Abstract
Background
In Thailand, almost one-quarter of strokes are related to atrial fibrillation (AF), and many could be prevented if AF were diagnosed and treated prior to the stroke. Therefore, we tested a novel strategy to screen large numbers of community residents using village health volunteers and primary care nurses.
Methods
Local primary care nurses and village health volunteers in Phetchaburi and Lopburi provinces, Thailand were trained to perform AF screening using a blood pressure device with AF algorithm (Microlife A200 AFib). 10% of residents aged ≥ 65 years were randomly selected for screening during home-visits. Participants with possible AF were given follow-up appointments for further testing, including 12-lead ECG and echocardiogram.
Results
Over two-months, 9.7% (13,864/143,478) of the target population were screened: mean age 73.2 ± 6.4 years, 32.4% male. The estimated AF prevalence (detected by Microlife A200 AFib) was 2.8% (95% CI, 2.6–3.1%) for age ≥ 65 years (i.e. 393/13,864 participants). Prevalence increased with age from 1.9% (65–69 years) to 5.0% (≥85 years) (p < 0.001). Only 58% (226/393) of participants with suspected AF attended the follow-up appointment (1–3 months after initial screen): mean CHA2DS2-VASc score 3.2 ± 1.2; 86.3% (195/226) had Class-1 oral anticoagulation recommendation, and 33% (75/226) had AF on 12-lead ECG.
Conclusions
In Thailand, large-scale AF screening in the community is feasible using trained volunteer health workers, allowing screening of large numbers in a short time-period. Further investigation of this strategy is warranted, ensuring mechanisms to obtain a timely rhythm strip or 12-lead ECG locally, and a designated pathway to treatment.
Keywords: Atrial fibrillation, Prevalence, Screening, Risk factors, Stroke risk
1. Introduction
Atrial fibrillation (AF) is the most common cardiac cause of ischemic stroke. Up to 33% of acute ischemic strokes are associated with AF in Sweden [1], and ~ 22% in Thailand [2]. Patients with AF-related stroke tend have more severe neurological deficits and higher mortality [3]. However, as many patients with AF have atypical or no symptoms [4], the initial presentation of AF may be hospitalization for a devastating stroke. Early detection of asymptomatic AF would offer an opportunity to initiate oral anticoagulant (OAC) therapy, warfarin or non-vitamin K antagonists, which can significantly reduce the risk of stroke, systemic embolism and all-cause mortality by two-thirds [5].
AF prevalence is known to be lower in Asian people compared to Caucasians [6], but the burden of AF and AF-related stroke is likely to increase in Asia in the coming decades. Globally the prevalence of AF is known to be increasing [6], and reported prevalence in China has increased 20-fold over 11 years with a 13-fold increase in AF-related strokes [7]. In Thailand, AF prevalence ranges from 0.4% for ages ≥ 30 years [8], to 1.9% for ages ≥ 65 years [9], with slightly higher prevalence of 2.2% in rural populations ≥ 60 years [10]. These studies have all utilized a 12-lead electrocardiogram (ECG) to identify AF. However, it is not feasible to screen a large population using 12-lead ECG, especially in rural or remote community settings.
For a screening method to be efficient in a large population, it must have a high positive predictive value, and be easy to perform at low cost. Opportunistic AF screening using pulse palpation or ECG rhythm strip is recommended in the European AF guidelines for people aged ≥ 65 years [11]. Lead-I ECG rhythm strip devices provide, on average, 93.9% sensitivity and 96.5% specificity [12], which is more accurate than pulse palpation. Automated oscillometric blood pressure (BP) monitors with AF detection algorithms also offer high sensitivity (97–100%) and specificity (89–93%) to detect AF [13], [14], [15], and are recommended by the UK National Institute for Health and Care Excellence (NICE) for screening patients ≥ 65 years during routine BP measurement in primary care [16]. Although these BP devices have been validated in many settings, the use and generalizability of these devices to detect AF in low-middle income countries remains unclear. Therefore, this study aimed to investigate the use of a BP monitor with AF detection algorithm to perform mass screening for AF in a real-world community setting in rural Thailand. The specific aims of the study were to a) determine the prevalence of AF in the population aged 65 years and over; and b) assess the feasibility of screening in the community in Thailand using a BP monitor with an AF detection algorithm.
2. Methods
2.1. Study design
This was a prospective multi-centre study investigating AF screening of residents aged 65 years, conducted in two provinces of Thailand: Lopburi province (March to May 2018) and Phetchaburi province (March to May 2019). The study was a collaboration between Chulalongkorn Comprehensive Stroke Center, the Medical Governance Certificate Program for Executives, King Narai Hospital, Ananda Mahidol Hospital, Phrachomklao Hospital, and the Primary healthcare unit, Ministry of Public Health, Lopburi and Phetchaburi Province. Ethical approval was granted by the Institutional Review Board of the Faculty of Medicine, Chulalongkorn University (COA no. 373/2018; IRB no. 124/61) and the study protocol conforms to the ethical guidelines of the 1975 Declaration of Helsinki.
2.2. Study population
The study recruited residents from Lopburi province in the central part of Thailand, and Phetchaburi province in the southern part of Thailand (Fig. 1). The study aimed to screen 10% of the population aged 65 years and over. This equated to 8,400 residents in Lopburi province (11 districts; population ≥ 65 years = 84,318), and 5,900 in Phetchaburi province (8 districts; population ≥ 65 years = 59,160) (Population statistics sourced from http://stat.dopa.go.th/stat/statnew/upstat_age_disp.php). In each of the districts, a random selection of villages was chosen to participate, ensuring a representation of rural and urban areas. Residents in these villages were eligible to be screened if they were aged ≥ 65 years and were able to provide informed consent. There were no other exclusion criteria.
Fig. 1.
Site map - Lopburi and Phetchaburi provinces, Thailand.
2.3. Screening intervention
2.3.1. Phase 1: Screening staff selection and training
The screening phase of the study was performed by local primary care nurses and village health volunteers in the provinces. Village health volunteers are part of the primary health care system in Thailand: they are selected by the villagers and trained by the Ministry of Public Health. Village health volunteers provide basic health services such as first aid, blood pressure monitoring, health consultations, liaison between villagers and the formal health sector, health promotion and sometimes transportation to the hospital. In the rural area, the ratio of village health volunteers is one per 8–15 households.
In collaboration with the study team, the Nurse Manager at the governmental hospital in each province identified suitable primary care nurses and Village health volunteers across the districts to participate in the screening intervention. These primary care nurses and Village health volunteers (185 from Lopburi and 202 from Phetchaburi) were invited to attend a 1-day training on AF screening and the study protocol. Training sessions were delivered by the research doctors and nursing staff and consisted of education on AF, associated stroke risk, the study protocol and data collection requirements (supplement 1); and practical sessions on screening for AF using both manual pulse palpation and an automatic oscillometric blood pressure monitor with AF detection algorithm (Microlife A200 AFib). Practical sessions were run in small groups (~10 people) to ensure each person was proficient in the assessment techniques.
2.3.2. Phase 2: Screening
Screening was performed over a two-month period in each province. The primary care nurses and village health volunteers offered screening to all eligible residents from their case load over the two-month study period. Written informed consent was obtained from those recruited to the study. Screening was performed in the participant’s home in combination with a routine health visit or as a specific screening visit. Socio-demographic data was collected including age, gender, past medical history, comorbidities, body mass index, alcohol intake, smoking status, physical activity level, and activities of daily living (i.e. Barthel Index) (supplement 1).
Following collection of demographic data, the radial pulse was palpated for 1-minute and results were recorded as ‘normal’, ‘minimally irregular’ or ‘irregular’. Blood pressure measurements and AF detection were then performed using the Microlife A200 AFib. The Microlife A200 AFib takes three sequential measurements and assesses pulse irregularity. The ‘Afib’ icon flashes on the screen if at least two of the three readings indicate AF, with reported 97–100% sensitivity and 89% specificity for AF detection [13], [14]. All participants with possible AF detected by the Microlife A200 AFib were given an appointment day to attend the designated study follow-up clinic for further testing. They were also given advice about AF and stroke risk and the importance of attending the follow-up review.
2.3.3. Phase 3: Follow-up assessment
The study follow-up clinic was located at Ananda Mahidol Hospital, Lopburi Province and Phrachomklao Hospital, Phetchaburi Province. They occurred on three specified dates in each province after completion of the screening phase. Thus, participants with AF attended the clinic 1–3 months after their initial screen. The clinic was run by a multi-disciplinary team consisting of neurologists, nurses, and medical assistance from Chulalongkorn comprehensive stroke center together with cardiologists, internists, and nurses from Ananda Mahidol Hospital and Phrachomklao Hospital with the support of Foundation for Good Governance on Medicine. The follow-up review included a 12-lead ECG, echocardiogram, blood tests (including cholesterol and blood sugar), physical examination including cardiac auscultation, assessment of current medication including OAC, assessment of stroke risk (CHA2DS2-VASc score [11]) and bleeding risk (HAS-BLED Score [11]), and determination of eligibility for OAC therapy. Participants with AF confirmed on the 12-lead ECG were referred to the hospital specialist physician (internist or cardiologist) for further review and treatment. These participants were provided with a comprehensive letter outlining their test results and summarizing their stroke and bleeding risk and OAC eligibility.
2.4. Outcomes
The primary outcome was the prevalence of AF (i.e. both new and known AF), with stratification according to age-group. Secondary outcomes were: a) The thrombo-embolic profile of those identified with AF: i.e. stroke risk calculated using CHA2DS2-VASc score, and bleeding risk calculated using HAS-BLED Score [11]; b) Comparison of AF incidence between Lopburi province and Phetchaburi province; and c) Accuracy of pulse palpation to detect AF in rural community in Thailand, compared to the Microlife A200 AFib.
2.5. Statistical analysis
Data were collected and analyzed by the Statistical Package for Social Sciences software (IBM© SPSS© Statistics version 25). Continuous variables are presented as mean ± standard deviation (SD), and categorical variables as numbers and percentages. Prevalence of AF is presented as cases per 100 person-years with accompanying binomial 95% CI calculated using Clopper–Pearson methodology. Comparisons between groups were assessed using analysis of variance (ANOVA) for continuous data and Chi-square tests for categorical data.
3. Results
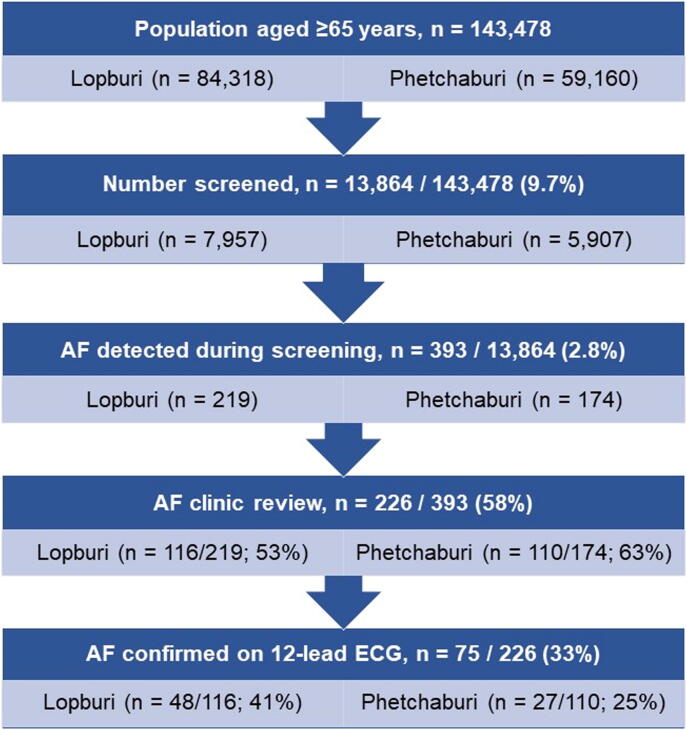
Over a two-month period in each province, 9.7% (13,864/143,478) of the population aged ≥ 65 years were recruited and screened: 7,957 in Lopburi province and 5,907 in Phetchaburi province (Fig. 2). The mean age of participants was 73.2 + 6.4 years and 32.4% were male (table 1). The main comorbidity was hypertension (65%) (table 1), with a measured systolic blood pressure > 130 mmHg in more than half the participants, and > 140 mmHg in a third of all participants (supplement 2). Additionally, dyslipidemia (49%) and diabetes mellitus (26%) were prevalent in the sample.
Fig. 2.
Study flow.
Table 1.
Characteristics of participants (n = 13,864).
| Total (n = 13,864) n (%) | AF * (n = 393) n (%) | No AF (n = 13,471) n (%) | P-value | |
|---|---|---|---|---|
| Age, years (mean ± SD) | 73.2 ± 6.4 | 75.44 ± 6.75 | 73.12 ± 6.42 | <0.001 |
| 65–69 | 4,989 (36) | 94 (24) | 4,895 (36) | |
| 70–74 | 3,628 (26) | 93 (24) | 3,535 (26) | |
| 75–79 | 2,726 (20) | 94 (24) | 2,632 (20) | |
| 80–84 | 1,665 (12) | 69 (18) | 1,596 (12) | |
| ≥ 85 | 856 (6) | 43 (11) | 813 (6) | |
| Male | 4,498 (32) | 157 (40) | 4,341 (32) | 0.001 |
| Hypertension | 8,950 (65) | 257 (65) | 8,693 (65) | 0.724 |
| Diabetes mellitus | 3,632 (26) | 91 (23) | 3,541 (26) | 0.164 |
| Dyslipidemia | 6,801 (49) | 183 (47) | 6,618 (49) | 0.316 |
| History of heart disease | 760 (6) | 77 (20) | 683 (5) | <0.001 |
| History of stroke | 474 (3) | 15 (4) | 459 (3) | 0.660 |
| Family history of stroke | 1,214 (9) | 34 (9) | 1,180 (9) | 0.940 |
| Smoker, current | 3,047 (22) | 108 (28) | 2,939 (22) | 0.940 |
| Alcohol intake | 3,220 (23) | 99 (25) | 3,121 (23) | 0.349 |
| Exercise, >3 days/week | 4,724 (35) | 115(29) | 4,609 (34) | 0.041 |
| QOL, Barthel index ≥ 60 | 12,887 (94) | 360 (92) | 12,527 (94) | 0.081 |
| BMI, kg/m2 (mean ± SD) | 23.6 ± 4.2 | 23.45 ± 4.3 | 23.7 ± 4.2 | 0.370 |
| SBP, mmHg (mean ± SD) | 133.5 ± 18.6 | 134.2 ± 20.6 | 133.5 ± 18.5 | 0.459 |
| DBP, mmHg (mean ± SD) | 73.1 ± 10.5 | 75 ± 12.1 | 73 ± 10.5 | <0.001 |
| Resting heart rate, bpm (mean ± SD) | 77.3 ± 12.5 | 78. ± 4.0 | 77.3 ± 12.5 | 0.094 |
AF = atrial fibrillation; * = detected during screening using the Microlife A200 AFib; SD = standard deviation; QOL = quality of life (Barthel index score interpretation: 0–20 “total” dependency, 21–60 “severe” dependency, 61–90 “moderate” dependency, and 91–99 “slight” dependency); BMI = body mass index; SBP = systolic blood pressure; DBP = diastolic blood pressure.
Participant characteristics differed slightly between the two provinces (supplement 3). Participants from Phetchaburi, compared to Lopburi, were slightly older and had statistically higher rates of hypertension (70% vs 61%), as well as diabetes (28% vs 25%), and dyslipidemia (53% vs 46%) (supplement 3). In Lopburi, compared to Phetchaburi, there were higher number of male participants (34% vs 30%); and significantly higher rates of heart disease (6.0% vs 4.8%), family history of stroke (9.6% vs 7.6%), and more participants regularly drank alcohol (26.5% vs 18.8%) (supplement 3).
3.1. Prevalence of AF
3.1.1. Microlife A200 AFib
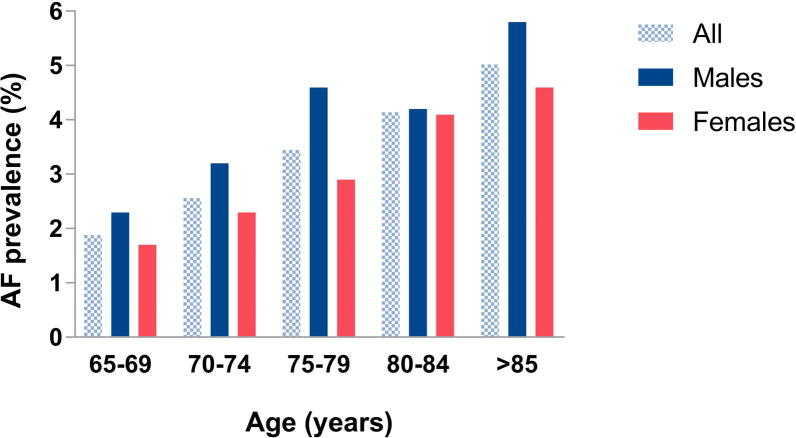
AF was detected in 393/13,864 participants using the Microlife A200 AFib, equating to a prevalence of 2.8% (95% CI, 2.6 – 3.1%) of the population screened, aged ≥ 65 years. Prevalence of AF significantly increased with age from 1.9% for age 65–69 years to 5.0% for age 85 years and older (p < 0.001) (Fig. 3). A similar prevalence was identified in Phetchaburi province (2.94%; 95% CI, 2.53 – 3.41%) and Lopburi province (2.75%; 95% CI, 2.40 – 3.14%) (supplement 3).
Fig. 3.
Atrial fibrillation prevalence by age and sex.
3.1.2. Pulse palpation
Manual radial pulse palpation, by the village health volunteers, detected an irregular pulse in 98 of the 393 cases detected by the Microlife A200 AFib. An irregular pulse was also noted in 15 cases where no AF was detected on the Microlife A200 AFib. The sensitivity of pulse palpation was 24.9% (95% CI, 20.7 – 29.5%), specificity 99.9% (95% CI, 99.8 – 99.9), positive predictive value 86.7% (95% CI, 79.3 – 91.8%), and negative predictive value 97.9% (95% CI, 97.7 – 98.0%).
3.1.3. AF follow-up clinic
Of the 393 participants with suspected AF, only 226 (58%) attended the follow-up clinic appointment, which occurred 1–3 months after the participants’ initial screen (Fig. 2). The 12-lead ECG results confirmed 75/226 (33%) were in AF at the time of the clinic visit, and these 75 participants were referred onwards to a hospital specialist physician for review and management.
Additionally, 12/226 (5.3%) were identified with valvular heart disease on the echocardiogram, of whom 4/12 were treated with OAC. People with AF on the 12-lead ECG, compared to those without AF on the 12-lead ECG, were statistically more likely to have valvular heart disease (13.3% vs. 1.3%; p < 0.001), past history of stroke (11% vs. 4%; p < 0.05), male gender (53% vs. 33%; p = 0.003), and a higher resting heart rate (85 bpm vs. 75 bpm; p < 0.001) (supplement 4).
3.2. Thromboembolic risk profile of people with AF
Stroke and bleeding risk were assessed on all 226 participants attending the follow-up clinic review. The mean CHA2DS2-VASc score of participants was 3.2 ± 1.2, and HAS-BLED score was 1.6 ± 0.8 (supplement 4 and supplement 5). In total, 195/226 (86.3%) had a Class-1 recommendation for OAC therapy (i.e. CHA2DS2-VASc score ≥ 2 males and ≥ 3 females); and 9/195 (4.6%) were already taking OAC (supplement 4).
For the participants who were in AF on the 12-lead ECG (n = 75), the mean CHA2DS2-VASc score was 3.3 ± 1.4, and the mean HAS-BLED score 1.7 ± 0.9; slightly higher, but not significantly different from those not in AF on the 12-lead ECG (supplement 4). Of these, 68/75 (91%) had a Class-1 recommendation for OAC therapy, and 8/68 (12%) were already taking OAC (supplement 4).
4. Discussion
This study demonstrates the successful use of a novel community-based AF screening strategy in Thailand. Almost 14,000 people were screened within only 2-months by village healthcare volunteers and local primary care nurses, equating to 10% of the registered population aged ≥ 65 years in those regions. The estimated prevalence of AF detected by the Microlife A200 AFib blood pressure device was 2.8%; and the majority (86%) of people with suspected AF had sufficiently high stroke risk score to recommend OAC therapy. Formal follow-up with 12-lead ECG occurred between 1 and 3 months later, at which time only 1/3 of the suspected AF cases were AF-positive on the 12-lead ECG, suggesting some cases may have been paroxysmal AF or false positives.
Our study identified a slightly higher AF prevalence (2.8%) than two earlier studies of community residents in Thailand which both screened ~ 1,000 residents each using 12-lead ECG. One of these studies identified an AF prevalence of 2.2%, however their participants were much younger than our sample, with a mean age of 69 years, and 56% of the sample aged only 60–69 years old [10]. The other study identified 1.9% prevalence, however their participants had substantially less comorbidities than our participants: including hypertension (48% vs 65%), diabetes (11% vs 26%); heart disease (2% vs 6%), and prior stroke (1% vs 3%) [9]. These sample differences may partially explain the higher prevalence noted in our study. In addition, hypertension is the most common risk factor for AF in Asian people, and diabetes is highly associated with AF [17]. If AF screening is focused on patients with hypertension in Thailand, a significantly higher prevalence of 3.46% is identified, despite the younger mean age of 63 years [18]. Therefore, it could also be argued that the high rate of hypertension (65%) in our sample may have also contributed to the higher AF prevalence identified in our study.
In Thailand, measurement of hypertension is a standard procedure performed by Village healthcare volunteers and primary care nurses during each home visit. Therefore, if health workers were supplied with an automatic oscillometric blood pressure monitor with an AF detection algorithm, a regular screen for AF could occur on all residents without increasing the health worker’s workload. Our study results indicate that using a blood pressure device for AF screening is more accurate than pulse palpation, when performed by a non-medical professional. Past research has shown that pulse palpation has a sensitivity of 92% (95% CI 85–96%) and specificity of 82% (95% CI 76 – 88%) to detect AF when performed by experienced qualified medical professionals [19]. However, the lower sensitivity of 24.9% (95% CI, 20.7 – 29.5%) noted in our study is almost identical to the sensitivity of 25% (95% CI 20 – 30%) noted in studies where non-medical professionals (i.e. patients) took their own pulse [20].
Although blood pressure devices have higher sensitivity and specificity for detecting AF, they do not provide sufficient information to confirm a diagnosis, and some false positives may be the result of pulse irregularity caused by ectopic beats. Therefore, it would be imperative to have an established pathway for all people with suspected AF to have a single- or 12-lead ECG within a few days, to obtain a timely confirmation of diagnosis, capture paroxysmal AF episodes, and enable early instigation of treatment. Because a timely 12-lead ECG recording may not be feasible for many in the community, recording of a single-lead ECG rhythm strip by the health worker at the time of BP recordings [21] could be a viable alternative, as this can provide sufficient diagnostic information due to the ability to review the presence of p-waves and identify ectopic beats causing pulse irregularity. The 2020 ESC Guidelines advise that a single- or 12-lead ECG is required if screening is undertaken using pulse palpation or devices reliant on photophlesmography, and that a 30-second single-lead ECG is sufficient to provide a definitive AF diagnosis [22].
Recently, the SMART-India study showed the feasibility and acceptability of village health workers in rural villages screening using a handheld single-lead ECG device [23]. In this study, residents ≥ 65 years from 50 rural villages received three screens over a five-day period, with 2,074 people screened over 12-months, finding a prevalence of 3.2% [23]. Screening multiple times took more time than a single screen, but identified higher rates of AF as only two-thirds of the AF cases were identified on the first screen [23]. It is therefore possible that future screening models in Thailand could consider using volunteer healthcare workers to screen all residents with the blood pressure machine with the AF algorithm, and then use a handheld ECG to collect a rhythm strip on all suspected AF cases. This strategy would also facilitate a timely specialist over-read of the ECG diagnosis. Further pilot studies would be required to determine if this is a feasible strategy to implement within Thailand. Furthermore, to be successful in reducing stroke risk, any future screening program would require a streamlined pathway to appropriate medical review, appropriate OAC treatment and ongoing management, which might again be facilitated by a timely ECG diagnosis.
Stroke prevention relies on patients receiving and taking ongoing guideline-based OAC treatment [11]. If Asian patients with AF and a class-1 OAC recommendation are undertreated, their ischaemic stroke risk is increased significantly (Hazard ratio 1.9) [24]. In general, OAC prescription rates are much lower in Asian countries compared to Western countries [17], with reported rates of 30% in Singapore [25], and only 4–28% in China in non-tertiary hospitals [26]. In a hospital setting, OAC treatment rates in Thailand are ~ 68% [24], however community rates are often significantly lower than this, so there is still much room for improvement. Often OAC is not prescribed in Asian patients due to fear of bleeding, however it has been recently shown that less intracranial bleeding is noted when using non-vitamin K antagonist OACs (NOAC) compared to warfarin in Asian patients [27]. The cost and availability of NOACs in low- and middle-income countries is a prohibitive factor to prescription compliance worldwide [28]. In Thailand, NOAC use remains limited (<10%) due to the high medication cost, with the majority of patients prescribed warfarin [29]. Compliance with warfarin follow up and INR testing is sub-optimal, leading to poor INR control and thus ineffective stroke prevention [29], [30]. Therefore, this is also a need for research into better management of stroke prevention strategies for those identified with AF in Thailand.
4.1. Strengths and limitations
The main strength of this study was the successful screening strategy which was able to screen 10% of the targeted population in only 2 months. Our study screened almost 14,000 people, which is the largest number screened in Thailand to date. This is a promising screening strategy which could enable widespread screening to be performed throughout Thailand in a limited resource setting.
However, the prevalence of AF identified from the Microlife A200 AFib in our study is an estimate. We are not able to calculate the sensitivity and specificity of the device as an ECG rhythm strip was not taken at the same time as the index test. Previous studies of the Microlife A200 AFib accuracy were conducted in Western countries, so we cannot assume these results are necessarily valid in a Thai population. It is likely that the initial screen may have identified some false positives. It is also likely that some cases of paroxysmal AF will not have been detected during the single screen.
Additionally, there was up to three-months delay between the incident screening test and the follow-up 12-lead ECG which confirmed AF in 75/226 (33%) participants. This suggests some people with paroxysmal AF may not have been in AF at the time of the 12-lead ECG recorded some months later. It is not possible to determine the proportion with paroxysmal AF in this study, and the rate in Thailand is not described in the literature. However, European studies report that up to 40% of known AF cases are classified as paroxysmal AF [31], and there are many additional cases of unknown asymptomatic AF that are only identified with repeated screening [32].
Although no participants withdrew from the study, only 58% attended the follow-up appointment. The follow-up clinic was centrally located, and thus some participants were required to travel 3–4 h to attend the clinic and travel issues precluded attendance in many cases. Furthermore, as the clinic follow-up was a half-day appointment (in addition to travel), this was a barrier to attendance for a number of participants. Therefore, if a definitive ECG test for AF could be performed locally in the village shortly after the screening test, the number receiving the definitive test would be higher and the accuracy of detecting AF would improve. These factors should be considered in the logistical planning of any future studies.
5. Conclusions
An estimated AF prevalence of 2.8% was identified through trained volunteer health workers and primary care nurses screening with a blood pressure monitor with an AF algorithm during home visits. This study demonstrates the feasibility of large-scale screening for AF in Thailand. This strategy can screen very large numbers of people in a very short time-period. Further investigation of this strategy is warranted, however future strategies need to ensure a mechanism to obtain a timely 12-lead ECG, or 30 s rhythm strip, and a clear pathway to treatment for those identified with AF.
Funding
This study received a grant from the Foundation for Good Governance on Medicine and the Medical Governance Certificate Program for Executives, Thailand.
Declaration of Competing Interest
BF reports prior fees and advisory board honoraria from Bayer Pharma AG, Boehringer Ingelheim, Daiichi-Sankyo, Omron and Pfizer/BMS, and grants to the institution for investigator-initiated studies from BMS and Pfizer.
Acknowledgements
We would like to acknowledge the valuable assistance of the Foundation for Good Governance on Medicine, Thailand as well as the primary care nurses and Village health volunteers from Lopburi and Phetchaburi Province, especially Mrs Saijai Loetwiriyaprapha, Mrs Pitchaya Suwanchart, Mrs Siraya Thasathan, and Mrs Saitip Jaipong.
Footnotes
All authors take responsibility for all aspects of the reliability and freedom from bias of the data presented and their discussed interpretation.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ijcha.2020.100709.
Appendix A. Supplementary material
The following are the Supplementary data to this article:
References
- 1.Friberg L., Rosenqvist M., Lindgren A., Terent A., Norrving B., Asplund K. High prevalence of atrial fibrillation among patients with ischemic stroke. Stroke. 2014;45:2599–2605. doi: 10.1161/STROKEAHA.114.006070. [DOI] [PubMed] [Google Scholar]
- 2.Sutamnartpong P., Dharmasaroja P.A., Ratanakorn D., Arunakul I. Atrial fibrillation and paroxysmal atrial fibrillation detection in patients with acute ischemic stroke. J. Stroke Cerebrovasc. Dis. 2014;23:1138–1141. doi: 10.1016/j.jstrokecerebrovasdis.2013.09.032. [DOI] [PubMed] [Google Scholar]
- 3.Lin H.J., Wolf P.A., Kelly-Hayes M., Beiser A.S., Kase C.S., Benjamin E.J., D'Agostino R.B. Stroke severity in atrial fibrillation. The Framingham Study. Stroke. 1996;27:1760–1764. doi: 10.1161/01.str.27.10.1760. [DOI] [PubMed] [Google Scholar]
- 4.Healey J.S., Connolly S.J., Gold M.R., Israel C.W., Van Gelder I.C., Capucci A., Lau C.P., Fain E., Yang S., Bailleul C., Morillo C.A., Carlson M., Themeles E., Kaufman E.S., Hohnloser S.H., Investigators A. Subclinical atrial fibrillation and the risk of stroke. N. Engl. J. Med. 2012;366:120–129. doi: 10.1056/NEJMoa1105575. [DOI] [PubMed] [Google Scholar]
- 5.Hart R.G., Pearce L.A., Aguilar M.I. Meta-analysis: Antithrombotic therapy to prevent stroke in patients who have nonvalvular atrial fibrillation. Ann. Intern. Med. 2007;146:857–867. doi: 10.7326/0003-4819-146-12-200706190-00007. [DOI] [PubMed] [Google Scholar]
- 6.Chugh S.S., Havmoeller R., Narayanan K., Singh D., Rienstra M., Benjamin E.J., Gillum R.F., Kim Y.H., McAnulty J.H., Jr., Zheng Z.J., Forouzanfar M.H., Naghavi M., Mensah G.A., Ezzati M., Murray C.J. Worldwide epidemiology of atrial fibrillation: a Global Burden of Disease 2010 Study. Circulation. 2014;129:837–847. doi: 10.1161/CIRCULATIONAHA.113.005119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Guo Y., Tian Y., Wang H., Si Q., Wang Y., Lip G.Y.H. Prevalence, incidence, and lifetime risk of atrial fibrillation in China: new insights into the global burden of atrial fibrillation. Chest. 2015;147:109–119. doi: 10.1378/chest.14-0321. [DOI] [PubMed] [Google Scholar]
- 8.Kiatchoosakun S., Pachirat O., Chirawatkul A., Choprapawan C., Tatsanavivat P. Prevalence of cardiac arrhythmias in Thai community. J. Med. Assoc. Thai. 1999;82:727–733. [PubMed] [Google Scholar]
- 9.Phrommintikul A., Detnuntarat P., Prasertwitayakij N., Wongcharoen W. Prevalence of atrial fibrillation in Thai elderly. J. Geriatr. Cardiol. 2016;13:270–273. doi: 10.11909/j.issn.1671-5411.2016.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Assantachai P., Panchavinnin P., Pisalsarakij D. An electrocardiographic survey of elderly Thai people in the rural community. J. Med. Assoc. Thai. 2002;85:1273–1279. [PubMed] [Google Scholar]
- 11.Kirchhof P., Benussi S., Kotecha D., Ahlsson A., Atar D., Casadei B., Castella M., Diener H.C., Heidbuchel H., Hendriks J., Hindricks G., Manolis A.S., Oldgren J., Popescu B.A., Schotten U., Van Putte B., Vardas P., Group ESCSD ESC Guidelines for the management of atrial fibrillation developed in collaboration with EACTS. Eur. Heart J. 2016;2016(37):2893–2962. doi: 10.1093/eurheartj/ehw210. [DOI] [PubMed] [Google Scholar]
- 12.Duarte R., Stainthorpe A., Greenhalgh J., Richardson M., Nevitt S., Mahon J., Kotas E., Boland A., Thom H., Marshall T., Hall M., Takwoingi Y. Lead-I ECG for detecting atrial fibrillation in patients with an irregular pulse using single time point testing: a systematic review and economic evaluation. Health Technol. Assess. 2020;24:1–164. doi: 10.3310/hta24030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stergiou G.S., Karpettas N., Protogerou A., Nasothimiou E.G., Kyriakidis M. Diagnostic accuracy of a home blood pressure monitor to detect atrial fibrillation. J. Hum. Hypertens. 2009;23:654–658. doi: 10.1038/jhh.2009.5. [DOI] [PubMed] [Google Scholar]
- 14.Wiesel J., Fitzig L., Herschman Y., Messineo F.C. Detection of atrial fibrillation using a modified microlife blood pressure monitor. Am. J. Hypertens. 2009;22:848–852. doi: 10.1038/ajh.2009.98. [DOI] [PubMed] [Google Scholar]
- 15.Wiesel J., Abraham S., Messineo F.C. Screening for asymptomatic atrial fibrillation while monitoring the blood pressure at home: trial of regular versus irregular pulse for prevention of stroke (TRIPPS 2.0) Am. J. Cardiol. 2013;111:1598–1601. doi: 10.1016/j.amjcard.2013.01.331. [DOI] [PubMed] [Google Scholar]
- 16.National Institute for Health and Clinical Excellence. Medical technologies guidance [MTG13]. WatchBP Home A for opportunistically detecting atrial fibrillation during diagnosis and monitoring of hypertension. Published date: 16 January 2013. Accessed 13 August 2020 from: https://www.nice.org.uk/guidance/mtg13/chapter/1-Recommendations.
- 17.Lip G.Y.H., Brechin C.M., Lane D.A. The global burden of atrial fibrillation and stroke: a systematic review of the epidemiology of atrial fibrillation in regions outside North America and Europe. Chest. 2012;142:1489–1498. doi: 10.1378/chest.11-2888. [DOI] [PubMed] [Google Scholar]
- 18.Krittayaphong R., Rangsin R., Thinkhamrop B., Hurst C., Rattanamongkolgul S., Sripaiboonkij N., Yindeengam A. Prevalence and associating factors of atrial fibrillation in patients with hypertension: a nation-wide study. BMC Cardiovasc. Disord. 2016;16:57. doi: 10.1186/s12872-016-0232-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Taggar J.S., Coleman T., Lewis S., Heneghan C., Jones M. Accuracy of methods for diagnosing atrial fibrillation using 12-lead ECG: A systematic review and meta-analysis. Int. J. Cardiol. 2015;184:175–183. doi: 10.1016/j.ijcard.2015.02.014. [DOI] [PubMed] [Google Scholar]
- 20.Ghazal F., Theobald H., Rosenqvist M., Al-Khalili F. Validity of daily self-pulse palpation for atrial fibrillation screening in patients 65 years and older: A cross-sectional study. PLoS Med. 2020;17 doi: 10.1371/journal.pmed.1003063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lowres N., Neubeck L., Salkeld G., Krass I., McLachlan A.J., Redfern J., Bennett A.A., Briffa T., Bauman A., Martinez C., Wallenhorst C., Lau J.K., Brieger D.B., Sy R.W., Freedman S.B. Feasibility and cost-effectiveness of stroke prevention through community screening for atrial fibrillation using iPhone ECG in pharmacies. The SEARCH-AF study. Thromb. Haemost. 2014;111:1167–1176. doi: 10.1160/TH14-03-0231. [DOI] [PubMed] [Google Scholar]
- 22.Hindricks G., Potpara T., Dagres N., Arbelo E., Bax J.J., Blomstrom-Lundqvist C., Boriani G., Castella M., Dan G.A., Dilaveris P.E., Fauchier L., Filippatos G., Kalman J.M., La Meir M., Lane D.A., Lebeau J.P., Lettino M., Lip G.Y.H., Pinto F.J., Thomas G.N., Valgimigli M., Van Gelder I.C., Van Putte B.P., Watkins C.L. Group ESCSD ESC Guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association of Cardio-Thoracic Surgery (EACTS) Eur. Heart J. 2020;2020 doi: 10.1093/eurheartj/ehaa612. [DOI] [PubMed] [Google Scholar]
- 23.Soni A., Karna S., Fahey N., Sanghai S., Patel H., Raithatha S., Thanvi S., Nimbalkar S., Freedman B., Allison J., McManus D.D. Age-and-sex stratified prevalence of atrial fibrillation in rural Western India: Results of SMART-India, a population-based screening study. Int. J. Cardiol. 2019;280:84–88. doi: 10.1016/j.ijcard.2018.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Krittayaphong R., Winijkul A., Kunjara-Na-Ayudhya R., Apiyasawat S., Siriwattana K., Kanjanarutjawiwat W., Dutsadeevettakul S., Lip G.Y.H., Investigators C.-A. Adherence to Anticoagulant Guideline for Atrial Fibrillation Improves Outcomes in Asian Population: The COOL-AF Registry. Stroke. 2020;51:1772–1780. doi: 10.1161/STROKEAHA.120.029295. [DOI] [PubMed] [Google Scholar]
- 25.Yap K.B., Ng T.P., Ong H.Y. Low prevalence of atrial fibrillation in community-dwelling Chinese aged 55 years or older in Singapore: a population-based study. J. Electrocardiol. 2008;41:94–98. doi: 10.1016/j.jelectrocard.2007.03.012. [DOI] [PubMed] [Google Scholar]
- 26.Chang S.S., Dong J.Z., Ma C.S., Du X., Wu J.H., Tang R.B., Xia S.J., Guo X.Y., Yu R.H., Long D.Y., Bai R., Liu N., Sang C.H., Jiang C.X., Liu X.H., Pan J.H., Lip G.Y. Current Status and Time Trends of Oral Anticoagulation Use Among Chinese Patients With Nonvalvular Atrial Fibrillation: The Chinese Atrial Fibrillation Registry Study. Stroke. 2016;47:1803–1810. doi: 10.1161/STROKEAHA.116.012988. [DOI] [PubMed] [Google Scholar]
- 27.Chan Y.H., Lee H.F., See L.C., Tu H.T., Chao T.F., Yeh Y.H., Wu L.S., Kuo C.T., Chang S.H., Lip G.Y.H. Effectiveness and Safety of Four Direct Oral Anticoagulants in Asian Patients With Nonvalvular Atrial Fibrillation. Chest. 2019;156:529–543. doi: 10.1016/j.chest.2019.04.108. [DOI] [PubMed] [Google Scholar]
- 28.Murphy A., Banerjee A., Breithardt G., Camm A.J., Commerford P., Freedman B., Gonzalez-Hermosillo J.A., Halperin J.L., Lau C.P., Perel P., Xavier D., Wood D., Jouven X., Morillo C.A. The World Heart Federation Roadmap for Nonvalvular Atrial Fibrillation. Glob Heart. 2017;12:273–284. doi: 10.1016/j.gheart.2017.01.015. [DOI] [PubMed] [Google Scholar]
- 29.Krittayaphong R., Winijkul A., Methavigul K., Wongtheptien W., Wongvipaporn C., Wisaratapong T., Kunjara-Na-Ayudhya R., Boonyaratvej S., Komoltri C., Kaewcomdee P., Yindeengam A., Sritara P., Investigators C.-A. Risk profiles and pattern of antithrombotic use in patients with non-valvular atrial fibrillation in Thailand: a multicenter study. BMC Cardiovasc Disord. 2018;18:174. doi: 10.1186/s12872-018-0911-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Krittayaphong R., Chantrarat T., Rojjarekampai R., Jittham P., Sairat P., Lip G.Y. Poor Time in Therapeutic Range Control is Associated with Adverse Clinical Outcomes in Patients with Non-Valvular Atrial Fibrillation: A Report from the Nationwide COOL-AF Registry. J Clin Med. 2020;9:1698. doi: 10.3390/jcm9061698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zoni-Berisso M., Lercari F., Carazza T., Domenicucci S. Epidemiology of atrial fibrillation: European perspective. Clin. Epidemiol. 2014;6:213–220. doi: 10.2147/CLEP.S47385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Svennberg E., Engdahl J., Al-Khalili F., Friberg L., Frykman V., Rosenqvist M. Mass Screening for Untreated Atrial Fibrillation: The STROKESTOP Study. Circulation. 2015;131:2176–2184. doi: 10.1161/CIRCULATIONAHA.114.014343. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.