Abstract
To study the effects of antibacterial peptides (ABPs) on feeding broilers, this experiment compared the 2 combinations of ABP with antibiotics by separately adding the supplement to the diet of 818 broilers as follows—antibiotics, Pratt and Full-tide, and Pratt and plant essential oil—and then the effect of them on production performance, immune function, antioxidant capacity, serum biochemical indicators, and microorganisms of the experimental flocks was investigated and compared. It was found that the aforementioned indicators among the 2 groups of ABP and the antibiotic group were close to or even better than those of antibiotics, and the combination added with plant essential oils had generally better effects. These results indicated that ABPs could improve economic benefits by promoting growth, preventing disease, and reducing the rate of death. This study deepened the research on the action mechanism of ABPs and not only explored the feasibility of ABPs as a novel feed additive for broilers but also provided experimental data and theoretical basis for the application of ABPs.
Key words: antibacterial peptide, broiler, performance, immune, intestinal health
Introduction
Veterinary antibiotics were vital for the control, prevention, and treatment of various infectious diseases (Chen et al., 2012). Feeding antibiotics not only can inhibit the growth of pathogenic microorganisms in the digestive tract of animals but also be beneficial to the absorption of nutrients and improve growth performance in animals. Over time, the misuse of antibiotics not only severely disrupted the microecological balance of the intestines of animals but also led to the bacterial antibiotic resistance (Stein et al., 2018). The widespread use of antibiotics and resistance of pathogenic strains have become increasingly serious, which has threatened the entire living nature. The search for new types of antibiotic substitutes is the most effective way to address these problems. Antibacterial peptides (ABPs) have obvious advantages and broad application prospects in replacing traditional antibiotics, which have become one of the hotspots in life science research.
ABPs, a type of peptides synthesized on the ribosome by coding genes, are an important part of the animal innate immune defense system and can exert a variety of resistant activities when the body is attacked by pathogens (Wang, 2007). They have the characteristics of stable physical and chemical properties, small molecular weight, antibacterial broad spectrum, and unique antibacterial mechanism (Van Cutsem JM and D, 1972; Ma, 2013). Antibiotics mainly specifically penetrate the cell membrane barrier, when resistance mutations occur to form mutant strains, and ABPs can penetrate the cell plasma membrane through various channels to kill bacteria (Nikaido, 1989). A common bacteriostatic mechanism of ABP is perforating the bacterial cell plasma membrane to form ion channels through charge attraction, resulting in the destruction of cell membrane structure, causing a large amount of intracellular materials to exudate and bacterial death. Therefore, resistant strains are not easily produced against antimicrobial peptides (Bernd et al., 1988).
The natural ABPs have a powerful and broad-spectrum effect against bacteria, fungi, viruses, and cancer cells and do not destroy the characteristics of normal cells. It is expected to bring revolutionary progress to the fields of clinical medicine and animal feeding if it could be developed and used well. So far, many scholars have isolated and purified more than 2,000 ABP products in succession. The discovery of these ABPs has undoubtedly provided brand new ideas and foundations for the development of innovative green antibacterial drugs.
During breeding of livestock and poultry, antimicrobial peptides are usually added to diet or drinking water, which can kill pathogenic microorganisms in feed and drinking water straightly and reduce the number of pathogenic bacteria exposed to animals. Besides, antimicrobial peptides can eliminate pathogens in the body or participate in immune responses promptly. It is reported that the addition of antimicrobial peptides to poultry-based diets can improve immune performance, improve the intestinal tissue structure and environment, promote growth, and so on (Chen et al., 2010; Xu et al., 2013).
Based on the previous discussion, ABPs have various effects such as improving the immunity, which is an ideal substitute for antibiotics. However, there are limited reports on the application effects of ABPs in broiler chicken production, especially about the effects on digestive and metabolic function in broilers. To this end, drawing on relevant theories and research methods, this study fed a batch of broiler chickens antibiotics and different combinations of antimicrobial peptides to compare production levels, disease resistance, and physiology of flocks. The significance of this study is to explore the mechanism and feasibility of ABPs as feed additives for broilers further by discussing the practical application.
Materials and methods
Preparation of Feed
The Pratt ABP is primarily composed of plectasin. The Full-tide ABP is mainly composed of cecropins. The original plant essential oil is mainly composed of carvacrol, thymol, and cinnamaldehyde. The 15% zinc bacitracin was used in the study. The synthetic peptides mentioned previously were provided by Guangdong Hinabiotech Co., Ltd.
Experimental Animals and Feeding
A total of 960 one-day-old healthy commercial 818 broiler chickens with no significant difference in body weight were provided by the Wens company (Guangdong, China). In this study conducted with one-factor completely randomized design, 960 chickens were randomly divided into 4 treatments, each with 5 replicates and 48 broilers per replicate. All the broilers were in routine feeding management with free intake and drinking water. The experimental period was 50 d. The grouping was indicated in Table 1. The use of animals for this study was approved by the South China Agricultural University Committee for Animal Experiments (SYXK [Guangdong] 2019-0136). All study procedures and animal care activities were carried out in accordance with the national and institutional guidelines for the care and use of laboratory animals.
Table 1.
The grouping of experiment.
| Group | Treatment | Number |
|---|---|---|
| Control group | Basal diet | 240 |
| Antibiotic group (ABG) | Basal diet + 100 mg/kg Zinc bacitracin | 240 |
| Antibacterial peptide group 1 (ABP1) | Basal diet + 100 g/t Pratt ABP + 100 g/t Full-tide ABP | 240 |
| Antibacterial peptide group 2 (ABP2) | Basal diet + 100 g/t Pratt ABP + 50 g/t Origin plant essential oil | 240 |
During the entire rearing period, broiler chickens in the control group were fed corn-soybean–based diets, those in the antibiotic group (ABG) were supplemented with zinc bacitracin in diets, those in the antibacterial peptide group 1 (ABP1) were supplemented with Pratt and Full-tide, and those in the antibacterial peptide group 2 (ABP2) were supplemented with Pratt and original plant essential oil. The composition and nutritional level of basal feeding reported according to the China's “NY/T33-2004 feeding standard of chicken” and the standard broiler diets are shown in Table 2.
Table 2.
Compositions and nutrient levels of the basal diets.
| Raw material (kg) | 1–21 d | 22–42 d | 43–64 d | Nutrient levels | 1–21 d | 22–42 d | 43–64 d |
|---|---|---|---|---|---|---|---|
| Corn | 600.58 | 606.33 | 616.39 | Moisture (%) | 13.34 | 13.12 | 13.04 |
| 46% Soybean | 340.61 | 312.98 | 294.40 | Crude protein (%) | 20.50 | 19.50 | 18.50 |
| Soybean oil Ⅳ | 18.87 | 39.64 | 53.33 | Crude fat (%) | 4.31 | 6.36 | 7.73 |
| Calcium hydrogen phosphate | 9.69 | 7.55 | 5.89 | Crude fiber (%) | 2.32 | 2.22 | 2.16 |
| Limestone | 14.71 | 14.89 | 14.69 | Ca (%) | 0.90 | 0.85 | 0.80 |
| Salt | 2.92 | 2.94 | 2.96 | P (%) | 0.54 | 0.49 | 0.40 |
| NaHCO3 | 1.50 | 1.50 | 1.50 | Nonphytate phosphorus (%) | 0.30 | 0.26 | 0.23 |
| 60% Choline chloride | 1.20 | 0.90 | 0.80 | Na (%) | 0.18 | 0.18 | 0.18 |
| Premix | 4.00 | 4.00 | 4.00 | K (%) | 0.86 | 0.81 | 0.77 |
| 70% Lys | 0.74 | 2.08 | 0.56 | Electrolyte balance (Meq/kg) | 234 | 222 | 210 |
| 98% Solid Met | 1.55 | 2.94 | 1.72 | Metabolizable energy (kcal/kg) | 2,900 | 3,050 | 3,150 |
| 98% Thr | 0.00 | 0.58 | 0.09 | Lys (%) | 1.17 | 1.10 | 1.03 |
| DLys-P (%) | 1.05 | 1.05 | 0.92 | ||||
| D(M + C)-P (%) | 0.71 | 0.82 | 0.68 | ||||
| DThr-P (%) | 0.67 | 0.68 | 0.61 | ||||
| DArg-P (%) | 1.22 | 1.14 | 1.08 | ||||
| DTrp-P (%) | 0.21 | 0.20 | 0.19 |
Measurements
Growth Performance
The weight of each chick and feed consumption of each group were recorded at 1 d, 21 d, 40 d, and 50 d, respectively. Growth performance was evaluated in terms of the average daily gain (ADG), average daily feed intake (ADFI), feed to gain ratio (F/G), and survival rate.
Serum Biochemical Indicator
Three 21-day-old broiler chickens in each group were randomly selected for sample collection. Individual blood samples were collected via the brachial vein puncture, and the serum was separated by centrifuging 3,000 rpm for 10 min to detect the serum biochemical indicators. The serum biochemical indicators were assessed in terms of aspartate aminotransferase (ALT), albumin (ALB), aminotransferase (AST), glucose (GLU), total protein (TP), and uric acid (UA).
Determination of Antibody Titers
The broiler chickens were vaccinated with the commercial inactivated vaccine of H9N2 AIV (cat. no. DL034; Beijing Biolab Technology Co. Ltd.) and Newcastle disease virus (NDV; cat. no. DL032; Beijing Biolab Technology Co. Ltd.) at 1 d of age via spray immunization and revaccinated with the same vaccine at 10 d of age via neck subcutaneous injection. Three broilers in each group were randomly selected at 21 d, 40 d, and 50 d. Individual blood sample was collected, and the serum was taken after centrifuging 3,000 rpm for 10 min to detect NDV and H9N2 AIV antibody levels.
Measurement of Intestinal Morphology
Three 21-day-old broiler chickens in each group were randomly selected for slaughter to take 1-cm-long intestinal samples from each duodenum, jejunum, and ileum. After carefully washing the intestinal cavity with PBS, it was fixed in a centrifuge tube configured with 10% formalin for hematoxylin and eosin staining and paraffin section preparation. The villus length and crypt depth of 10 intact villi were measured, and the average values of each tissue were calculated.
Assay of Intestinal Flora
At 21 d, 3 broiler chickens in each group were randomly selected for slaughter. The contents of the ileocecal junction were retrieved in a sterile environment and then stored in a 2-mL cryopreserved tube treated with DEPC RNase-free water. At last, the samples were sent to Shanghai Majorbio Co., Ltd., for 16S sequencing.
Detection of Intestinal Immune Factor
Three broiler chickens in each group were randomly selected for slaughter at 21 d. A section of the ileum was intercepted in a sterile environment and then stored in a 2-mL grinding tube containing 1 mL of RNAiso. RNA of intestinal tissues was extracted by RNAiso, and the quantitative real-time polymerase chain reaction was used to detect the transcription levels of intestinal tissue protein genes (ZO-1, claudin-3, Muc-2) and immune factors (IL-17A, INF-α, INF-γ, TGF-β, IL-10). Data were analyzed by the 2−ΔΔCt method and normalized using the expression of the internal control 16S gene expression level. The primer pairs used for PCR were as follows: ZO-1, forward 5′-GCCTGAATCAAACCCAGCAA-3′, reverse 5′-TATGCGGCGGTAAGGATGAT-3’; Claudin-3, forward 5′-GAAGGGCTGTGGATGAACTG-3′, reverse 5′-GAGACGATGGTGATCTTGGC-3’; Muc-2, forward 5′-TTCATGATGCCTGCTCTTGTC-3′, reverse 5′-CCGTAGCCTTGGTACATTCTTGT-3’; IL-17A, forward 5′-AGATGCTGGATGCCTAACCC-3′, reverse 5′-ACTGGGCATCAGCAACCAAG-3’; IFN-α, forward 5′-CCAGCACCTCGAGCAAT-3′, reverse 5′-GGCGCTGTAATCGTTGTCT-3’; IFN-γ, forward 5′-ATCATACTGAGCCAGATTGTTTCG-3′, reverse 5′-TCTTTCACCTTCTTCACGCCAT-3’; TGF-β, forward 5′-CGGGACGGATGAGAAGAA-3′, reverse 5′-TCGGCGCTCCAGATGTAC-3’; IL-10, forward 5′-CACGCGGAGGGCGTTAAA-3′, reverse 5′-CAGGTGAAAGTCAGCCCGT-3’; and 16S, forward 5′-GTAACGCAAGCGATCNCG-3′, reverse 5′-AACCGCGACGCTTTCCAA-3’.
Determination of Immune Organ Index
Three broiler chickens in each group were randomly selected for slaughter at 21 d, 40 d, and 50 d, and the spleen, bursa, and thymus were separated to weigh and calculate the immune organ index. The formula is shown below:
Detection of Intestinal Antioxidant Capacity
One-centimeter-long intestinal samples were collected from each duodenum, jejunum, and ileum at the 21 days of age. After carefully washing the intestinal cavity with PBS, they were placed in a 2-mL grinding tube containing physiological saline. Then, the samples were under cryogrinding and centrifuged at 8,000 rpm for 5 min, and the supernatant was collected to detect the antioxidant capacity of intestinal tissue in terms of catalase (CAT), glutathione peroxidase activity (GSH-Px), malondialdehyde (MDA), superoxide dismutase activity (SOD), and total antioxidant capacity (T-AOC).
Statistical Analysis
Using the SPSS 20.0 software for one-way ANOVA analysis and LSD for multiple comparisons, the experimental data were expressed as mean ± SEM. P value < 0.05 was considered significant.
Results
Effects of Different Treatments on Growth Performance
The effects of different treatments on ADG, ADFI, F/G, and survival rate of broiler chickens at the age of 1∼21 d, 22∼40 d, 41∼50 d, and the overall period of 1∼50 d are shown in Table 3.
Table 3.
Effects of different treatments on growth performance of broiler chickens.
| Stage | Indicator | Control group | ABG | ABP1 | ABP2 |
|---|---|---|---|---|---|
| 1∼21 d | ADG | 18.38 ± 0.33 | 19.06 ± 0.23 | 18.42 ± 0.35 | 18.42 ± 0.32 |
| ADFI | 28.26 ± 0.36 | 28.80 ± 0.34 | 28.20 ± 0.50 | 28.24 ± 0.45 | |
| F/G | 1.538 ± 0.011a | 1.512 ± 0.006b | 1.532 ± 0.007 | 1.538 ± 0.009a | |
| Survival rate | 93.34 ± 1.52 | 90.01 ± 1.66 | 90.84 ± 3.25 | 94.58 ± 2.52 | |
| 22∼40 d | ADG | 42.86 ± 0.70 | 42.92 ± 1.02 | 41.92 ± 1.272 | 44.5 ± 0.57 |
| ADFI | 81.74 ± 1.54 | 82.72 ± 1.47 | 80.74 ± 1.65 | 82.4 ± 0.98 | |
| F/G | 1.908 ± 0.020a | 1.928 ± 0.015a | 1.902 ± 0.025 | 1.854 ± 0.004b | |
| Survival rate | 91.60 ± 2.79a | 94.61 ± 1.21 | 94.96 ± 0.97 | 97.24 ± 1.36b | |
| 41∼50 d | ADG | 49.56 ± 1.71 | 48.70 ± 0.77 | 49.62 ± 1.68 | 50.84 ± 0.97 |
| ADFI | 114.37 ± 2.12 | 109.34 ± 1.77 | 108.06 ± 2.21 | 106.72 ± 0.87 | |
| F/G | 2.38 ± 0.067a | 2.27 ± 0.050a,c | 2.152 ± 0.026b,c | 2.10 ± 0.025b | |
| Survival rate | 92.82 ± 3.14 | 97.26 ± 1.22 | 96.09 ± 2.16 | 96.88 ± 2.03 | |
| 1∼50 d | ADG | 34.68 ± 0.54 | 34.39 ± 0.55 | 34.18 ± 0.70 | 35.04 ± 0.22 |
| ADFI | 68.17 ± 0.48a | 66.47 ± 0.80 | 64.74 ± 1.10b | 65.73 ± 0.41b | |
| F/G | 1.97 ± 0.020a | 1.93 ± 0.018a,c | 1.89 ± 0.013b,c | 1.88 ± 0.011b | |
| Survival rate | 80.42 ± 4.97 | 83.33 ± 2.27 | 82.92 ± 2.90 | 89.584 ± 2.37 |
a-cIn the same line, the same letter superscripts mean no significant difference (P > 0.05), and the different letter superscripts mean significant difference (P < 0.05).
Abbreviations: ABG, antibiotic group; ABP1, antibacterial peptide group 1; ABP2, antibacterial peptide group 2; ADG, average daily gain; ADFI, average daily feed intake; F/G, feed to gain ratio.
As shown in Table 3, at 1∼21 d, F/G of ABG was significantly lower than that of control group and ABP2. Compared with control group, ADG of ABG, ABP1, and ABP2 were increased by 3.40, 0.22, and 0.22%, respectively. At 22∼40 d, F/G in ABP2 was significantly lower than that in the control group and ABG (P < 0.05), and the survival rate of ABP2 was markedly higher than that in the control group (P < 0.05). Compared with control group, the survival rate in other groups were increased by 3.28, 3.67, and 6.26%, respectively. At 41∼50 d, F/G of ABP1 was significantly lower than that of the control group (P < 0.05), and F/G of ABP2 was significantly lower than that of the control group and ABG (P < 0.05). Compared with the control group, the survival rate of other groups increased by 4.78, 3.52, and 4.37% and the F/G decreased by 4.62, 9.58, and 11.76%, respectively. In the overall period of feeding, that is, 1∼50 d, ADFI of ABP1 and ABP2 was noticeably lower than that of the control group (P < 0.05). F/G of ABP1 was remarkably lower than that of the control group (P < 0.05), and that of ABP2 was significantly lower than that of the control group and ABG (P < 0.05). Besides, compared with the control group, the survival rate of ABG, ABP1, and ABP2 were increased by 3.62, 3.11, and 11.39%, and F/G reduced by 2.03, 4.06, and 4.57%, respectively. And there was no significant difference in each index in other treatment groups (P > 0.05). The aforementioned results showed that ABG, ABP1, and ABP2 can affect the growth performance and have the effect of improving survival rate and reducing F/G. Two kinds of ABP combinations had a greater advantage than ABG in improving F/G. Furthermore, F/G and survival rate of the ABP2 were significantly better than those of the control group and ABG.
Effects of Different Treatments on Blood Biochemical Indicator
As shown in Table 4, GLU in ABP1 was significantly lower than that in the control group. Compared with the control group, UA and GLU of ABG, ABP1, and ABP2 had a tendency to decrease, and TP and ALT had a tendency to increase, while showing an increasing trend in ABP1 and ABP2. The results presented that antibiotics and ABP combinations can affect the serum biochemical indicators of broiler chickens.
Table 4.
The blood biochemical indicator of broiler chickens in different treatment groups.
| Indicator | Control group | ABG | ABP1 | ABP2 |
|---|---|---|---|---|
| Alanine aminotransferase (ALT, U/L) | 3.6 ± 0.40 | 4.5 ± 0.28 | 4.0 ± 0.31 | 4.25 ± 0.25 |
| Albumin (ALB, g/L) | 15.54 ± 0.49 | 15.03 ± 0.81 | 16.70 ± 0.95 | 16.35 ± 1.24 |
| Aspartate aminotransferase (AST, U/L) | 386.6 ± 33.7 | 352.3 ± 13.3 | 347.0 ± 25.5 | 389.0 ± 14.6 |
| Glucose (GLU, mmol/L) | 10.12 ± 0.18a | 9.43 ± 0.61 | 8.00 ± 1.07b | 8.33 ± 1.33 |
| Total protein (TP, g/L) | 35.80 ± 1.19 | 36.68 ± 2.32 | 43.0 ± 2.95 | 42.4 ± 5.89 |
| Uric acid (UA, mmol/L) | 0.860 ± 0.050 | 0.775 ± 0.048 | 0.775 ± 0.075 | 0.833 ± 0.133 |
a,bIn the same line, the same letter superscripts mean no significant difference (P > 0.05), and the different letter superscripts mean significant difference (P < 0.05).
Abbreviations: ABG, antibiotic group; ABP1, antibacterial peptide group 1; ABP2, antibacterial peptide group 2.
Effects of Different Treatments on Antibody Titers
The serum antibody levels of broilers at various stages after immunization against H9N2 avian influenza virus (H9N2 AIV) and NDV vaccine were shown in Table 5 and Table 6.
Table 5.
Serum antibody levels of H9N2 AIV at various stages.
| Stage | Control group | ABG | ABP1 | ABP2 |
|---|---|---|---|---|
| 21 d | 6.11 ± 0.11 | 6.11 ± 0.16 | 6.06 ± 0.18 | 6.22 ± 0.22 |
| 40 d | 4.77 ± 0.18a | 4.55 ± 0.39a | 6.67 ± 0.21b | 6.78 ± 0.07b |
a,bIn the same line, the same letter superscripts mean no significant difference (P > 0.05), and the different letter superscripts mean significant difference (P < 0.05).
Abbreviations: ABG, antibiotic group; ABP1, antibacterial peptide group 1; ABP2, antibacterial peptide group 2.
Table 6.
Serum antibody levels of ND at various stages.
| Stage | Control group | ABG | ABP1 | ABP2 |
|---|---|---|---|---|
| 21 d | 4.22 ± 0.25a | 5.00 ± 0.36b | 4.56 ± 0.19 | 4.33 ± 0.21 |
| 40 d | 4.78 ± 0.18a | 4.56 ± 0.39a | 4.79 ± 0.25a | 5.78 ± 0.25b |
a,bIn the same line, the same letter superscripts mean no significant difference (P > 0.05), and the different letter superscripts mean significant difference (P < 0.05).
Abbreviations: ABG, antibiotic group; ABP1, antimicrobial peptide group 1; ABP2, antimicrobial peptide group 2.
According to Table 5, at 21 d, there was no significant difference in the serum antibody levels of H9N2 AIV in each group (P > 0.05), while at 40 d, the levels in the ABP1 and ABP2 were significantly higher than those in the control group and ABG. In Table 6, the serum NDV antibody level in ABG was remarkably higher than that in the control group, but at 40 d, the serum NDV antibody level in ABP2 was noticeably higher than that in the other 3 groups. Compared with the control group, ABP1 and ABP2 could improve the serum NDV antibody level at 21 d and 42 d. These results indicated that antibiotics and 2 ABP combinations have the ability to enhance the immune function of the broiler chickens. For instance, they could improve the serum H9N2 AIV and NDV antibody levels in vivo to a certain extent, and the increase degrees was as follows: ABP2 > ABP1 > ABG.
Effects of Different Treatments on Intestinal Morphology
Intestinal Villus Length and The Ratio of Villi Length to Crypt Depth (V/C)
In Table 7, compared to the ileum of the control group, the V/C of ABP2 increased significantly (P < 0.05). Compared with the control group, the crypt depth in ABG, ABP1, and ABP2 decreased by 1.74, 5.23, and 5.81%, respectively, and the V/C increased by 1.83, 1.32, and 14.61%, respectively. At the jejunum level, the villus length of ABP2 was markedly higher than that in the control group and ABP1, and V/C in ABP2 was significantly higher than that in ABP1. Compared with the control group, the villus length in ABG, ABP1, and ABP2 increased by 2.69, 0.15, and 0.13%, respectively. At the duodenum level, compared with the control group, the crypt depth of other treatment groups was noticeably reduced (P < 0.05), while the V/C of ABP2 was significantly increased (P < 0.05). From a numerical point of view, the V/C of the other treatment groups increased by 8.39, 3.93, and 15.00%, respectively. These results suggested that antibiotics and ABP combinations can cause changes in intestinal morphology such as increase in villi length and decrease in crypt depth. Moreover, ABP2 had the best effect on all 3 intestinal tissues. Tissue sections of each intestine is shown in Figure 1.
Table 7.
The intestinal morphology of 21-day-old broiler chickens in different treatment groups.
| Position | Indicator | Control group | ABG | ABP1 | ABP2 |
|---|---|---|---|---|---|
| Ileum | Villus length | 0.997 ± 0.035 | 1.025 ± 0.071 | 0.984 ± 0.056 | 1.088 ± 0.045 |
| Crypt depth | 0.172 ± 0.016 | 0.169 ± 0.008 | 0.163 ± 0.009 | 0.162 ± 0.013 | |
| V/C | 6.02 ± 0.75a | 6.13 ± 0.56 | 6.10 ± 0.23 | 6.90 ± 0.76b | |
| Jejunum | Villus length | 0.669 ± 0.083a | 0.687 ± 0.069 | 0.670 ± 0.036a | 0.756 ± 0.044b |
| Crypt depth | 0.154 ± 0.006 | 0.157 ± 0.021 | 0.163 ± 0.015 | 0.149 ± 0.012 | |
| V/C | 4.49 ± 0.49 | 4.47 ± 0.34 | 4.13 ± 0.17a | 5.18 ± 0.43b | |
| Duodenum | Villus length | 1.151 ± 0.078 | 1.186 ± 0.053 | 1.083 ± 0.066 | 1.218 ± 0.067 |
| Crypt depth | 0.214 ± 0.009a | 0.187 ± 0.08b | 0.187 ± 0.021b | 0.189 ± 0.01b | |
| V/C | 5.60 ± 0.35a | 6.07 ± 0.27 | 5.82 ± 0.30 | 6.44 ± 0.35b |
a,bIn the same line, the same letter superscripts mean no significant difference (P > 0.05), and the different letter superscripts mean significant difference (P < 0.05).
Abbreviations: ABG, antibiotic group; ABP1, antibacterial peptide group 1; ABP2, antibacterial peptide group 2; V/C, the ratio of villi length to crypt depth.
Figure 1.
Section of different tissue (40×) to see villi length and crypt depth. (A) The section of ileum tissue (40×). (B) The section of jejunum tissue (40×). (C) The section of duodenal tissue (40×). a: Control group, broilers were fed with corn-soybean–based diets; b: antibiotic group (ABG), broilers were fed with corn-soybean–based diets and zinc bacitracin antibiotic supplement; c: antibacterial peptide group 1 (ABP1), broilers were fed with corn-soybean–based diets and Pratt and Full-tide antibacterial peptide; d: antibacterial peptide group 2 (ABP2), broilers were fed with corn-soybean–based diets and Pratt and original plant essential oil antibacterial peptide. All the broilers were in routine feeding management with 50-D experimental period.
Effects of Different Treatments on Intestinal Immune Factor and Tight Junction Protein
Effect of Different Treatments on Immune Factor
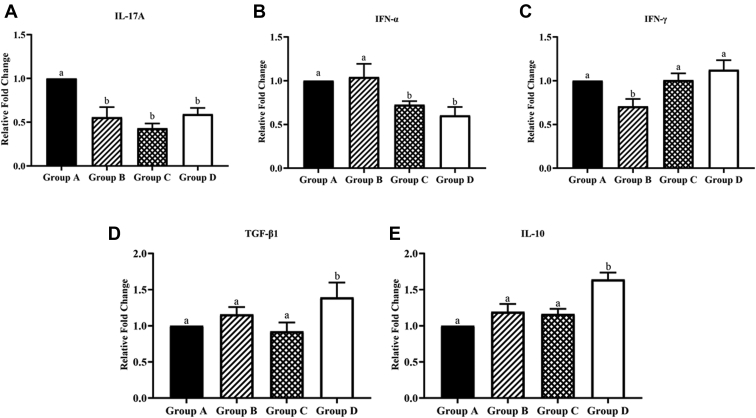
In Figure 2A, compared with the control group, the expression of IL-17A in each treatment group was significantly decreased, in which the decrease in degree of ABP1 was greater than that of ABG and ABP2. As shown in Figure 2B, the expression of IFN-α in ABP1 and ABP2 was significantly lower than that in the control group and ABG. In Figure 2C, the expression of IFN-β in ABG was markedly lower than that in the other 3 groups. In Figure 2D, the expression of TGF-β1 in ABP2 was noticeably higher than that of the other 3 groups. Compared with the control group, the expression of TGF-β1 in ABG had an upward trend. In Figure 2E, the expression of IL-10 in ABP2 was considerably higher than that in the other 3 groups. Compared with the control group, the expression of TGF-β1 in ABG and ABP1 had an upward tendency. The aforementioned results indicated that antibiotics and ABP combinations can affect the expression of intestinal immune factors in broiler chickens.
Figure 2.
Effects of different treatments on different intestinal immune factors of broilers aged 21 d. (A) The effect of different treatments on IL-17A. (B) The effect of different treatments on IFN-α. (C) The effect of different treatments on IFN-γ. (D) The effect of different treatments on TGF-β. (E) The effect of different treatments on IL-10. In all figures, the horizontal axis represents different groups. Group A: Control group, broilers were fed with corn-soybean–based diets; group B: antibiotic group (ABG), broilers were fed with corn-soybean–based diets and zinc bacitracin antibiotic supplement; group C: antibacterial peptide group 1 (ABP1), broilers were fed with corn-soybean–based diets and Pratt and Full-tide antibacterial peptide; group D: antibacterial peptide group 2 (ABP2), broilers were fed with corn-soybean–based diets and Pratt and original plant essential oil antibacterial peptide. The same letter superscripts mean no significant difference (P > 0.05), and the different letter superscripts mean significant difference (P < 0.05). Data are presented as the mean ± SEM of 5 independent experiments. The differences between groups were analyzed using one-way ANOVA and LSD.
Effects of Different Treatments on Tight Junction Protein
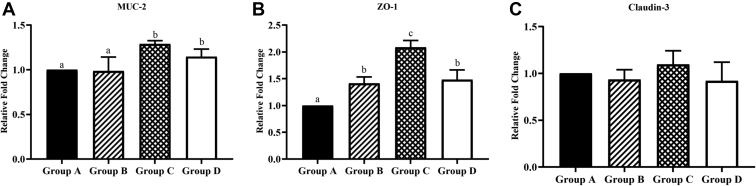
In Figure 3A, at 21 d, the expression level of Muc-2 in ABP1 and ABP2 were significantly higher than those in the control group and ABG. In Figure 3B, compared with the control group, the expression of ZO-1 in each treatment group was remarkably increased, and the expression of ZO-1 in ABP1 was noticeably higher than that in ABG and ABP2. In Figure 3C, compared with the control group, Claudin-3 of ABP1 had an upward trend, but ABG and ABP2 had a downward trend. These results indicated that antibiotics and ABP combinations could increase the gene expression of intestinal tight junction protein to vary degrees, among which ABP1 was the best, followed by ABP2.
Figure 3.
Effects of different treatments on tight-junction protein. The quantitative real-time polymerase chain reaction detected the transcription levels of intestinal tissue protein genes and immune factors. (A) The expression level of Muc-2. (B) The expression level of ZO-1. (C) The expression level of Claudin-3. The horizontal axis represents different groups. Group A: Control group, broilers were fed with corn-soybean–based diets; group B: antibiotic group (ABG), broilers were fed with corn-soybean–based diets and zinc bacitracin antibiotic supplement; group C: antibacterial peptide group 1 (ABP1), broilers were fed with corn-soybean–based diets and Pratt and Full-tide antibacterial peptide; group D: antibacterial peptide group 2 (ABP2), broilers were fed with corn-soybean–based diets and Pratt and original plant essential oil antibacterial peptide. The same letter superscripts mean no significant difference (P > 0.05), and the different letter superscripts mean significant difference (P < 0.05). Data are presented as the mean ± SEM of 5 independent experiments. The differences between groups were analyzed using one-way ANOVA and LSD.
Effects of Different Treatments on Immune Organ Index
As shown in Table 8, at 21 d, the spleen index of ABP1 was significantly higher than that of the control group and ABG. The index of bursa in 3 different treatment groups was higher than that in the control group. At 40 d, compared with the control group, the spleen index in ABP1 was significantly lower, the bursa index in ABP2 was considerably higher, and the thymus index in ABP1 and ABP2 was significantly higher than that in ABG. At 50 d, compared with the control group, the bursal index of ABG and ABP2 increased remarkably, and the thymus index of treatment groups increased significantly. The results suggested that antibiotics and the 2 ABPs combinations can affect the immune organs and improve the development of bursa and thymus.
Table 8.
The immune organ index of broiler chickens in different treatment groups.
| Stage | Indicator | Control group | ABG | ABP1 | ABP2 |
|---|---|---|---|---|---|
| 21 d | Spleen | 1.63 ± 0.11a | 1.63 ± 0.10a | 2.26 ± 0.14b | 2.05 ± 0.045 |
| Bursal | 1.68 ± 0.11 | 2.64 ± 0.66 | 2.06 ± 0.66 | 2.23 ± 0.26 | |
| Thymus | 2.94 ± 0.44 | 3.33 ± 0.32 | 3.50 ± 0.30 | 3.32 ± 0.30 | |
| 40 d | Spleen | 1.96 ± 0.27a | 1.57 ± 0.31 | 1.20 ± 0.10b | 1.51 ± 0.09 |
| Bursal | 1.03 ± 0.21a | 1.10 ± 0.17 | 1.73 ± 0.32 | 2.03 ± 0.45b | |
| Thymus | 1.94 ± 0.42 | 1.54 ± 0.21a | 2.97 ± 0.30b | 3.14 ± 0.56b | |
| 50 d | Spleen | 1.57 ± 0.28 | 1.49 ± 0.15 | 1.39 ± 0.07 | 1.31 ± 0.08 |
| Bursal | 1.12 ± 0.22a | 1.89 ± 0.21b | 1.57 ± 0.12 | 1.91 ± 0.16b | |
| Thymus | 2.21 ± 0.21a | 3.46 ± 0.21b | 3.17 ± 0.20b | 3.339 ± 0.17b |
a,bIn the same line, the same letter superscripts mean no significant difference (P > 0.05), and the different letter superscripts mean significant difference (P < 0.05).
Abbreviations: ABG, antibiotic group; ABP1, antibacterial peptide group 1; ABP2, antibacterial peptide group 2.
Effects of Different Treatments on Intestinal Antioxidant Capacity
As shown in Table 9, compared with the control group and ABG, GSH-Px, and CAT in ABP2 showed a rising trend, MDA in ABP1 and ABP2 showed a downward trend, and SOD showed a significant upward trend. T-AOC of each treatment group was higher than that of the control group. These results indicated that antibiotics and ABP combinations have a tendency to improve the intestinal antioxidant capacity, and the combination with essential oil is more effective.
Table 9.
The intestinal antioxidant capacity of broiler chickens in different treatment groups.
| Indicator | Control group | ABG | ABP1 | ABP2 |
|---|---|---|---|---|
| CAT (U/mL) | 6.69 ± 0.56 | 7.32 ± 0.86 | 6.57 ± 0.48 | 7.41 ± 0.81 |
| GSH-Px (U/mL) | 2,388.6 ± 78.2 | 2,332.7 ± 95.7 | 2,347.8 ± 111.2 | 2,498.0 ± 129.1 |
| MDA (nmol/mL) | 17.04 ± 1.70 | 17.17 ± 1.28 | 16.46 ± 1.75 | 16.35 ± 1.43 |
| SOD (U/mL) | 23.74 ± 1.23 | 23.40 ± 0.65 | 26.27 ± 0.81 | 26.40 ± 1.19 |
| T-AOC (unit/mL) | 42.72 ± 1.29 | 43.44 ± 2.04 | 43.18 ± 1.48 | 43.33 ± 1.41 |
In the same line, no letter superscripts mean no significant difference (P > 0.05).
Abbreviations: ABG, antibiotic group; ABP1, antibacterial peptide group 1; ABP2, antibacterial peptide group 2; GSH-Px, glutathione peroxidase activity; CAT, catalase; MDA, malondialdehyde; SOD, superoxide dismutase activity; T-AOC, total antioxidant capacity.
Effects of Different Treatments on the Ileum Microbial Composition
As can be observed in the Alpha diversity index in Table 10, the number of valid reads in ABP1 and ABP2 was significantly higher than that in the control group. Compared with the control group and ABG, the Chao index and Shannon index of ABP1 and ABP2 showed an increasing tendency, and Simpson index showed a decreasing trend. These results showed that the ileum microbial richness and diversity of ABP1 and ABP2 have improved.
Table 10.
Alpha diversity index of broiler chickens in different treatment groups.
| Treatment | Valid reads | Chao | ACE | Simpson | Shannon |
|---|---|---|---|---|---|
| Control group | 42,121 ± 4,308a | 257.7 ± 55.9 | 270.0 ± 50.0 | 0.365 ± 0.057 | 1.76 ± 0.57 |
| ABG | 47,374 ± 1,982 | 277.6 ± 65.6 | 287.7 ± 44.8 | 0.364 ± 0.094 | 1.84 ± 0.53 |
| ABP1 | 55,560 ± 2,714b | 289.6 ± 49.5 | 303.7 ± 49.7 | 0.321 ± 0.089 | 2.02 ± 0.44 |
| ABP2 | 53,080 ± 2,074b | 325.6 ± 8.3 | 333.3 ± 17.6 | 0.350 ± 0.119 | 2.05 ± 0.25 |
In the same line, the same letter superscripts mean no significant difference (P > 0.05), and the different letter superscripts mean significant difference (P < 0.05).
Abbreviations: ABG, antibiotic group; ABP1, antibacterial peptide group 1; ABP2, antibacterial peptide group 2.
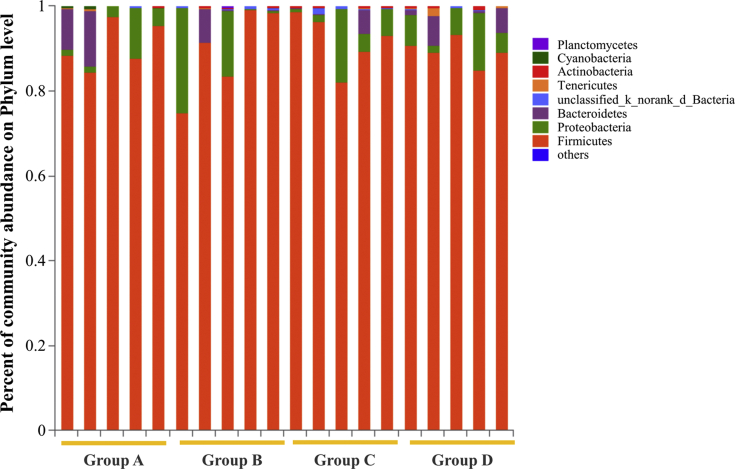
As shown in Figure 4 and Figure 5, for phylum horizontal microbial abundance statistics, the Firmicutes, Proteobacteria, and Bacteroidetes were the principal flora, and there was no significant difference among the groups. Compared with the control group, the Proteobacteria in the other treatment group showed an increasing tendency. In contrast, the Bacteroidetes decreased correspondingly, and the ABP2 was relatively balanced.
Figure 4.
The phylum horizontal microbial abundance of ileal flora in different treatment groups. The vertical axis represents percent of community abundance on phylum level. The horizontal axis represents different groups. Group A: Control group, broilers were fed with corn-soybean–based diets; group B: antibiotic group (ABG), broilers were fed with corn-soybean–based diets and zinc bacitracin antibiotic supplement; group C: antibacterial peptide group 1 (ABP1), broilers were fed with corn-soybean–based diets and Pratt and Full-tide antibacterial peptide; group D: antibacterial peptide group 2 (ABP2), broilers were fed with corn-soybean–based diets and Pratt and original plant essential oil antibacterial peptide.
Figure 5.
The changes in abundance of the 3 major microflora in different treatment groups. The vertical axis represents relative abundance. The horizontal axis represents different flora. Group A: Control group, broilers were fed with corn-soybean–based diets; group B: antibiotic group (ABG), broilers were fed with corn-soybean–based diets and zinc bacitracin antibiotic supplement; group C: antibacterial peptide group 1 (ABP1), broilers were fed with corn-soybean–based diets and Pratt and Full-tide antibacterial peptide; group D: antibacterial peptide group 2 (ABP2), broilers were fed with corn-soybean–based diets and Pratt and original plant essential oil antibacterial peptide. Data are presented as the mean ± SEM of 5 independent experiments. The differences between groups were analyzed using one-way ANOVA and LSD.
Discussion
Researches showed that ABPs can improve the growth performance of broiler chickens, in which the researchers found the addition of cecropin ABP to diets can significantly reduce F/G of broiler chickens at 5 to 6 wks (Wang, 2007). Du et al. added 12,000 U/kg ABP to the diet of broiler chickens to reduce the F/G of broiler chickens (Du et al., 2010). The addition of 0.1% full-tide ABP in the diet can significantly reduce F/G of broiler chickens and improve growth performance (Han et al., 2014). Liang et al. found that feeding broilers with cecropin ABPs can increase broiler chicken survival rate and reduce the incidence (Liang et al., 2009). Consistent with these research studies, in this study, the 2 kinds of ABP combinations could reduce ADFI of broilers, while increasing the feed conversion efficiency and survival rate. This may be because ABPs and plant essential oil not only could enhance the intestinal barrier function and immune performance and balance the intestinal microbial community but also could improve the ability to digest and absorb nutrients.
Blood biochemical indexes can reflect the absorption and utilization rate of nutrients to a certain extent. TP, composed of albumin and globulin, reflects the organism's protein absorption and the relationship with humoral immunity (Tian et al., 2009). When this index increases, it could enhance the tissue protein deposition to promote the growth of tissues and organs. ALB is the subject of the plasma colloid osmotic pressure and the transportation vector of low-water-soluble substances in the blood, which plays a key role in the liver in protein metabolism (Xu et al., 2013). UA is the main product of protein metabolism in poultry, and content change of it could reflect the utilization of nutrients such as protein and amino acids of poultry indirectly. The lower the content, the higher the utilization rate of nitrogen-contained nutrients and the stronger the anabolism of nutrients such as protein in vivo. GLU is a substance that is directly oxidized and oxidized and energized in animals. ALT, mainly distributed in the liver, plays an important role in the intermediary metabolism of glucose and amino acids. AST mainly exists in tissues such as myocardial cells and liver. When hepatocyte injury or permeability increases, ALT and AST are released into the blood, which increases serum ALT and AST activity. Therefore, serum ALT and AST activity are sensitive indicators of hepatocyte injury (Zhang, 2011). In this study, ABP1 and ABP2 could promote energy absorption and transformation and maintain the balance of tissue protein and blood glucose synthesis. Similar to the results of Sa et al. and Zhang et al., ABPs, as a peptide with broad-spectrum antibacterial function, can improve the liver's ability to synthesize proteins (Sa et al., 2006; Zhang et al., 2012).
Animal immunity can be divided into cellular immunity and humoral immunity. In humoral immunity, B lymphocytes proliferate and differentiate into effector B cells through stimulation of antigens. B cells synthesize and secrete antibody proteins, combine with antigens to form complexes, and are subsequently cleared by phagocytes. Thus, the antibody titer in the serum reflects the strength of humoral immune function and the body's health under certain conditions. Yuan et al. found that ABPs could increase the levels of avian influenza and NDV antibodies in specific pathogen free chickens (Yuan et al., 2016). In this experiment, after immunizing NDV and H9N2 AIV vaccine at 10 d in broilers, ABP1 and ABP2 could considerably increase the H9 serum antibody levels, and ABP2 can significantly increase NDV serum antibody levels in the medium-late stage. ABP1 and ABP2 can maintain the antibodies in the blood for a long time and exert a lasting immunity effect.
The small bowel is the main position of nutrient absorption and transport. The villi length can reflect the ability of the small intestine to absorb nutrients. Only mature villus cells can absorb nutrients. The intestinal crypts are intestinal epithelial cells with secretion and absorption functions, which can replenish the intestinal villi epithelial cells that fall off the small bowel quickly, and the crypts become shallow gradually. The ratio of V/C reflects the functional status of the small intestine comprehensively. As the ratio increases, the mucosa is repaired, digestion and absorption are enhanced, and growth is accelerated, while the decline of the ratio will result in the opposite effect (Yao et al., 2003). Similar to the results of Liu et al. and Lu et al., Yao et al. reported that the cecropin ABP could significantly increase V/C in the duodenum and jejunum of chicken (Liu et al., 2008; Lv et al., 2014; Yao et al., 2014). Consistent with the reports of Cao et al., Liu et al. found that the diet adding 100 mg/kg cinnamaldehyde can substantially increase the villi length of the jejunum and duodenum, the V/C ratio, to promote the intestinal development of chickens (Cao et al., 2012; Liu et al., 2013). In this study, ABP1 and ABP2 improved the intestinal tissue structure and the ability to absorb and digest of broilers. The possible reason that ABP2 is better than ABP1 is that the plant essential oil, as a secondary metabolite in the plant, can better promote cell metabolism and increase the production and maturing rate of villus cells.
Immune factors promote the growth of intestinal beneficial bacteria and achieve the flora balance, so as to improve the ability to resist disease. IL-17 has a strong ability to recruit and activate neutrophils, mediating the stimulating process of neutrophil mobilization, thereby expanding the inflammation (Stark et al., 2005; Chen et al., 2007). IL-17 A is highly expressed at the location of lesions, and its expression is positively correlated with the severity of the disease (Li et al., 2012). IFN-α and IFN-γ have key roles in antiinfection and immune regulation. IFN-α belongs to type I interferon, and IFN-γ belongs to type II interferon. Type I interferon is a major component of innate immunity and can resist virus replication in the body. Type II interferon is a major immune response molecule and can activate immune cells (Plachý et al., 1999). When the body is exposed to pathogens, the immune cells will produce interferons to regulate the immune response to resist virus replication in the body. In the intestine, there is a positive correlation between the expression of interferon and the extent of inflammation in the body. TGF-β1 can inhibit macrophage activation, T- and B-cell apoptosis, and the expression and secretion of inflammatory mediators such as TLR4, IL-1, IL-6, and IFN-γ (Bickerstaff and Orosz, 2002). TGF-β1 can also upregulate the expression of tight junction protein in intestinal epithelial cells, maintain the transmembrane potential balance, and strengthen the intestinal mucosal barrier (Howe et al., 2005). IL-10, secreted by regulative T cells, plays a major negative regulatory role in the immune response. It can inhibit the activation of natural killer cells and macrophages and downregulate the expression of MHC II molecules and costimulatory molecules by antigen presenting cells to play an immunosuppressive role indirectly. IL-10 can combine with TGF-β to produce a wide range of nonspecific anti-inflammatory effects and contact the inhibition of the Th1 and Th2 immune response (Yue et al., 2012). In this experiment, ABP1 and ABP2 can decline the expression of promotion inflammation such as cytokine IL-17A and IFN-α to reduce the occurrence of intestinal inflammation and provide the function of digestion and absorption of nutrients. ABP2 can significantly enhance the expression of anti-inflammatory cytokines such as TGF-β and IL-10. Ocaña et al. found that the anti-inflammatory mechanism of origanum oil is through the inhibition of human monocyte macrophage activity to decrease the synthesis of TNF-α, IL-1β, and IL-6 and increasing the secretion of IL-10 (Ocaña-Fuentes et al., 2010). Zhou et al. and Wang have similar reports (Zhou et al., 2015; Wang, 2017).
The largest peripheral immune organ of poultry is the spleen, which participates in humoral immunity and can secrete specific immune antibodies and generate lymphocytes. The thymus is chiefly involved in cellular immunity. The unique central immune organ of poultry is the bursal, which is the site for the growth and differentiation of cells that form the serum antibody system and is mainly involved in humoral immunity. The growth and development status of immune organs can reflect the level of immunity of the entire body to a certain extent. In this study, ABP1 and ABP2 could increase the spleen index of 21-day-old chickens and the thymus and bursal index of 40- and 50-day-old chickens. The results are similar to those of Qu et al. and Peng et al. (Qu et al., 2006; Peng et al., 2014). In terms of the bursal index, Wang reported that thymus and bursal indexes of broilers reached the maximum at 18 d and the spleen reached the maximum at 35 d. As to spleen index, ABP1 and ABP2 did not differ significantly between 40- and 50-day-old broilers. This may be because the thymus and the bursal are the primary immune organs of birds, and the spleen serves as its secondary immune organ to assist in the immune response.
Free radicals generated by oxidative metabolism are eliminated by the antioxidant defense system. Excessive free radicals could damage biological macromolecules such as proteins and nucleic acids and cause tissue and cell damage, affecting the relative stability of the internal environment of the organism (Singh et al., 2019). SOD, GSH-Px, and CAT can catalyze the lipid peroxides or H2O2 to alcohol, whose activity can reflect the ability to scavenge free radicals indirectly (Chen, 12011). MDA, a kind of relatively stable lipid peroxides, content in blood and tissues not only reflects the degree of lipid peroxidation mediated by oxygen-free radicals but also reflects the degree of oxidative damage in the organism indirectly (Shi et al., 2013). In this study, ABP1 and ABP2 could improve the antioxidative capacity of the intestine in broilers, which was shown to increase the intestinal SOD activity and reduce the MDA content of intestinal tissues. ABPs and plant essential oils can enhance the intestinal antioxidant defense system, promote peroxide decomposition, and reduce excess free radicals, reducing oxidative damage to intestinal tissues (Chen et al., 2010; Du et al., 2010; Ruan et al., 2013; Xie and Zhang, 2014). The principle might be that ABPs and plant essential oils increase the richness and diversity of intestinal microorganisms and balance the intestinal microbial flora and pH.
Alpha diversity index reflects the diversity of microorganisms, including richness index (Chao1 and ACE) and diversity index (Shannon and Simpson index). The larger the Chao1 or ACE, the higher the community richness. And the larger the Shannon or the smaller the Simpson, the higher the community diversity. A large number of microorganisms inhabit the poultry intestines, which constitute the microbial flora, mainly made up of bacteria. Once the flora is imbalanced, it will cause a series of diseases (Deriu et al., 2016), such as colitis, fowl typhoid, pullorum disease, and so on. Antibiotics and ABPs have a broad-spectrum antibacterial function, which can inhibit the growth of intestinal microorganisms and have a strong regulatory effect on the microflora (Park et al., 2004, 2006). In this study, ABPs can enrich the species' diversity of microorganisms and enhance the balance ability. And the synergistic effect of ABPs and plant essential oil can reduce the negative effect on the intestinal microflora and promote the coordination between various microorganisms.
Intestinal barriers include physical barriers, mucus barriers, and immune barriers, which work together to resist the external environmental stimulation of the intestinal. A variety of proteins are involved in the formation of tight junctions, which consisted of claudin, occludin, junction adhesion molecules, and the ZO family (Ballabh et al., 2004). Claudin is the main protein with specificity that guarantees tight junction permeability (Ballabh et al., 2004). ZO-1 maintains and regulates the function of the fence and barrier of the epithelium, participates in transport, and maintains the polarity of the epithelium (Bauer et al., 2014). Mucus barrier, the main physiological component is mucin, which performs dual functions of nutrition and immunity (Pinto-De-Sousa et al., 2002). Muc-2, the main mucin of the intestinal mucosa, is involved in the nutrient absorption and defense against the invasion of bacteria, viruses, parasites, and enteritis (Zhang et al., 2009). The quantity and distribution of Muc-2 are molecular manifestations of the intestinal mucosal morphology. This study showed that ABP1 and ABP2 can significantly increase the expression of Muc-2 and ZO-1. This is similar to the results of Huan et al. (Huan et al., 2015). This may be due to the broad-spectrum antibacterial properties of ABPs, which can effectively inhibit harmful bacteria and upregulate the expression of tight-junction protein in intestinal epithelium (Segawa et al., 2011; Miyauchi et al., 2012).
In conclusion, the 2 combinations of ABPs added in broiler's basal diets can promote broiler growth and improve feed conversion rate instead of antibiotics. And the effect of antimicrobial peptide with plant essential oil was better than that of the compound antimicrobial peptide group. The use of plant essential oil had a positive influence on growth performance, the level of serum antibody, intestinal antiinfection ability, intestinal tissue structure, and the body immunity. Through the practical application of antimicrobial peptides in feed additives for broilers, this study further deepened the mechanism and feasibility of antimicrobial peptides as feed additives for broilers.
Acknowledgments
This work was supported by the Chief Expert Project of Agricultural Industry Technology System in Guangdong Province (grant no. 2019KJ128), Creation of Triple Chimeric Vaccine (rIBV-ND-H9) based on attenuated Avian Infectious bronchitis virus D90 (grant no. 2017KZDM008), Special project of national modern agricultural industrial technology system (grant no. CARS-41), and Guangdong Provincial Promotion Project on Preservation and Utilization of Local Breed of Livestock and Poultry.
Conflict of Interest Statement: The authors declare that there are no conflicts of interest.
References
- Ballabh P., Braun A., Nedergaard M. The blood-brain barrier: an overview: structure, regulation, and clinical implications. Neurobiol. Dis. 2004;16:1–13. doi: 10.1016/j.nbd.2003.12.016. [DOI] [PubMed] [Google Scholar]
- Bauer H.C., Krizbai I.A., Bauer H., Traweger A. "You Shall Not Pass"-tight junctions of the blood brain barrier. Front Neurosci. 2014;8:392. doi: 10.3389/fnins.2014.00392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernd C., Juergen F., R B M., David M. Channel-forming properties of cecropins and related model compounds incorporated into planar lipid membranes. PNAS. 1988;85:5072–5076. doi: 10.1073/pnas.85.14.5072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bickerstaff A., Orosz C. Evidence for a limited contribution of immune regulation to cardiac allograft acceptance. Hum. Immunol. 2002;63:935–947. doi: 10.1016/s0198-8859(02)00447-0. [DOI] [PubMed] [Google Scholar]
- Cao F.L., Zhang X.H., Yu W.W., Zhao L.G., Wang T. Effect of feeding fermented Ginkgo biloba leaves on growth performance, meat quality, and lipid metabolism in broilers. Poult. Sci. 2012;91:1210–1221. doi: 10.3382/ps.2011-01886. [DOI] [PubMed] [Google Scholar]
- Chen X.A. Effects of Exercise and nutritional Supplementation on free radical metabolism. Sci. Technology Inf. 2011:441–453. [Google Scholar]
- Chen Y., Zhang H., Luo Y., Song J. Occurrence and assessment of veterinary antibiotics in swine manures: a case study in East China. Chin. Sci. Bull. 2012;57:606–614. [Google Scholar]
- Chen J.X., Zhang R.Y., Wang Q.X., Fu Z.C., Zheng Q.X., Wang C.K. Effects of Bacillus licheniformis on growth performance, antioxidant Indices and blood biochemical Parameters of broiler chickens. Chin. J. Anim. Nutr. 2010;22:1019–1023. [Google Scholar]
- Chen Z., Tato C.M., Muul L., Laurence A., O'Shea J.J. Distinct regulation of interleukin-17 in human T helper lymphocytes. Arthritis Rheum. 2007;56:2936–2946. doi: 10.1002/art.22866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deriu E., Boxx G.M., He X., Pan C., Benavidez S.D., Cen L., Rozengurt N., Shi W., Cheng G. Influenza virus affects intestinal Microbiota and secondary Salmonella infection in the Gut through type I interferons. PLoS Pathog. 2016;12:e1005572. doi: 10.1371/journal.ppat.1005572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du H.M., Lu Z.X., Wang T. Effect of antimicrobial peptides on antioxidant capacity and biochemical indicators in broilers. Anim. Husbandry Vet. Med. 2010;42:8–13. [Google Scholar]
- Han X.J., Ma G.Y., Zhou Y.Y., Zhang Y.Z., Yao T.Y., Ma Q.G., Ji C., Zhao L.H. Effects of antibacterial peptides on growth performance and immune function of broiler chickens. Chin. J. Anim. Sci. 2014;50:70–74. [Google Scholar]
- Howe K.L., Reardon C., Wang A., Nazli A., Mckay D.M. Transforming growth factor-beta regulation of epithelial tight junction proteins enhances barrier function and blocks enterohemorrhagic Escherichia coli O157:H7-induced increased permeability. 2005;167:1587–1597. doi: 10.1016/s0002-9440(10)61243-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huan H.L., Bai J.Y., Zhou W.R., Yan J.S., Xu X.M., Yang Y., Wen C., Zhou Y.M. Effects of antimicrobial peptides on serum biochemical Indices, intestinal mucosa morphology and the Relative expression level of tight junction protein gene of jejunum of Piglets. Chin. J. Anim. Nutr. 2015;27:3797–3804. [Google Scholar]
- Li C.G., Yang P.H., Sun Y., Li T.S., Wang C., Wang Z., Zou Z., Yan Y.W., Wang W., Chen Z.W., Xing L., Tang C., Ju X.W., Guo F., Deng J.J., Zhao Y., Yang P., Tang J., Wang H.L., Zhao Z.P., Yin Z.N., Cao B., Wang X.L., Jiang C.Y. IL-17 response mediates acute lung injury induced by the 2009 pandemic influenza A (H1N1) virus. Cell Res. 2012;22:528–538. doi: 10.1038/cr.2011.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang J., Li Y.N., Dong J.X. Application of cecropin in chicken feeding. Mod. Food Sci. Technology. 2009;25:416–419. [Google Scholar]
- Liu T., She R., Wang K., Bao H., Zhang Y., Luo D., Hu Y., Ding Y., Wang D., Peng K. Effects of Rabbit Sacculus Rotundus antimicrobial peptides on the intestinal mucosal immunity in chickens. Poult. Sci. 2008;87:250–254. doi: 10.3382/ps.2007-00353. [DOI] [PubMed] [Google Scholar]
- Liu Y., Zang X.M., Li T.Z., Yang J., Guo J.J., Zhao W.X. Effects of cinnamaldehyde on intestinal flora, intestinal structure and nutrient Digestibility of broilers. Chin. J. Anim. Sci. 2013;49:65–68. [Google Scholar]
- Lv J.X., Kuang Z.S., Yang J.B., Zhao X.J., Zhang B.L., Huang G.L. Effects of antimicrobial peptides on serum biochemical indexes, cytokine levels and intestine mucosal morphology of Weaning Piglets. Chin. J. Anim. Sci. 2014;50:45–49. [Google Scholar]
- Ma Q. Jilin University; 2013. Effects of Antibacterial Peptide on Growth and IGF-1 mRNA Expression in Liver and Breast Muscle of Luhua Chicken. Master: 77. Accessed Sept. 2020. http://kns.cnki.net/KCMS/detail/detail.aspx?FileName=1013194020. [Google Scholar]
- Miyauchi E., Morita M., Rossi M., Morita H., Suzuki T., Tanabe S. Effect of D-Alanine in Teichoic acid from the Streptococcus thermophilus cell Wall on the barrier-Protection of intestinal epithelial cells. Biosci. Biotechnol. Biochem. 2012;76:283–288. doi: 10.1271/bbb.110646. [DOI] [PubMed] [Google Scholar]
- Nikaido H. Outer membrane barrier as a mechanism of antimicrobial resistance. Antimicrob. Agents Chemother. 1989;33:1831–1836. doi: 10.1128/aac.33.11.1831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ocaña-Fuentes A., Arranz-Gutiérrez E., Señorans F.J., Reglero G. Supercritical fluid extraction of oregano (Origanum vulgare) essentials oils: anti-inflammatory properties based on cytokine response on THP-1 macrophages. Food Chem. Toxicol. 2010;48:1568–1575. doi: 10.1016/j.fct.2010.03.026. [DOI] [PubMed] [Google Scholar]
- Park Y., Kim H.J., Hahm K. Antibacterial synergism of novel antibiotic peptides with chloramphenicol. Biochem. Bioph Res. Co. 2004;321:109–115. doi: 10.1016/j.bbrc.2004.06.113. [DOI] [PubMed] [Google Scholar]
- Park Y., Park S.N., Park S., Shin S.O., Kim J., Kang S., Kim M., Jeong C., Hahm K. Synergism of Leu–Lys rich antimicrobial peptides and chloramphenicol against bacterial cells. Biochim. Biophys. Acta (Bba) - Proteins Proteomics. 2006;1764:24–32. doi: 10.1016/j.bbapap.2005.10.019. [DOI] [PubMed] [Google Scholar]
- Peng X., Sun Q.Y., Li J., Xu B., Wei F.X., Wang L.Y., Bai J., Lu M., Li S.Y. Effects of antimicrobial peptide and Curcumin on growth performance and immune function of broilers aged from 1 to 21 Days. Chin. J. Anim. Nutr. 2014;26:474–481. [Google Scholar]
- Pinto-de-Sousa J., David L., Reis C.A., Gomes R., Silva L., Pimenta A. Mucins MUC1, MUC2, MUC5AC and MUC6 expression in the evaluation of differentiation and clinico-biological behaviour of gastric carcinoma. Virchows Arch. 2002;440:304–310. doi: 10.1007/s00428-001-0548-y. [DOI] [PubMed] [Google Scholar]
- Plachý J., Weining K.C., Kremmer E., Puehler F., Hala K., Kaspers B., Staeheli P. Protective effects of type I and type II interferons toward Rous sarcoma virus-induced tumors in chickens. Virology. 1999;256:85–91. doi: 10.1006/viro.1999.9602. [DOI] [PubMed] [Google Scholar]
- Qu J.M., Chen P.J., Li W.P., Qu X.C., Tang B.G., Huang T.R. Extraction and Efficacy Colibacillosis in vivo an in Vitro of antibacterial peptides of Musca Domestica. Chin. J. Anim. Sci. 2006;33:56–58. [Google Scholar]
- Ruan Y., Hou Y.D., Chen Y.Z., Lu Y.D. Study on the antioxidant activity of Rosemary antioxidant. Yunnan Chem. Technology. 2013;40:25–28. [Google Scholar]
- Sa R.N., Zhang Q., Gu C.T., Tong J.M. Effects of microbial feed additives on E.coli inhibition and blood biochemical Barameters in broilers. Feed Research. 2006:4–8. [Google Scholar]
- Segawa S., Fujiya M., Konishi H., Ueno N., Kobayashi N., Shigyo T., Kohgo Y. Probiotic-derived polyphosphate enhances the epithelial barrier function and maintains intestinal homeostasis through integrin-p38 MAPK pathway. PLoS One. 2011;6:e23278. doi: 10.1371/journal.pone.0023278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi D.H., Chen J.F., Zhao L.S., Xu Y.H., Wang J.L. Effects of Lamiaceae plant Extracts on antioxidant function of serum and lipid oxidation in chicken. Chin. J. Anim. Sci. 2013;49:63–67. [Google Scholar]
- Singh A., Kukreti R., Saso L., Kukreti S. Oxidative stress: a key Modulator in Neurodegenerative diseases. Molecules. 2019;24:1583. doi: 10.3390/molecules24081583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stark M.A., Huo Y., Burcin T.L., Morris M.A., Olson T.S., Ley K., Ley A.K. Phagocytosis of Apoptotic neutrophils regulates Granulopoiesis via IL-23 and IL-17. Immunity. 2005;22:285–294. doi: 10.1016/j.immuni.2005.01.011. [DOI] [PubMed] [Google Scholar]
- Stein K., Farmer J., Singhal S., Marra F., Sutherland S., Quiñonez C. The use and misuse of antibiotics in dentistry: a scoping review. J. Am. Dent Assoc. 2018;149:869–884. doi: 10.1016/j.adaj.2018.05.034. [DOI] [PubMed] [Google Scholar]
- Tian Y.B., Zhou J.R., Li Q.H., Jia J.J., Huang R.H. Effects of Atractylodes Polysaccharide on growth performance and serum biochemical Parameters in Piglets. Chin. J. Anim. Sci. 2009;45:45–48. [Google Scholar]
- Van Cutsem J.M., D T. Miconazole, a broad-spectrum antimycotic agent with antibacterial activity. Chemotherapy. 1972;6:392–404. doi: 10.1159/000220875. [DOI] [PubMed] [Google Scholar]
- Wang J. Shandong Agricultural University; 2007. Effects of Antibacterial Peptides on the Performance and Immune Function of Broilers. Master: 66. Accessed Sept. 2020. http://kns.cnki.net/KCMS/detail/detail.aspx?FileName=2007135266.nh&DbName=CMFD2008. [Google Scholar]
- Wang Z.T. Shihezi University; 2017. Antimicrobial Peptides in Housefly Larvae (Musca Domestica) Affect Intestinal Mucosal Immune in Salmonella. Accessed Sept. 2020. https://kns.cnki.net/kcms/detail/detail.aspx?dbcode=CMFD&dbname=CMFD201801&filename=1018810552.nh&v=7r5VEtGVvrJMAhWQqkrbs9zo96NZCKIVaHcece78rfzH6%25mmd2B82Dff3IBdhPpXa7fvZ. [Google Scholar]
- Xie Q.X., Zhang J.M. Effects of compound Probiotics Gu Benkang on growth performance , cecal microflora, immune Indices and Antioxid ant capacity of broilers. Chin. J. Anim. Sci. 2014;50:58–62. [Google Scholar]
- Xu L.H., Qi R.X., Wang C.K., Wang Q.X., Xie L.Q., Lin L.H., Chen Q.D. Effects of fermented soybean meal on growth performance, serum biochemical Indices, mucosal immune function and microorganism in Yellow-Feathered broilers. Chin. J. Anim. Nutr. 2013;25:840–848. [Google Scholar]
- Yao Y., Kuang W., Huang Z.Y., Xi Z.L., Wang B.X., He Z.L., Wang R.Z. Effect of antibacterial peptide cecropin on growth performance, intestinal mucosal morphology, Caecal microflora and immune function of chickens. Jiangsu J. Agr. Sci. 2014;30:331–338. [Google Scholar]
- Yao L.Q., Sa R.N., Tong J.M., Huo Q.G. Effect of Apramycin on intestinal flora and intestinal morphology of Piglets. Acta Veterinaria et Zootechnica Sinica. 2003;34:250–257. [Google Scholar]
- Yuan Y., Li H.P., Xing X.Y., Hu Y.H. Effect of vaccine antibody levels based on Fermentation antimicrobial peptide of small molecule on inactivated vaccine against avian. Mod. J. Anim. Husbandry Vet. Med. 2016:8–14. [Google Scholar]
- Yue W.J., Liu Y., Xu W., Dong L., Luo X.T., Jiang W.R., Sun X., Zhong L., Liu J. The expression of IL-2, IL-4, IL-17 and IL-10 in ulcerative colitis (UC) mucosa and its relation with disease activity. Fudan Univ. J. Med. Sci. 2012;39:454–459. [Google Scholar]
- Zhang X. Application of serum total bile acid, Alanine aminotransferase and serum aspartate aminotransferase in liver Cirrhosis. Jilin Med. J. 2011;32:4840–4841. [Google Scholar]
- Zhang A.Z., Jiang N., Zhang T., Song J.F. Different Housefly Larvae products affect nutrient Availability, intestinal flora and serum biochemical Indices in Yellow-Feathered broilers. Chin. J. Anim. Nutr. 2012;24:911–917. [Google Scholar]
- Zhang X., Li F., Hou F., Qi C., Wu Y., Ding Y. Expression and significance of Muc-1, Muc-2, Muc5AC, Muc-6 in hyperplastic polyp, globoid dysplasia, gastric signet ring cell carcinoma. Mod. Med. J. China. 2009;11:20–23. [Google Scholar]
- Zhou Y., Liu W.D., Sun B.Q., Wu C.R. Effect of Qiwei Baizhu san and its Extracts on expressions of IF N -α, IL -4 and IL -10 in small intestinal epithelial cell of enteric Dysfunctions in Mice. Chin. J. Exp. Traditional Med. Formulae. 2015;21:112–117. [Google Scholar]