.jcyt.2015.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mehdizadeh M, Sheykhhasan M, Qomi RT, Kalhor N, Ghiasi M. Evaluation of the ability of natural and synthetic scaffolds in providing an appropriate environment for growth and chondrogenic differentiation of adipose-derived mesenchymal stem cells. Indian J Orthop. 2015;49(5):561–568. doi: 10.4103/0019-5413.164043. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 48.Musumeci G, Mobasheri A, Kalamegam G, Batt ME. Chondrocyte and mesenchymal stem cell-based therapies for cartilage repair in osteoarthritis and related orthopaedic conditions. Maturitas. 2014;78(3):188–198. doi: 10.1016/j.maturitas.2014.04.017. [DOI] [PubMed] [Google Scholar]
- 49.Correia CR, Reis RL, Mano JF. Nanostructured capsules for cartilage tissue engineering. Methods Mol Biol. 2015;1340:181–189. doi: 10.1007/978-1-4939-2938-2_13. (Clifton, N.J.) [DOI] [PubMed] [Google Scholar]
- 50.Sedlaczek J, Leslie SK, Cohen DJ, Hyzy SL, Dosier CR, Nicolini A. Microencapsulated rabbit adipose stem cells initiate tissue regeneration in a rabbit ear defect model. Journal of tissue engineering and regenerative medicine. 2018;12(7):1742–1753. doi: 10.1002/term.2702. [DOI] [PubMed] [Google Scholar]
- 51.Chan D, Li YY, Cheng HW, Cheung KM, Chan BP. Mesenchymal stem cell-collagen microspheres for articular cartilage repair: cell density and differentiation status. Acta Biomater. 2014;10(5):1919–1929. doi: 10.1016/j.actbio.2014.01.002. [DOI] [PubMed] [Google Scholar]
- 52.Jing H, Sun Q, Zhang L, Xu T, Ying J, Xia B. Combined use of adipose derived stem cells and TGF-β3 microspheres promotes articular cartilage regeneration in vivo. Biotech Histochem : official publication of the Biological Stain Commission. 2018;93(3):168–176. doi: 10.1080/10520295.2017.1401663. [DOI] [PubMed] [Google Scholar]
- 53.Fang J, Zhang Y, Yan S, Liu Z, He S, Cui L. Poly(L-glutamic acid)/chitosan polyelectrolyte complex porous microspheres as cell microcarriers for cartilage regeneration. Acta Biomater. 2014;10(1):276–288. doi: 10.1016/j.actbio.2013.09.002. [DOI] [PubMed] [Google Scholar]
- 54.Lim SW, Park JS, Lim HJ, Park KH. Stem cell differentiation-related protein-loaded PLGA microspheres as a novel platform micro-typed scaffold for chondrogenesis. Biomed Mater. 2016;11(5) doi: 10.1088/1748-6041/11/5/055003. (Bristol, England) [DOI] [PubMed] [Google Scholar]
- 55.Lu S, Lam J, Trachtenberg JE, Lee EJ, Seyednejad H, van den Beucken JJJP. Dual growth factor delivery from bilayered, biodegradable hydrogel composites for spatially-guided osteochondral tissue repair. Biomaterials. 2014;35(31):8829–8839. doi: 10.1016/j.biomaterials.2014.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Xu M, Wang X, Yan Y, Yao R, Ge Y. An cell-assembly derived physiological 3D model of the metabolic syndrome, based on adipose-derived stromal cells and a gelatin/alginate/fibrinogen matrix. Biomaterials. 2010;31(14):3868–3877. doi: 10.1016/j.biomaterials.2010.01.111. [DOI] [PubMed] [Google Scholar]
- 57.Wei Y, Yin F, Cai J, Zen W, Zhou W, Yuan F. Cartilage regeneration of adipose-derived stem cells in the TGF-β1-immobilized PLGA-gelatin scaffold. Stem cell reviews and reports. 2015;11(3):453–459. doi: 10.1007/s12015-014-9561-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kurakazu I, Akasaki Y, Hayashida M, Tsushima H, Goto N, Sueishi T. FOXO1 transcription factor regulates chondrogenic differentiation through transforming growth factor β1 signaling. J Biol Chem. 2019;294(46):17555–17569. doi: 10.1074/jbc.RA119.009409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Garnier C, Zykwinska A, Marquis M, Godin M, Marchand L, Sinquin C. Microcarriers based on glycosaminoglycan-like marine exopolysaccharide for TGF-β1 long-term protection. Mar Drugs. 2019;17(1) doi: 10.3390/md17010065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ab-Rahim S, Selvaratnam L, Raghavendran HR, Kamarul T. Chondrocyte-alginate constructs with or without TGF-β1 produces superior extracellular matrix expression than monolayer cultures. Mol Cell Biochem. 2013;376:11–20. doi: 10.1007/s11010-012-1543-0. [DOI] [PubMed] [Google Scholar]
- 61.Claros S, Rodríguez-Losada N, Cruz E, Guerado E, Becerra J, Andrades JA. Characterization of adult stem/progenitor cell populations from bone marrow in a three-dimensional collagen gel culture system. Cell Transplant. 2012;21(9):2021–2032. doi: 10.3727/096368912X636939. [DOI] [PubMed] [Google Scholar]
- 62.Gaissmaier C, Koh JL, Weise K. Growth and differentiation factors for cartilage healing and repair. Injury. 2008:S88–S96. doi: 10.1016/j.injury.2008.01.035. [DOI] [PubMed] [Google Scholar]
- 63.Veilleux NH, Yannas IV, Spector M. Effect of passage number and collagen type on the proliferative, biosynthetic, and contractile activity of adult canine articular chondrocyte in type I and II collagen-glycosaminoglycan matrices in vitro. Tissue Eng. 2004;10:119–127. doi: 10.1089/107632704322791763. [DOI] [PubMed] [Google Scholar]
- 64.Gavenis K, Heussen N, Hofman M, Andereya S, Schneider U, Schmidt-Rohlfing B. Cell-free repair of small cartilage defects in the Goettingen minipig: the effects of BMP-7 continuously released by poly(lactic-co-glycolide acid) microspheres. J Biomater Appl. 2014;28(7):1008–1015. doi: 10.1177/0885328213491440. [DOI] [PubMed] [Google Scholar]
- 65.Ghosh P, Gruber SMS, Lin CY, Whitlock PW. Microspheres containing decellularized cartilage induce chondrogenesis in vitro and remain functional after incorporation within a poly(caprolactone) filament useful for fabricating a 3D scaffold. Biofabrication. 2018;10(2) doi: 10.1088/1758-5090/aaa637. [DOI] [PubMed] [Google Scholar]
- 66.Mikos AG, Freed JE, Marquis JC, Nohria A, Emmanual J, Langer R. Neocartilage formation in vitro and in vivo using cells cultured on synthetic biodegradable polymers. J Biomed Mater Res. 1993;27(1):11–23. doi: 10.1002/jbm.820270104. [DOI] [PubMed] [Google Scholar]
- 67.Zhou Y, Yan Z, Zhang H, Lu W, Liu S, Huang X. Expansion and delivery of adipose-derived mesenchymal stem cells on three microcarriers for soft tissue regeneration. Tissue Eng. 2011;17:2981–2997. doi: 10.1089/ten.tea.2010.0707. [DOI] [PubMed] [Google Scholar]
- 68.Hu JC, Huwe LW, Sullan GK, Athanasiou KA. Using costal chondrocytes to engineer articular cartilage with applications of passive axial compression and bioactive stimuli. Tissue Eng. 2018;24:516–526. doi: 10.1089/ten.tea.2017.0136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Lam J, Clark EC, Fong EL, Lee EJ, Lu S, Tabata Y. Evaluation of cell-laden polyelectrolyte hydrogels incorporating poly(L-Lysine) for applications in cartilage tissue engineering. Biomaterials. 2016;83:332–346. doi: 10.1016/j.biomaterials.2016.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Petersen N, Gatenholm P. Bacterial cellulose-based materials and medical devices: current state and perspectives. Appl Microbiol Biotechnol. 2011;91(5):1277–1286. doi: 10.1007/s00253-011-3432-y. [DOI] [PubMed] [Google Scholar]
- 71.Morille M, Toupet K, Montero-Menei CN, Jorgensen C, Noël D. PLGA-based microcarriers induce mesenchymal stem cell chondrogenesis and stimulate cartilage repair in osteoarthritis. Biomaterials. 2016;88:60–69. doi: 10.1016/j.biomaterials.2016.02.022. [DOI] [PubMed] [Google Scholar]
- 72.Filová E, KuŽelová Košťáková E, Jakubcová B, Danilová I, Jarošíková T, Chernyavskiy O. Polycaprolactone foam functionalized with chitosan microparticles - a suitable scaffold for cartilage regeneration. Physiol Res. 2016;65(1):121–131. doi: 10.33549/physiolres.932998. [DOI] [PubMed] [Google Scholar]
- 73.Zhou Y, Chen M, Zhou M, Ye Z, Tan WS. Ectopic osteogenesis of macroscopic tissue constructs assembled from human mesenchymal stem cell-laden microcarriers through in vitro perfusion culture. PloS One. 2014;9(10) doi: 10.1371/journal.pone.0109214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Yao R, Zhang R, Luan J, Lin F. Alginate and alginate/gelatin microspheres for human adipose-derived stem cell encapsulation and differentiation. Biofabrication. 2012;4(2) doi: 10.1088/1758-5082/4/2/025007. [DOI] [PubMed] [Google Scholar]
- 75.Zhao R, Boudou T, Wang WG, Chen CS, Reich DH. Decoupling cell and matrix mechanics in engineered microtissues using magnetically actuated microcantilevers. Advanced materials (Deerfield Beach, Fla.) 2013;25(12):1699–1705. doi: 10.1002/adma.201203585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Schrobback K, Klein TJ, Schuetz M, Upton Z, Leavesley DI, Malda J. Adult human articular chondrocyte in a microcarrier-based culture system: expansion and redifferentiation. J Orthop Res : official publication of the Orthopaedic Research Society. 2011;29(4):539–546. doi: 10.1002/jor.21264. [DOI] [PubMed] [Google Scholar]
- 77.Gurer B, Cabuk S, Karakus O, Yilmaz N, Yilmaz C. In vivo cartilage tissue engineering. J Orthop Surg Res. 2018;13(1):107. doi: 10.1186/s13018-018-0823-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Sivandzade F, Mashayekhan S. Design and fabrication of injectable microcarriers composed of acellular cartilage matrix and chitosan. J Biomater Sci Polym Ed. 2018;29(6):683–700. doi: 10.1080/09205063.2018.1433422. [DOI] [PubMed] [Google Scholar]
- 79.Woo E, Park H, Lee KY. Shear reversible cell/microsphere aggregate as an injectable for tissue regeneration. Macromol Biosci. 2014;14(5):740–748. doi: 10.1002/mabi.201300365. [DOI] [PubMed] [Google Scholar]
- 80.Liao J, Peng J, Wang B, Huang Y, Qu Y, Qian Z. Injectable Alginate hydrogel cross-linked by calcium gluconate-loaded porous microspheres for cartilage tissue engineering. ACS Omega. 2017;2(2):443–454. doi: 10.1021/acsomega.6b00495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Go G., Han J, Zhen J, Zheng S, Yoo A, Jeon MJ. A magnetically actuated microscaffold containing mesenchymal stem cells for articular cartilage repair. Advanced healthcare materials. 2017;6(13) doi: 10.1002/adhm.201601378. [DOI] [PubMed] [Google Scholar]
- 82.Park CH, Thomas RG, Unnithan AR, Moon MJ, Surendran SP, Batgerel T. Electromagnetic manipulation enabled calcium alginate Janus microsphere for targeted delivery of mesenchymal stem cells. Int J Biol Macromol. 2018;110:465–471. doi: 10.1016/j.ijbiomac.2018.01.003. [DOI] [PubMed] [Google Scholar]
- 83.Arami H, Khandhar A, Liggitt D, Krishnan KM. In vivo delivery, pharmacokinetics, biodistribution and toxicity of iron oxide nanoparticles. Chem Soc Rev. 2015;44(23):8576–8607. doi: 10.1039/c5cs00541h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Wang J, Yang Q, Cheng N, Tao X, Zhang Z, Sun X. Collagen/silk fibroin composite scaffold incorporated with PLGA microsphere for cartilage repair. Materials science & engineering. C, Materials for biological applications. 2016;61:705–711. doi: 10.1016/j.msec.2015.12.097. [DOI] [PubMed] [Google Scholar]
- 85.Zhu W, Castro NJ, Castro X, Castro M, Zhang LG. Cold atmospheric plasma modified electrospun scaffolds with embedded microspheres for improved cartilage regeneration. PloS One. 2015;10(7) doi: 10.1371/journal.pone.0134729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Wang J, Sun X, Zhang Z, Wang Y, Huang C, Yang C. Silk fibroin/collagen/hyaluronic acid scaffold incorporating pilose antler polypeptides microspheres for cartilage tissue engineering. Materials science & engineering. C, Materials for biological applications. 2019;94:35–44. doi: 10.1016/j.msec.2018.09.017. [DOI] [PubMed] [Google Scholar]
- 87.Kutlusoy T, Oktay B, Apohan NK, Süleymanoğlu M, Kuruca SE. Chitosan-co-Hyaluronic acid porous cryogels and their application in tissue engineering. Int J Biol Macromol. 2017;103:366–378. doi: 10.1016/j.ijbiomac.2017.05.067. [DOI] [PubMed] [Google Scholar]
- 88.Xia Q, Zhang YT, Li Z, Zhang K, Zhang HY, He ZH. Co-delivery of evodiamine and rutaecarpine in a microemulsion-based hyaluronic acid hydrogel for enhanced analgesic effects on mouse pain models. Int J Pharm. 2017;528:100–106. doi: 10.1016/j.ijpharm.2017.05.064. [DOI] [PubMed] [Google Scholar]
- 89.Lin J, Xiu Z, Zhaoyang WU. Effect OF pilose antler polypeptides ON the apoptosis OF rabbit marrow mesenchymal stem cells differentiated into chondrogenic phenotype IN vitro. Chin J Reparative Reconstr Surg. 2006;20(4):427–430. [PubMed] [Google Scholar]
- 90.Zhao D, Li Y, Zhou X, Yang Z. Peripheral blood mesenchymal stem cells combined with modified demineralized bone matrix promote pig cartilage defect repair. Cells Tissues Organs. 2018;206:26–34. doi: 10.1159/000493210. [DOI] [PubMed] [Google Scholar]
- 91.Xu J, Han L, Wang M, Li P, Gan D, Yan L. Mussel-inspired tissue-adhesive hydrogel based on the polydopamine-chondroitin sulfate complex for growth-factor-free cartilage regeneration. ACS Appl Mater Interfaces. 2018;10(33):28015–28026. doi: 10.1021/acsami.8b05314. [DOI] [PubMed] [Google Scholar]
- 92.Mohan N, Gupta V, Sridharan BP, Mellott AJ, Easley JT, Palmer RH. Microsphere-based gradient implants for osteochondral regeneration: a long-term study in sheep. Regen Med. 2015;10(6):709–728. doi: 10.2217/rme.15.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Goto N, Okazaki K, Akasaki Y, Ishihara K, Murakami K, Koyano K. Single intra-articular injection of fluvastatin-PLGA microspheres reduces cartilage degradation in rabbits with experimental osteoarthritis. J Orthop Res : official publication of the Orthopaedic Research Society. 2017;35(11):2465–2475. doi: 10.1002/jor.23562. [DOI] [PubMed] [Google Scholar]
- 94.Tellier LE, Treviño EA, Brimeyer AL, Reece DS, Willett NJ, Guldberg RE. Intra-articular TSG-6 delivery from heparin-based microparticles reduces cartilage damage in a rat model of osteoarthritis. Biomaterials science. 2018;6(5):1159–1167. doi: 10.1039/C8BM00010G. [DOI] [PubMed] [Google Scholar]
- 95.Amirian J, Jung A, Makkar P, Lee BT. A novel hybrid multichannel biphasic calcium phosphate granule-based composite scaffold for cartilage tissue regeneration. J Biomater Appl. 2018;32(6):775–787. doi: 10.1177/0885328217741757. [DOI] [PubMed] [Google Scholar]
- 96.Wang J, Wang Y, Sun X, Liu D, Huang C, Wu J. Biomimetic cartilage scaffold with orientated porous structure of two factors for cartilage repair of knee osteoarthritis. Artificial cells, nanomedicine, and biotechnology. 2019;47(1):1710–1721. doi: 10.1080/21691401.2019.1607866. [DOI] [PubMed] [Google Scholar]
- 97.Du Y, Liu H, Yang Q, Wang S, Wang J, Ma J. Selective laser sintering scaffold with hierarchical architecture and gradient composition for osteochondral repair in rabbits. Biomaterials. 2017;137:37–48. doi: 10.1016/j.biomaterials.2017.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Wang K, Cao Y, Xiong D, Niu Y. Semi-degradable porous poly (vinyl alcohol) hydrogel scaffold for cartilage repair: evaluation of the initial and cell-cultured tribological properties. Journal of the mechanical behavior of biomedical materials. 2017;68:163–172. doi: 10.1016/j.jmbbm.2017.02.001. [DOI] [PubMed] [Google Scholar]
- 99.Ghosh P, Gruber SMS, Lin CY, Whitlock PW. Microspheres containing decellularized cartilage induce chondrogenesis in vitro and remain functional after incorporation within a poly(caprolactone) filament useful for fabricating a 3D scaffold. Biofabrication. 2018;10(2) doi: 10.1088/1758-5090/aaa637. [DOI] [PubMed] [Google Scholar]
- 100.Sutherland AJ, Detamore MS. Bioactive microsphere-based scaffolds containing decellularized cartilage. Macromol Biosci. 2015;15(7):979–989. doi: 10.1002/mabi.201400472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Vayas R, Reyes R, Rodríguez-Évora M, Del Rosario C, Delgado A, Évora C. Evaluation of the effectiveness of a bMSC and BMP-2 polymeric trilayer system in cartilage repair. Biomed Mater. 2017;12(4) doi: 10.1088/1748-605X/aa6f1c. (Bristol, England) [DOI] [PubMed] [Google Scholar]
- 102.Zhou Y, Gao HL, Shen LL, Pan Z, Mao LB, Wu T. Chitosan microspheres with an extracellular matrix-mimicking nanofibrous structure as cell-carrier building blocks for bottom-up cartilage tissue engineering. Nanoscale. 2016;8(1):309–317. doi: 10.1039/c5nr06876b. [DOI] [PubMed] [Google Scholar]
- 103.Sun X, Wang J, Wang Y, Zhang Q. Collagen-based porous scaffolds containing PLGA microspheres for controlled kartogenin release in cartilage tissue engineering. Artificial cells, nanomedicine, and biotechnology. 2018;46(8):1957–1966. doi: 10.1080/21691401.2017.1397000. [DOI] [PubMed] [Google Scholar]
- 104.Wen YT, Dai NT, Hsu SH. Biodegradable water-based polyurethane scaffolds with a sequential release function for cell-free cartilage tissue engineering. Acta Biomater. 2019;88:301–313. doi: 10.1016/j.actbio.2019.02.044. [DOI] [PubMed] [Google Scholar]
- 105.Zurriaga Carda J, Lastra ML, Antolinos-Turpin CM, Morales-Román RM, Sancho-Tello M, Perea-Ruiz S. A cell-free approach with a supporting biomaterial in the form of dispersed microspheres induces hyaline cartilage formation in a rabbit knee model. J Biomed Mater Res B Appl Biomater. 2019;108(4):1428–1438. doi: 10.1002/jbm.b.34490. [DOI] [PubMed] [Google Scholar]
- 106.Hopkins RA, Beck EC, Barragan M, Libeer TB, Kieweg SL, Converse GL. Chondroinduction from naturally derived cartilage matrix: a comparison between devitalized and decellularized cartilage encapsulated in hydrogel pastes. Tissue Eng. 2016;22:665–679. doi: 10.1089/ten.tea.2015.0546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Reyes R, Solis R., Delgado A, Sanchez E, Hernandez A, San Roman J. Cartilage repair by local delivery of transforming growth factor-β1 or bone morphogenetic protein-2 from a novel, segmented polyurethane/polylactic-co-glycolic bilayered scaffold. J Biomed Mater Res. 2014;102(4):1110–1120. doi: 10.1002/jbma.34769. [DOI] [PubMed] [Google Scholar]
- 108.Reyes R., Delgado A, Sánchez E, Fernández A, Hernández A, Evora C. Repair of an osteochondral defect by sustained delivery of BMP-2 or TGFβ1 from a bilayered alginate-PLGA scaffold. Journal of tissue engineering and regenerative medicine. 2014;8(7):521–533. doi: 10.1002/term.1549. [DOI] [PubMed] [Google Scholar]
- 109.Reis RL, Correia CR, Gil S, Mano JF. A closed chondromimetic environment within magnetic-responsive liquified capsules encapsulating stem cells and collagen II/TGF-β3 microparticles. Advanced healthcare materials. 2016;5(11):1346–1355. doi: 10.1002/adhm.201600034. [DOI] [PubMed] [Google Scholar]
- 110.Zhang Z, Gupte MJ, Jin X, Ma PX. Injectable peptide decorated functional nanofibrous hollow microspheres to direct stem cell differentiation and tissue regeneration. Adv Funct Mater. 2015;25(3):350–360. doi: 10.1002/adfm.201402618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Huang J, Wang S, Zhang X, Li G, Ji P, Zhao H. Experimental study on loading naringin composite scaffolds for repairing rabbit osteochondral defects. Zhongguo xiu fu chong jian wai ke za zhi = Zhongguo xiufu chongjian waike zazhi = Chinese journal of reparative and reconstructive surgery. 2017;31(4):489–496. doi: 10.7507/1002-1892.201611112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Daley EL, Coleman RM, Stegemann JP. Biomimetic microbeads containing a chondroitin sulfate/chitosan polyelectrolyte complex for cell-based cartilage therapy. J Mater Chem B. 2015;3(40):7920–7929. doi: 10.1039/C5TB00934K. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Morille M, Venier-Julienne MC, Montero-Menei CN. Scaffolds for controlled release of cartilage growth factors. Methods Mol Biol. 2015;1340:171–180. doi: 10.1007/978-1-4939-2938-2_12. (Clifton, N.J.) [DOI] [PubMed] [Google Scholar]
- 114.Hilt JZ, Clark A, Milbrandt TA, Puleo DA. Retention of insulin-like growth factor I bioactivity during the fabrication of sintered polymeric scaffolds. Biomed Mater. 2014;9(2) doi: 10.1088/1748-6041/9/2/025015. (Bristol, England) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Crecente-Campo J, Borrajo E, Vidal A, Garcia-Fuentes M. New scaffolds encapsulating TGF-β3/BMP-7 combinations driving strong chondrogenic differentiation. Eur J Pharm Biopharm : official journal of Arbeitsgemeinschaft fur Pharmazeutische Verfahrenstechnik e.V. 2017;114:69–78. doi: 10.1016/j.ejpb.2016.12.021. [DOI] [PubMed] [Google Scholar]
- 116.Kim JE, Kang ML, Ko JY, Im GL. Intra-articular delivery of kartogenin-conjugated chitosan nano/microparticles for cartilage regeneration. Biomaterials. 2014;35(37):9984–9994. doi: 10.1016/j.biomaterials.2014.08.042. [DOI] [PubMed] [Google Scholar]
- 117.Jin GZ, Kim HW. Porous microcarrier-enabled three-dimensional culture of chondrocyte for cartilage engineering: a feasibility study. Tissue engineering and regenerative medicine. 2016;13(3):235–241. doi: 10.1007/s13770-016-0038-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Dang PN, Solorio LD, Alsberg E. Driving cartilage formation in high-density human adipose-derived stem cell aggregate and sheet constructs without exogenous growth factor delivery. Tissue Eng. 2014;20:3163–3175. doi: 10.1089/ten.tea.2012.0551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Ren B, Hu X, Cheng J, Huang Z, Wei P, Shi W. Synthesis and characterization of polyphosphazene microspheres incorporating demineralized bone matrix scaffolds controlled release of growth factor for chondrogenesis applications. Oncotarget. 2017;8(69):114314–114327. doi: 10.18632/oncotarget.23304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Liao HT, Shalumon KT, Sheu C, Fong YT, Chen JP. Microsphere-based hierarchically juxtapositioned biphasic scaffolds prepared from poly(lactic-co-glycolic acid) and nanohydroxyapatite for osteochondral tissue engineering. Polymers. 2016;8(12) doi: 10.3390/polym8120429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Park E, Hart ML, Rolauffs B, Stegemann JP, T Annamalai R. Bioresponsive microspheres for on-demand delivery of anti-inflammatory cytokines for articular cartilage repair. J Biomed Mater Res. 2020;108(3):722–733. doi: 10.1002/jbm.a.36852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Boyan BD, Lee CS, Nicolini AM, Watkins EA, Burnsed OA, Schwartz Z. Adipose stem cell microbeads as production sources for chondrogenic growth factors. J Stem Cells Regen Med. 2014;10(2):38–48. doi: 10.46582/jsrm.1002007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Hobbs AJ, Peake NJ, Pavlov AM, D’Souza A, Pingguan-Murphy B, Sukhorukov GB. Controlled release of C-type natriuretic peptide by microencapsulation dampens proinflammatory effects induced by IL-1β in cartilage explants. Biomacromolecules. 2015;16(2):524–531. doi: 10.1021/bm501575w. [DOI] [PubMed] [Google Scholar]
- 124.He P, Nie X, Chuah YJ, Wang DA. Engineering a multiphasic, integrated graft with a biologically developed cartilage-bone interface for osteochondral defect repair. J Mater Chem B. 2019;7(42):6515–6525. doi: 10.1039/c9tb00822e. [DOI] [PubMed] [Google Scholar]
- 125.Chan JK, Lam AT, Li J, Toh JP, Sim EJ, Chen AK. Biodegradable poly-ε-caprolactone microcarriers for efficient production of human mesenchymal stromal cells and secreted cytokines in batch and fed-batch bioreactors. Cytotherapy. 2017;19(3):419–432. doi: 10.1016/j.jcyt.2016.11.009. [DOI] [PubMed] [Google Scholar]
- 126.Ansboro S, Hayes JS, Browne S, Barron V, Howard L, Greiser U. A chondromimetic microsphere for in situ spatially controlled chondrogenic differentiation of human mesenchymal stem cells. J Contr Release : official journal of the Controlled Release Society. 2014;179:42–51. doi: 10.1016/j.jconrel.2014.01.023. [DOI] [PubMed] [Google Scholar]
- 127.Guo XE, Stefani RM, Lee AJ, Tan AR, Halder SS, Hu Y. Sustained low-dose dexamethasone delivery via a PLGA microsphere-embedded agarose implant for enhanced osteochondral repair. Acta Biomater. 2020;102:326–340. doi: 10.1016/j.actbio.2019.11.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Kawazoe N, Nanda HS, Chen S, Zhang Q, Chen G. Collagen scaffolds with controlled insulin release and controlled pore structure for cartilage tissue engineering. BioMed Res Int. 2014;2014:623805. doi: 10.1155/2014/623805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Hirayama K, Okitsu T, Teramae H, Kiriya D, Onoe H, Takeuchi S. Cellular building unit integrated with microstrand-shaped bacterial cellulose. Biomaterials. 2013;34(10):2421–2427. doi: 10.1016/j.biomaterials.2012.12.013. [DOI] [PubMed] [Google Scholar]
- 130.Mrakovcic M, Absenger M, Riedl R, Smole C, Roblegg E, Fröhlich LF. Assessment of long-term effects of nanoparticles in a microcarrier cell culture system. PloS One. 2013;8(2) doi: 10.1371/journal.pone.0056791. [DOI] [PMC free article] [PubMed] [Google Scholar]