Abstract
During simultaneous liver-kidney transplantation (SLK) in highly sensitized patients, donor specific anti-human leukocyte antigen antibodies (DSA, HLA) can be present prior to transplant leading to positive crossmatch, yet these recipients have relatively low incidences of acute rejection. The mechanisms and timing underlying immunologic changes that occur intra-operatively remain largely unknown. Therefore, we measured the intra- and peri-operative kinetics of anti-HLA antibodies in highly sensitized SLK recipients. In this study, pre- and post-operative blood samples were obtained from sensitized SLK candidates with documented DSA. Intra-operative samples were obtained from a sub-group of SLK recipients. Pretransplant anti-HLA antibody profiles were created and flow cytometry and anti-human globulin complement-dependent cytotoxic crossmatches were performed. Significant reductions in anti-HLA class I and II DSA were seen intra-operatively shortly after reperfusion of the liver allograft. This effect was most pronounced for anti-HLA class I DSA (mean change, −85%, p < 0.05); changes to anti-HLA class II DSA were less robust (mean change, −47%, p = 0.15). Importantly, non-DSA anti-HLA antibodies remained unchanged throughout the perioperative period, suggesting the mechanism(s) by which the liver lowers DSA levels are specific to the DSA. These data demonstrate the immunologic benefit of performing SLK is lasting and occurs very shortly after liver reperfusion.
Keywords: Simultaneous liver-kidney transplant, Donor specific antibody, Sensitized
1. Introduction
Kidney transplant recipients with pre-formed donor specific antibodies (DSA) have a higher incidences of acute and chronic antibody mediated rejection (AMR) [[1], [2], [3], [4]]. The presence of DSA can lead to worse renal allograft outcomes and, in the pretransplant setting, may be considered a contraindication to transplant [[1], [2], [3], [4]]. However, at least in the short-term, the liver appears to be “immunologically privileged” compared to the kidney, in that the liver appears to be refractory to high levels of DSA. Furthermore, the liver may provide “immunological cover” in dual organ recipients with high levels of circulating anti-human leukocyte antigen (HLA) antibodies. The duration and exact mechanism(s) of this protection are largely unknown, but the immunologic protection from the liver can overcome some core immunologic tenets of transplantation, allowing for successful transplantation in the face of both a positive anti-human globulin complement-dependent cytotoxic (AHG-CDC) crossmatch and a high level of DSA without requiring prior desensitization [5,6].
Although AMR is occasionally reported in liver recipients, it is widely accepted that high levels of DSA are not a contraindication to transplant [7]. However, the importance of humoral immune sensitization in liver transplants is still controversial. There is compelling evidence that detection of circulating DSA in liver recipients serum is a contributing factor to short- and long-term graft dysfunction [[8], [9], [10], [11]]. Unfortunately, there is very little or no routine immunological assessment performed for liver or simultaneous liver-kidney transplants (SLK) mainly due to the lack of hyperacute rejection and the well-known effect the liver has on lowering the humoral immune response risk [7,10,[12], [13], [14], [15], [16]]. Also, another factor that greatly complicates the impact of DSA in liver allograft dysfunction has been the variable induction therapies used between centers, which range from the use of a full course of anti-thymocyte immunoglobulin to steroid-only protocols [8,11,17].
There are two classical HLA antigens, HLA class I and class II, that are the most recognized antigens to be involved in transplant. Importantly in terms of transplant, antibodies against these HLA antigens can be DSA or non-DSA. Interestingly, recent studies have described the liver as providing preferential protection against anti-HLA class I DSA, but persistent anti-HLA class II DSA could be associated with rejection, suggesting that the liver may not be impervious to alloantibodies [18,19].
Although transplant centers have embraced the immune-privileged state of the liver, to our knowledge, there are very few studies assessing the impact of perioperative management and surgical techniques on pre-transplant DSA levels. Importantly, the optimal timing of the renal transplant during SLK has been debated from an immunologic standpoint with some programs delaying kidney transplant from 6 to 24 h, while other programs perform the transplant with no delay [6]. The decision to delay transplant may lead to increased cold/ischemia time on the kidney, increased undue stress on the patient, and increased inflammation, all leading to decreased allograft survival. In this study, in addition to demonstrating the safety of avoiding strong induction immunosuppression, we aim to show that delay is unnecessary when transplanting the kidney into a highly-sensitized recipient in a sequential liver-kidney transplant.
In creating an evidence-based approach to the practice of delaying kidney transplant in highly sensitized patients, we focused on understanding the immediacy and extent of the protective effect of the liver during SLK in this pilot study. To this end, we identified highly sensitized SLK candidates and monitored the level of anti-HLA antibodies peri- and intra-operatively to assess the kinetics of anti-HLA antibody levels during allograft reperfusion.
2. Methods
2.1. Patient selection
Within the study period (September 2016 – December 2018), subjects were included if they were actively a candidate for SLK and had documented DSA at the time of organ offer (n = 7). Inclusion criteria mandated that patients receive SLK from the same donor. All patients had peri- and post-transplant serum samples drawn (n = 7). Intra-operative samples were obtained on a subset of SLK recipients identified prospectively (n = 4). Antibody screening and SAB identification (specificity) were performed on each serum sample collected. Antibody assessment was performed by the HLA laboratory to determine if the patient had DSA (>1000 MFI) by SAB against their donor. In addition, each patient included had at least a retrospective flow cytometry and AHG-CDC crossmatches performed using sera obtained pre-, peri- and post-transplant against their donor's cells. All subjects were followed from the time of transplant to either death or last known follow up. Organ function was assessed by routine serum chemistry analysis at 30 days, 6 months, and 1 year post-transplant. All subjects signed informed research consents in addition to the standard surgical consent.
2.2. Immunosuppression management
All patients received induction immunosuppression with weight-based intravenous hydrocortisone. The pediatric patient additionally received basiliximab at the discretion of the nephrology team. No peri-operative desensitization maneuvers were employed, e.g. intravenous immunoglobulin, plasmapheresis, or rituximab. A triple-drug maintenance immunosuppression regimen was used for all patients consisting of tacrolimus, prednisone, and delayed introduction of mycophenolic acid.
2.3. Sample collection
Pre- (PRE-OP) and post-operative (POD1) blood samples were obtained from sensitized SLK candidates with documented DSA (n = 7). Intra-operative samples were obtained: after reperfusion of the liver allograft, during completion of the choledococholedocostomy (POST-LIVER), after reperfusion of the renal allograft, and during completion of the ureterocystostomy (POST-KIDNEY). Biopsies were obtained for cause only during the study period. Triggers for biopsy of an allograft were elevated organ-specific serum chemistries.
2.4. Alloantibody detection, crossmatching and HLA typing
Antibody screening was performed by FlowPRA®Screening and acquired on a FACSCanto II (Becton-Dickinson, San Jose, CA), and then reflexed to LabScreen® Single Antigen™ Beads (SAB) (One Lambda, Canoga Park, CA) and acquired on the LABScan 200 instrument (Luminex Corp., Austin, TX). Sera were treated with ethylenediaminetetraacetic acid (EDTA) prior to SAB testing. Specificities were assigned for bead reactions ≥1000 mean fluorescence intensity (MFI) units (raw values). Crossmatching was performed by flow cytometry for T and B cell and AHG-CDC crossmatches. All positive crossmatches were repeated using blood from the POST-LIVER reperfusion sample, if available, or the POD1 sample. The donor and patient were HLA typed using molecular methods at low/intermediate resolution for HLA A, B, Bw4/Bw6, C, DRB1, DRB3, 4, and 5, DQA1, DQB1, DPA1, and DPB1 loci. Recipient HLA typing was performed at the Baylor College of Medicine Immune Evaluation Laboratory using sequence specific oligonucleotide probes and/or next generation sequencing (Immucor, Inc., Norcross, GA). Deceased donor HLA typing was retrieved from United Network for Organ Sharing.
2.5. Statistics
Continuous variables were reported as means ± standard deviations and compared using the Student's t-test. Contingency table analysis was used to compare categorical variables. Results were considered significant at a p-value <0.05 and all reported p-values were two-sided.
3. Results
3.1. Inclusion criteria and patient demographics
The patient demographics are listed in Table 1. All patients had at least one pre-transplant DSA (>1000 MFI) detected by SAB. Only anti-HLA DSA were tested for. A subset of patients had a pre-transplant positive flow cytometry (n = 4/7; 57%) and/or AHG-CDC crossmatch (n = 2/7; 29%) (Table 1). Intra-operative sample collection did not begin until after the conclusion of the first three of the seven SLK cases. Therefore, intra-operative samples were obtained from four of the seven SLK recipients. Mean intra-operative blood transfusion was 900 +/− 634 mL of packed red blood cells and 286 mL +/− 419 mL of fresh frozen plasma. Transfusion requirements of individual subjects are listed in Table 2.
Table 1.
Patient demographics and crossmatch results.
| Patient | age | liver dz | kidney dz | Sex | Prior Txp | ABO, recipient | ABO, donor | PRA-I | PRA-II | Flow B cell | Flow T cell | CDC | CIT |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 56 | HCV¥ | HTN | M | YES | B | O | 0 | 41 | NEG | NEG | NEG | 8 h 38 min |
| 2 | 48 | NASH | DM, HTN |
M | NO | O | O | 5 | 0 | NEG | NEG | NEG | 6 h 59 min |
| 3 | 56 | HCV₤ | HTN, DM |
F | YES | A | A | 99 | 80 | POS | POS | POS | 14 h 34 min |
| 4 | 17 | Hyper-oxaluria | Hyper-oxaluria | M | NO | B | O | 0 | 0 | NEG | NEG | NEG | 9 h 25 min |
| 5 | 70 | NASH | DM | M | NO | B | B | 93 | 50 | POS | POS | NEG | 12 h 0 min |
| 6 | 57 | NASH | DM | F | NO | B | B | 87 | 22 | POS | POS | NEG | 10 h 51 min |
| 7 | 63 | HCV¥ | DM | F | NO | A | A | 100 | 80 | POS | POS | POS | 14 h 3 min |
Dz, disease; txp, transplant; NASH, non-alcoholic steatohepatitis; HCV, hepatitis C virus; DM, diabetes mellitus; HTN, hypertension; txp, transplant; ABO, ABO blood group system; PRA, panel reactive antibody; CDC, complement-dependent cytotoxic crossmatch (retrospective, pre-transplant); *prior kidney transplant, ₤untreated; ¥treated with sustained viral response.
Table 2.
Anti-HLA antibody specificities and intensities.
| Anti-HLA Antibodiesa |
||||||
|---|---|---|---|---|---|---|
| MFI |
||||||
| Antibody | PRE-OP | POST-LIVER | POST-KIDNEY | POD1 | Transfusion RBC/FFP |
|
| Patient 1 | A*01:01 | 12,072 | 311 | 258 | 241 | 700/1000 |
| A*02:01 | 6851 | 8703 | 8651 | 9252 | ||
| B*07:02 | 13,702 | 14,305 | 12,964 | 12,186 | ||
| B*35:01 | 14,378 | 285 | 229 | 168 | ||
| B*41:01 | 14,794 | 2771 | 2461 | 1662 | ||
| C*17:01 | 3653 | 482 | 383 | 242 | ||
| DRB1*08:01 | 1279 | 366 | 316 | 226 | ||
| DRB1*04 | 9546 | 6849 | 6102 | 5357 | ||
| Patient 2 | B*57:01 | 3181 | 581 | 405 | 338 | 1050/0 |
| Patient 3 | DQ8 | 6935 | 5424 | 3698 | 2119 | 700/0 |
| Patient 4 | A*24:01 | 2145 | 785 | 984 | 556 | 0/0 |
| Bw4 | Present | Absent | Absent | Absent | ||
| Patient 5 | A*68:01 | 9020 | 12,003 | 2100/750 | ||
| B*07:02 | 11,220 | 75 | ||||
| B*49:01 | 8115 | 11,015 | ||||
| Patient 6 | A*01:01 | 14,720 | 195 | 1050/250 | ||
| A*24:01 | 6796 | 11,062 | ||||
| B*44:03 | 10,479 | 119 | ||||
| B*57:01 | 12,133 | 3242 | ||||
| Patient 7 | A*33:01 | 19,308 | 2274 | 700/0 | ||
| B*53:01 | 19,201 | 1158 | ||||
| B*15:03 | 23,082 | 2431 | ||||
| C*02:02 | 10,725 | 1697 | ||||
| DRB1*14:01 | 2963 | 3772 | ||||
| DPB1*13:01 | 3400 | 1355 | ||||
Bold indicates non-DSA anti-HLA.
HLA, human leukocyte antigen complex; MFI, mean fluorescent intensity; PRE-OP, pre-transplant sample; POST-LIVER, post-liver reperfusion sample; POST-KIDNEY, post-kidney reperfusion sample; POD1, first post-operative day sample; transfusion, intra-operative transfusion requirements during transplant; RBC, packed red blood cells (mL); FFP, fresh frozen plasma (mL).
3.2. Anti-HLA antibody assessment during transplant/immunologic assessment
Three patients had a prior kidney transplant in which they had DSA against the previous donor, but these were not shared mismatched antigens with the current donor (Table 2; non-DSA anti-HLA). All positive crossmatches became negative after reperfusion of the liver allograft at either the POST-LIVER sample (4 patients who were fully assessed) or POD1 sample (3 patients that were not assessed intra-operatively) (Table 2).
3.3. Do anti-HLA antibody levels go down: POD1 vs PRE-OP?
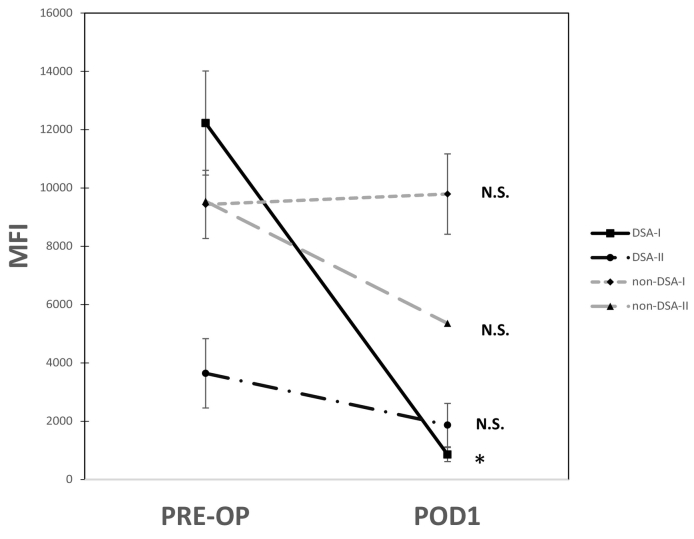
To determine if non-immunological factors (e.g. transfusions, saline, etc.) influenced the detection of anti-HLA antibodies peri- and post-transplant, DSA and non-DSA MFI levels (>1000 MFI) were compared between PRE-OP vs POD1 samples. All patients had a significant reduction or marked percent change in anti-HLA class I DSA (mean percent change: −92%, p < 0.01) and anti-HLA class II DSA (mean percent change: −46%, p = 0.20) (Fig. 1). Importantly, there was no significant percent change in the anti-HLA class I antibodies that were not donor specific: non-DSA-I (mean change: +14%, p = 0.87) (Fig. 1).
Fig. 1.
Perioperative changes in anti-human leukocyte antigen (HLA) antibodies are donor specific. Mean fluorescence intensity (MFI) values are the average values >1000 MFI, for anti-HLA antibodies detected pre-transplant (PRE-OP) and post-operatively (POD1). Donor specific antibodies (DSA) for HLA class I and II (DSA-I and DSA-II) had a marked drop in MFI values in the POD1 samples (percent change: DSA-I and DSA-II PREOP vs POD1, −92% and − 46%, respectively). The anti-HLA antibodies that were not donor specific (non-DSA-I and non-DSA-II) did not have marked change in MFI values (percent change: non-DSA-1 and non-DSA-II, +14% and − 14%, respectively). *p < 0.05.
3.4. How long does it take for the liver to remove DSA as assessed by intra-operative anti-HLA antibody measurements?
The PRE-OP sample (n = 7) was collected in the operating room prior to administration of induction immunosuppression. The first intra-operative sample (n = 4, POST-LIVER) was obtained an average of 56 min (+/− 28 min) after liver reperfusion. The kidney was reperfused at an average of 3 h 19 min (+/− 36 min) after liver reperfusion. The second intra-operative sample (n = 4, POST-KIDNEY) was obtained an average of 4 h 44 min (+/− 40 min) after liver reperfusion, 1 h 25 min (+/− 39 min) after kidney reperfusion. The POD1 sample (n = 7) was collected during the first post-operative day, at least 24 h after the PRE-OP sample was obtained. Each sample collected was screened for anti-HLA antibodies (MFI > 1000) using SAB assays.
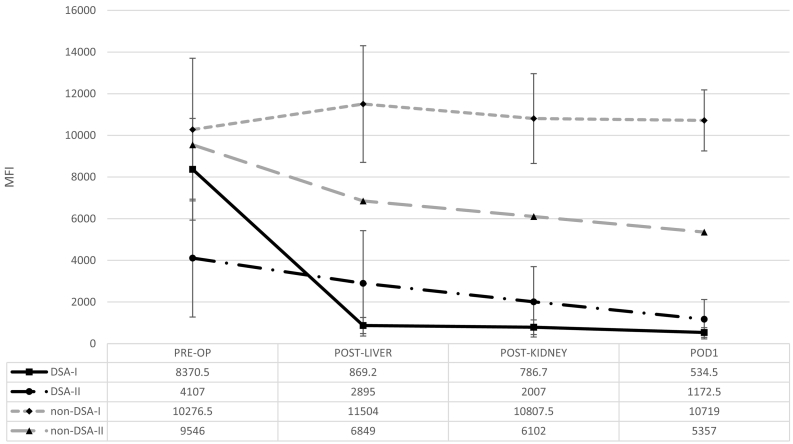
In the subgroup (n = 4) of SLK recipients in whom intra-operative samples were obtained, anti-HLA class I DSA MFI values had a significant decrease within 56 min after liver reperfusion (mean percent change: PRE-OP vs POST-LIVER, −85%, p < 0.05) and remained low through POD1 (percent change: PRE-OP vs POD1, −90%, p < 0.05 (Fig. 2). Interestingly, the MFI changes in anti-HLA class II DSA were modest when compared to class I, and were not significantly lower by POD1 (anti-HLA class II percent change: PRE-OP vs POST-LIVER, −47%, p = 0.15; PRE-OP vs POD1, −76%, p = 0.36 (Fig. 2), suggesting the liver is not able to absorb and/or neutralize anti-HLA class II antibodies as effectively as anti-HLA class I antibodies.
Fig. 2.
Intra-operative kinetics of anti-HLA antibodies in simultaneous liver-kidney transplantation (SLK). Data is representative of 4 patients. MFI values are the average MFI results of DSA and non-DSA values.
3.5. Graft/patient survival ˗ How do these results correlate with rejection and outcome?
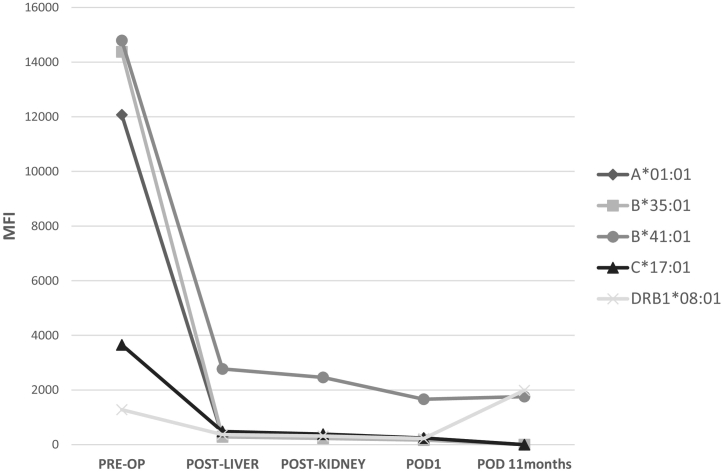
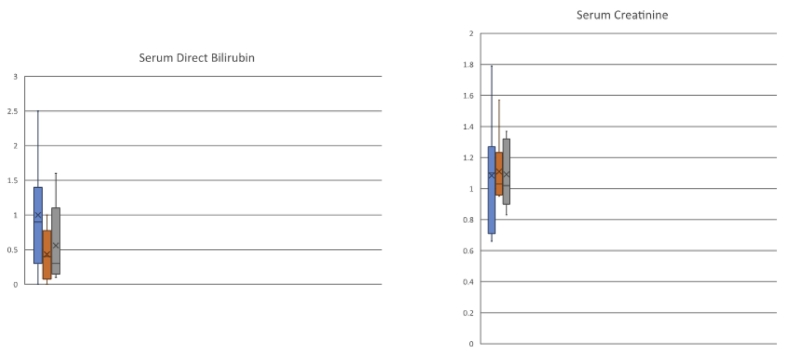
One patient presented with acute kidney injury at 11 months after SLK and underwent DSA assessment at that time. This highly sensitized patient had multiple PRE-OP class I and II DSA which fell intra-operatively and remained low at 11 months post-operatively (Fig. 3 and Table 2, Patient 1). One patient (Patient 5) died of sepsis during the study period (index admission, post-operative day 39). Another patient (Patient 3) experienced delayed graft function of the renal allograft requiring outpatient dialysis after discharge with eventual gain of function. All surviving patients had functioning allografts (liver and kidney) at the time of last follow-up (Fig. 4). Overall, 1-year Kaplan-Meier patient and graft survivals were 86% and 86%. Of patients surviving to discharge from the index admission, patient and graft survivals were 100% with a median follow up of 517 days (range: 302–1847 days). Five for cause allograft biopsies were obtained for varying degrees of graft dysfunction during the study period. There were no episodes of biopsy-proven or empirically treated rejection during the study period (Table 3).
Fig. 3.
DSA do not rebound in one Patient 11 months post-transplant. The MFI values do not increase or rebound in the one representative patient with both class I and II DSA and non-DSA (Table 2, Patient 1).
Fig. 4.
1-, 6-, and 12-month serum biochemical tests of allograft function. Mean (mg/dL) +/− standard deviation.
Table 3.
Biopsy results/characteristics.
| Allograft type, patient # | POD | Findings |
|---|---|---|
| Liver, 3 | 54 | Recurrent HCV |
| Kidney, 3 | 12 | ATN |
| Liver, 5 (death) | 21 | Interface hepatitis, changes c/w sepsis |
| Liver, 6 | 29 | 20% steatosis |
| Liver, 7 | 30 | Centrilobular necrosis |
POD, post-operative day; HCV, hepatitis C virus; ATN, acute tubular necrosis.
4. Discussion
This study comprises a group of immunologically high-risk patients that have maintained excellent dual graft function after successful SLK with minimal immunologic induction. Not only were these patients selected based on the presence of pre-formed DSA, 63% also had positive crossmatches with their donors. Induction immunosuppression followed a “liver-centric” approach with only steroids administered prior to allograft implantation, except for the one pediatric patient who received basiliximab as well. The crossmatches from this patient (patient 4) were negative pre-operatively.
Although the immunosuppression in this series was fairly homogeneous, the optimal immunosuppression regimen for SLK is still a topic of debate and the lack of standardization is evident from the literature. For example, in the largest reported series of SLK recipients with DSA, induction therapies included both monoclonal and polyclonal antibodies with tacrolimus used in the maintenance immunosuppression regimen only 59% of the time [8]. This heterogeneity is likely due to the 25 year duration of the study spanning multiple immunosuppression eras. In the current series, maintenance immunosuppression in all patients was with a triple-drug regimen with tacrolimus, prednisone, and mycophenolic acid. Tacrolimus was introduced during POD1 and mycophenolic acid was introduced near the time of discharge. The effect of the choice of immunosuppression after SLK on the fate of DSA is not clear, but an increased index of suspicion in cases where there is pre-formed DSA (anti-HLA class I or II) is likely warranted [11]. Taken together, there is a clear need to determine risk factors in the post-transplant liver patients to help guide clinicians on management of these patients in the era of personalized medicine. A confounding factor in the understanding of humoral immune responses following SLK is the immunomodulated state accompanying a current or prior HCV infection, including the presence of lymphoproliferative disorders and cryoglobulinemia. The three patients in this series with HCV liver-failure had significant heterogeneity in their DSA repertoires and so the role of HCV infection in post-transplant DSA kinetics needs further study. Additionally, a weakness of this study was the inability to consider potential donor characteristics beyond ABO blood type, including degree of donor-recipient matching, as contributors to DSA clearance.
The accuracy in measuring short-term changes in circulating antibody levels in a dynamic environment like SLK can be questioned given the potential confounders of intra-operative blood product transfusions and the use of continuous dialysis. The average transfusion requirements for this cohort of subjects did not appear to differ significantly between those that experienced a drastic or moderate antibody response. The single SLK recipient who did not receive exogenous blood transfusion had a noticeable drop in DSA MFI. In this study, three highly sensitized patients, with anti-HLA antibodies against their previous donors but not specific to the current SLK donors, had high levels of anti-HLA non-DSA persisting in the intra- and perioperative periods, thereby serving as an internal control. Indeed, in a previously published case report of a single highly sensitized SLK recipient, DSA, but not anti-HLA non-DSA, fell significantly shortly after reperfusion of the liver allograft [20].
In this series of SLK recipients with pre-formed DSA, we found a marked reduction in DSA within one hour of reperfusion of the liver allograft which most-likely prevented hyperacute rejection of the renal allograft and may provide a long lasting effect with seemingly very little rebound of DSA. Although the long-term fate of DSA in this study was not fully assessed, one patient had a repeat DSA assessment at 11 months post-transplant. This patient (Patient 1) was the most-highly sensitized patient of the series with high levels of both anti-HLA class I and II DSA and anti-HLA class I and II non-DSA. De novo DSA were not detected post-transplant in this patient and the anti-HLA class I DSA suppression persisted. A low-level class II DSA was no longer detected and the anti-HLA non-DSA remained present at high levels. This adds more evidence to observations made by other investigators about the inconsistent nature of the clearance of class II DSA during SLK [6,18,19,21]. Clearly, more work is needed to confirm these results that we observed in one patient.
Similar to others, we found that anti-HLA class II DSA were not cleared as readily as anti-HLA class I DSA, which fell to undetectable levels soon after reperfusion of the liver allograft [18,19]. The significance of the intra-operative kinetics of anti-HLA class II DSA in our study is complicated by the low incidence of class II pre-transplant DSA, which were only present in clinically significant levels in three patients. In these three patients, there were reductions in anti-HLA class II DSA of 82%, 69%, and 19%. The question of incomplete penetrance of the protective effect of the liver allograft against anti-HLA class II DSA may have its answer in the concept of epitope- rather than allelic-level matching, but clarification of the liver's role in potentially neutralizing anti-HLA class II antibodies is needed [22].
In kidney transplant, a positive crossmatch (flow cytometry and AHG-CDC) in the presence of detectable DSA by SAB assays is a contraindication to transplant. Although the developments over the past decade in antibody detection technologies and improved resolution of HLA typing have clearly led to more appropriate identification of DSA, there is still no agreement in the field as to what an appropriate MFI value by SAB assays is for delineating high risk from contraindication to transplant [[23], [24], [25], [26]]. Regardless of the MFI value, we tandemly ran the flow cytometry crossmatch and AHG-CDC crossmatch to assess avidity and cytotoxicity, respectively. These cell-based assays are the “gold-standard” by which we evaluate our SAB results. We found that two of the patients with positive AHG-CDC crossmatches in PRE-OP serum were negative post-transplant, suggesting that the liver is able to neutralize significantly high levels of DSA. A positive AHG-CDC crossmatch in kidney alone transplant would most-likely result in hyperacute rejection. Taken together, the results of this small but informative prospective study provide important insight on how quickly DSA are neutralized post-liver transplant, even when the patient has high levels of DSA in their serum.
In summary, this group of immunologically high-risk patients represent a prohibitive degree of sensitization that would severely decrease access to transplant if unacceptable antigens were delineated in the allocation process. They have maintained excellent dual graft function after successful SLK with minimal immunologic induction, and so the potential risk of SLK in the presence of strong DSA must be considered against the risk of death on the waitlist. Although the ability to detect a long-term effect of DSA on allograft and patient survival is limited by our intermediate median length of follow up of 1.4 years, the presence of stable graft function without any episodes of treated rejection suggests a favorable outcome is likely. Vigilance in the post-operative period is of course needed as these immunologically high-risk dual organ recipients need well-informed and well-executed surveillance and long-term care.
Acknowledgments
We would like to thank Jane E. Libbey, MS, for copyediting and proofreading of the manuscript and the Texas Heart Institute anesthesiology team for assistance in obtaining intra-operative samples and caring for the patients.
Contributor Information
M. Kueht, Email: mlkueht@utmb.edu.
M.F. Cusick, Email: mfcusick@med.umich.edu.
References
- 1.Wiebe C. Evolution and clinical pathologic correlations of de novo donor-specific HLA antibody post kidney transplant. Am. J. Transplant. 2012;12(5):1157–1167. doi: 10.1111/j.1600-6143.2012.04013.x. [DOI] [PubMed] [Google Scholar]
- 2.Willicombe M. De novo DQ donor-specific antibodies are associated with a significant risk of antibody-mediated rejection and transplant glomerulopathy. Transplantation. 2012;94(2):172–177. doi: 10.1097/TP.0b013e3182543950. [DOI] [PubMed] [Google Scholar]
- 3.Hidalgo L.G. De novo donor-specific antibody at the time of kidney transplant biopsy associates with microvascular pathology and late graft failure. Am. J. Transplant. 2009;9(11):2532–2541. doi: 10.1111/j.1600-6143.2009.02800.x. [DOI] [PubMed] [Google Scholar]
- 4.Everly M.J. Incidence and impact of de novo donor-specific alloantibody in primary renal allografts. Transplantation. 2013;95(3):410–417. doi: 10.1097/TP.0b013e31827d62e3. [DOI] [PubMed] [Google Scholar]
- 5.Olausson M. Successful combined partial auxiliary liver and kidney transplantation in highly sensitized cross-match positive recipients. Am. J. Transplant. 2007;7(1):130–136. doi: 10.1111/j.1600-6143.2006.01592.x. [DOI] [PubMed] [Google Scholar]
- 6.Paterno F. Successful simultaneous liver-kidney transplantation in the presence of multiple high-titered class I and II antidonor HLA antibodies. Transplant Direct. 2016;2(12):e121. doi: 10.1097/TXD.0000000000000633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Barth R.N. Antibody-mediated rejection of renal allograft in combined liver-kidney transplant. Clin. Transpl. 2010;24(5):685–690. doi: 10.1111/j.1399-0012.2009.01161.x. [DOI] [PubMed] [Google Scholar]
- 8.O’Leary J.G., Klintmalm G.B. Impact of donor-specific antibodies on results of liver transplantation. Curr. Opin. Organ Transplant. 2013;18(3):279–284. doi: 10.1097/MOT.0b013e3283614a10. [DOI] [PubMed] [Google Scholar]
- 9.Askar M. Combined liver-kidney transplants: allosensitization and recipient outcomes. Transplantation. 2011;91(11):1286–1292. doi: 10.1097/TP.0b013e3182184181. [DOI] [PubMed] [Google Scholar]
- 10.Koch M. Four-year allograft survival in a highly sensitized combined liver-kidney transplant patient despite unsuccessful anti-HLA antibody reduction with rituximab, splenectomy, and bortezomib. Transpl. Int. 2013;26(8):e64–e68. doi: 10.1111/tri.12120. [DOI] [PubMed] [Google Scholar]
- 11.AbdulRahim N. Lack of benefit and potential harm of induction therapy in simultaneous liver-kidney transplants. Liver Transpl. 2019;25(3):411–424. doi: 10.1002/lt.25390. [DOI] [PubMed] [Google Scholar]
- 12.Singer A.L., Segev D.L. Transplantation: alloantibodies in simultaneous liver-kidney transplantation. Nat. Rev. Nephrol. 2013;9(7):373–374. doi: 10.1038/nrneph.2013.84. [DOI] [PubMed] [Google Scholar]
- 13.Iwatsuki S. Successful liver transplantation from crossmatch-positive donors. Transplant. Proc. 1981;13(1 Pt 1):286–288. [PMC free article] [PubMed] [Google Scholar]
- 14.Takaya S. The adverse impact on liver transplantation of using positive cytotoxic crossmatch donors. Transplantation. 1992;53(2):400–406. doi: 10.1097/00007890-199202010-00026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jucaud V. Prevalence and impact of De novo donor-specific antibodies during a multicenter immunosuppression withdrawal trial in adult liver transplant recipients. Hepatology. 2019;69(3):1273–1286. doi: 10.1002/hep.30281. [DOI] [PubMed] [Google Scholar]
- 16.Yazawa M., Cseprekal O., Helmick R.A., Talwar M., Balaraman V., Podila P.S.B., Agbim U.A., Maliakkal B., Fossey S., Satapathy S.K., Sumida K., Kovesdy C.P., Nair S., Eason J.D., Molnar M.Z. Association between post-transplant donor-specific antibodies and recipient outcomes in simultaneous liver-kidney transplant recipients: single-center, cohort study. Transpl Int. 2020 Feb;33(2):202–215. doi: 10.1111/tri.13543. Epub 2019 Dec 17. PMID: 31647143. [DOI] [PubMed] [Google Scholar]
- 17.Kamal L., Yu J.W., Reichman T.W., Kang L., Bandyopadhyay D., Kumar D., King A., Gautam U., Bhati C., Yakubu I., Lacy K., Levy M., Gupta G. Impact of induction immunosuppression strategies in simultaneous liver/kidney transplantation. Transplantation. 2020 Feb;104(2):395–403. doi: 10.1097/TP.0000000000002768. PMID: 31022149. [DOI] [PubMed] [Google Scholar]
- 18.Dar W. Donor-directed MHC class I antibody is preferentially cleared from sensitized recipients of combined liver/kidney transplants. Am. J. Transplant. 2011;11(4):841–847. doi: 10.1111/j.1600-6143.2011.03467.x. [DOI] [PubMed] [Google Scholar]
- 19.O’Leary J.G. Class II alloantibody and mortality in simultaneous liver-kidney transplantation. Am. J. Transplant. 2013;13(4):954–960. doi: 10.1111/ajt.12147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Key T. The kinetics of donor HLA class I-specific antibody absorption following a combined split liver and kidney transplant. NDT Plus. 2010;3(6):579–581. doi: 10.1093/ndtplus/sfq160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dar W.A. Ischaemia reperfusion injury in liver transplantation: cellular and molecular mechanisms. Liver Int. 2019;39(5):788–801. doi: 10.1111/liv.14091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cusick M.F., Jindra P.T. Human leukocyte antigen epitope matching in solid organ transplantation. Clin. Lab. Med. 2018;38(4):595–605. doi: 10.1016/j.cll.2018.07.004. [DOI] [PubMed] [Google Scholar]
- 23.Wiebe C., Nickerson P. Posttransplant monitoring of de novo human leukocyte antigen donor-specific antibodies in kidney transplantation. Curr. Opin. Organ Transplant. 2013;18(4):470–477. doi: 10.1097/MOT.0b013e3283626149. [DOI] [PubMed] [Google Scholar]
- 24.Cusick M.F., Tambur A.R. Advancing histocompatibility testing for solid organ transplantation - what is needed? A personal opinion. Clin. Transpl. 2015;31:193–201. [PubMed] [Google Scholar]
- 25.Reed E.F. Comprehensive assessment and standardization of solid phase multiplex-bead arrays for the detection of antibodies to HLA. Am. J. Transplant. 2013;13(7):1859–1870. doi: 10.1111/ajt.12287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tambur A.R. Sensitization in transplantation: assessment of risk (STAR) 2017 working group meeting report. Am. J. Transplant. 2018;18(7):1604–1614. doi: 10.1111/ajt.14752. [DOI] [PubMed] [Google Scholar]