Infective endocarditis (IE) complicated with ST-segment elevation myocardial infarction (STEMI) is a rare clinical condition usually due to coronary embolism, only rarely related to obstruction or external compression by abscess formation [1]. Despite modern diagnostic and therapeutic options, 1-year mortality has not improved and is still around 30% [2]. Acute coronary syndrome (ACS) has been reported in only 3% of all the patients with IE and septic embolization, particularly from a prosthetic valve is a very uncommon event, accounting only for < 1% of IE complications [3]. Herein, we report a case of fatal concomitant septic coronary embolism and extrinsic coronary compression by abscess, in a man with aortic prosthetic valve endocarditis (PVE).
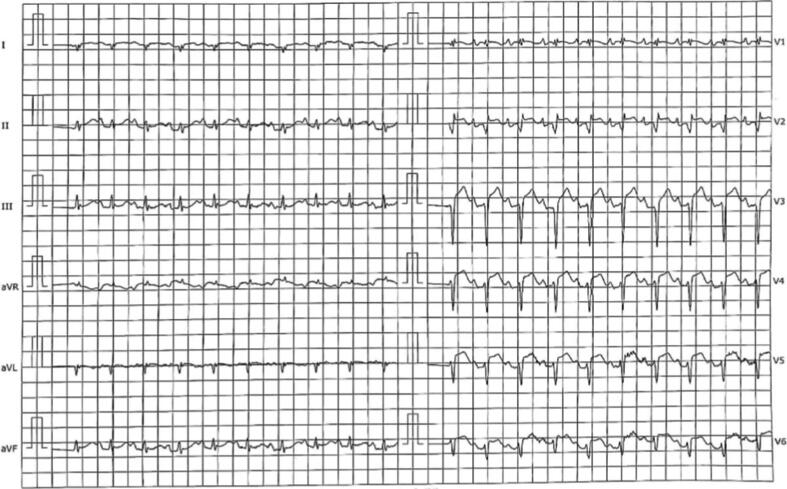
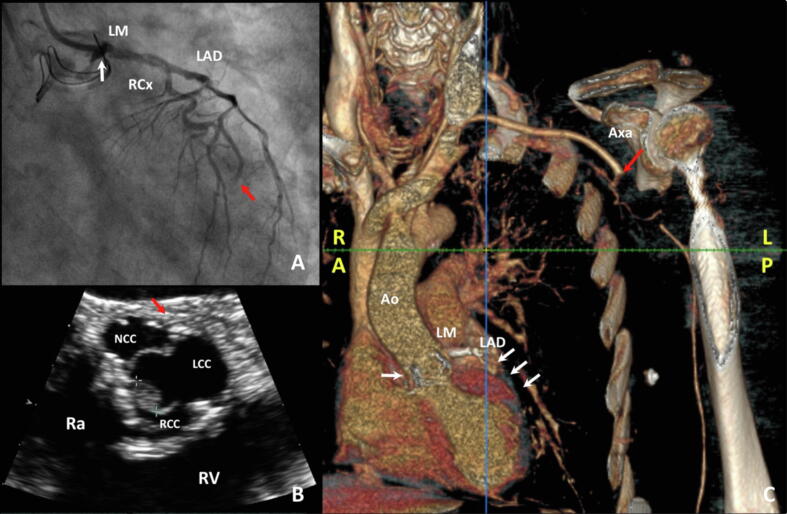
A 77-year-old man has been admitted to our hospital for dyspnoea and fever three months after a surgical aortic valve replacement (SAVR) with bio-prosthesis for symptomatic severe aortic stenosis (AS). Physical examination and Chest X-Ray were unremarkable but laboratory tests showed increased inflammatory markers (CRP 20 mg/l) and Staphylococcus aureus isolation in blood cultures; thus, an empirical broad-spectrum antibiotic therapy was initiated. Trans-thoracic echocardiography (TTE) showed no vegetation and trans-oesophageal echocardiography (TOE) was planned in a few days. Shortly thereafter, the patient presented sudden onset of left superior arm weakness: an urgent Head CT scan was performed, showing only minimal chronic ischaemic vascular signs. Rapidly, the patient developed progressive hypoperfusions signs of left arm (cold, pale and disappearance of arterial pulses) and typical chest pain. The 12-lead ECG showed elevation up to 3 mm in V3-V5 (Fig. 1), and TTE showed newly detected apical and septal akinesia with globally reduced systolic function (left ventricular ejection fraction (LVEF) = 35%). The patient underwent, indeed, to emergency coronary angiography which showed contrast medium stasis in the left cusp just below left main ostium and thrombotic occlusion of a diffusely diseased left anterior descending artery (Fig. 2 Panel A). Despite multiple aspirations, angioplasty with drug eluting stent implantation and eptifibatide infusion, the control angiography displayed only a partial reperfusion. TOE with 3D reconstruction revealed a 15 mm perivalvular abscess with an 8 mm vegetation on the right aortic cusp, suggesting an IE (Fig. 2 Panel B). A CT scan confirmed, finally, IE of the prosthetic aortic valve with left main involvement and thrombotic occlusion of the left axillary artery, as result of septic embolization (Fig. 2 Panel C). The rapidly worsening clinical conditions did not allow a surgical evaluation and the patient died on the same day from septic shock. This is a rare case of fatal concomitant septic coronary embolism and extrinsic coronary compression by abscess.
Fig. 1.
12-lead ECG with ST-segment elevation in V3-V5.
Fig. 2.
Multimodality imaging; (Panel A): Coronary angiography: thrombotic occlusion of distal LAD (red arrow) and contrast medium stasis in the left cusp (white arrow); (Panel B): TOE: short-axis view of aortic valve with evidence of vegetation (green crosses) in the right cusp and peri-valvular abscess (red arrow); (Panel C): Angio-CT scan: prosthetic valve deterioration by IE (white arrow), re-occlusion of LAD (white arrows) and embolization of left axillary artery (red arrow); Ao: aorta; Axa: axillary artery; LAD: left anterior descending; LCC: left coronary cusp; LM: left main; LP: left posterior; NCC: non-coronary cusp; RA: right anterior; Ra: right atrium; RCC: right coronary cusp; RCx: ramus circumflexus artery; RV: right ventricle. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
Infective Endocarditis (IE) is the fourth most common life-threatening infection; thus, a multidisciplinary management, as suggested by current international Guidelines, is mandatory to outcome improvement [4]. Risk factor for IE is a pre-existing valvular heart disease, such as mitral or aortic regurgitations, as result of ageing population; a notably proportion of IE comes from the presence of a prosthetic valve, which accounts for 10–30% of total IE cases and involves both biological and mechanical, particularly for these latter within the first 3 months after surgery [5]. Diagnosis is based on integration of clinical, laboratory, imaging and microbiological elements and current Guidelines recommend modified Duke Criteria, that define IE diagnosis as Definite, Possible, or Rejected [6].
Clinical presentation of IE, particularly in PVE, is frequently aspecific with fever, loss of appetite, a new heart murmur, left bundle branch block, heart failure, or embolic events as most frequent symptoms and signs. Echocardiography may be negative in PVE and does not rule out the diagnosis, but can identify a new leak involving the prosthetic valve (major criterion), and eventually motivate additional imaging modalities (such as TOE and CT scan) [7].
Myocardial infarction from septic embolization is a rare but serious complication of IE, conferring an increase mortality risk, if present [8]. Clinical presentation in this population may influence physician to apply traditional management strategies for atherosclerotic disease with poor results [9].
There are no current practice guidelines for management of STEMI in IE setting, but thrombolytics (Tissue Plasminogen Activator) appear to be harmful, leading to significant bleeding and embolic risks. Surgical intervention, with debridement of necrotic and infected tissue, drainage of abscess and reconstruction of cardiac morphology with repair or replacement of diseased valve is often the only chance for these patients, burdened, however, by a high risk of mortality [10].
Our case report describes a particularly rare event: the combination of septic coronary embolism and extrinsic coronary compression by abscess, in a patient with prosthetic valve endocarditis.
Declaration of Competing Interest
The authors report no relationships that could be construed as a conflict of interest.
References
- 1.Singh M., Mishra A., Kaluski E. Acute ST-elevation myocardial infarction due to septic embolism: a case report and review of management options. Catheter. Cardiovasc. Interv. 2015;85:E166–71. doi: 10.1002/ccd.25829. [DOI] [PubMed] [Google Scholar]
- 2.Cahill T.J., Prendergast B.D. Infective endocarditis. Lancet. 2016;387:882–893. doi: 10.1016/S0140-6736(15)00067-7. [DOI] [PubMed] [Google Scholar]
- 3.Habib G., Lancellotti P., Antunes M.J. 2015 ESC Guidelines for the management of infective endocarditis. Eur. Heart J. 2015 doi: 10.1136/heartjnl-2015-308791. [DOI] [PubMed] [Google Scholar]
- 4.Baddour L.M., Wilson W.R., Bayer A.S. Infective endocarditis in adults: Diagnosis, antimicrobial therapy, and management of complications: A scientific statement for healthcare professionals from the American Heart Association. Circulation. 2015 doi: 10.1161/CIR.0000000000000296. [DOI] [PubMed] [Google Scholar]
- 5.Østergaard L., Valeur N., Ihlemann N. Incidence and factors associated with infective endocarditis in patients undergoing left-sided heart valve replacement. Eur. Heart J. 2018;39:2668–2675. doi: 10.1093/eurheartj/ehy153. [DOI] [PubMed] [Google Scholar]
- 6.Li J.S., Sexton D.J., Mick N. Proposed modifications to the Duke criteria for the diagnosis of infective endocarditis. Clin. Infect. Dis. 2000;30:633–638. doi: 10.1086/313753. [DOI] [PubMed] [Google Scholar]
- 7.Habib G. Management of infective endocarditis. Heart. 2006;92:124–130. doi: 10.1136/hrt.2005.063719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Roux V., Salaun E., Tribouilloy C. Coronary events complicating infective endocarditis. Heart. 2017;103:1906–1910. doi: 10.1136/heartjnl-2017-311624. [DOI] [PubMed] [Google Scholar]
- 9.Overend L., Rose E. Uncertainties in managing myocardial infarction associated with infective endocarditis. Exp. Clin. Cardiol. 2012;17(3):144–145. [PMC free article] [PubMed] [Google Scholar]
- 10.Alexis S.L., Malik A.H., George I. Infective endocarditis after surgical and transcatheter aortic valve replacement: A state of the art review. J. Am. Heart Assoc. 2020;9(16):e017347. doi: 10.1161/JAHA.120.017347. [DOI] [PMC free article] [PubMed] [Google Scholar]